Abstract
Rationale
Research indicates that genetics influence methamphetamine self-administration as well as sensitization to the psychomotor stimulating effects of methamphetamine (MA). Other studies have suggested that heightened levels of impulsivity, including low levels of behavioral inhibition, are associated with the use of drugs, including MA.
Objectives
The current study examined whether lines of mice selected for traits associated with a heightened risk of developing MA dependence would also exhibit low levels of drug-naïve inhibition, and whether administration of MA would result in different levels of inhibition in animals selected to consume or respond more to MA.
Methods
A Go/No-go task was used to assess inhibition in male and female mice selected for low or high levels of MA consumption or selected for high or low levels of locomotor sensitization to repeated injections of MA.
Results
Mice selected for MA sensitization differed in false alarms, precue response rates, (measures of behavioral inhibition) but also hits (measure of operant responding). Mice selected for MA consumption did not differ in measures of behavioral inhibition, though hits differed. When MA was administered prior to the task, false alarms, precue response rates, and hits decreased for mice from all selected lines. Female high drinking mice were particularly resistant to MA's effects on hits, but not precue response rate or false alarms.
Conclusions
These data suggest a shared, but complex, genetic association between inhibition processes, general levels of operant responding and MA sensitization or consumption.
Keywords: inhibition, Go/No-go, behavioral sensitization, self-administration, selected lines, methamphetamine, impulsivity
Introduction
Methamphetamine (MA) is a commonly used drug of abuse. However, only a small portion of the population that tries MA ever develops an addiction to this drug, and it is important to identify factors that differentiate individuals prone to addiction from those that can sustain casual use. Although environmental factors likely play important roles in risk for MA addiction, there is also evidence that genes contribute to risk (Agrawal et al. 2004; Kendler et al. 2003; Uhl et al. 1995; Vetulani 2001). Genetic research using animal models of traits thought to be important for risk may offer a means for identifying specific genetic elements, as well as behavioral characteristics, that influence susceptibility to addiction and relapse (for review see Phillips et al., 2008).
Genes affecting one particular trait of interest can also affect other traits, i.e., have pleiotropic effects (Crabbe et al. 1990; Phillips & Belknap 2002; Phillips et al. 2002), and this has been shown to be the case with MA-related traits. For example, Wheeler et al. (2009) selectively bred two lines of mice to orally self-administer either higher (MA high drinkers, MAHDR) or lower amounts of MA (MA low drinkers, MALDR; both lines are known collectively as MADR mice). Compared to MALDR mice, the MAHDR mice were more sensitive to the rewarding effects of MA and less sensitive to the aversive effects of MA. These lines have also been used to examine the state-dependent conditioned rewarding effects of multiple MA doses (Shabani et al., 2011).
In another study, Scibelli et al. (2011) selectively bred two lines of mice to exhibit either high sensitization (MA high sensitization, MAHSENS) or relatively low sensitization (MA low sensitization, MALSENS; both lines are known collectively as MASENS mice) to the locomotor-activating effects of repeated MA injection. The MALSENS mice were less sensitive to the locomotor activating effects of acute MA and consumed higher amounts of MA. Together these studies suggest that there is overlap of sets of genes that influence several MA-related behaviors, and, most notably, that these behavioral traits are all associated with level of MA self-administration.
The finding that some of the same genes affect several different MA-related behaviors led us to extend the search for pleiotropic gene effects to another trait that has been associated with MA abuse in human populations: impulsivity. Impulsivity is thought to have multiple distinct forms (Evenden 1999), and one of the most commonly examined is behavioral inhibition. A large body of evidence has shown that impulsivity is both a risk factor for and a consequence of drug abuse (see Moeller & Dougherty 2002 and Carroll et al. 2010, for reviews), and MA abusers have been found to have decreased behavioral inhibition in a stop-signal task (Monterosso et al. 2005). Unfortunately, human studies have difficulty dissociating the separate roles of genes and environment. Animal research accomplishes this more easily, but little research has examined the relationship between MA and impulsivity in animal models. However, research with related psychostimulants such as d-amphetamine and methylphenidate has reported some effects. Both d-amphetamine and methylphenidate administered acutely have generally decreased behavioral inhibition in the 5 choice serial reaction time task (5CSRTT) in rats (Cole & Robbins 1987; Cole & Robbins 1989; Harrison et al. 1997; van Gaalen et al. 2006; but see Bizarro & Stolerman 2003; Bizarro et al. 2004). Conversely, these two drugs generally increased behavioral inhibition in the stop-signal task (de Wit et al. 2000; de Wit et al. 2002; Eagle & Robbins 2003; Eagle et al. 2007; Feola et al. 2000) and Go/No-Go tasks (de Wit et al. 2002; Vaidya et al. 1998; but see Fillmore et al. 2003; Loos et al. 2010). Such disparate findings likely reflect differences in the underlying processes that the tasks themselves measure, as shown by studies indicating different neuroanatomical and neuropharmacological correlates of performance on different tasks (for reviews see Eagle & Baunez 2010; Eagle et al. 2008; Perry & Carroll 2008; Winstanley et al. 2010).
