Abstract
The dopamine system is involved in motivation, reward and learning, and dysfunction in this system has been implicated in several disorders, including Parkinson’s disease (PD) and schizophrenia. Key progress in our understanding of its functions has come from extracellular in vivo electrophysiological recordings from midbrain dopamine neurons. Numerous studies have used a defined set of criteria to identify dopamine neurons electrophysiologically. However, a few recent studies have suggested that a minority population of non-dopamine neurons may not be readily distinguishable from dopamine neurons, raising questions as to the reliability of past findings. We provide an overview of the key findings related to this controversy and assess the criteria used for the electrophysiological identification of dopamine neurons in the substantia nigra pars compacta (SNC) and ventral tegmental area (VTA).
Keywords: midbrain, reward, basal ganglia, VTA, substantia nigra
Introduction
Midbrain dopamine neurons located in the SNC and VTA (Fig. 1a,b) play key roles in a broad range of behaviours, and are implicated in regulating goal-directed behaviour and response to reward. In addition, dopamine is believed to be central to a number of clinical disorders, including PD, schizophrenia, drug addiction, and attention deficit hyperactivity disorder [1–5]. Progress in our understanding of the role of the dopamine system in normal function and in disease states has been made on several fronts, including anatomical studies, neurochemical investigations, and electrophysiological recordings. Indeed, crucial insights into their function have come from extracellular in vivo electrophysiological recordings. However, for such recordings to be applicable to the broad literature on the dopamine system, one must be confident that the neuron being recorded is indeed a dopamine-containing and -releasing neuron. Such an endeavour can be complicated when one must penetrate several millimetres into the brain with an electrode, and be able to segregate electrical signals coming from dopamine neurons from those arising from other cell types in the same brain regions (Fig. 1c,d). For example, midbrain dopamine neurons are located within the mesencephalon in regions including the SNC and VTA, which also contain GABAergic neurons and glutamatergic neurons. Although dopamine neurons represent the majority [around 70%, although there is subregional variation; GABA around 30%; and glutamatergic 2–3% in VTA only (see below for more detailed discussion)] [6–9], they are intermingled with neurons representing these other neurochemical types. Consequently, in order to draw valid conclusions relating to dopamine neuron activity, it is important to be confident that one is indeed recording from this specific neuron class.
Figure 1.
Anatomical features of the VTA and SNC in rodents. (a) Schematic diagram illustrating the location of the VTA and SNC in the rodent brain and two major dopaminergic projections to cortex and striatum. (b) In situ hybridization of TH mRNA in the mouse brain (coronal section) showing the position of dopamine neurons (purple) in the VTA and SNC. Image adapted from Allen Mouse Brain Atlas (http://mouse.brain-map.org). (c) Approximately 70% of VTA/SNC neurons are dopaminergic, around 30% are GABAergic, and a remaining small population are glutamatergic (in the VTA). However, it should be noted that there is some variability in these proportions, which is especially the case in distinct sub-regions of the VTA/SNC (see [8]). Some dopamine neurons also co-release glutamate [62, 76]. (d) A brain slice from the VTA triple labelled for tyrosine hydroxylase (TH, red), glutamic acid decarboxylase (GAD)-GFP (labels GABAergic neurons, green) and (C) NeuN (a neuronal nuclei marker, blue). The merged image illustrates how most neurons in the VTA are either dopaminergic (i.e., TH+ve) or GABAergic (i.e., GAD-GFP+ve; exceptions (presumably glutamatergic) are indicated by the white arrows). Note that the different neuronal groups are intermingled. Reproduced, with permission, from [48].
To address this issue, a series of studies have established a method to identify putative dopamine neurons by their unique broad waveform characteristics, irregular or bursting firing pattern, and slow firing rate (Box 1). This approach has generated a substantial and influential body of work examining the rapid, reward-coding properties of these neurons in primates and rodents (e.g., [10–19]). In addition, this has permitted the exploration of how dopamine neuron firing may be involved in disorders including PD [20], schizophrenia [21, 22] and addiction [23]. Although these criteria have proven sufficient to identify dopamine neurons in the SNC, in the VTA there have been reports of a minority of neurons (within the range of 0–37%; average = 11.6%) [24–28] that share some of these electrophysiological characteristics but could not be identified as dopaminergic using juxtacellular labelling and immunofluorescence for tyrosine hydroxylase (TH; the rate limiting enzyme in dopamine synthesis) [24–26, 28, 29]. The suggestion that some recordings, once thought to have been made from dopamine neurons, may have mis-identified the neurons in question is an important issue, since it raises doubt as to the comparability of these data with established neurochemical and behavioural investigations. This has implications for understanding, for example, how dopamine neurons respond to aversive events, because some of these non-dopamine neurons are excited by such events (and may have been assumed to be dopaminergic in previous studies), which would not appear to be consistent with the suggestion that dopamine neurons are selectively activated by reward-related events (reviewed in [30]).
