Abstract
There are well-documented changes in thyroid hormone metabolism that accompany heart failure (HF). However, the frequency of thyroid hormone abnormalities in HF with preserved ejection fraction (HFpEF) is unknown, and no studies have investigated the association between triiodothyronine (T3) and markers of HF severity (B-type natriuretic peptide [BNP] and diastolic dysfunction [DD]) in HFpEF. We prospectively studied 89consecutive patients with HFpEF, defined as symptomatic HF with LV ejection fraction >50% and LV end-diastolic volume index < 97 ml/m2. Patients were dichotomized into two groups based upon median T3 levels, and clinical, laboratory, and echocardiographic data were compared between groups. Univariable and multivariable linear regression analyses were performed to determine whether BNP and DD were independently associated with T3 level. We found that 22% of HFpEF patients had reduced T3. Patients with lowerT3 levels were older, more symptomatic, more frequently had hyperlipidemia and diabetes, and had higher BNP levels. Severe (grade 3) DD, higher mitral E velocity, shorter deceleration time, and higher pulse pressure/stroke volume ratio were all associated with lower T3 levels. T3 was inversely associated with both log BNP (p=0.004) and severity of DD (p=0.039). On multivariable analysis, T3 was independently associated with both log BNP (β=−4.7 [95% CI −9.0, −0.41]ng/dl, p=0.032) and severe DD (β=−16.3 [95% CI −30.1, −2.5]ng/dl, p=0.022). In conclusion, T3 is inversely associated with markers of HFpEF severity (BNP and DD). Whether reduced T3 contributes to or is a consequence of increased severity of HFpEF remains to be determined.
Keywords: thyroid, brain natriuretic peptide, diastolic heart failure, transthoracic echocardiography
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is common and associated with high morbidity and mortality.1,2 Finding dedicated treatments for HFpEF has been challenging, and novel therapeutic pathways are necessary. Given the hormonal alterations that occur in HF, interest in endocrine dysregulation as a potential avenue for intervention is growing. Thyroid hormone, specifically triiodothyronine (T3), is known to have beneficial cardiovascular effects including facilitating myocardial relaxation and lowering peripheral vascular resistance.3 Derangement of the thyroid axis can thus lead to a spectrum of cardiovascular manifestations. This relationship has been studied in HF with reduced EF,4,5 but the frequency of thyroid hormone abnormalities in HFpEF is unknown. Furthermore, no prior studies have investigated the association between T3 and markers of HF severity (e.g., B-type natriuretic peptide [BNP] and left ventricular (LV) diastolic dysfunction [DD]) in patients with HFpEF. We therefore sought to (1) define the prevalence of thyroid axis abnormalities in HFpEF and (2) investigate associations between T3 and laboratory and echocardiographic data in HFpEF. We hypothesized that reduced T3 is common in HFpEF and may independently contribute to increased BNP and worse DD in these patients, similar to that observed in classical hypothyroidism.3
METHODS
Consecutive patients were prospectively enrolled from the outpatient clinic of the Northwestern University HFpEF Program as part of a systematic observational study of HFpEF (ClinicalTrials.gov identifier #NCT01030991). Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital using the following search criteria: (1) diagnosis of HF or the words “heart failure” anywhere in the hospital notes; or (2) BNP >100 pg/ml; or (3) administration of 2 or more doses of intravenous diuretics. The list of patients generated was screened daily, and only those patients who had LV ejection fraction (EF) > 50% and who meet Framingham criteria for HF6 were offered post-discharge follow-up in a specialized HFpEF outpatient program. Once evaluated as an outpatient in the HFpEF clinic, the diagnosis of HF was confirmed by a board-certified HF specialist. All study participants gave written, informed consent, and the institutional review board at Northwestern University approved the study.
We defined HFpEF as symptomatic HF with both an LVEF > 50% and an LV end-diastolic volume index < 97 ml/m2. Based on previously published guidelines,7 we additionally required evidence of either significant diastolic dysfunction (grade 2 or 3) on echocardiography or evidence of elevated LV filling pressures. In patients in whom diastolic dysfunction was graded as normal or grade 1 (mild), we performed invasive hemodynamic testing and required evidence of elevated LV end-diastolic pressure (i.e., pulmonary capillary wedge pressure > 15 mmHg or LV end-diastolic pressure > 15 mmHg). In line with a large population-based study in HFpEF,8 patients with hemodynamically significant valvular disease (defined as greater than moderate in severity), prior cardiac transplantation, prior history of overt LV systolic dysfunction (LVEF < 40%), or a diagnosis of constrictive pericarditis were excluded. Patients were also excluded if they had known hypothyroidism or hyperthyroidism.
