Abstract
Duplications leading to functional disomy of chromosome Xq28, including MECP2 as the critical dosage-sensitive gene, are associated with a distinct clinical phenotype in males, characterized by severe mental retardation, infantile hypotonia, progressive neurologic impairment, recurrent infections, bladder dysfunction, and absent speech.
Female patients with Xq duplications including MECP2 are rare. Only recently submicroscopic duplications of this region on Xq28 have been recognized in four females, and a triplication in a fifth, all in combination with random X-chromosome inactivation (XCI). Based on this small series, it was concluded that in females with MECP2 duplication and random XCI, the typical symptoms of affected boys are not present. We present clinical and molecular data on a series of five females with an Xq28 duplication including the MECP2 gene, both isolated and as the result of a translocation, and compare them with the previously reported cases of small duplications in females. The collected data indicate that the associated phenotype in females is distinct from males with similar duplications, but the clinical effects may be as severe as seen in males.
Keywords: MECP2 duplication, Xq28 duplication, X-inactivation, Mental retardation in females, IRAK1
1. Introduction
Duplications leading to functional disomy of chromosome Xq28 are associated with a distinct clinical phenotype in males, characterized by severe mental retardation, infantile hypotonia, progressive neurologic impairment, recurrent infections, bladder dysfunction, and absent speech (see [1] and [2] for reviews). This combination of features is known as the Xq28 microduplication syndrome or Lubs syndrome and was first described by Pai et al. and Lubs et al.[3,4], followed by many others. The reported Xq28 duplications vary in size and location, however the majority are intrachromosomal duplications ranging from 0.3 to 2.3 Mb. Alternatively, duplications may be the result of another mechanism, such as rearrangements between Xq and Xp, or between Xq and the Y chromosome [5]. The methyl-CpG binding protein (MECP2) gene on Xq28 is proposed to be the critical dosage-sensitive gene responsible for the severe phenotypes observed in patients with this duplication. In males, over 140 cases of Xq28 duplications including MECP2 have been described to date [5–24].
Female patients with an Xq28 duplication are rare. In families with X-linked pedigrees, female carriers of an Xq duplication are usually asymptomatic, due to skewed X-chromosome inactivation (XCI) pattern with preferential inactivation of the rearranged chromosome. Until recently, only three symptomatic females had been reported with large, cytogenetically visible Xq28 duplications (summarized in [21]). Clinically, these females presented with severe developmental delay and other features similar to those observed in affected males. In all cases the duplication was the result of an unbalanced X-autosome translocation, explaining the absence of skewing of the aberrant X-chromosome and the effect on the phenotype. After introduction of array CGH analysis, smaller, submicroscopic duplications of this region on Xq28 have been detected in four females, all in combination with random XCI [25–27]. The patient with a MECP2 duplication described by Ariani et al. [28] should not be included in this series, as the patient turned out to have a 47,XXX karyotype (F. Ariani, personal communication). Based on this small series, it was concluded that in females with MECP2 duplication and random XCI, the typical symptoms of affected boys are not present [26]. Instead, the clinical signs in female patients consist of unspecific mild to moderate mental retardation, combined with variable symptoms (autistic features, recurrent infections in early childhood, constipation, and late-onset neurological features). Recently, a girl with a de novo complex X-chromosomal rearrangement was described, with a triplication of a fragment containing MECP2. She was severely mentally retarded and had seizures, but no other symptoms as seen in boys with a duplication [29].
Here we present clinical and molecular data on a series of five females with an Xq28 duplication including the MECP2 gene and these are compared with the previously reported cases of small duplications in females. In patient 1, a MECP2 duplication was the result of a rare insertion duplication of a small segment of Xq28 into an autosome. Patients 2 and 3 are functionally disomic for region Xq28 due to an unbalanced X-autosome translocation. The fourth patient is a mildly affected obligate carrier from an X-linked mental retardation (XLMR) family. We detected a familial, intrachromosomal Xq duplication including MECP2 in her, but unlike previously reported carrier females in X-linked families, she showed random X-inactivation. Patient 5 carries a small intrachromosomal de novo duplication of Xq28, including MECP2. In contrast to the previously reported series of affected females, our series includes a female patient with the typical symptoms of affected boys, therefore expanding the phenotypic spectrum of small Xq28 duplications including MECP2 in females.
2. Methods
2.1. Patients
Patients were evaluated in a diagnostic setting because of mental retardation (in the family). Standard karyotyping was performed by G-banding. Genomic DNA was extracted from whole blood using standard procedures.
2.2. Array platforms
Duplications were either characterized by Affymetrix Genome-Wide SNP Array 6.0 (Affymetrix Inc, Santa Clara, California, USA; Patients 1 and 5) or Agilent 44K array (Agilent, Santa Clara, California, USA; patient 2 and 3) according to the manufacturer's instructions. The array design for patient 2 was modified for constitutional cytogenetic applications (see design specification at: http://www.ngrl.org.uk/wessex/arraycgh.htm). Data analysis was carried out with Genotyping Console 3.0.2 (patient 1) or Agilent's Analytics (patient 2 and 3). Patient 4 was characterized using an X-chromosome specific tiling-path array as described previously [30].
