Abstract
Background
Little is known about treatment outcomes for children who have end stage renal disease (ESRD) after treatment for Wilms tumor (WT).
Methods
Time-to-transplant, graft failure and survival outcomes were examined for 173 children enrolled on the National Wilms Tumor Study who developed ESRD.
Results
Fifty-five patients whose ESRD resulted from progressive bilateral WT (PBWT) experienced high early mortality from WT that limited their opportunity for transplant (47% at 5 years) and survival (44% at 10 years) in comparison with population controls. The 118 patients whose ESRD was due to other causes (termed “chronic kidney disease”), many of whom had WT associated congenital anomalies, had transplant (77% at 5 years) and survival (73% at 10 years) outcomes no worse than those for population controls. Graft failure following transplant was comparable for the 2 groups. Minority children had twice the median time to transplant as non-Hispanic whites and twice the mortality rates, also reflecting population trends.
Conclusions
In view of the continuing high mortality in patients with ESRD, and the dramatic improvement in outlook following kidney transplantation, re-evaluation of current guidelines for a 2 year delay in transplant following WT treatment may be warranted.
Keywords: Kidney Failure, Chronic, Transplantation, Graft Failure
Introduction
The past half century witnessed dramatic progress in the treatment and survival of children with Wilms tumor (WT).(1;2) Since the late 1980’s, 5-year survival rates have remained consistently above 90%, exceeding those for most other childhood cancers.(3) Attention is now focused on medical conditions related to the disease and its treatment that may adversely affect quality of life for the increasing number of survivors. End stage renal disease (ESRD) is one of the most serious late sequelae due to the morbidity of treatment and high mortality.
In view of the cardiovascular damage, impaired cognitive development and growth retardation associated with long term dialysis, pediatric patients with ESRD have had high priority for renal transplantation, particularly since 2005.(4;5) However, early reports recommended delaying transplant for 1–2 years following WT treatment because of deaths due to sepsis and tumor recurrences observed in patients undergoing transplant sooner.(6;7) European best practice guidelines for renal transplantation recommended a 2 year waiting period.(8) Since then options for prevention and treatment of acute graft rejection, and for dealing with consequences of immunosuppression, have improved dramatically.(9;10) Re-evaluation of the recommended waiting time may be warranted.
Kidney replacement therapy (dialysis or renal transplant) for children with ESRD has been used for several decades and data on long term survival are available.(11;12) However, little is known about WT patients and survivors treated for ESRD. While it is believed that improvements in dialysis and transplantation offer the potential for marked improvement in prognosis, such conclusions are based on relatively small numbers of patients.(7;13) The largest study to date of renal transplantation in children with WT involved just 43 patients(14). The National Wilms Tumor Study (NWTS) clinical trials thus constitute an exceptional resource for elucidation of these issues.
The goal of this study was to describe the characteristics and clinical course of NWTS patients with ESRD. We considered separately ESRD that results from progressive bilateral WT (PBWT) and ESRD ascribed to other causes, termed collectively chronic kidney disease (CKD). Our earlier study noted that the risk of ESRD due to PBWT was largely confined to the first 3 years following the onset of bilateral disease, whereas incidence rates of ESRD due to CKD continued to increase for 20–25 years from WT diagnosis.(15) One focus of the present study was to investigate the timing of transplant in relation to WT treatment, with a view towards possible reconsideration of the recommended waiting times. Overall survival and transplant outcomes in comparison with those for a general pediatric and young adult population with ESRD, and comparisons of treatments and outcomes by ethnicity, were also investigated.
Methods
Patient populations
The base population consisted of 9,162 subjects with a WT diagnosed before 15 years of age who were enrolled from North American institutions on 1 of 5 NWTS clinical trials between October, 1969 and April, 2002. Informed consent was obtained from the family prior to enrollment by each participating institution. Approval for conduct of the study was given by the institutional review board of the Fred Hutchinson Cancer Research Center. The 173 patients known to have received kidney replacement therapy for ESRD by the closing date, December 31, 2008, constituted the study population for this report. We did not consider here 7 patients included in the risk factor study who died of ESRD before therapy was instituted.(15)
For comparison of the NWTS experience with that of the general population, 2 disjoint stratified random samples were drawn from the public use records of the United States Renal Data System (USRDS).(16) The samples were frequency matched 20:1 to the ages at onset of ESRD-PBWT and ESRD-CKD NWTS participants, respectively, in 5 year categories of age. A few USRDS patients were excluded whose ESRD reportedly occurred prior to 15 years of age as the result of a childhood renal neoplasm, since such patients could have been enrolled by NWTS. USRDS subjects whose latest record indicated a transplant prior to 2009 were considered to be alive at closing.
Types of ESRD
ESRD was categorized as due to PBWT if its onset, the initiation of chronic dialysis or renal transplant, coincided with surgical removal of all kidney tissue as a consequence of progressive bilateral WT. ESRD occurring in patients with unilateral WT, or in those with bilateral WT who had been in complete remission for a least 1 year with no intervening relapse, was categorized as due to CKD. One patient diagnosed with bilateral WT by biopsy the day after dialysis was started for renal failure was also categorized as CKD. The most frequent causes of CKD were the Denys-Drash (DDS) or Wilms tumor, aniridia, genitourinary anomalies, retardation (WAGR) congenital malformation syndromes, focal segmental glomerulosclerosis and radiation nephritis (Table 1).
Table 1.