No studies have examined the genetic relationship between MA self-administration, MA sensitization and impulsivity. Nonetheless, research with other drugs of abuse suggests that such a relationship may exist (cocaine: Anker et al., 2008; Dalley et al., 2007; ethanol: Wilhelm et al., 2007). To assess this, we performed two experiments. Using a Go/No-go task, we examined basal levels of behavioral inhibition for both sets of MADR and MASENS lines to establish if there were genetic associations. Because higher impulsivity is associated with a higher likelihood of developing an addiction, we expected that the MAHDR mice, which self-administered more MA than the MALDR mice, would also exhibit lower levels of behavioral inhibition than the MALDR mice. For the same reason, we expected that the MALSENS mice would have lower behavioral inhibition than the MAHSENS mice. We also examined the effects of MA on behavioral inhibition in these sets of lines. We hypothesized that MA would increase behavioral inhibition, as related psychostimulants have increased inhibition in Go/No-go tasks (de Wit et al. 2002; Vaidya et al. 1998). de Wit et al (2002) showed this effect to be limited to individuals with low basal behavioral inhibition. Therefore, we expected that MA would increase inhibition more in MAHDR mice than in MALDR mice and would also increase inhibition more in MALSENS mice than in MAHSENS mice.
Methods
Subjects
Subjects were male and female mice from lines that were selectively bred for high or low levels of voluntary consumption of water containing MA when it was offered versus plain tap water (MAHDR and MALDR respectively; selection as described by Wheeler et al. 2009). Male and female mice selectively bred for high or low levels of locomotor sensitization induced by repeated treatment with 1 mg/kg of MA were also used (MAHSENS and MALSENS respectively; selection as described by Scibelli et al. 2011). The mice used in the current studies were from S5G6 (where S5 refers to the number of selected generations and G6 refers to the total number of generations that have elapsed since selection began) and were obtained from the Methamphetamine Abuse Research Center Animal Core within the VA Medical Center, Portland OR.
Mice were housed 2–5 per cage under a 12:12-h light: dark cycle (lights on at 6 am) in a temperature-controlled vivarium (21.7 ± 1°C), and maintained according to guidelines provided by the Oregon Health & Science University's Department of Comparative Medicine. The Institutional Animal Care and Use Committee approved all procedures.
Mice were weighed for 5 days to obtain their free-feeding weights. MADR and MASENS mice were tested in separate experiments. At the beginning of the study, MADR mice were 48.98 ± 1.18 days old and weighed 20.90 ± 0.61 g, and MASENS mice were 92.48 ± 2.64 days old and weighed 29.22 ± 0.69 g. However, within each selected line, MALSENS and MAHSENS mice did not differ in weight or age, and MALDR and MAHDR mice did not differ in weight or age. The first day of behavioral training occurred after a minimum of 48 hours on a food-restricted diet, the mice were then maintained at approximately 90% of their free-feeding weights with laboratory mouse chow. The food-restricted diet was imposed to ensure that animals responded in the Go/No-go task, which used sucrose solution as a reinforcer.
A total of 45 MADR mice and 64 MASENS mice participated. Of these, 41 MADR and 50 MASENS mice completed the baseline Go/No-go task and all subsequently received four doses of MA, including placebo, according to a Latin square design (see Procedures), while completing the same Go/No-go task.
Apparatus
The test chambers were identical to those described in Gubner et al. (2010). Briefly, behavior was assessed in 16 Med-Associates (St. Albans, VT) operant conditioning chambers, inside boxes designed to attenuate external noise and containing fans for ventilation. A 2.8 W house light was mounted in the panel to the left of the door of each chamber (the “back panel”). The panel to the right of each door (the “front panel”) contained three nose poke holes, each of which contained a nose poke detector and a reward cup for liquid rewards. This panel also contained two yellow LED lights, which served as the cue lights; each light was centered 1.91 cm above the left- or right-most nose poke hole. Eighteen-gauge stainless steel pipes connected by plastic tubing attached to a syringe secured in a Med-Associates pump allowed 10% (w/v) sucrose solution to be delivered into the reward cup.
Procedure
Go/No-go Task
The Go/No-go task was identical to that described in Gubner et al. (2010), which was modeled after McDonald et al. (1998). Sessions ended after 60 trials were completed or 40 minutes had passed. Each trial began with a 9–24 second variable-duration precue period, which was signaled by the house light being lit. Responses made during the last 3 seconds of the pre-cue period reset the trial to prevent on-going responding at the end of the precue period being mistakenly classified as a response during the cue. “Go” trials were signaled by the cue light being lit above the left or right nose poke hole (side counterbalanced between subjects, but constant throughout the study for an individual subject). A nose-poke response during a Go trial terminated the Go cue and was reinforced with 20 μl of 10% sucrose solution. A “click” signaled the delivery of the reward and the start of the 3-second reward period. If no response occurred during the Go cue, the cue and the house light were switched off after 5 seconds, and a 10-second inter-trial interval (ITI) period began. “No-go” trials were signaled by a continuous 65-dB, 2.9 kHz tone. If no nose-poke response was made during the No-go cue (5 s), 20 μl sucrose solution was delivered at the end of the period, signaled by a click. Both Go and No-go reinforcers were delivered to the same aperture. After a 3-second reward period, the 10-second ITI began. If a nose-poke response was made during the No-go period, the tone and house light were turned off and the ITI began. Go and No-go trials were scheduled in a random order for each session, with the proviso that there would be 30 of each.