Box 1. Key electrophysiological features of SNC and VTA dopamine neurons (see text for discussion of caveats).
Slow (2–10 Hz) firing rate, which may include burst firing (i.e., series of 2–10 spikes of decreasing amplitude, increasing duration, and interspike interval of 80–160 ms within a burst [83]) (Figure Ia).
In the unfiltered waveform, a biphasic (positive-negative) action potential, typically with a “notch” in the rising phase and a prominent negative component; duration >2.2 ms overall. Spikes typically vary in shape within a train from a single neuron due to interaction between slow depolarization preceding spike and prominent inhibitory synaptic input [10, 35] (Figure Ib).
For action potentials recorded with high-pass filter settings of 50 Hz or greater, biphasic or triphasic action potentials with a duration of ≥1.1 ms (measured from spike initiation to the maximal negative phase of the action potential) [24] (Figure Ib).
Inhibition by systemic administration of low doses of dopamine (as illustrated in Fig. Ic) or dopamine receptor agonists (e.g., approx. 30 ug/kg apomorphine i.v.) for nigrostriatal or mesolimbic dopamine neurons; this is not applicable to the mesocortical dopamine neurons [42, 55]. Also note that some non-dopamine neurons in VTA brainslices exhibit D2R-mediated inhibition [32].
Antidromic activation from target sites with slow (0.5 m/s) conduction velocity; typically only the spike initial segment is activated due to the absence of a slow depolarization (for detailed discussion see [39]).
Hyperpolarisation-activated inward current (Ih) (as illustrated in Fig. Id). Although, it should be noted that meso-cortical dopamine neurons recorded from acute slices of the VTA do not exhibit Ih [55], and some non-dopaminergic neurons do ([32]).
This issue has unfortunately generated confusion in the field about the reliability of criteria for the in vivo identification of dopamine neurons in both the VTA and SNC, and the relevance of previous literature that relied on such identification procedures. This also represents a significant challenge for those currently working on midbrain dopamine neurons. Moreover, a similar controversy has emerged concerning the electrophysiological criteria used for identifying dopamine neurons in brain slices [31–33]. Here, we review the relevant literature and assess criteria used to identify dopamine neurons in the VTA and SNC. We suggest that, as initially defined, identification should be based on more than just action potential duration, and that relatively straightforward and reliable criteria for the identification of dopamine neurons in vivo in both SNC and VTA are available. We also discuss the identification of dopamine neurons during brain slice recordings, and some outstanding questions for future resolution. The issues raised here are illustrative of challenges faced when trying to elucidate in vivo neuronal function in the brain, where most regions contain intermingled neurochemically heterogeneous neurons.
In vivo identification of SNC dopamine neurons
Dopamine neurons were first identified in the SNC based on pharmacological criteria (such as inhibition by dopamine receptor agonists and activation by dopamine receptor antagonists [11]), loss of neurons with this electrophysiological phenotype after neurochemically specific lesions [11, 20], and antidromic activation from terminal regions [34]. Subsequent in vivo intracellular recording and labelling studies (see Glossary) in anesthetised rats established that neurochemically-identified SNC dopamine neurons exhibit broad action potential waveforms (i.e., 2–3 ms) with a prominent negative phase, and slow (typically 1–10 Hz) firing rates with an irregular or burst firing pattern (Fig. 2a–c) [10, 35]. These studies also established that approximately 50% of dopamine neurons are silent (i.e., do not fire action potentials spontaneously) in vivo. Indeed, alteration in the number of dopamine neurons firing spontaneously has been quantified by counting the number of spontaneously active neurons recorded as an electrode tracks through the SNC/VTA (e.g., [36–38]).
Figure 2.