We collected and analyzed demographics, clinical data, including comorbidities and medications, and laboratory data including BNP, thyroid stimulating hormone (TSH), free T4 (fT4), and total T3. Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet in Renal Disease equation. Hypertension was defined by systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg, physician-documented history of hypertension, or current use of antihypertensive medications. Diabetes mellitus was defined by the presence of physician-documented history of diabetes or use of oral hypoglycemic agents or insulin for the treatment of hyperglycemia. Coronary artery disease (CAD) was defined by the presence of physician-documented history of CAD, known coronary stenosis > 50%, prior history of myocardial infarction, percutaneous intervention, coronary artery bypass grafting, or abnormal stress test consistent with myocardial ischemia. Obesity was defined by a body mass index > 30 kg/m2. Chronic kidney disease was defined as eGFR < 60 ml/min/1.73m2.
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging. All standard echocardiographic views were obtained. Echocardiography was performed using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500, Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp., Waukesha, WI). Cardiac structure and systolic function were quantified as recommended by the American Society of Echocardiography.9,10 Diastolic function was graded in accordance with criteria published previously.11 All echocardiographic measurements were made by an experienced research sonographer blinded to all other clinical data using ProSolv 4.0 echocardiographic analysis software (ProSolvCardioVascular; Indianapolis, IN) and were verified by a board-certified echocardiographer.
For descriptive purposes, we dichotomized participants into 2 groups based on the median T3 level (T3≥108 ng/dl [N=46] vs. T3 < 108 ng/dl [N=43]). The reference range for normal T3 values in the Northwestern Memorial Hospital laboratory is 87–178ng/dl. We chose to dichotomize study participants by median T3 level (108ng/dl) instead of the clinical cut-point for reduced T3 (87 ng/dl) in order to preserve statistical power in our 2 comparison groups.
Demographics, clinical characteristics, laboratory data, and echocardiographic parameters were compared between groups using t-tests for normally distributed continuous variables (or non-parametric equivalent when appropriate). Chi-squared tests (or Fisher’s exact test when appropriate) were used to compare categorical variables between groups. A two-sided p-value < 0.05 was considered statistically significant. Continuous data with a normal distribution were displayed as mean ± standard deviation. Right-skewed data were log-transformed and presented as median and 25th–75th percentile.
To determine associations between thyroid function tests and cardiac data (echocardiographic variables and BNP), we performed Pearson correlation analyses to determine the strength of correlation and significance for any bivariate associations (variables which were right-skewed [i.e., TSH, BNP] were log-transformed prior to analysis). Next we performed univariable and multivariable linear regression analyses to determine whether BNP (independent variable) was associated with T3 level (dependent variable). We performed similar analyses to determine the association between severe (grade 3) DD and T3. Candidate covariates were selected for inclusion into our multivariable linear regression models if they differed between the T3≥108 ng/dl versus T3 < 108 ng/dl groups at a significance level of p < 0.05. Further, because obesity correlates inversely with BNP and beta-blocker use has been linked to worsening HFpEF severity,12,13 these variables were also included in the multivariable analyses. All analyses were performed using Stata v.10.1 (StataCorp, College Station, TX).
RESULTS
Demographic and clinical characteristics of the 89 study participants were consistent with previous epidemiologic and large observational studies of HFpEF,2,14 with an observed sample mean age of 67±14 years and female predominance (69%). Ethnicities represented a mixed urban population:49% were white, 42% were African-American, and 9% were other ethnicities. The majority of patients (52%) had NYHA functional class III or IV symptoms, and comorbidities were common. The distribution of medication use reflected standard medical therapy in those with HFpEF who have multiple cardiovascular comorbidities. Notably, only 6% of patients were taking amiodarone at the time of thyroid function testing. Echocardiographic variables were consistent with expected findings in HFpEF: preserved LVEF, normal LV end-diastolic volume index, increased left atrial volume index, and increased E/e′ ratio reflective of increased LV filling pressures. In addition, the majority of patients had either moderate or severe DD.