Baseline
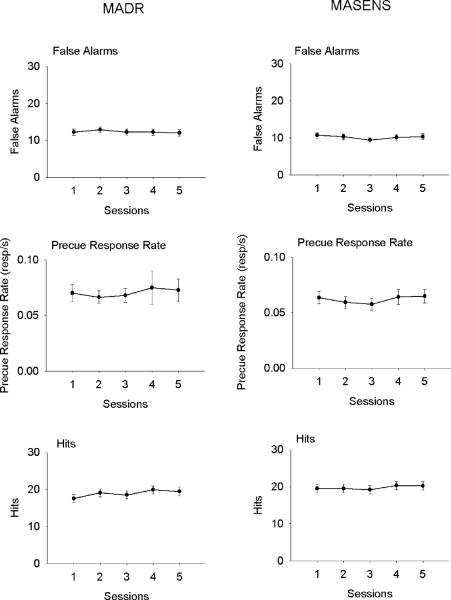
Following training (see Table 1 for training data), mice performed the task for 15 sessions. Beginning at this point, the most recent five sessions for each individual were assessed for stability in hits (responses during Go cue), false alarms (responses during No-go cue) and precue response rate by visually examining plots of these measures (Perone 1999), and if no general trends were present in all three measures, the subject's performance was considered stable (Figure 1). If performance was stable, the mice advanced to the drug administration phase of the study. If measures were not stable, mice continued in the baseline phase until the criteria were met (see Table 1 for number of sessions required to attain stable performance under baseline conditions). Because performance measures were assessed on a subject-by-subject basis, formal statistic techniques were not used. The final five sessions were averaged for use in analyses of baseline performance.
Table 1.
Mean ± SEM number of sessions to complete training phases 1 and 2 for Go/No-go task and to attain stable performance under baseline conditions
| Strain | Na | Phase lb | Phase 2c | Baselined | |
|---|---|---|---|---|---|
| MALDR | Male | 9 | 5.44 ± 0.73 | 5.00 ± 0.68 | 19.00 ± 0.94 |
| Female | 12 | 5.75 ± 0.63 | 2.75 ± 0.58 | 20.33 ± 0.82 | |
| MAHDR | Male | 11 | 6.00 ± 0.66 | 4.18 ± 0.61 | 20.64 ± 0.85 |
| Female | 9 | 5.00 ± 0.73 | 3.22 ± 0.67 | 21.44 ± 0.94 | |
| MALSENS | Male | 12 | 2.58 ± 0.56 | 2.00 ± 0.71 | 28.33 ± 1.72 |
| Female | 14 | 3.00 ± 0.52 | 2.14 ± 0.65 | 28.79 ± 1.59 | |
| MAHSENS | Male | 11 | 4.18 ± 0.59 | 2.91 ± 0.74 | 28.55 ± 1.80 |
| Female | 13 | 4.92 ± 0.54 | 5.46 ± 0.68 | 23.62 ± 1.65 |
Notes.
Mice that failed to acquire the task at any training phase are excluded (see Results section for number of failures).
During Phase 1 there were 60 Go trials and 0 No-Go trials. Each Go cue lasted for 30 seconds. Mice advanced to the Phase 2 of training by responding on at least 30 of the 60 Go trials within 40 minutes for two consecutive sessions. MALSENS mice advanced after fewer sessions that the MAHSENS mice (F(1, 46) = 10.19,p = .003.) No other differences were found.
During Phase 2 the Go cue was shortened to 10 s, and mice again had to respond on at least 30 of 60 Go trials within 40 minutes for two consecutive sessions. Again, MALSENS mice advanced more quickly than MAHSENS mice (F(1, 46) = 9.24, p = .004.)
The baseline phase consisted of 30 Go trials and 30 No-go trials (see Procedure for full description). Mice attained stable behavior at similar rates, and no significant line differences were observed.
Fig. 1.
Mean (± SEM) indices of inhibition in the five days before entering the injection phase of the experiment. There was a small, but significant increase in hits across the five days for the MADR mice (F(4,148) = 4.27, p = .003), but no other measure. It should be noted that this effect for hits was no longer significant if the data point for the first session was removed from the data.
Drug administration
After the baseline phase, all mice received four doses of methamphetamine (0.0, 1.0, 2.0, 4.0 mg/kg s.c. for MASENS mice and 0.0, 0.5, 1.0, 2.0 mg/kg s.c. for MADR mice, dissolved in 0.9% physiological saline solution). Injections occurred on Tuesdays and Fridays, with each dose given on four occasions in a Latin square design. On other sessions (Monday, Wednesday and Thursday), no injections were given. Thus, this phase of the study required 8 weeks to complete.
Excluded subjects and sessions
18 mice were excluded from the experiment because they either did not complete Phase 1 or 2 of Go/No-go training, died, or had to be euthanized (MAHDR male = 1, MAHDR female = 2, MALDR female = 1, MAHSENS female = 5, MAHSENS male = 3, MALSENS female = 2, MALSENS male = 4). Out of a total of 1,456 injections, 20 were considered unreliable due to subject movement that led them to not receive the full dose (MAHDR male = 5, MAHDR female = 6, MALDR male = 5 MALDR female = 4; MAHSENS male = 1, MAHSENS female = 1, MALSENS male = 3, MALSENS female = 1). In these instances, the three other injections at that specific dose were averaged and the mean score was used to replace the missing data.
Data Analysis
False alarms and precue response rate were the dependent variables used to measure behavioral inhibition. False alarms were operationally defined as responses made during the No-go cue. Precue response rate was defined as the total number of responses made during the precue periods divided by the total precue time. Additional measures of interest were hits (i.e. responses during the Go cue), which may reflect motivation for the sucrose reward or general operant activity level, and response latency following the onset of a Go or No-Go cue. In this manuscript, we interpret changes in measures of behavioral inhibition to indicate changes in the neurophysiological correlates of inhibition only if these changes are not accompanied by similar, proportional changes in other response indices like hits.