In vivo electrophysiological characteristics of substantia nigra pars compacta (SNC) and ventral tegmental area (VTA) dopamine neurons. (a) A typical waveform of an extracellular action potential (top panel) and an intracellular action potential and its first differential (i.e., predicted extracellular waveform) as recorded in the SNC. Open arrow indicates initial segment spike; solid arrows indicate peak intracellular voltage deflection. (b) Neurons are often spontaneously active and often fire bursts (shown in right-hand traces; top, extracellular recording; bottom, intracellular recording) that occur during a slow depolarisation (seen in intracellular recordings). Reproduced, with permission, from [10]. (c) An intensely fluorescent dopamine neuron (bright green-white) following intracellular injection of L-DOPA (L-3,4-dihydroxyphenylalanine), surrounded by neurons exhibiting normal dopamine fluorescence (green) in the SNC of a rat. This is the first direct histochemical identification of a single DA neuron labelled during electrophysiological recordings. The brain slice was treated with glyoxylic acid, which causes catecholamines to fluoresce [77]; hence, the higher the catecholamine content, the brighter the fluorescence. Only DA neurons, which have the capability to convert L-DOPA to DA and store it intracellularly, will fluoresce more brightly when injected with L-DOPA. Reproduced, with permission, from [35]. (d) In vivo characteristics of VTA GABAergic neurons. The extracellular waveform (top panel) is narrow compared to dopamine neurons, and, unlike DA neurons, they fire at high frequency (bottom panel), or in burst-pause patterns (middle panel) under halothane anaesthesia. Reproduced, with permission, from [78]. (e) Extracellular waveform and firing characteristics of a VTA dopamine neuron (upper panel) and a non-dopamine neuron (lower panel). Images show dual immunofluorescence for a single neuron juxtacellularly-labelled with neurobiotin (NB; blue) and immunolabelled for tyrosine hydroxylase (TH; red). Note that the non-dopamine neuron has a narrower waveform, particularly with respect to the duration from start to the trough. Reproduced, with permission, from [24]. (f), Illustration of how the start and trough (represented by solid blue lines) of the extracellular waveform (lower trace) correspond to the intracellular waveform (upper trace). Dashed lines show initial segment spike and peak of the intracellular action potential, respectively.
To understand this issue, one must first assess what an extracellularly recorded neuronal action potential waveform means. Intracellular waveforms reflect the changes in transmembrane voltage that are recorded during an action potential. In contrast, extracellular waveforms represent the voltage drop that these action potential-related transmembrane ion fluxes, or currents, produce across the extracellular electrode resistance (i.e., current, or dV/dt). Thus, the shape of the extracellular waveforms can be derived from the first differential of the intracellular waveforms, and these correspond to the waveforms recorded with extracellular electrodes (Fig. 2a). The distinct waveform of dopamine neurons is particularly apparent when observed in the absence of high-pass filtering (e.g., Figure Ib in Box I). Indeed, the often-reported triphasic dopamine neuron action potential is actually reflective of a filtering artefact, since with open filters dopamine neuron action potentials are biphasic when recorded extracellularly (see Box 1b) [39]. Such filters (e.g., 300 Hz) are commonly used in vivo to eliminate cardiac related signals and thereby aid in the detection of neuronal action potentials. Therefore, high-pass filters are not typically used ex vivo. It should also be noted that extracellular waveform features can vary depending on electrode characteristics and distance from the neuron. It is therefore advisable to position the electrode as close to the neuron as possible without altering its activity, and at least in a consistent position from recording to recording. Because single-cell labelling is extremely challenging in vivo, and does not lend itself well to studies of large numbers of dopamine neurons -particularly when recorded in individual animals - the field has subsequently relied on these findings to identify dopamine neurons in vivo, particularly in awake animals, including primates.
Box Figure I.
Representative traces illustrating some of the key electrophysiological features of dopamine neurons. (a) Examples of rat VTA dopamine neurons spontaneous firing in vivo in regular (top trace) or irregular (middle trace) patterns, including bursts (lower traces). (b) Extracellular waveforms of SNC dopamine neurons in rats recorded with two different filter settings in vivo. ((c) Inhibition of a VTA dopamine neuron by dopamine, in a mouse brain slice. Reproduced, with permission, from [25] (a), [40] (b), [55] (c) and (d). d) Hyperpolarisation-activated inward current as seen in membrane sag during hyperpolarising pulse in current-clamp recording from a VTA dopamine neuron in mouse brain slice.
Recently, the reliability of these criteria for identifying SNC dopamine neurons has been confirmed using juxtacellular labelling in anesthetised rats [40]. In this case, an extracellular recording electrode is used to record from and label single neurons [41]. Using this approach, it was reported that neurons with broad waveforms and slow firing rates were uniformly dopaminergic in the SNC [40]. On the basis of these studies, it seems reasonable to conclude that reliable criteria are available for the extracellular electrophysiological identification of dopamine neurons in the SNC in vivo, and that in past studies investigators have generally used them appropriately to conclude that they are recording from dopamine neurons. For this reason, the remainder of this article, except where mentioned, focuses on identification of VTA dopamine neurons.