Based on clinical cut-offs, T3 was low (i.e., T3 < 87 ng/dl) in 20/89 (22%) of patients and elevated (i.e., T3 > 178 ng/dl) in 3/89 (3%) of patients. In addition, 6/89 (7%) had low fT4 (<0.7 ng/dl), 2/89 (2%) had elevated fT4 (> 1.5 ng/dl), 12/89 (13%) had low TSH (< 0.4 μIU/ml) and 9/89 (10%) had elevated TSH (> 4 μIU/ml). Patients with lower T3 levels were more likely to be older, were more symptomatic with higher NYHA functional class, more frequently had hyperlipidemia and diabetes, and had higher BNP levels (Table 1). These patients also had lower diastolic blood pressure, but this association was no longer significant after adjusting for age (P=0.26).3,15 No differences in sex, ethnicity, medication use (including amiodarone), systolic blood pressure, body-mass index, hemoglobin, TSH, or fT4 values were observed compared to those with T3 ≥108 ng/dl. Patients with lower T3 levels had a higher prevalence of grade 3DD, higher early mitral inflow velocity, and shorter early mitral inflow deceleration time (Table 2). A higher pulse pressure/stroke volume ratio, indicative of increased arterial stiffness, was also noted. There were no observed differences in LV structure, LVEF, E/A ratio, orseptal e′ velocity. Patients with lowerT3 had higher values of E/e′ ratio (i.e., higher LV filling pressures), and longer isovolumic relaxation times (i.e. lusitropic impairment), though these findings were not statistically significant (p = 0.14 and 0.24, respectively).
Table 1.
Clinical Characteristics of the Study Sample
| Characteristic | All patients (N=89) | T3 (ng/dl)
|
P-value | |
|---|---|---|---|---|
| ≥ 108 (N=46) | < 108 (N=43) | |||
| T3 (ng/dl) | 111±31 | 134±24 | 86 ±14 | |
|
| ||||
| Age (years) | 67±14 | 63±14 | 70±13 | 0.012 |
| Women | 61 (69%) | 32 (70%) | 29 (67%) | 0.83 |
| Ethnicity | 0.74 | |||
| • White | 44 (49%) | 23 (50%) | 21 (49%) | |
| • African-American | 37 (42%) | 20 (44%) | 17 (40%) | |
| • Other | 8 (9%) | 3 (7%) | 5 (12%) | |
| NYHA functional class | 0.014 | |||
| I or II | 43 (48%) | 28 (61%) | 15 (35%) | |
| III or IV | 46 (52%) | 18 (39%) | 28 (65%) | |
| • Coronary artery disease | 31 (35%) | 15 (33%) | 16 (37%) | 0.65 |
| • Hypertension | 73 (82%) | 36 (78%) | 37 (86%) | 0.34 |
| • Hyperlipidemia | 49 (55%) | 20 (44%) | 29 (67%) | 0.023 |
| • Diabetes mellitus | 28 (32%) | 9 (20%) | 19 (44%) | 0.012 |
| • Chronic kidney disease | 35 (39%) | 14 (30%) | 21 (49%) | 0.08 |
| • Current smoker | 35 (39%) | 18 (39%) | 17 (40%) | 0.97 |
| • Atrial fibrillation | 23 (26%) | 10 (22%) | 13 (30%) | 0.36 |
| • Obesity | 53 (60%) | 28 (61%) | 25 (58%) | 0.79 |
| • Chronic obstructive pulmonary disease or asthma | 21 (24%) | 10 (22%) | 11 (26%) | 0.67 |
| • Heart rate (bpm) | 74±15 | 75±16 | 73±14 | 0.55 |
| • Systolic blood pressure (mm Hg) | 128±21 | 129±20 | 128±23 | 0.85 |
| • Diastolic blood pressure (mm Hg) | 72±13 | 74±12 | 69±13 | 0.043 |