Because MADR and MASENS mice were generated using different selection criteria, their data were collected at different times, and they had differences in age and weight, data for the two sets of selection lines were analyzed separately. Baseline data were examined using Analysis of Variance (ANOVA) techniques with sex (2: male, female) and line (2: MALDR, MAHDR or MALSENS, MAHSENS) as separate factors. Injection data were analyzed by using a 2-level factor (injection) that included each injection session and the preceding, non-injection session, resulting in a factor sensitive to fluctuations in performance independent of the injection itself. MA injection data were analyzed with a 2 × 2 × 2 × 4 ANOVA (line × sex × injection × dose). For measures also showing a line or sex difference, we performed these post hoc tests separately for each line or sex. Where there were violations of sphericity we report Huynh-Feldt adjusted degrees of freedom.
Drug-associated changes in measures of behavioral inhibition (false alarms and precue response rate) could be due to changes in either a process uniquely associated with behavioral inhibition or a process associated with more general changes in behavior such as motivation or behavioral activation. As a first step in disambiguating these possibilities we compared the proportions for hits, false alarms, and precue response rate calculated relative to each measure's value on the preinjection saline day for each animal. Two-way repeated measures ANOVAs were used to analyze the differences in MA's effects on the three measures of interest (false alarms, precue response rate and hits) for the MA doses. ANOVAs were conducted separately for each line and each sex. Significant measure × dose effects were examined further using simple ANOVAs at each dose and Bonferroni post hoc tests between specific doses to identify doses at which the proportion measures differed.
Results
Baseline
Experiment 1: MADR mice
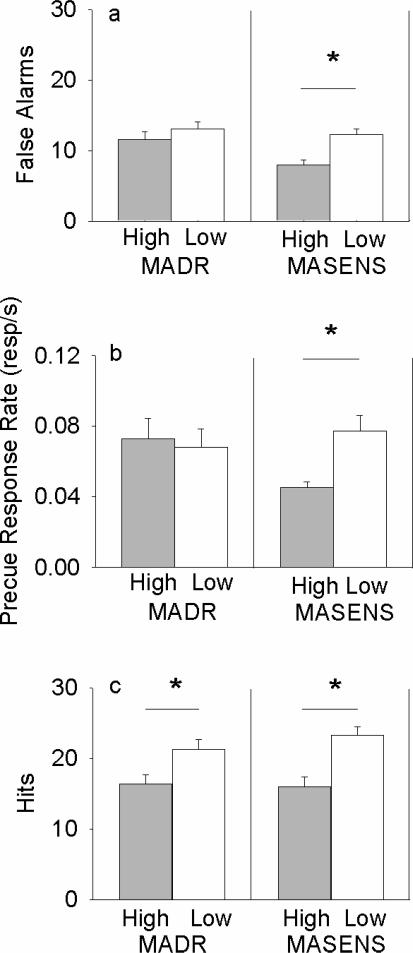
There were no differences in false alarms or precue responding between the high and low lines (Figure 2A and 2B). However, MALDR mice had more hits (F(1, 37) = 6.08, p = .018) than did MAHDR mice (Figure 2C) , suggesting that the similarities in these measures of behavioral inhibition between lines were independent of differences in general levels of responding. Animals did not differ in latency to respond to either cue.
Fig. 2.
Mean (± SEM) number of trials on which mice responded during the No-go cue (a: false alarms) or the Go cue (c: hits) and the rate of response during the precue period in responses/second (b: precue response rate). * p < .05
Experiment 2: MASENS mice
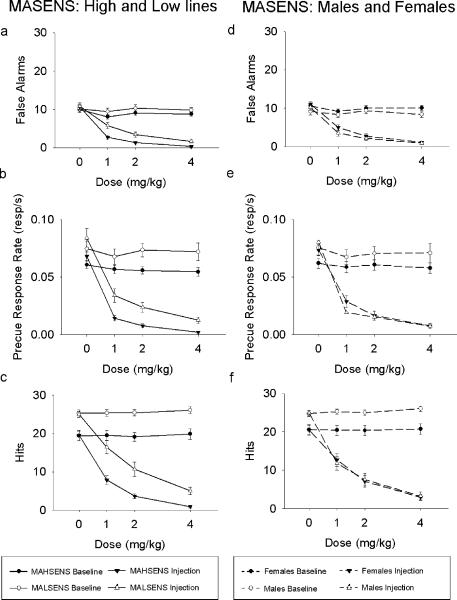
MALSENS mice made more false alarms (F(1, 46) = 15.30, p < .001), and a higher precue response rate (F(1,46) = 12.21, p = .001), but also more hits (F(1, 46) = 19.21, p < .001), than did MAHSENS mice (Figure 2). Therefore, although measures of behavioral inhibition differed between lines, this appeared to be part of a general difference in responding rather than a difference specific to inhibitory processes. MASENS males had more hits than females (F(1, 46) = 13.59, p = .001), and responded more quickly to the Go cue than females (F(1,46) = 12.78, p = .001). MALSENS mice also responded more quickly than MAHSENS mice to the Go cue (line: F(1,46) = 34.75, p < .001), and female MAHSENS mice responded more quickly to the No-Go cue than other MASENS mice (line × sex: F(1,46) = 4.54, p = .039).