In vivo identification of VTA dopamine neurons
Similar to the SNC, extracellular recordings in the VTA report two types of electrophysiologically-distinct neuronal groups: broad-waveform, slow-firing neurons (i.e., < 10 Hz) and narrow-waveform, fast-firing neurons (Fig. 2d,e). This first type of neuron is typically assumed to be dopaminergic based on their similarity to SNC dopamine neurons, histochemical identification with intracellular staining [42], and recordings in in vitro brain slices, where neurochemical identity has been confirmed following single-cell filling (but see discussion below). In addition, the criterion of a prominent negative component to the unfiltered waveform combined with the aforementioned criteria was sufficient to identify mesocortical/mesolimbic VTA dopamine neurons in a reliable manner using intracellular electrodes (for discussion, see [2]). It is clear that at least one class of non-dopamine neurons exhibiting a short-duration action potential, rapid firing rate, and activation by stimulation of the internal capsule have been identified as GABA ergic in intracellular single-cell labelling studies in vivo [43] (Fig. 2d). However, there is increasing evidence (particularly in cases in which identification may be ambiguous due to required filter settings or multi-unit recordings of multiple neurons) that suggests that some non-dopamine neurons in the VTA may be misidentified as dopaminergic. For example, combined extracellular recording and single-cell juxtacellular labelling in vivo showed that, in some cases, using the criteria of a broad-waveform and slow firing rate alone is not always adequate to identify dopamine neurons in the VTA [24] (Fig. 2e). The neurochemical identity of these non-dopamine neurons is unknown, although several possibilities exist, including, for example, that they are glutamatergic [8, 9, 44]. The precise proportion of putative glutamatergic neurons in the VTA and the degree to which they co-express TH varies dramatically (i.e., between 2% overall and 60% in medial VTA) among publications, and therefore is a matter of contention. Nonetheless, when found, these neurons are mostly located in rostral and medial parts of the VTA [8, 9, 44, 45]. It is therefore important to note that in vivo recordings commonly target more central and lateral regions of the VTA (in an attempt the avoid the medially-placed sagittal sinus), and therefore are less likely to sample this population of neurons.
Consistent with the absence of non-dopamine neurons with broad waveforms and slow firing rates in the SNC, glutamatergic neurons are generally located in the VTA and not in the SNC [8, 9]. Importantly, though, these non-dopamine neurons had narrower waveforms compared to dopamine neurons (Fig. 2e). Interestingly, it has also been noted [46] that very slow firing rates (i.e., < 1 Hz) are rarely reported for dopamine neurons (in either the SNC or VTA, but are included in the range for firing rates seen in these non-dopaminergic neurons (e.g., [24]). It should also be noted, that mesocortical dopamine neurons differ from other dopamine neurons in having higher average firing rates (≈9Hz) and not exhibiting dopamine D2-receptor mediated auto-inhibition [42]. In addition, these meso-cortical dopamine neurons are located more medially, and so less frequently sampled during in vivo recordings.
A more conservative criterion of ≥1.1 ms from start to trough of the waveform was found to be adequate to identify putative dopamine neurons even with filtering [24]. Indeed, applying this criterion to in vivo juxtacellular labelling studies shows that ≈ 90% of neurons fitting this criterion are dopaminergic [24–28, 47] (Table 1). This criterion has also been shown to be effective for extracellular recordings from spontaneously firing neurons in the VTA of brain slices [48]. In addition, juxtacellular labelling of VTA neurons in awake, immobilised rats found that slow-firing non-dopamine neurons have narrower waveforms compared to dopamine neurons (as measured either from the peak to trough, or from start to end) [29]. This extracellular waveform criterion is based on the duration of the intracellular waveform from the initiation of the positive phase to the peak rate-of-change of the falling phase (Fig. 2f), and therefore takes into account both the sodium and the initial calcium components of this waveform [49]. This approach, of course, leaves open the possibility that some dopamine neurons with narrower waveforms will be excluded, which has been suggested to be the case in one report of recordings made during the dark phase of the diurnal cycle (recordings are typically made during the light phase; see Table 1) [26].
Table 1.
Studies involving juxtacellular labelling and electrophysiological in vivo recordings of neurons in the rat VTA. For each study the number (and percentage) of cells fitting the start-to-trough waveform criterion ≥1.1 ms that are dopaminergic (i.e., tyrosine hydroxylase immunopositive, TH+ve) are listed.
| Topic of study | TH+ve cells ≥1.1 ms | Refs | |
|---|---|---|---|
| TH+ve/total | %TH+ve | ||
| Aversive events (dopamine vs. non-dopamine) | 9/9 | 100% | [24] |
| Diurnal cycle | 11/13a | 84.6% | [26] |
| Aversive events (dorsal vs. ventral VTA) | 5/8b | 62.5% | [25] |
| Orexin/hypocretin regulation | 8/8 | 100% | [28] |
| Hippocampal regulation | 5/6 | 83.3% | [27] |
| Orbitofrontal cortex regulation | 6/6b | 100% | [47] |
|
| |||
| Total number of cells | 44/50 | (88%) | |
| Mean % TH+ve (per study) | 88.4% | ||
These authors conducted some of their recordings during the dark phase of the diurnal cycle and found 3/5 neurons ≥1.1 ms were TH+ve, and that 6/16 thin-spike (< 2 ms total waveform duration) neurons were TH+ve, suggesting that identification may be more challenging during the dark phase. However, this deserves further investigation before firm conclusions can be drawn (for example, note that in [28] 6/6 neurons ≥1.1 ms were TH+ve in the dark phase).