| • Pulse pressure (mm Hg) | 57±17 | 55±15 | 59±19 | 0.20 |
| • Body-mass index (kg/m2) | 33.0±8.3 | 34.0±9.0 | 32.0±7.5 | 0.27 |
| • Serum sodium (mEq/L) | 138±3 | 138±3 | 139±3 | 0.48 |
| • Blood urea nitrogen (mg/dl) | 25±17 | 22±18 | 27±16 | 0.19 |
| • Serum creatinine (mg/dl) | 1.56±1.34 | 1.51±1.45 | 1.63±1.22 | 0.68 |
| • Estimated glomerular filtration rate (ml/min per 1.73m2) | 57±26 | 61±27 | 52±25 | 0.10 |
| • Serum glucose (mg/dl) | 119±53 | 117±61 | 121±44 | 0.71 |
| • Hemoglobin (g/dl) | 12.0±1.8 | 12.3±1.9 | 11.6±1.6 | 0.07 |
| • B-type natriuretic peptide (pg/ml)* | 214 (66–603) | 133 (46–318) | 326 (115–874) | 0.009 |
| • Thyroid stimulating hormone (μIU/ml)* | 1.7 (0.9–2.6) | 1.6 (0.9–2.6) | 1.9 (1.0–2.9) | 0.48 |
| • Free T4 (ng/dl) | 0.97±0.27 | 0.93±0.23 | 1.02±0.30 | 0.11 |
| Medications | ||||
| • Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 56 (63%) | 30 (65%) | 26 (61%) | 0.64 |
| • Beta-blocker | 60 (67%) | 27 (59%) | 33 (76%) | 0.07 |
| • Calcium channel blocker | 30 (34%) | 17 (37%) | 13 (30%) | 0.50 |
| • Loop diuretic | 54 (61%) | 25 (54%) | 29 (67%) | 0.21 |
| • Thiazide diuretic | 21 (24%) | 11 (24%) | 10 (23%) | 0.94 |
| • Statin | 43 (48%) | 19 (41%) | 24 (56%) | 0.17 |
| • Aspirin | 31 (35%) | 18 (39%) | 13 (30%) | 0.38 |
| • Warfarin | 18 (20%) | 7 (15%) | 11 (26%) | 0.22 |
| • Amiodarone | 5 (6%) | 2 (4%) | 3 (7%) | 0.59 |
NYHA = New York Heart Association; hypertension was defined by systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg, physician-documented history of hypertension, or current use of antihypertensive medications; hyperlipidemia was defined by a physician-documented history of hyperlipidemia or current use of lipid lowering medications
Median (25th–75th percentile) shown and Wilcoxon rank-sum test performed because these variables were right-skewed.
Table 2.
Echocardiographic Parameters of the Study Sample
| Parameter | All patients (N=89) | T3 (ng/dl)
|
P-value | |
|---|---|---|---|---|
| ≥ 108 (N=46) | < 108 (N=43) | |||
| LV end-systolic volume index (ml/m2) | 15±6 | 16±7 | 15±5 | 0.46 |
| LV end-diastolic volume index (ml/m2) | 40±11 | 41±13 | 39±9 | 0.44 |
| LV ejection fraction (%) | 62±7 | 62±7 | 62±7 | 0.85 |
| LV mass index (g/m2) | 105±37 | 104±45 | 106±26 | 0.77 |
| Stroke volume (ml) | 93±63 | 91±27 | 96±87 | 0.70 |
| Cardiac index (L/min/m2) | 3.4±2.3 | 3.3±1.1 | 3.5±3.2 | 0.65 |
| Pulse pressure/stroke volume ratio (mm Hg/ml) | 0.69±0.25 | 0.64±0.22 | 0.75±0.28 | 0.03 |
| LV diastolic function | ||||
| • Normal | 8 (9%) | 5 (11%) | 3 (7%) | 0.71 |
| • Grade 1 (impaired relaxation) | 8 (9%) | 5 (11%) | 3 (7%) | 0.71 |
| • Grade 2 (pseudonormal) | 41 (46%) | 24 (52%) | 17 (40%) | 0.19 |
| • Grade 3 (restrictive) | 29 (33%) | 10 (22%) | 19 (44%) | 0.027 |
| • Indeterminate | 3 (3%) | 2 (4%) | 1 (2%) | 0.99 |
| Left atrial volume index (ml/m2) | 33±13 | 31±11 | 35±15 | 0.23 |