Causes of end stage renal disease (ESRD in Patients with Wilms Tumor
| Assigned ESRD Cause | No. of Patients |
|---|---|
| Progressive bilateral Wilms tumor | 55 |
| Drash syndrome | 27 |
| WAGR syndrome | 10 |
| Polycystic kidney disease | 2 |
| Hemolytic uremic syndrome | 1 |
| Immune complex glomerulonephritis | 5 |
| Infection | 2 |
| Hydronephrosis | 2 |
| Chemotherapy | 2 |
| Radiation nephritis | 12 |
| Surgical complication | 5 |
| Focal segmental glomerulosclerosis | 18 |
| Hypertension | 7 |
| Diabetes | 1 |
| SMN not WT in remaining kidney | 1 |
| Dysplastic kidney, including horseshoe | 3 |
| Chronic kidney disease etiology unknown | 16 |
| Preemptive Transplant | 1 |
| Unknown | 3 |
| Total | 173 |
WAGR Wilms tumor, aniridia, genitourinary anomalies, retardation; SMN secondary malignant neoplasms; WT Wilms tumor
Statistical methods
Patient survival and mortality rates in different subgroups were analyzed using standard methods for time-to-event data subject to right censorship.(17–19) Relapse-free survival times were calculated as the time from ESRD to subsequent relapse or progression of WT, non-tumor death or loss-to-follow-up, whichever came first. For a few patients whose ESRD onset was prior to WT diagnosis, survival times were left truncated.(20) Results of Cox regression analyses were expressed as hazard ratios (HR), with 95% confidence intervals (CI) and p-values based on score (logrank) or non-proportionality tests. To assess its effect on mortality, transplantation was treated as a time-dependent covariate. (21) The cumulative probability of receiving a renal transplant, accounting for the fact that some patients could not because of prior death, was estimated using competing risks methods.(22) For patients who received a renal transplant, time to graft failure was estimated as the time to subsequent dialysis, second transplant or death, whichever came first. Graft failure rates were analyzed in the same manner as for mortality. Frequency distributions were summarized by the median and inter-quartile range (IQR). Two-way contingency tables were analyzed using exact tests for heterogeneity and trend. All statistical analyses were conducted with the R package. (23)
Results
Patient characteristics and treatments by type of ESRD
The 2 categories of ESRD were similar in gender and ethnicity but differed markedly otherwise (Table 2). Patients whose ESRD was the result of PBWT had younger ages at onset of ESRD (median 4 years, IQR 2–7, vs. 15, IQR 4–22, for CKD) and over 2/3 of their deaths were due to progressive WT or its treatment. For CKD patients 19 deaths, or over 60% of the total, were ascribed to ESRD. Three of these 19 deaths occurred after transplant, 16 while the patient was on dialysis, and they were accompanied primarily by cardiovascular events or sepsis. Nearly half the CKD patients had a WT1 associated congenital syndrome or anomaly -- DDS, WAGR, hypospadias or cryptorchidism -- vs. 15% for PBWT. Six of 28 DDS patients with CKD were treated for ESRD before diagnosis of WT, as was 1 patient on dialysis for hemolytic uremic syndrome at the time of WT diagnosis. Patients with PBWT were more likely to have received peritoneal dialysis than hemodialysis for their initial treatment, consistent with their younger ages at ESRD onset. All 6 cases of anaplastic WT had developed ESRD due to PBWT. Among CKD patients 26 (22%) had bilateral disease at WT diagnosis vs. 37 (67%) among those with PBWT. Fifty (91%) of the PBWT patients developed ESRD within 5 years of WT diagnosis. For CKD only 37 (31%) developed ESRD within this period, 26 of whom had DDS, whereas 30 (25%) developed ESRD more than 20 years following WT.
Table 2.
National Wilms Tumor Study (NWTS) patient characteristics by end stage renal disease (ESRD) type