Cell numbers were obtained from authors; relation to criterion not explicitly stated in paper. It should be noted that in [25] this criterion was not used as a strict guide for selecting cells for recording because cells were labelled and neurochemically identified.
The balance of evidence from these single-cell labelling studies indicates that the classically defined criteria for dopamine neuron identification in the VTA in vivo, if applied carefully, can be used to identify dopamine neurons based on their electrophysiological characteristics. Importantly, misidentification is a potential issue using this method, but studies to date suggest that it should only occur in a minority of cases, and this can be largely avoided by employing an additional conservative waveform duration criterion, as discussed above. This suggests that, in many cases, a sufficiently large sample size would dilute the impact of such potential misidentification, particularly where the sampled population appears functionally homogeneous. However, in cases in which smaller numbers of neurons are studied or when there is clear functional diversity in the sampled population, more stringent criteria (and/or establishing neurochemical identity through labelling) may be required to establish the identity reliably (e.g., [24, 25]).
We conclude, therefore, that the use of all of the available extracellular criteria, including waveform, firing pattern, firing rate, and location appear to be adequate to identify dopamine neurons in the SNC, and are sufficient to identify dopamine neurons in the majority of cases in the VTA in vivo. The waveform criteria are derived from the unique membrane electrophysiology of the dopamine neurons. However, there is still a possibility that some neurons may be mis-identified. The impact of such a potential confound can be mitigated by recording from large numbers of neurons and careful application of additional identification criteria such as peak-to-trough duration.
Is the same identification issue present when recording in vitro?
Dopamine neurons can be recorded in vitro in midbrain slices (coronal, horizontal or sagittal) containing the VTA and SNC, and in this preparation also generally exhibit broad action potential waveforms and slow firing rates, similar to those seen in vivo (Fig. 3). On the other hand, dopamine neurons are devoid of most of their inputs in brain slices, due to transaction of afferent fibers. As a result, dopamine neurons recorded in vitro do not exhibit burst firing and irregular firing observed in vivo, likely due to loss of these afferent inputs (for discussion see [49, 50]). In addition, during intracellular (whole cell or sharp electrode) recordings they commonly exhibit a hyperpolarization-activated inward current (Ih), which is perhaps the most common feature used to infer their neurochemical identity (Box 1 and Fig. 3). As observed in vivo, these electrophysiological criteria appear to reliably identify SNC dopamine neurons [51, 52]. However, single-cell labelling studies have revealed that in the VTA the presence of Ih is not always consistent with a dopaminergic identity [53, 54], and moreover dopamine neurons in the medial VTA have very small or no Ih [33, 55]. There is, it should be noted, quite a bit of variability between reports concerning the reliability of Ih as a marker (e.g., ranging from 55% dopaminergic [31, 53] to 94% [56] and 100% [57]). This may be related to a number of factors, including the age of the animal, the slicing procedure itself (which may differentially affect the survival of different cell types), the orientation and thickness of the slices (which is likely to affect survival of long dendritic processes in which many of these conductances originate) and experimenter biases (not necessarily explicit) towards targeting certain types of neurons.
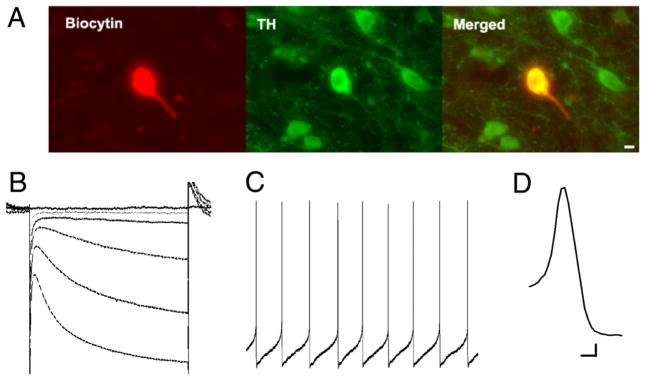
Figure 3.
In vitro electrophysiological characteristics of VTA dopamine neurons in rodent brain slices. A, Immunolabelling (TH, tyrosine hydroxylase – green) of a recorded dopamine neuron (filled with biocytin – red). Scale bar: 10 μm. B, Example of Ih current during hyperpolarising steps. C, Action potential activity, showing slow regular firing, and D, relatively broad waveform. Scale bar: B, 80 ms, 80 pA; C, 350 ms, 6.25 mV; D, 1 ms, 10 mV. Reproduced, with permission, from [56].