| Early mitral inflow, E velocity (cm/s) | 101±33 | 93± 27 | 110±36 | 0.012 |
| Late mitral inflow, A velocity (cm/s) | 86±31 | 86±30 | 86±33 | 0.98 |
| E/A ratio | 1.4±0.8 | 1.2±0.6 | 1.5±1.0 | 0.14 |
| E deceleration time (ms) | 219±48 | 231±47 | 206±47 | 0.016 |
| Isovolumic relaxation time (ms) | 88±24 | 85±21 | 91±27 | 0.24 |
| Septal e′ velocity (cm/s) | 6.6±2.0 | 6.5±2.1 | 6.8±2.1 | 0.59 |
| E/e′ ratio | 16.8±8.6 | 15.5±6.2 | 18.2±10.4 | 0.14 |
LV = left ventricular
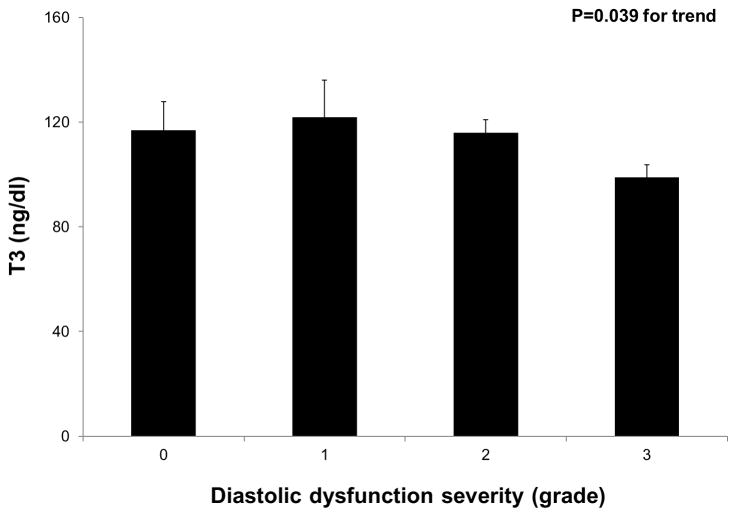
Table 3 displays the correlation coefficients and p-values for the bivariate association of thyroid function tests with BNP and markers of DD. Compared to fT4and TSH, T3 correlated more closely with markers of elevated filling pressures and abnormal diastolic function. Figure 1 displays a scatter plot of T3 and log-transformed BNP. Log BNP was independently associated with T3 after adjustment for age, hyperlipidemia, diabetes mellitus, NYHA class, and diastolic blood pressure on multivariable analysis; per 1-unit increase in log BNP, there was a corresponding 4.7 ng/dl decrease in T3 (95% CI −9.0 to −0.41, P=0.032). The independent association between log BNP and T3 persisted after further adjustment for log TSH (P=0.045) and subsequent additional adjustment for obesity and beta-blocker use (P=0.049). Figure 2 demonstrates an overall decrease in T3levels with worsening DD (P=0.039 for the trend). When individual groups of DD were compared to the remaining groups, only the presence of grade 3 DD was associated with reduced T3 levels (P=0.011). Multivariable analysis demonstrated that the association between reduced T3 and grade 3 DD persisted after adjusting for age, hyperlipidemia, diabetes mellitus, NYHA class, and diastolic blood pressure; the presence of grade 3 DD was associated with a 16.3 ng/dl decrease in T3levels (95% CI −30.1 to −2.5, P=0.022). The independent association between grade 3 DD and T3 persisted after further adjustment for log TSH (P=0.026) and subsequent additional adjustment for obesity and beta-blocker use (P=0.034).
Table 3.
Correlation of Thyroid Hormones with B-Type Natriuretic Peptide and Diastolic Parameters
| Parameter | T3 (ng/dl) | Free T4 (ng/L) | Log TSH (μIU/ml) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| R | P-value | R | P-value | R | P-value | |
| Log B-type natriuretic peptide (pg/ml) | −0.30 | 0.003 | 0.12 | 0.01 | 0.24 | 0.02 |
| Early mitral inflow velocity (m/s) | −0.23 | 0.03 | −0.05 | 0.65 | −0.01 | 0.94 |
| Early mitral inflow deceleration time (ms) | 0.37 | 0.0004 | −0.07 | 0.55 | −0.10 | 0.36 |
| Septal e′ velocity (cm/s) | −0.10 | 0.35 | 0.27 | 0.01 | −0.06 | 0.59 |
| Grade 3 diastolic dysfunction | −0.27 | 0.01 | −0.06 | 0.63 | 0.06 | 0.59 |
Figure 1. Scatterplot of T3 versus Log B-type Natriuretic Peptide in Heart Failure with Preserved Ejection Fraction.