Effects of MA administration
Experiment 1: MADR mice
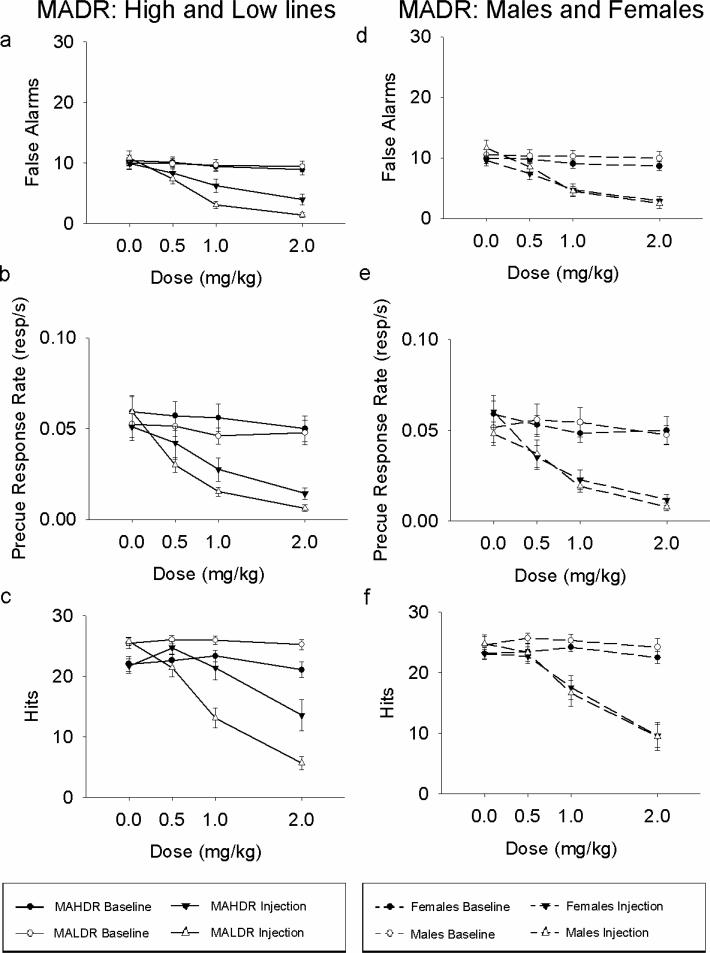
MA dose-dependently decreased responding. There were fewer false alarms (Dose × Injection: F(3, 111) = 39.70, p < .001), a lower precue response rate (Dose × Injection: F(2.97, 110.05) = 23.34, p < .001) but also fewer hits (Dose × Injection: F(2.8, 103.61) = 69.27, p < .001) in both lines. Therefore, although MA led to a decrease in our measures of behavioral inhibition, this decline appeared to be attributable to a general decline in activity.
In addition to the main effects of dose, there were differences in how responding in the High and Low lines altered as a function of dose (Figure 3). MALDR mice had significantly fewer false alarms than MAHDR mice at doses 1.0 and 2.0 mg/kg (Dose × Injection × Line: F(3,111) = 6.30, p = .001, with follow-up Bonferroni post hoc tests). A similar pattern was observed for precue response rate (Dose × Injection × Line: F(2.97, 110.05) = 2.83, p = .042), although there were no significant line differences at any dose. Despite having more hits on days prior to receiving an injection (Injection × Line: F(1,37) = 29.04, p < .001), which was consistent with the line difference at baseline (Figure 2), MALDR mice had significantly fewer hits than did MAHDR mice at doses 1.0 and 2.0 mg/kg (Dose × Injection × Line: F(2.80, 103.61) = 12.69, p < .001, with follow-up Bonferroni post hoc tests). Bonferroni post hoc tests showed that hits, false alarms, and precue response rate were significantly lower at all doses when compared to saline for MALDR mice. However, dose effects were less evident for MAHDR mice, which showed reduced false alarms and precue response rate at 1.0 mg/kg and 2.0 mg/kg, but reduced hits only at 2.0 mg/kg. Notice that the effects for each dose were also significantly different when compared to their respective pre-injection data. These data therefore suggest that MA decreased measures of behavioral inhibition without affecting hits in MAHDR mice at a dose of 1.0 mg/kg. Female MAHDR mice appeared particularly resistant to MA's effects on hits, although there were no significant differences between the female and male MAHDR mice at any dose (Dose × Injection × Line × Sex: F(2.80,103.61) = 3.10, p = .033). There were no other sex differences nor interactions in any other measures (see Figures 3d, 3e, and 3f), nor were there any effects of MA dose on latency to either the Go or No-go cue for these lines (all ps >.05).
Fig. 3.
Mean (± SEM) indices of inhibition as a function of MA dose for the MADR mice. Left, comparing high and low lines: (a) the number of trials on which mice responded during the No-go cue and (b) the rate of response during the precue period. (c) shows the number of Go trials on which the mouse made a response (hits) as a function of MA dose. Right, comparing males and females: (d) the number of trials on which mice responded during the No-go cue and (e) the rate of response during the precue period. (f) shows the number of Go trials on which the mouse made a response (hits) as a function of MA dose.