It may also be that species-specific differences are important: non-dopamine neurons with Ih are more commonly found in brain slices from guinea pigs [54] and rats [53], than in brain slices from mice [30–33]. In addition, during prolonged whole-cell recordings there are increased chances that TH will be washed out, leading to false negatives in studies in which TH is localized immunohistochemically to identify the neurons [33]. This appears to be particularly dependent on the internal solution used in the electrode, with high concentrations of Cl− leading to washout, whereas the use of other solution compositions can substantially increase the reliability of this identification method [31].
Consequently, these studies indicate that some caution should be observed in the VTA when inferring neurochemical identity from intracellular electrophysiological characteristics in brain slices. Although it would be ideal to label every cell recorded, it is not always possible to successfully recover individual neurons and immunolabelling is often not sufficiently robust to allow neurochemical identification. An alternative approach that has been adopted by several groups is to conduct a batch of single-cell labelling experiments to establish the reliability of their own particular methods, which would appear to be a sufficiently conservative approach particularly when immature animals, transgenic mice, or non-standard slicing procedures are used.
Alternatively, several mouse lines are available that selectively express enhanced green fluorescent protein (eGFP) in dopamine neurons in the SNC and VTA and are already commonly used to conduct targeted recordings. For example, in heterozygous mice in which the eGFP reporter gene is inserted into the promoter region of the paired-like homeobox transcription factor (Pitx3; a transcription factor important for dopamine neuron differentiation and development)-, GFP fluorescence is observed selectively in essentially 100% of VTA and SNC dopamine neurons (as identified with TH-immunohistochemical labeling)) [58]. In comparison, only around 80% of dopamine neurons have been reported to express GFP in the VTA in TH-GFP transgenic mice (although it should be noted that GFP expression appeared stronger in those cells that were labelled, and all GFP cells were dopaminergic) [58, 59]. One further approach that will no doubt become more routine in the future is to use optogenetics [60] to selectively activate dopamine neurons with light pulses and thereby confirm neurochemical identity [61–64] (N.B., it has not yet been determined if such an approach may alter the electrophysiological properties of the SNC/VTA neuron in which the channels are inserted).
Identification of distinct subpopulations within the VTA and SNC
It is well-established that there is diversity amongst SNC and VTA dopamine neurons with respect to their projection targets (e.g., striatal versus cortical; [65–67]), pharmacology (e.g., dopamine D2 receptor auto-inhibition; [42, 55]), molecular markers (e.g., calbindin-, Girk2 (G protein-gated inwardly rectifying K+ channel)-, and otx2 (orthodenticle homeobox 2 transcription factor)-positive neurons [68]) and neuropeptide co-release (e.g., neurotensin; [69]). Currently there are no electrophysiological criteria to help distinguish between these subgroups in vivo (except of course antidromic activation from projection targets). However, in brain slices, recent evidence suggests reasons to be optimistic: VTA neurons projecting to different regions (established with injections of retrograde markers prior to brain slicing) can differ in their waveform features, Ih magnitude, pharmacological responses to opioids and D2 receptor agonists, as well as plasticity in response to cocaine and stress [55, 70–73]. For example, κ-opioid receptor agonists inhibit VTA dopamine neurons projecting to the prefrontal cortex, but not those projecting to the nucleus accumbens [73]. Moreover, it is also possible to distinguish between dopaminergic and non-dopaminergic sub populations in some cases. For example, once projection targets are established, neurochemical identity can be accurately inferred for nucleus accumbens- and amygdala- projecting dopamine neurons which have significantly broader waveforms compared to non-dopamine neurons projecting to the same regions [71] [Refs]. However these findings (whilst certainly encouraging) do not translate immediately to in vivo criteria and will need to be examined in future in vivo single-cell labelling studies [55, 70–72]. Subpopulations of VTA and SNC dopamine neurons also show functional diversity, particularly with respect to coding for aversive events (e.g., [15, 25, 74, 75]). For example, some of these latter studies have also shown that the anatomical position of dopaminergic neurons within the SNC and VTA relates to their functional properties. Such a finding will be particularly relevant for studies in awake-behaving animals, where single-cell labelling is problematic.
Summary
The firing activity of midbrain dopamine neurons codes for reward- and punishment-related information, and changes in firing activity may contribute to the pathophysiology of a number of disorders, including schizophrenia and addiction. In most cases, studies have relied upon electrophysiological criteria for the identification of dopamine neurons in both the VTA and SNC. However, some confusion has arisen in the field concerning the reliability of electrophysiological criteria used for the identification of midbrain dopamine neurons during both in vivo and in vitro brain slice recordings. Here, we have addressed these concerns by reviewing those criteria and have evaluated the evidence that has been cited in support of their reliability.