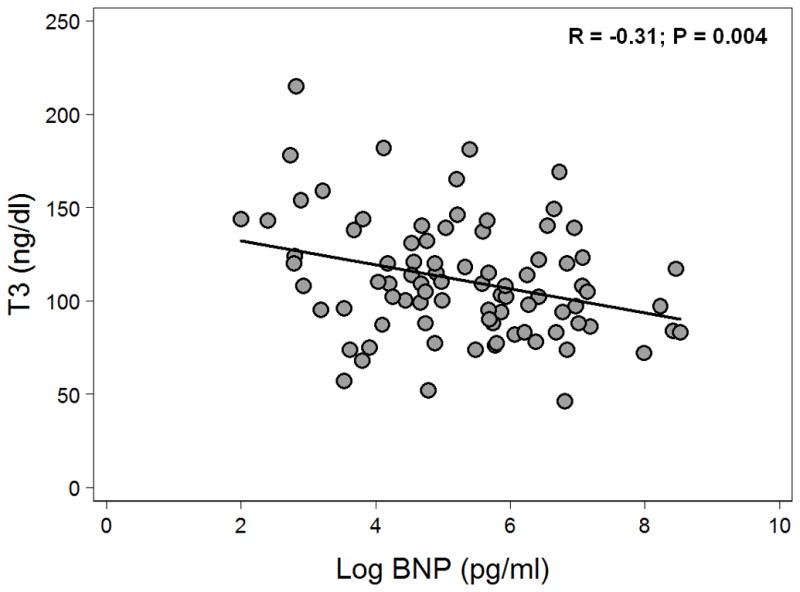
T3 is inversely associated with log B-type natriuretic peptide in heart failure with preserved ejection fraction.
Figure 2. Inverse Relationship of T3 with Left Ventricular Diastolic Dysfunction Severity in Heart Failure with Preserved Ejection Fraction.
P-value for trend was calculated using linear regression. T3 levels were also compared to individual diastolic dysfunction groups using linear regression; only severe (grade 3) diastolic dysfunction was significantly associated with T3levels (p=0.011).
DISCUSSION
In our prospective, detailed analysis of 89 consecutive patients with HFpEF without a prior diagnosis of thyroid dysfunction, we found that low T3was relatively common (22% prevalence), consistent with prior studies in HF with reduced EF.4,5,16 Increased BNP and severe (grade 3) DD were both independently associated with lower T3levels. To our knowledge, our study is the first to examine thyroid function in HFpEF.
Only a few studies have previously examined the relationship between BNP and thyroid function. These studies typically excluded patients with HF, and their findings are mixed.17–20 Similar to our findings, one study identified an inverse correlation between log BNP and log T3 (r= −0.37, P<0.0001); however, the study population was characterized by non-ischemic LV systolic dysfunction in the absence of overt HF.18 Paradoxically, in patients with both overt and subclinical thyroid dysfunction without known cardiac disease, NT-pro-BNP (the inactive portion of the BNP precursor molecule, known to correlate highly with BNP), was positively correlated to both T4and T3levels. Thyroid treatment in these dysthyroid states resulted in increased NT-pro-BNP in the hypothyroid patients and reduced NT-pro-BNP in the hyperthyroid patients,20 perhaps reflecting the direct effect of T3 on myocyte BNP gene expression, independent of the changes observed with HF.21 The results of the present study suggest that the hemodynamic stimulus to BNP release may override this genomic effect. This difference in positive versus negative correlation of T3 to natriuretic peptides may also highlight the important molecular and structural derangements that occur in the pathophysiology of HF, which appears to represent a relative thyroid hormone deficient state. Thus, our study provides a novel association between BNP and T3levels in those with HFpEF.