| Patient characteristic | ESRD-PBWT | ESRD-CRF | All ESRD | ||||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | ||
| Gender | |||||||
| Male | 28 | 50.9 | 54 | 45.8 | 82 | 47.4 | |
| Female | 27 | 49.1 | 64 | 54.2 | 91 | 52.6 | |
| Ethnicity | |||||||
| White, non-Hispanic | 37 | 67.3 | 80 | 67.8 | 117 | 67.6 | |
| African | 11 | 20.0 | 16 | 13.6 | 27 | 15.6 | |
| Hispanic | 6 | 10.9 | 16 | 13.6 | 22 | 12.7 | |
| Other | 1 | 1.8 | 6 | 5.1 | 7 | 4.0 | |
| Age at ESRD (years) | |||||||
| 0–4 | 33 | 50.0 | 32 | 27.1 | 65 | 37.6 | |
| 5–9 | 16 | 29.1 | 11 | 9.3 | 27 | 15.6 | |
| 10–14 | 3 | 5.5 | 16 | 13.6 | 19 | 11.0 | |
| 15–19 | 2 | 3.6 | 17 | 14.4 | 19 | 11.0 | |
| 20–24 | 1 | 1.8 | 26 | 22.0 | 27 | 15.6 | |
| 25+ | 0 | 0.0 | 16 | 13.6 | 16 | 9.2 | |
| Congenital syndrome | |||||||
| DDS | 1 | 1.8 | 28 | 23.7 | 29 | 16.8 | |
| WAGR | 1 | 1.8 | 14 | 11.9 | 15 | 8.8 | |
| GU | 6 | 10.9 | 13 | 11.0 | 19 | 11.0 | |
| None | 47 | 85.5 | 63 | 53.4 | 110 | 63.6 | |
| Onset of ESRD | |||||||
| Before WT diagnosis | 0 | 0.0 | 8 | 6.8 | 8 | 4.6 | |
| During WT treatment | 27 | 49.1 | 16 | 13.6 | 43 | 30.9 | |
| During remission | 28 | 50.9 | 94 | 79.7 | 122 | 70.5 | |
| Laterality of WT | |||||||
| Unilateral | 0 | 0.0 | 89 | 75.4 | 89 | 51.4 | |
| Synchronous bilateral | 37 | 67.3 | 26 | 22.0 | 63 | 36.4 | |
| Metachronous bilateral | 18 | 32.7 | 3 | 2.5 | 21 | 12.1 | |
| Initial ESRD treatment | |||||||
| Peritoneal dialysis | 24 | 42.9 | 41 | 34.7 | 65 | 37.6 | |
| Hemodialysis | 28 | 51.8 | 56 | 47.5 | 84 | 48.6 | |
| Dialysis (unspecified) | 3 | 5.4 | 3 | 2.5 | 6 | 3.7 | |
| Preemptive Transplant | 0 | 0.0 | 18 | 15.2 | 18 | 10.4 | |
| Cause of death | |||||||
| WT/Rx | 23 | 41.8 | 7 | 5.9 | 30 | 17.3 | |
| ESRD | 7 | 12.7 | 19 | 16.1 | 26 | 15.0 | |
| Other | 3 | 5.5 | 4 | 3.4 | 7 | 4.0 | |
| Still living | 22 | 40.0 | 88 | 74.5 | 110 | 63.6 | |
PBWT progressive bilateral Wilms tumor; CRF chronic renal failure; DDS Denys-Drash syndrome; WAGR Wilms tumor, aniridia, genitourinary anomalies, retardation; WT Wilms tumor; GU genitourinary
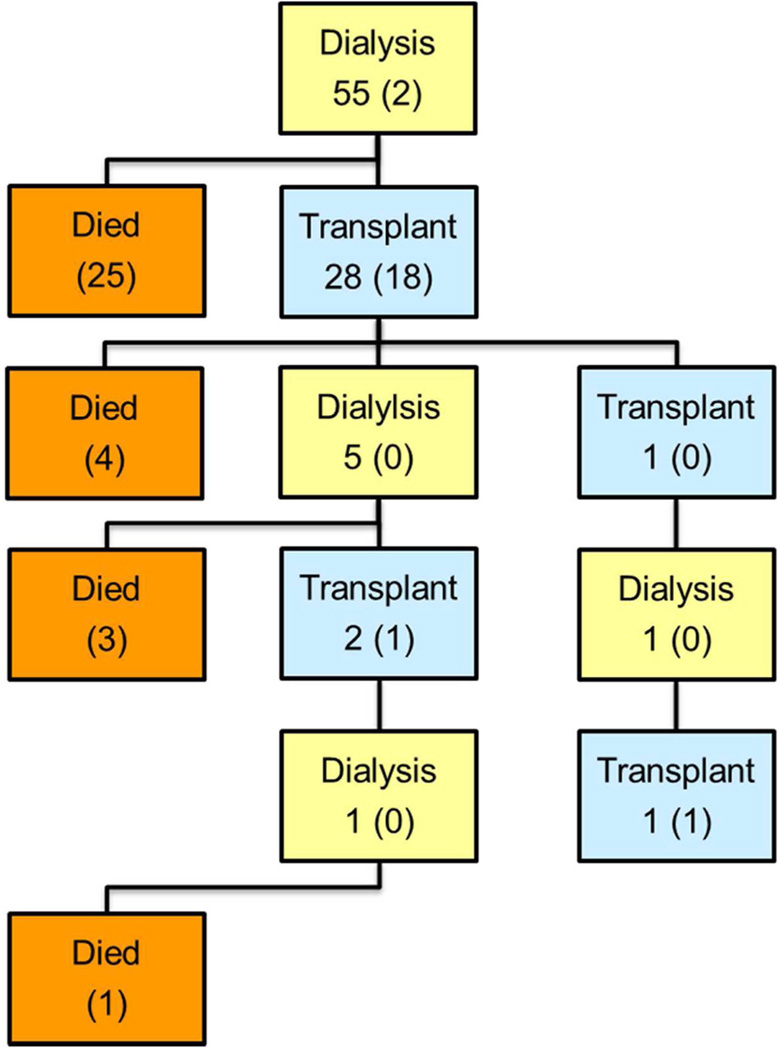
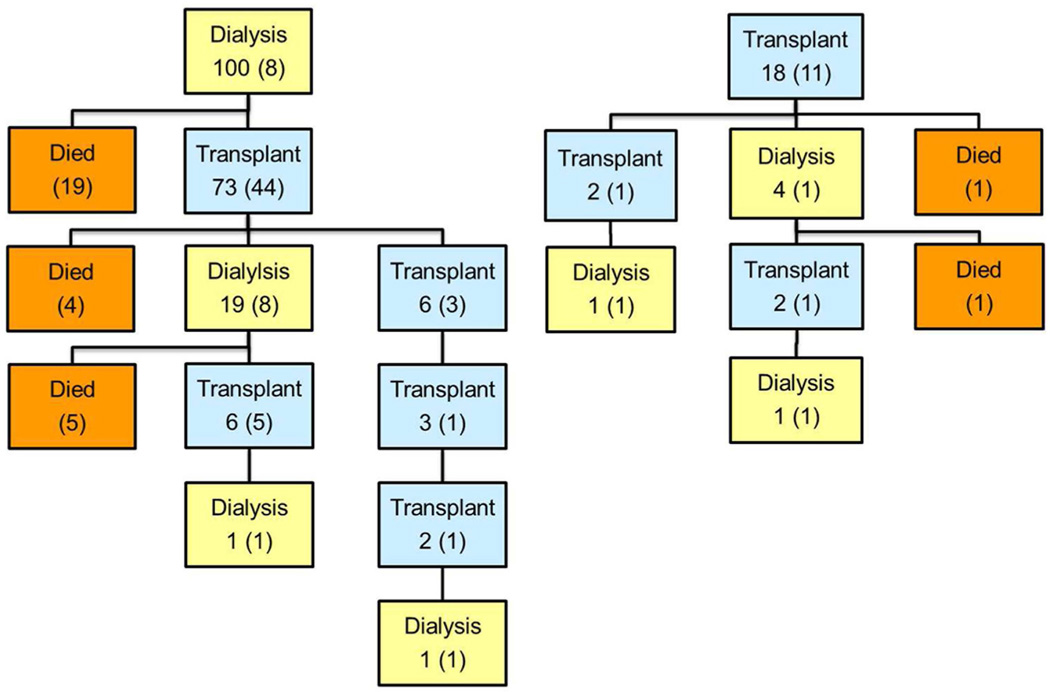
Figure 1 charts the flow of patients through the various stages of treatment. The numbers in parentheses at each stage represent those who remained there at the end of follow-up. The rest moved on to subsequent stages. All 55 patients with PBWT started with dialysis and 2 continued on dialysis until their last follow-up. Twenty-five died before transplantation, at a median of 0.6 years (IQR 0.3–0.8) since ESRD onset, and 28 received a transplant. By summing the numbers in parentheses in each transplant (blue) box, one may calculate that 20 patients with PBWT had a functioning renal transplant at last follow-up whereas 33 died. Eighteen of the 118 patients with CKD received a preemptive transplant and, at the time of last follow-up or closing, 11 of these were still living with the original graft at a median of 7 years since transplant (IQR 2–10). Of 100 CKD patients who started on dialysis, 73 (73%) survived long enough for a transplant and 44 of these were still living with the initial graft at a median of 7 years (IQR 3–10). At last follow-up, 54 of the 100 had a functioning renal transplant, 28 had died and 18 remained on dialysis. Among all 173 NWTS patients, 54 deaths occurred while on dialysis whereas only 9, all following the initial graft, occurred during a period of transplantation.
Figure 1.
Flow charts showing sequence of treatments and survival outcomes for National Wilms Tumor Study (NWTS) patients with end stage renal disease (ESRD). Numbers in parentheses in each box refer to patients who remained in the state indicated by that box at the conclusion of follow-up. A: ESRD due to progressive bilateral Wilms tumor; B: ESRD due to chronic kidney disease.