Single-cell labelling studies indicate that the established criteria used in the SNC have proven to be reliable indicators of dopaminergic identity, both in vivo and in brain slices. For VTA brain slice recordings, it appears that due to the nature of the preparation, some caution is warranted, particularly when working with rats compared to mice. For in vivo recordings in the VTA, traditional criteria, which have proven to be effective to identify dopamine neurons, can be further refined (e.g., by using start to trough duration measurements). As a result, investigators can be reasonably confident of selective dopaminergic identity, even when recording from small numbers of neurons or when more restrictive filter settings are necessary. More generally, these studies illustrate the challenge of dissecting out neuronal function in functionally- and anatomically-heterogeneous brain regions, and that progress relies on an iterative process drawing on a range of techniques. In essence, as with most approaches, no technique is without its drawbacks, but confounds related to neuronal identification can be effectively limited to enhance reliability in the observed experimental findings.
Box 2. Outstanding questions.
Is it possible that some TH-negative neurons in the VTA with a “dopamine-like” electrophysiological signature are indeed dopamine neurons that are not currently expressing TH? If not, what is the identity of these cells? Do they correspond to glutamatergic neurons in this region?
Are differences in reported proportions of non-dopamine neurons in VTA brain slices related to species differences or different laboratory protocols?
Do dopamine neuron subgroups have distinct electrophysiological signatures in vivo?
Acknowledgments
We thank Paul Bolam, Frederic Brischoux, Howard Fields and Elyssa Margolis for comments on earlier versions. Preparation of this article was supported by grant U120085816 from the U.K. Medical Research Council (MRC; to M.A.U), a University Research Fellowship from The Royal Society (to M.A.U.), and National Institutes of Health grants MH57440 and DA15408 (to A.A.G.).
Glossary
- Extracellular recording
This is the most technically-feasible approach that is available for reliably recording dopamine neurons in vivo, particularly in awake, freely-moving animals (e.g., [13, 15, 16]), including humans [79]. It allows for recordings of responses from single neurons as well as populations of neurons. Typically, only action potentials can be detected, which often limits recordings to spontaneously active neurons
- Fluorescent targeting
Brain slices can be prepared from mice expressing green fluorescent protein (GFP) in dopamine neurons [58, 59]. GFP-expressing neurons (identified with a fluorescence microscope) can then be selectively targeted with the recording electrode
- Intracellular sharp-electrode recording
A sharp glass micro-electrode is used to penetrate the neuron and record the membrane potential and properties of the impaled neuron. By introducing substances such as dyes into the electrode solution, the neuron can be labelled intracellularly, which can be effective in neurochemically identifying the recorded neuron when combined with immunocytochemical localization of a transmitter-specific substance (e.g., TH in the case of dopamine neurons). This is readily applicable to recordings in brain slices [51, 80], and, although challenging, has been done to identify dopamine neurons in vivo [10, 35, 39, 81, 82]
- Juxtacellular labelling
Can be used to intracellularly label a neuron with a dye (usually neurobiotin) when recording extracellularly [41]. Current pulses modulate firing in the neuron, ensuring close proximity of the electrode to the neuron and ensuring that the tracer labels only the neuron that is being recorded. Allows for the correlation of extracellular activity with neurochemical identity, but is technically challenging and success rates can be low with dopamine neurons [24–29, 40] For this reason it is essential that only cases in which a single neuron is labelled are used to prevent false positive identification
- Optogenetic activation
Optogenetic tools are genetically-encoded light sensitive proteins that can depolarise or hyperpolarise neurons [60]. Such genes can be selectively expressed in dopamine neurons thereby allowing the neuron to be definitively identified as dopaminergic based on its activation with a laser light pulse [61, 64]
- Whole-cell recording
The most commonly used technique to record from dopamine neurons in brain slices. A tight seal is formed between the recording electrode and the neuron, followed by subsequent rupture of the membrane, allowing intracellular access for recording and labelling [33, 53]
References
- 1.Schultz W. Behavioral dopamine signals. Trends in Neurosciences. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Grace AA, et al. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein M, Deutch AY. Dopaminergic mechanisms in the pathogenesis of schizophrenia. Faseb J. 1992;6:2413–2421. [PubMed] [Google Scholar]
- 5.Hyman SE, et al. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 6.Ford B, et al. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. The Journal of comparative neurology. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- 7.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 8.Nair-Roberts RG, et al. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi T, et al. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 11.Bunney BS, et al. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- 12.Waelti P, et al. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 13.Tobler PN, et al. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 14.Roesch MR, et al. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyland BI, et al. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS One. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobler PN, et al. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris G, et al. Midbrain dopamine neurons encode decisions for future action. Nat Neurosci. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- 20.Hollerman JR, Grace AA. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 1990;533:203–212. doi: 10.1016/0006-8993(90)91341-d. [DOI] [PubMed] [Google Scholar]