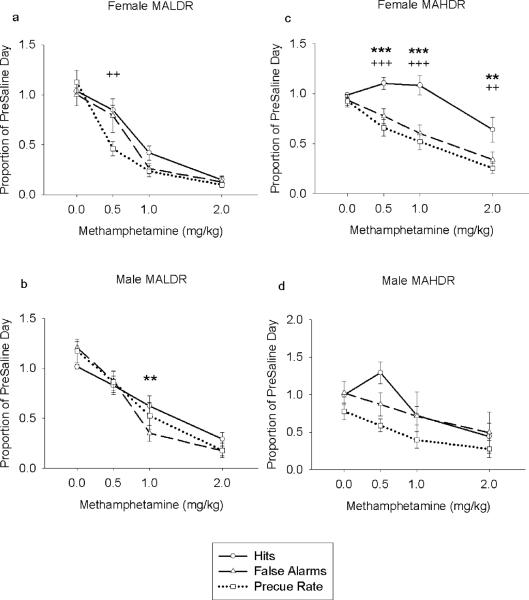
Comparing proportions of hits, false alarms, and precue rate to each other, revealed a significant difference in the effect of MA on the three measures for female MALDR, male MALDR, and female MAHDR mice (Dose × Measure: female MALDR: F(3.95,39.47) = 4.70, p = .003; male MALDR: F(6,54) = 3.97, p = .002; female MAHDR: F(6,72) = 6.75, p < .001; see Fig. 4). Simple effects ANOVAs for each dose accompanied with Bonferroni post hocs test show that, proportionally, female MAHDR had a lower precue response rate and fewer false alarms than hits at all doses except for saline, with a few additional differences for other sexes and lines at other doses (see Fig. 4). This suggests that, although changes in measures in behavioral inhibition were often accompanied by changes in measures in operant activity, these changes were sometimes more pronounced in measures of behavioral inhibition, particularly in female MAHDR mice.
Fig. 4.
Mean proportional (± SEM) indices of inhibition as a function of MA dose for the MADR mice. Each data point represents the proportion of the values obtained for each measure on the preinjection saline day. (a) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for female MALDR mice across dose, (b) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for male MALDR mice across dose. (c) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for female MAHDR mice across dose, (d) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for male MAHDR mice across dose. * p < .05, ** p < .01, *** p < .001 comparing false alarms and hits. + p < .05, ++ p < .01, +++ p < .001 comparing precue response rate and hits.
Experiment 2: MASENS mice
As shown in Figure 5, MA dose-dependently decreased behavior: there were fewer false alarms (Dose × Injection: F(2.66, 122.23) = 104.23, p < .001), hits (Dose × Injection: F(2.88, 132.51) = 160.85, p < .001), and a lower precue response rate (Dose × Injection: F(2.65, 122.08) = 89.13, p < .001) in both lines. Therefore, although MA led to a decrease in measures of behavioral inhibition, the effects appeared attributable to general decreases in responding in the task.
Fig. 5.
Mean (± SEM) indices of inhibition as a function of MA dose for the MASENS mice. Left, comparing high and low lines: (a) the number of trials on which mice responded during the No-go cue and (b) the rate of response during the precue period. (c) shows the number of Go trials on which the mouse made a response (hits) as a function of MA dose. Right, comparing males and females: (d) the number of trials on which mice responded during the No-go cue and (e) the rate of response during the precue period. (f) shows the number of Go trials on which the mouse made a response (hits) as a function of MA dose.
There were no line differences in any measure (Figure 5a, 5b, and 5c), nor effects of MA on latency to either the Go or the No-go cue for these lines (all ps >.05). However, there were main effects of sex. Males and females did not differ in number of false alarms, but males showed a significantly lower precue response rate (F(1, 46) = 8.55, p = .005) and made fewer responses during the Go cue (F(1, 46) = 7.42, p = .009) compared to females (Figure 5e and 5f). For hits, there was also a significant Dose × Injection × Sex interaction for hits (F(2.88, 132.51) = 4.08, p = .009) that Bonferroni post hoc tests indicated was due to males responding more to the Go cue following the saline dose. Therefore, unlike the mice lines (MALSENS and MAHSENS), whose pre-injection day differences in hits were maintained across doses of MA, the difference between males and females disappeared upon administration of MA (Figure 5c and 5f). Bonferroni post hoc tests showed that hits, false alarms, and precue response rate were significantly lower at all MA doses when compared to saline for both males and females, suggesting MA's effects on these animals were due to a general reduction in responding, rather than a specific effect on measures of behavioral inhibition.
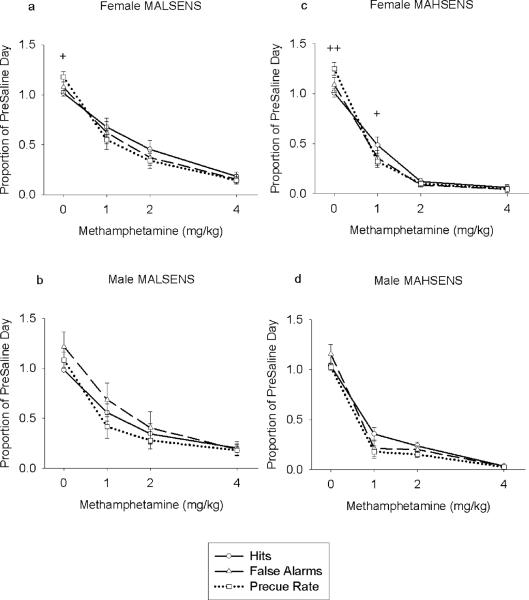
Analyses of the proportions of hits, false alarms, and precue rate indicated a significant difference in the effect of MA on the three measures for female MALSENS and female MAHSENS mice (Dose × Measure: female MALSENS: F(6,84) = 4.10, p = .001; female MAHSENS: F(3.06,36.72) = 8.75, p < .001; see Fig. 6). Simple effects ANOVAs for each dose accompanied with Bonferroni post hocs test showed few differences at isolated doses (see Fig. 6). This suggests that changes in measures of behavioral inhibition were largely accompanied by changes in of general operant behavior.