Time to transplant from ESRD onset
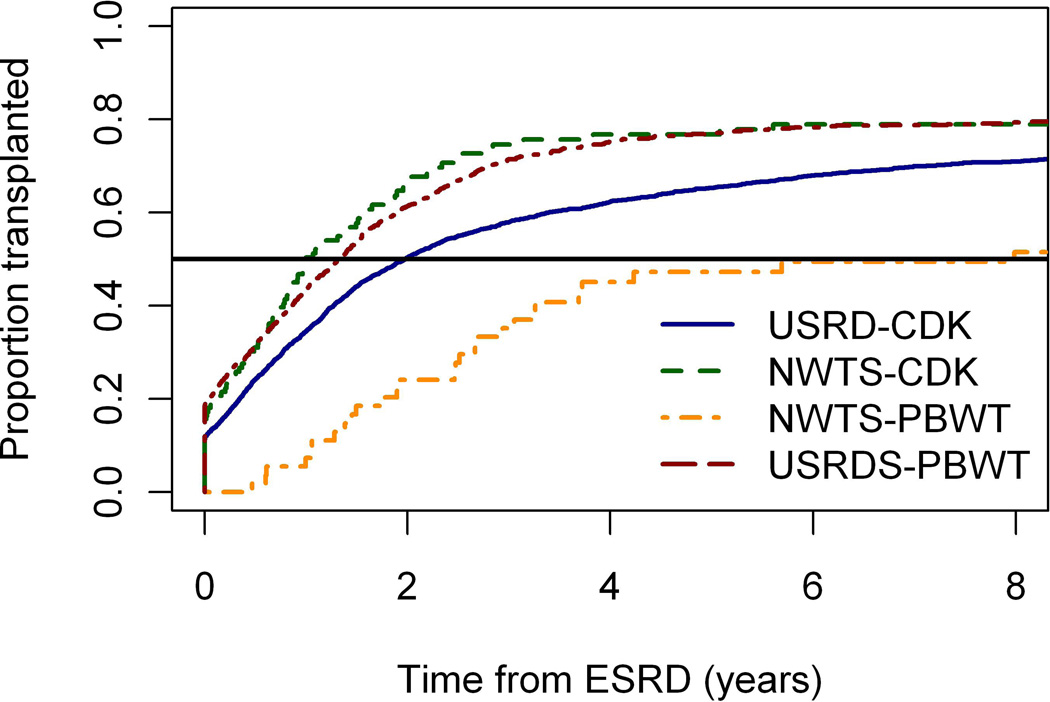
Two DDS patients who received transplants before diagnosis of WT were excluded, leaving 117 who eventually received transplants and 54 who did not for analyses of time from ESRD onset to transplant. Figure 2 graphs the cumulative proportion of patients who received a transplant by time since ESRD onset for 4 comparison groups: the 2 NWTS groups according to type of ESRD (CKD vs PBWT) and the 2 USRDS groups matched on ages at onset of the corresponding NWTS group. The 18 NWTS CKD patients who received preemptive transplants at onset of ESRD, and comparable fractions of the age-matched USRDS patients, account for the jumps at time 0. By 5 years from onset of ESRD, 77% of NWTS CKD but only 47% of NWTS PBWT patients were estimated to have received a transplant. This difference was partially explained by the high early mortality among PBWT patients, 46% of whom were estimated to have died without transplant by 5 years as compared with 16% for NWTS CKD and 13–14% for USRDS. Median time to transplant was longer for the USRDS patients age-matched to the older NWTS CKD cases than for those age matched to the younger NWTS PBWT cases, reflecting the priority given to children in selection of transplant patients. Except for the comparison of NWTS-CKD with USRDS-PBWT, differences in transplant rates between all 4 groups were highly statistically significant (p<0.0001).
Figure 2.
Cumulative incidence curves showing proportions of patients who received an initial transplant by time since onset of end stage renal disease (ESRD). NWTS-CKD and NWTS-PBWT refer to National Wilms Tumor Study (NWTS) patients whose ESRD was due to progressive bilateral Wilms tumor (PBWT) and chronic kidney disease (CDK), respectively. USRD-PBWT and USRD-CKD refer to stratified samples of USRDS (United States Renal Data System) patients that were frequency matched on age at ESRD onset to the corresponding NWTS populations.
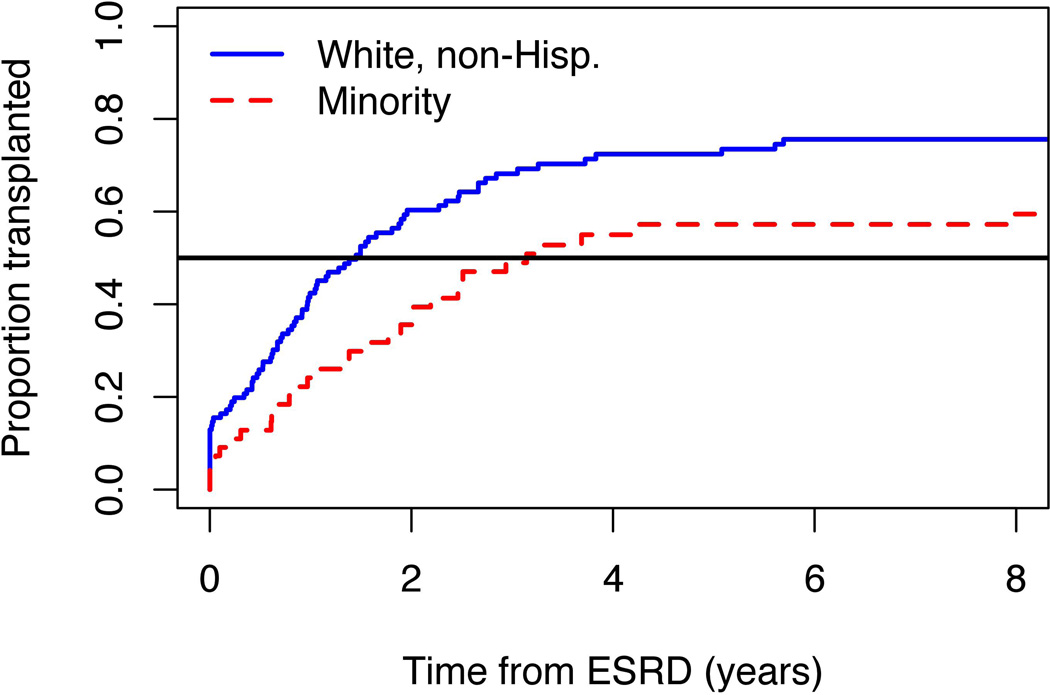
Median time to transplant from ESRD onset was 1.5 years for NWTS non-Hispanic whites and 3.1 for minorities (Figure 3). The transplant (“hazard”) rate ratio was 1.7 (95% CI 1.1–2.6, p=0.011) for whites vs. minorities. Excluding 19 patients for whom donor type (living vs. cadaveric) was unknown, non-Hispanic whites were more likely to have received an initial transplant from a living donor (56/73=77%) than were minorities (15/25=60%), but this difference was not statistically significant (p=0.12). Similar, highly statistically significant ethnic disparities in timing of transplantation and donor type were observed for USRDS patients (data not shown).