Our study found several clinical and echocardiographic parameters associated with T3in HFpEF. The association between advanced NYHA functional class and lower levels of T3 was confirmed in the present study.4,5,22 Novel associations between echocardiographic parameters and lower T3levels included severe (grade 3) DD, mitral E velocity, early mitral inflow deceleration time, and pulse pressure/stroke volume ratio. An increase in the pulse pressure /stroke volume ratio is indicative of reduced arterial compliance, consistent with the known effects of T3on decreasing systemic vascular resistance and increasing arterial compliance.3,22 In line with two previous studies, we did not find a correlation between T3and LVEF.19,23 Our study provides a more robust echocardiographic analysis, which may account for the discovery of these novel associations not previously observed.
Although we collected our data prospectively, because of its cross-sectional design we cannot determine whether the HF syndrome results in low T3levels or vice versa. It is possible that the HFpEF syndrome may cause lower T3 levels which in turn exacerbate the HF syndrome, creating a vicious cycle.3,4 Several prior studies have investigated the mechanisms of low T3 in HF, including upregulation of type III deiodinase enzyme, which convertsT3to its inactive counterpart, in a rat model and increased levels of pro-inflammatory cytokines in human subjects.16,24 Even when levels of T3 are “normal” in individuals with HF, the downstream effects of T3may be blunted by the upregulation of certain repressor proteins; for example, increased FOG-2 downregulates expression of SERCA2, a thyroid hormone-dependent calcium pump in the sarcoplasmic reticulum that facilitates myocardial diastolic relaxation.25
Conversely, there are plausible mechanisms by which low T3can lead to the exacerbation of HF in the absence of overt structural remodeling. For example, low T3decreases expression SERCA2 and alpha-sarcomericactinin, leading to phenotypic and structural distortion at the molecular level with subsequent impaired relaxation.26 These pathophysiologic processes may underlie the association between worse DD and lower T3 observed in our study, although our findings are more consistent with an association between reduced T3 levels and elevated LV filling pressures (grade 3 DD and BNP) rather than impaired relaxation per se.
Restoring the thyroid axis in HFpEF patients with low T3remains an under explored territory. In a rat model of myocardial infarction-induced HF, replacement T3 therapy resulted in a trend toward improvement in diastolic function.27 Hamilton et al. demonstrated in hospitalized patients with advanced HF and reduced EF, T3 replacement results in increases in cardiac output and decreases in systemic vascular resistance without untoward effects on blood pressure and arrhythmogenesis.28 Pingitore et al. showed that short term replacement doses of T3improvedLV stroke volume in patients with dilated cardiomyopathy and even mild symptoms.17 However, these studies are limited by their small size, lack of randomization, and short treatment duration, highlighting the need for more robust investigation. Our study further supports the growing evidence that lower levels of T3 may contribute to the impaired cardiac performance of HF in general and of HFpEF in particular.
Several limitations should be considered when interpreting our results. First, our sample size is modest, which may have impaired the detection of more subtle associations between clinical and echocardiographic variables andT3 levels. However, to our knowledge, our study is the largest investigation of thyroid function in HFpEF. Second, our findings are limited to a single center analysis, and should be confirmed by a larger, multicenter study. Third, since we restricted our sample to HFpEF patients only, our findings showing a relationship between low T3 and severe DD cannot be extrapolated to HF with reduced EF. Further studies of patients with HF and reduced EF will be necessary to examine the association between low T3 and severe DD in these patients. In addition, while we specifically sought to determine the factors associated with low T3 within a group of patients with HFpEF, the lack of a non-HF control group for comparison could be viewed as a potential limitation. Finally, as noted previously, we cannot determine a cause-and-effect relationship between T3 and HF severity given the cross-sectional nature of our study.