Fig. 6.
Mean proportional (± SEM) indices of inhibition as a function of MA dose for the MASENS mice. Each data point represents the proportion of the values obtained for each measure on the preinjection saline day. (a) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for female MALSENS mice across dose, (b) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for male MALSENS mice across dose. (c) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for female MAHSENS mice across dose, (d) the proportion of the preinjection saline day hits, false alarms, and precue response rate obtained for male MAHSENS mice across dose. * p < .05, ** p < .01, *** p < .001 comparing false alarms and hits. + p < .05, ++ p < .01, +++ p < .001 comparing precue response rate and hits.
Discussion
During the baseline condition, mice exhibited different response profiles in the Go/No-go task. The MADR lines did not differ in measures of behavioral inhibition. However, MALDR mice had more hits than MAHDR mice, suggesting that they were more responsive to the Go cue. Because a light was used as the Go cue for all mice, it is possible that this cue was more salient for MALDR mice. Additional research would be needed to address this possibility. Drug naïve MAHSENS mice exhibited lower levels of operant activity across all measures than did drug naïve MALSENS mice. This line difference in operant activity is not likely to be due to a difference in sucrose preference or motivation because these lines do not differ in preference for another sweet substance, saccharin (Scibelli et al. 2011). However, it may be that the lower rates of operant responding reflected differences in general activity, as MAHSENS mice have been shown to have lower basal locomotor activity than MALSENS mice (Scibelli et al. 2011).
We did not directly compare the MADR mice to the MASENS mice for several reasons. This was primarily because differences in selection criteria prevent meaningful comparisons. Additionally, differences in the age and weight for these two sets of lines could have influenced some of our findings. Walter & Giovanni (2003) showed that age can influence some measures of impulsivity in mice. In our study increased age was correlated with more false alarms in MASENS mice (r = .26, p = .046). Also, increased weight was correlated with more false alarms and more hits in MASENS mice (r = .38, p = .003, r = .38, p = .003). There were no significant relationships for MADR mice, which entered into the study at a younger age.
MA had marked effects on all lines of mice tested, and resulted in a dose-dependent decrease in false alarms, precue response rate, and hits. It seems likely that, certainly at the higher doses, the effects of MA on each of these measures were due to a general reduction in responding in the task. Importantly, our results at the higher doses are similar to a recent finding that amphetamine decreased responding in mice in the Go/No-go task while having no effects specific to measures of behavioral inhibition (Loos et al 2010). Interestingly, these authors also found that the same doses of amphetamine increased premature responding in the 5CSRTT without affecting responding on other measures in mice. This suggests that the effects of amphetamine on behavioral inhibition are task-specific. Nonetheless, it is possible that lower doses of MA would yield additionally interesting data in the Go/No-go task.
Although MA dose-dependently decreased responding in all mice, the exact nature of this effect varied. In MASENS mice, MA decreased responding regardless of the dependent measure examined. However, in MADR mice, MA dose-dependently decreased precue response rate and false alarms more than hits. This effect was particularly robust in female MAHDR mice. Since MADR mice do not differ by either sex or line in sensitivity to the locomotor activating effects of acute or repeated treatment with doses of MA up to those used here (Shabani et al., 2011), it seems unlikely that locomotor differences are the cause of differences in measures of behavioral inhibition. Our finding for MADR mice is consistent with previous findings of other studies that found psychostimulants to increase behavioral inhibition in the Go/No-go task (de Wit et al. 2002; Vaidya et al. 1998). MA may be increasing behavioral inhibition via its actions on several different neurotransmitter systems, as it has been shown to increase levels of norepinephrine (NE), dopamine (DA), and serotonin (5-HT) (Rothman et al 2001). Both NE and 5-HT have been shown to alter behavioral inhibition in the Go/No-go task (Ma et al. 2003; Harrison et al. 1999, respectively). More interestingly, a study by Frank et al. (2007) showed that individuals with ADHD had lower accuracy on a probabilistic selection task, and that subjects that were on medication (methylphenidate) increased their accuracy for the Go signal in the task, but not the No-go signal. The authors suggest that because individuals with ADHD have lower striatal DA (Sagvolden et al. 2005), methylphenidate is having its effects on the Go process by increasing striatal DA. Indeed, another study by the same group found that low doses of haloperidol (which increases DA in the striatum at low doses; Garris et al. 2003) increased number of hits in a go/no-go task (Frank & O'Reilly 2006). This research is consistent with our finding that MA increased hits in MAHDR mice at the lowest dose, although it should be noted that this effect did not survive the Bonferonni post hoc correction for significance.