Figure 3.
Cumulative incidence curves showing proportions of National Wilms Tumor Study (NWTS) patients who received an initial transplant by time since onset of end stage renal disease (ESRD), separately for non-Hispanic Whites and all other ethnic groups.
Timing of transplant in relation to chemotherapy
Eighty-five patients, 52 PBWT and 33 CKD, developed ESRD within 2 years of chemotherapy for WT and thus might have been considered for kidney transplant within that period. One patient received a transplant while being treated for WT, 9 within 1 year after chemotherapy and 17 more within 2 years, A variety of extenuating circumstances may have influenced the decision to transplant during or soon after chemotherapy. One early NWTS patient received chemotherapy and radiation for “relapse” to the contralateral kidney. Two months later a radical nephrectomy revealing completely necrotic tumor rendered the patient anephric. Chemotherapy was continued for 15 months, during which period a transplant was performed. Four patients received transplants within 6 months of ending chemotherapy. For 2 of these, transplants were performed following, respectively, respiratory arrest and congestive heart failure that occurred while dialysis and chemotherapy were administered simultaneously. One DDS patient was transplanted following hospital admission for aggressive fungal treatment and problems with catheter management while on dialysis; she died 7 years later of viral pneumonia.
Graft failure
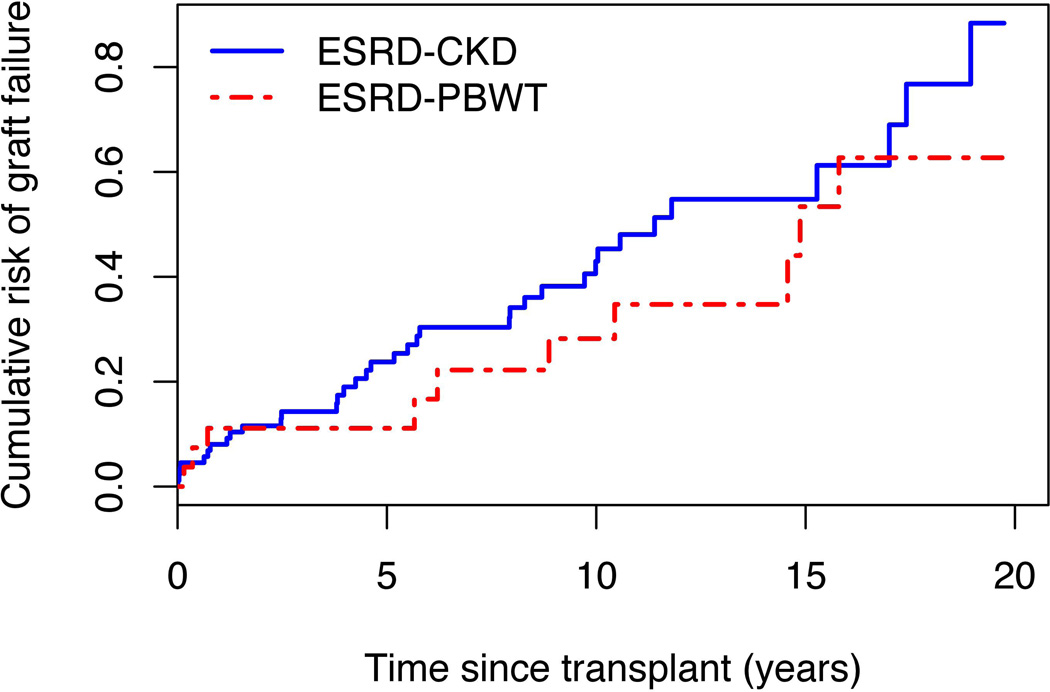
One NWTS patient lost to follow-up immediately upon transplantation was excluded, leaving 118 for this analysis. Graft failure occurred by virtue of subsequent dialysis for 28 patients, a 2nd transplant for 9 and death for 9. Three deaths, 1 following treatment for a late WT relapse, were ascribed to infection, 3 to ESRD and 1 each to congestive heart failure, myocardial infarction and cystic fibrosis. CKD patients had moderately higher rates of graft failure compared to PBWT (HR=1.5, 95% CI 0.7–3.0, p=0.29) but the difference was not statistically significant (Figure 4). Differences in time to graft failure according to donor type were dramatic, with an estimated median of 16 years for patients who received their transplant from a live donor vs. 8 years for cadaveric grafts or those of unknown donor type (p=0.004). Only 22 (19%) first transplants were recorded prior to 1990 as opposed to 48 (41%) during the 1990’s and 48 (41%) between 2000 and closing. Although the graft failure rates declined by 20% on average from one period to the next, the differences were not statistically significant (HR=0.8 per period, 95% CI 0.5–1.2, p-trend=0.29) The risk of graft failure by 5 years from transplant for those who received their transplant after 2000, namely 24.8% (95% CI 4.0–41.1), was actually higher than the corresponding risks for those transplanted during the 1990’s (23.5%, CI 10.3–34.8) or earlier (14.3%, CI 0–28.0). However, the confidence intervals were very wide and overlapping, indicating the limitations of the data to evaluate this question.
Figure 4.
Proportions of transplanted National Wilms Tumor Study (NWTS) patients whose grafts failed by time since transplant, separately for patients whose end stage renal disease (ESRD) was due to progressive bilateral Wilms tumor (PBWT) and chronic kidney disease (CKD).
Minorities had higher rates of graft failure than non-Hispanic whites (HR=1.9, 95 % CI 1.0–3.5, p=0.049). When adjusted for the discrepancy in donor type, however, the HR for minorities dropped to 1.5 (95% CI 0.8–3.0) and was no longer statistically significant (p=0.19).
Omitting 1 transplanted patient with DDS who never received chemotherapy, the number of graft failures vs. number expected by logrank analysis were 7 (1 death, 6 dialysis) vs 7.25 expected for those receiving transplants before 1 year after chemotherapy, 4 (2 deaths, 2 re-transplanted) vs. 7.57 for those transplanted between 1 and 2 years, and 34 (6 deaths, 22 dialysis, 6 re-transplanted) vs. 30.2 for those transplanted later (p=0.33). The HR for the first 2 categories relative to the 3rd were 0.9 (95% CI 0.4–2.0) and 0.5 (0.2–1.3), respectively.