Acknowledgments
Funding: This work was supported by grants from the American Heart Association (#0835488N) and the National Institutes of Health (R01 HL107557) (both to S.J.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 4.Ascheim DD, Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid. 2002;12:511–515. doi: 10.1089/105072502760143908. [DOI] [PubMed] [Google Scholar]
- 5.Opasich C, Pacini F, Ambrosino N, Riccardi PG, Febo O, Ferrari R, Cobelli F, Tavazzi L. Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure. Eur Heart J. 1996;17:1860–1866. doi: 10.1093/oxfordjournals.eurheartj.a014804. [DOI] [PubMed] [Google Scholar]
- 6.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 7.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Farasat SM, Bolger DT, Shetty V, Menachery EP, Gerstenblith G, Kasper EK, Najjar SS. Effect of Beta-blocker therapy on rehospitalization rates in women versus men with heart failure and preserved ejection fraction. Am J Cardiol. 2010;105:229–234. doi: 10.1016/j.amjcard.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 13.McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, Steg PG, Omland T, Knudsen CW, Sandberg KR, McCullough PA. Breathing Not Properly Multinational Study I. Relationship between obesity and B-type natriuretic peptide levels. Arch Intern Med. 2004;164:2247–2252. doi: 10.1001/archinte.164.20.2247. [DOI] [PubMed] [Google Scholar]
- 14.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 15.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 16.Abo-Zenah HA, Shoeb SA, Sabry AA, Ismail HA. Relating circulating thyroid hormone concentrations to serum interleukins-6 and -10 in association with non-thyroidal illnesses including chronic renal insufficiency. BMC Endocr Disord. 2008;8:1. doi: 10.1186/1472-6823-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pingitore A, Galli E, Barison A, Iervasi A, Scarlattini M, Nucci D, L’Abbate A, Mariotti R, Iervasi G. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:1351–1358. doi: 10.1210/jc.2007-2210. [DOI] [PubMed] [Google Scholar]
- 18.Pingitore A, Iervasi G, Barison A, Prontera C, Pratali L, Emdin M, Giannessi D, Neglia D. Early activation of an altered thyroid hormone profile in asymptomatic or mildly symptomatic idiopathic left ventricular dysfunction. J Card Fail. 2006;12:520–526. doi: 10.1016/j.cardfail.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Pingitore A, Landi P, Taddei MC, Ripoli A, L’Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med. 2005;118:132–136. doi: 10.1016/j.amjmed.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 20.Schultz M, Faber J, Kistorp C, Jarlov A, Pedersen F, Wiinberg N, Hildebrandt P. N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) in different thyroid function states. Clin Endocrinol (Oxf) 2004;60:54–59. doi: 10.1111/j.1365-2265.2004.01941.x. [DOI] [PubMed] [Google Scholar]
- 21.Liang F, Webb P, Marimuthu A, Zhang S, Gardner DG. Triiodothyronine increases brain natriuretic peptide (BNP) gene transcription and amplifies endothelin-dependent BNP gene transcription and hypertrophy in neonatal rat ventricular myocytes. J Biol Chem. 2003;278:15073–15083. doi: 10.1074/jbc.M207593200. [DOI] [PubMed] [Google Scholar]
- 22.Galli E, Pingitore A, Iervasi G. The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev. 2010;15:155–169. doi: 10.1007/s10741-008-9126-6. [DOI] [PubMed] [Google Scholar]
- 23.Koga H, Kaku T, Hashiba K. Primary hypothyroidism in severe chronic heart failure. Jpn J Med. 1988;27:42–48. doi: 10.2169/internalmedicine1962.27.42. [DOI] [PubMed] [Google Scholar]
- 24.Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, Simonides WS. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology. 2002;143:2812–2815. doi: 10.1210/endo.143.7.8985. [DOI] [PubMed] [Google Scholar]
- 25.Rouf R, Greytak S, Wooten EC, Wu J, Boltax J, Picard M, Svensson EC, Dillmann WH, Patten RD, Huggins GS. Increased FOG-2 in failing myocardium disrupts thyroid hormone-dependent SERCA2 gene transcription. Circ Res. 2008;103:493–501. doi: 10.1161/CIRCRESAHA.108.181487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forini F, Paolicchi A, Pizzorusso T, Ratto GM, Saviozzi M, Vanini V, Iervasi G. 3,5,3′-Triiodothyronine deprivation affects phenotype and intracellular [Ca2+]i of human cardiomyocytes in culture. Cardiovasc Res. 2001;51:322–330. doi: 10.1016/s0008-6363(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 27.Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure. Circ Heart Fail. 2009;2:243–252. doi: 10.1161/CIRCHEARTFAILURE.108.810747. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, Chopra IJ, Moriguchi JD, Hage A. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol. 1998;81:443–447. doi: 10.1016/s0002-9149(97)00950-8. [DOI] [PubMed] [Google Scholar]