As stated above, the high and low MASENS lines did not markedly differ in their response to MA. Nonetheless, the MASENS mice did differ by sex. MA significantly reduced hits in the males more than in the females. Previous research has shown female MASENS mice to have a higher preference for saccharin than males (Scibelli et al. 2011). Thus, it is possible that this higher preference is protective against any effects MA may have on the appetitive value of sucrose, which may explain the results we see. It is difficult to compare these sex differences with other literature, in part due to the lack of studies examining sex effects. In the studies examining the effects of methylphenidate or d-amphetamine on go/no-go performance, two studies had no females (Vaidya et al. 1998; Loos et al. 2010), one study did not appear to include sex in their analysis (Fillmore et al. 2003), and the final study found no effect of sex (de Wit et al. 2002). In terms of other behavioral inhibition tasks, to our knowledge no gender differences have been reported in MA abusers (e.g. Monterosso et al, 2005; Tabibnia et al, 2011; Vergedo-Garcia et al, 2006), but these studies did not explicitly include sex in their analysis. Therefore, it is difficult to see how such findings relate to human studies.
In conclusion, we did not find any differences in behavioral inhibition at baseline, but we did find that MA decreased false alarms and precue response rate to varying degrees depending on the mice tested. These changes were often accompanied by decreases in hits, suggesting a general decline in operant activity was responsible. However, decreases in hits did not accompany decreases in measures of behavioral inhibition under all doses for MADR mice, implying increased behavioral inhibition following these particular doses of MA. This effect was particularly strong in female MAHDR mice, suggesting that a combination of sex and selection for MA drinking can influence MA's effects on behavioral inhibition. Finally, this study highlights the importance of concurrent measures of activity to interpret alterations in measures of behavioral inhibition and the need, in future research, to delineate the components that contribute to the expression of behavioral inhibition.
Acknowledgements
SHM and TJP designed the study, TMM and KAS collected the data with help from Noah Gubner and Ryan McLaughlin, data analysis was performed by TMM, KAS and SHM, all authors were involved in data interpretation and manuscript preparation.
This research was supported by The Methamphetamine Abuse Research Center (MARC; P50 DA018165), the Department of Veterans Affairs and the Portland Alcohol Research Center (P60 AA10760). TMM was supported by NIAAA T32 training grant AA007468. All research was conducted in compliance with laws in the United States of America.
References
- Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: comorbid use and abuse/dependence in males and females. Behav Genet. 2004;34:217–228. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–629. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levelsof motivation. Pyschopharmacology. 2003;170:271–277. doi: 10.1007/s00213-003-1543-6. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine, and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL. Delay discounting as a predictor of drug abuse. In: Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. American Psychological Association; Washington DC: 2010. pp. 243–271. [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: New evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology. 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: Implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: Interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-Amphetamine and Ethanol on a Measure of Behavioral Inhibition in Humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser MA, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson E, Theobald D, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft M, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Feola TW, de Wit H, Richards JB. Effects of d-Amphetamine and Alcohol on a Measure of Behavioral Inhibition in Rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Santamaria A, O'Reilly RC, Willcutt E. Testing Computational Models of Dopamine and Noradrenaline Dysfunction in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology. 2007;32:1583–1599. doi: 10.1038/sj.npp.1301278. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O'Reilly RC. A mechanistic account of striatal dopamine function in human cognition: Psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PEM, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin Exp Res. 2010;34:1353–1362. doi: 10.1111/j.1530-0277.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Doubly dissociable effects of median- and dorsalraphé lesions on the performance of the five-choice serial reaction time test of attention in rats. Behav Brain Res. 1997;89:135–149. doi: 10.1016/s0166-4328(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res. 1999;100:99–112. doi: 10.1016/s0166-4328(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Loos M, Stall J, Schoffelmeer ANM, Smit A, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Ma C-L, Qi X-L, Peng J-Y, Li B-M. Selective deficit in no-go performance induced by blockade of prefrontal cortical [alpha]2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng S, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone β1 receptor gene. Learn Mem. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moeller GF, Dougherty DM. Impulsivity and substance abuse: what is the connection? Addict Disord Their Treat. 2002;1:3–10. [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Perone M. Statistical inference in behavior analysis: Experimental control is better. Behav Analyst. 1999;22:109–116. doi: 10.1007/BF03391988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK, Hitzemann RJ, Buck KJ, Cunningham CL, Crabbe JC. Harnessing the mouse to unravel the genetics of human disease. Genes Brain Behav. 2002;1:14–26. doi: 10.1046/j.1601-1848.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK. Complex-trait genetics: emergence of multivariate strategies. Nat Rev Neurosci. 2002;3:478–485. doi: 10.1038/nrn847. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Kamens HM, Wheeler JM. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev. 2008;32:707–759. doi: 10.1016/j.neubiorev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:23–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder, (adhd) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Scibelli AC, McKinnon CS, Reed C, Burkhart-Kasch S, Li N, Baba H, Wheeler JM, Phillips TJ. Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology. 2011;214:791–804. doi: 10.1007/s00213-010-2086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different Forms of Self-Control Share a Neurocognitive Substrate. J Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Elmer GI, Labuda MC, Pickens RW. Genetic influences on drug abuse. In: Bloom F, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven Press; New York: 1995. pp. 1793–1806. [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer ANM, Vandershuren LJMJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiat. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor E, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Vetulani J. Drug addiction. Part II. Neurobiology of addiction. Pol J Pharmacol. 2001;53:303–317. [PubMed] [Google Scholar]
- Walter A, Giovanni L. Elevated Levels of Impulsivity and Reduced Place Conditioning With d-Amphetamine: Two Behavioral Features of Adolescence in Mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Cunnigham CL, Janowsky A, Franken FH, et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31:1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]