Secondary Malignant Neoplasms
Seven secondary malignant neoplasms (SMN) were identified among the 173 patients in this study. Three occurred prior to onset of ESRD: a basal cell skin cancer diagnosed in the radiation field a year prior to onset of ESRD and 29 years following WT diagnosis; a papillary/follicular thyroid carcinoma that occurred 2 years prior to ESRD onset and 7 years following diagnosis and treatment with 27 Gy to the left flank of an anaplastic WT; and a rhabdomyosarcoma of the kidney that was an incidental finding following removal of the 2nd kidney 1.5 years after WT diagnosis due to PBWT. Two additional papillary thyroid carcinomas were diagnosed 8.7 and 4.6 years following ESRD onset, both following transplant at 1.3 and 0 years, respectively, as was a lymphoma diagnosed at 1.9 years after ESRD onset and 0.4 years after transplant. A PNET tumor of the ribs occurred 9.8 years after ESRD and 1.2 years prior to transplant. There was no evidence, based on these very small numbers, that the SMN rate increased following transplant (HR=0.7, 95% CI 0.1–6.9, p=0.80).
Relapse-free and overall survival
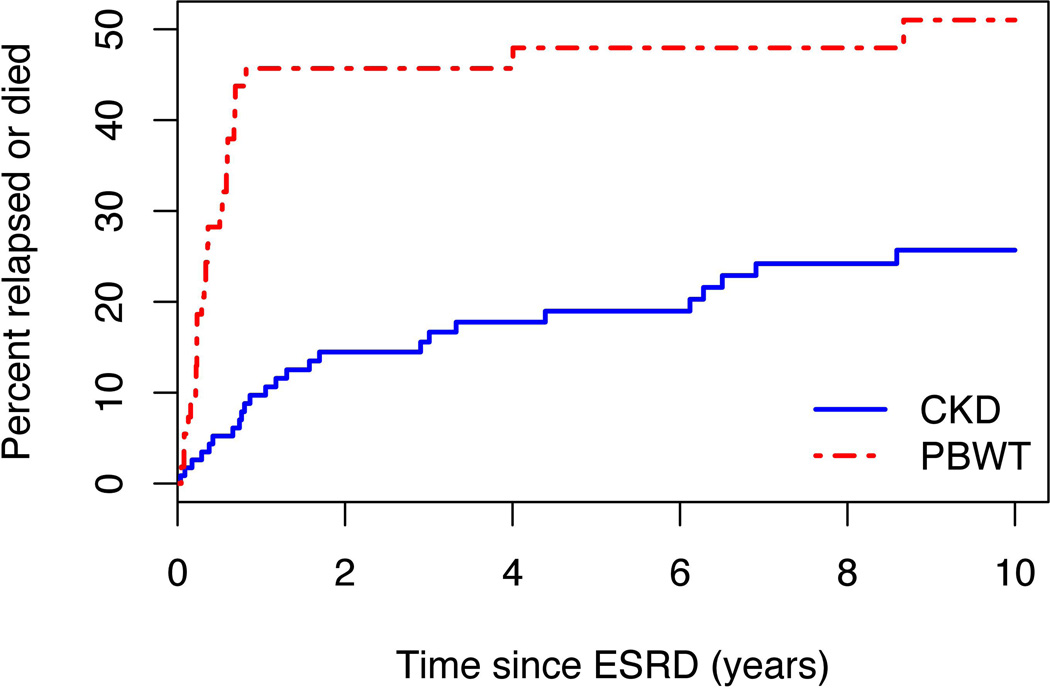
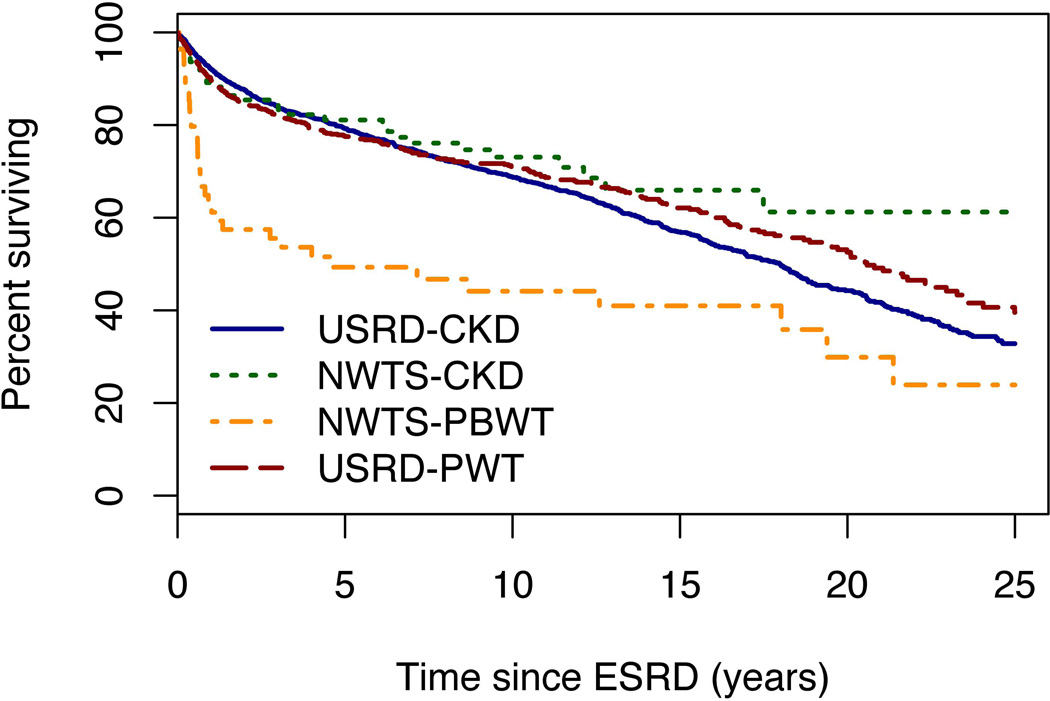
NWTS patients with ESRD due to PBWT had substantially higher relapse and mortality rates than those with ESRD due to CKD (Figures 5 and 6). Hazard ratios were 3.0 and 2.7, respectively, and highly statistically significant (p<0.0001). All but 1 of the 24 events that occurred among PBWT patients prior to 1 year from ESRD (Figure 5) were relapse or progression of WT; a single non-tumor death was due to SMN. All 5 events after 1 year were non-tumor deaths, 1 from ESRD at 4 years and 4 more beyond 5 years. For CKD patients, by contrast, only 4 of the 11 events prior to 1 year from ESRD were relapse or progression of WT. Estimated survival proportions at 5 and 10 years, respectively, were 81% and 73% for CKD vs. 49% and 44% for PBWT (Figure 6). The survival curves for the USRDS patients were both close to that for NWTS patients with ESRD due to CKD, and none of the pairwise differences between these 3 curves was statistically significant.