- 21.Valenti O, et al. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31:12330–12338. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. The Journal of Neuroscience. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungless MA, et al. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 25.Brischoux F, et al. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo AH, et al. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27:408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- 27.Luo AH, et al. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mileykovskiy B, Morales M. Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear. J Neurosci. 2011;31:7471–7476. doi: 10.1523/JNEUROSCI.5731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungless MA. Dopamine: the salient issue. Trends Neurosci. 2004;27:702–706. doi: 10.1016/j.tins.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Margolis EB, et al. Reliability in the identification of midbrain dopamine neurons. PLoS One. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margolis EB, et al. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? The Journal of physiology. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang TA, et al. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- 35.Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with L-dopa injection and histofluorescence. Science. 1980;210:654–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- 36.Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23:1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- 37.Floresco SB, et al. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 38.Floresco SB, et al. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--2. Action potential generating mechanisms and morphological correlates. Neuroscience. 1983;10:317–331. doi: 10.1016/0306-4522(83)90136-7. [DOI] [PubMed] [Google Scholar]
- 40.Brown MT, et al. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci. 2009;29:2915–2925. doi: 10.1523/JNEUROSCI.4423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 42.Chiodo LA, et al. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- 43.Steffensen SC, et al. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawano M, et al. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi T, et al. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chenu F, et al. An enhancement of the firing activity of dopamine neurons as a common denominator of antidepressant treatments? Int J Neuropsychopharmacol. 2011:1–3. doi: 10.1017/S1461145711001362. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi YK, et al. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat Neurosci. 2011;14:1590–1597. doi: 10.1038/nn.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chieng B, et al. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. The Journal of physiology. 2011;589:3775–3787. doi: 10.1113/jphysiol.2011.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grace AA. Evidence for the functional compartmentalization of spike generating regions of rat midbrain dopamine neurons recorded in vitro. Brain Res. 1990;524:31–41. doi: 10.1016/0006-8993(90)90488-w. [DOI] [PubMed] [Google Scholar]
- 50.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandecasteele M, et al. Spike frequency adaptation is developmentally regulated in substantia nigra pars compacta dopaminergic neurons. Neuroscience. 2011;192:1–10. doi: 10.1016/j.neuroscience.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Margolis EB, et al. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cameron DL, et al. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- 55.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Mao D, et al. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanat MJ, et al. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao S, et al. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]
- 59.Matsushita N, et al. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem. 2002;82:295–304. doi: 10.1046/j.1471-4159.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- 60.Yizhar O, et al. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stuber GD, et al. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2011;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tecuapetla F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2011;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen JY, et al. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 66.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 67.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Salvio M, et al. Otx2 expression is restricted to dopaminergic neurons of the ventral tegmental area in the adult brain. Int J Dev Biol. 2010;54:939–945. doi: 10.1387/ijdb.092974ms. [DOI] [PubMed] [Google Scholar]
- 69.Jayaraman A, et al. Cholecystokinin and neurotensin mRNAs are differentially expressed in subnuclei of the ventral tegmental area. The Journal of comparative neurology. 1990;296:291–302. doi: 10.1002/cne.902960209. [DOI] [PubMed] [Google Scholar]
- 70.Lammel S, et al. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Margolis EB, et al. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ford CP, et al. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Margolis EB, et al. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joshua M, et al. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valenti O, et al. Aversive Stimuli Alter Ventral Tegmental Area Dopamine Neuron Activity via a Common Action in the Ventral Hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tecuapetla F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindvall O, et al. Application of the glyoxylic acid method to vibratome sections for the improved visualization of central catecholamine neurons. Histochemie. 1973;35:31–38. doi: 10.1007/BF00303662. [DOI] [PubMed] [Google Scholar]
- 78.Lee RS, et al. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1757–1766. doi: 10.1523/JNEUROSCI.21-05-01757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaghloul KA, et al. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--3. Evidence for electrotonic coupling. Neuroscience. 1983;10:333–348. doi: 10.1016/0306-4522(83)90137-9. [DOI] [PubMed] [Google Scholar]
- 82.Tepper JM, et al. Electrophysiologically identified nigral dopaminergic neurons intracellularly labeled with HRP: light-microscopic analysis. J Neurosci. 1987;7:2794–2806. doi: 10.1523/JNEUROSCI.07-09-02794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]