Figure 5.
Cumulative risk of subsequent relapse, disease progression or death, whichever came first, for Wilms tumor (WT) patients from onset of end stage renal disease (ESRD). Progressive bilateral Wilms tumor (PBWT) refers to ESRD due to progressive bilateral WT, chronic kidney disease (CKD) to ESRD due to chronic kidney disease.
Figure 6.
Survival curves (proportions of surviving patients) by time since onset of end stage renal disease (ESRD) for four groups. NWTS-CKD and NWTS-PBWT refer to National Wilms Tumor Study (NWTS) patients whose ESRD was due to progressive bilateral Wilms tumor (PBWT) and chronic kidney disease (CKD), respectively. USRD-PBWT and USRD-CKD refer to stratified samples of USRDS (United States Renal Data System) patients that were frequency matched on age at ESRD onset to the corresponding NWTS populations.
Three, 2 and 14 deaths occurred, respectively, for patients who received transplants within 0–1, 1–2 and 2+ years of chemotherapy. The HR for the first 2 categories relative to the 3rd were 0.9 (95% CI 0.3–3.3) and 0.6 (0.1–2.6), respectively (p=0.78).
Only 1 patient relapsed following transplantation, to the original tumor bed and liver at 5 years from transplant and 17 years from WT diagnosis, and he died 2 years later. Eighteen additional deaths (12 from ESRD) occurred in transplanted patients. Nonetheless, mortality rates were reduced by 84% (HR=0.16, 95% CI 0.07–0.38, p=0.0003) following the initial transplant compared with those of the same ESRD type (PWBT or CKD) who remained on dialysis. The reduction in mortality was about the same when only deaths due to ESRD were considered (HR=0.18, 95% CI 0.05–0.60, p=0.021). Mortality following transplant was greater (HR=1.7, 95% CI 0.7–4.3, p=0.13) for patients with ESRD due to PBWT compared to those with ESRD due to CKD, but there were only 8 and 11 post-transplant deaths in these 2 groups, respectively, and the difference was not statistically significant.
Minorities had higher overall mortality than non-Hispanic whites (HR=1.9, 95% CI 1.2–3.1, p=0.01), a result not affected by stratification on ESRD type (PBWT vs. CKD). A similarly high HR for minorities was observed for USRDS patients (data not shown). When adjusted for their delayed times to transplant, however, the mortality rate ratio for NWTS minorities was reduced (HR=1.7, 95% CI 1.0–2.7, p=0.04) and of borderline statistical significance.
Discussion
Treatments and outcomes differed dramatically between WT patients who developed ESRD following surgical removal of all renal tissue to treat progressive disease (PBWT) and those who developed ESRD as a result of other pathologies (CKD). Most patients with PBWT developed their ESRD while still receiving or shortly after completion of chemotherapy. A large fraction (25/55=45%) died while on their initial ESRD treatment with dialysis (Figure 1a), all but 4 of these deaths due to WT. Fewer than half lived long enough to receive a transplant (Figures 1a and 2) and half had died within 5 years of onset of ESRD (Figure 6). By contrast, patients who developed ESRD due to CKD, in most cases long after they had been cured of WT, had a good opportunity to receive a transplant: an estimated 66% within 2 years and 79% within 5 years of ESRD onset (Figures 1b and 2). Their overall survival was estimated to be 81% at 5 years and 73% at 10 years (Figure 6). Once a transplant had been performed, however, the differences in outcomes between the CKD and PBWT groups were markedly reduced. In fact the CKD group had slightly higher risk of graft failure (Figure 4) but better post-transplant survival, neither comparison being statistically significant. Once the WT has been “cured”, the risk of graft failure due to recurrent disease has been removed. Since very few WT relapses occur following transplant, both groups face similar challenges.
Patients with ESRD due to PBWT also had markedly worse early mortality, most of it due to progressive WT, in comparison with age-matched population controls (Figure 6). This resulted in many fewer of them receiving transplants (Figure 2). Patients with ESRD due to CKD, by contrast, were a bit more likely than their population counterparts to receive a transplant and their survival experience was comparable to that of controls. Age matching of the controls to the respective NWTS group was important in view of the dramatic effects of age-at-onset on treatment and survival of patients with ESRD.(24) The youngest USRDS patients have relatively high mortality rates which then decline but increase progressively with age at ESRD above 10 years. The situation is similar for time to transplant. Median time to transplant for USRDS is about 1.7 years for children aged 0–4 and 10–19 years at ESRD onset, under 1 year for those aged 5–9 and approximately 5 years for young adults aged 20 and above. Transplant for the youngest patients is often delayed until they have grown large enough to accommodate a live adult graft, while those 18 or older no longer have priority for a cadaveric graft. These results may also be influenced by the differential mortality such that some of those with the highest early mortality, notably in the 0–4 and 20+ age groups, never have the opportunity for a transplant. More detailed study of the USRDS population has been conducted by others.(12) The USRDS data were used here only to provide an age-matched control group for patients with WT.
NWTS patients with ESRD who belonged to a minority group had nearly twice the graft failure and mortality rates of non-Hispanic Whites and their median time to first transplant was twice as long (Figure 3). This finding is consistent with general population trends. African, Asian and Hispanic Americans with ESRD all have lower rates of transplantation than non-Hispanic whites.(25;26) African Americans with ESRD have higher rates of overall mortality than whites, a result confirmed by analysis of the USRDS controls used in this study.(27) While the disparity between mortality rates for non-Hispanic whites and minorities in the NWTS population was reduced after adjusting for time to initial transplant, and was no longer statistically significant, it did not disappear. This may reflect continuing medical and social disparities in access to management of ESRD.
Comparisons of graft failure and mortality rates following the initial transplant did not reveal any differences according to the time between cessation of chemotherapy for WT and the transplant. However, very small numbers of patients were available: 10 who received a transplant within 1 year and 17 transplanted between 1–2 years following chemotherapy. The numbers of events, deaths or graft failures, within these groups were even smaller. Furthermore, patients who received early transplants could well have been selected on the basis of characteristics associated with their treatment outcomes. While it would be unwise, therefore, to attempt to draw firm conclusions from these data alone as regards the suitability of the current guidelines that mandate a 1 or 2 year waiting period, there is also nothing in the study data to suggest a markedly adverse prognosis associated with early transplant. Patients with ESRD following “low risk” WT, i.e. Stage I or II with “favorable histology”(28) who remained disease free for 6 months following treatment, might be considered candidates for transplant. Indeed, such a criterion was explicitly mentioned in clinical notes to justify an early transplant for 1 patient whose disease-free contralateral kidney had been severely damaged during surgery. Since relapses beyond 1 year from ESRD were non-existent in patients with PBWT, and rare in those with CKD, earlier transplants might be considered also in higher risk patients. Unfortunately, however, firm statistical evidence to support early transplant is lacking due to the small numbers of patients who have received early transplant to date.
Too few patients were available for meaningful analysis of the effect of transplant on subsequent rates of SMN. Confidence limits for the HR (0.1–6.9) were compatible with a wide range of values, including the 2.1 fold SMN increase recently reported for US solid organ transplant recipients in comparison with the general population.(29)
In summary, except as explained by the high risk of early death from progressive disease in patients whose ESRD was due to PBWT, access to renal transplantation for NWTS patients who developed ESRD was better than for age-matched population controls and long term survival was at least as good. The well known and dramatic improvement in survival prospects following transplantation was confirmed to hold also for the small population of WT survivors. Since mortality for ESRD patients in general, and NWTS-ESRD patients in particular, remains substantial, these findings suggest that WT patients who subsequently have ESRD can immediately be considered candidates for kidney transplant and not subject to a 2 year wait time
Acknowledgment
This study was supported in part by grant CA054498 from the United States National Cancer Institute. We thank investigators of the Children’s Oncology Group and the health professionals who managed the care of children entered in the National Wilms Tumor Study.
Footnotes
Notice: Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Reference List
- 1.Ehrlich PF. Wilms tumor: Progress and considerations for the surgeon. Surg Oncol. 2007;16:157–171. doi: 10.1016/j.suronc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Dome JS, Perlman EJ, Ritchey ML, Coppes MJ, Kalapurakal JA, Grundy PE. Renal Tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Fifth ed. Philadelphia: Lippincott, Williams & Wilkins; 2005. pp. 905–932. [Google Scholar]
- 3.Smith MA, Seibel NL, Altekruse SF, Ries LAG, Melbert DL, O'Leary M, Smith FO, Reaman GH. Outcomes for Children and Adolescents With Cancer: Challenges for the Twenty-First Century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groothoff JW. Long-term outcomes of children with end-stage renal disease. Pediatr Nephrol. 2005;20:849–853. doi: 10.1007/s00467-005-1878-9. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro R, Sarwal MM. Pediatric kidney transplantation. Pediatr Clin North Am. 2010;57:393–400. doi: 10.1016/j.pcl.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Demaria JE, Hardy BE, Brezinski A, Churchill BM. Renal transplantation in patients with bilateral Wilms tumor. J Pediatr Surg. 1979;14:577–579. doi: 10.1016/s0022-3468(79)80143-8. [DOI] [PubMed] [Google Scholar]
- 7.Penn I. Renal transplantation for Wilms tumor - Report of 20 cases. J Urol. 1979;122:793–794. doi: 10.1016/s0022-5347(17)56607-0. [DOI] [PubMed] [Google Scholar]
- 8.EBPG Expert Group. SECTION IV: Long-term management of the transplant recipient. Nephrol Dial Transplant. 2002;17:1–67. [PubMed] [Google Scholar]
- 9.Nankivell BJ, Kuypers DRJ. Organ Transplantation 3 Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378:1428–1437. doi: 10.1016/S0140-6736(11)60699-5. [DOI] [PubMed] [Google Scholar]
- 10.Geissler EK. Can Immunosuppressive Strategies Be Used to Reduce Cancer Risk in Renal Transplant Patients? Transplant Proc. 2010;42:S32–S35. doi: 10.1016/j.transproceed.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 11.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 12.Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA. Change in Mortality Risk Over Time in Young Kidney Transplant Recipients. Am J Transplant. 2011;11:2432–2442. doi: 10.1111/j.1600-6143.2011.03691.x. [DOI] [PubMed] [Google Scholar]
- 13.Rudin C, Pritchard J, Fernando ON, Duffy PG, Trompeter RS. Renal transplantation in the management of bilateral Wilms' tumour (BWT) and of Denys-Drash syndrome (DDS) Nephrol Dial Transplant. 1998;13:1506–1510. doi: 10.1093/ndt/13.6.1506. [DOI] [PubMed] [Google Scholar]
- 14.Kist-van Holthe JE, Ho PL, Stablein D, Harmon WE, Baum MA. Outcome of renal transplantation for Wilms' tumor and Denys-Drash syndrome: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant. 2005;9:305–310. doi: 10.1111/j.1399-3046.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 15.Lange J, Peterson SM, Takashima JR, Grigoriev YA, Ritchey ML, Shamberger RC, Beckwith JB, Perlman E, Green DM, Breslow NE. Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J Urol. 2011;186:378–386. doi: 10.1016/j.juro.2011.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. pp. 1–421. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:456–481. [Google Scholar]
- 18.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Roy Stat Soc A. 1972;135:185–206. [Google Scholar]
- 19.Cox DR. Regression models and life-tables (with discussion) J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.Woodroofe M. Estimating a distribution function with truncated data. Ann Statist. 1985;13:163–177. [Google Scholar]
- 21.Crowley J, Hu M. Covariance analysis of heart transplant survival data. J Am Stat Assoc. 1977;72:27–36. [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 24.US Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. Pediatric ESRD; pp. 325–334. [Google Scholar]
- 25.Stolzmann KL, Bautista LE, Gangnon RE, McElroy JA, Becker BN, Remington PL. Trends in kidney transplantation rates and disparities. J Natl Med Assoc. 2007;99:923–932. [PMC free article] [PubMed] [Google Scholar]
- 26.Gadegbeku C, Freeman M, Agodoa L. Racial disparities in renal replacement therapy. J Natl Med Assoc. 2002;94:45S–54S. [PMC free article] [PubMed] [Google Scholar]
- 27.US Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. Morbidity and Mortality; pp. 269–280. [Google Scholar]
- 28.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumor. Cancer. 1978;41:1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]