2.3. Multiplex Ligation Probe Amplification (MLPA)
MLPA was performed with a specific kit for Rett syndrome (Salsa P015, MRC Holland, Amsterdam, The Netherlands) according to the manufacturer's instruction. Data analysis was performed with GeneMarker software (Softgenetics, USA).
2.4. Fluorescent In Situ Hybridisation (FISH)
FISH analysis was carried out by standard procedures, using BAC clones selected within the duplicated and deleted regions (patients 1, 2, 5) and TelVision subtelomeric Xq probe (Vysis) (patient 3).
2.5. X-inactivation studies
In patients 1, 2, 4, and 5, X-chromosome inactivation was studied using the highly polymorphic small tandem repeat within the human androgen receptor gene, as described by Allen et al. [31]. Inactivation was considered to be random if the ratio of active to inactive X was less than 75:25. Extreme skewing of X inactivation was defined as the preferential inactivation of one X chromosome in 90–95% of cells [32].
3. Results
Table 1 provides a summary of the cytogenetic and phenotypic characteristics on five female patients with a MECP2 duplication. All patients showed a normal karyotype by G-banding.
Table 1.
Comparison of genotype and phenotype in males and females with submicroscopic Xq28 duplications, including MECP2.
| Case ID | Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 142a | Auber et al., 2009[25] | Makrythanasis et al., 2010[27] | Reardon et al., 2010[20] |
Grashoff et al., 2011[26] |
Mayo et al., 2011[29] | This study |
||||||
| III-4 in family 1 | Patient 1 | Patient 2 | 1 | 2 | 3 | 4 | 5 | |||||
| Cytogenetic characteristics | ||||||||||||
| Size of Xq duplication | 3.81 Mb | 129 kb | 0.7 Mb (minimum) | 266 kb | 478 kb | 300 kb | 279 kb | 1.69 Mb | 2.10 Mb | 700 kb | 107.5 kb | |
| Chromosomal rearrangementb | t(X; A) | X → A | dup X | dup X | dup X | complex (trip X/dup X) |
X → A | t(X; A) | t(X; A) | dup X | dup X | |
| Inheritance | d.n. | d.n. | familial | d.n. | d.n. | d.n. | d.n. | d.n. | d.n. | familial | d.n. | |
| Parental origin | mat | pat | pat | pat | pat | mat | ||||||
| 2nd alteration | del 17p13.3 (d.n.) | – | – | dup 2q37.3 (d.n.) |
– | – | dup 3q (d.n.) | del 21q (d.n.) | – | – | dup 2q (pat) | |
| X-inactivation | Random | 70:30 | 39:61 | 29:71 | 75:25 | 65:35 | 63:37 | 84:16 | ||||
| Clinical features | ||||||||||||
| Age at last examination (y) | 9 10/12 | 19 | 12 | 7 6/12 | 20 | 7 | 9 | 4 6/12 | 6 5/12 | 26 | 7 | |
| Mental retardation | 141/142 | + | + | + | + | + | + | + | + | + | – | + |
| Learning difficulty | + | |||||||||||
| Hypotonia | 102/111 | + | + | – | – | + | – | + | – | + | ||
| Absent/delayed speech | 82/93 | + | – | – | + | + | + | + | + | – | + | |
| Lack/loss of ambulation | 32/90 | – | – | – | – | – | + | – | – | – | ||
| Recurrent infections | 99/120 | + | – | – | + | – | + | + | + | – | + | |
| Breathing abnormalities | 7/19 | + | – | – | – | + | – | |||||
| Stereotyped hand movements | 23/47 | + | – | – | + | + | – | – | ||||
| Autistic features/autism | 16/21 | – | + | + | – | + | + | – | ||||
| Epilepsy | 65/132 | + | – | – | – | – | + | + | – | – | – | – |
| GU abnormalities | 29/67 | + | + | + | – | |||||||
| Death < 25 y | 27/73 | – | ||||||||||
| Spasticity | 46/76 | – | – | – | + | – | – | |||||
| Ataxia | 22/41 | – | – | + | + | – | ||||||
| GER | 18/31 | – | – | |||||||||
| Swallowing difficulties/feeding problems | 29/57 | + | + | – | + | – | ||||||
| IPO or constipation | 33/45 | – | + | – | + | – | + | + | + | |||
| Dysmorphic features | 53/57 | + | – | – | – | – | + | + | – | + | – | – |
| Laryngomalacia | + | + | ||||||||||
d.n., de novo; +, present; –, absent (as stated in paper, or deduced from normal results); empty field, no data; mat, maternal; pat, paternal; GU, genitourinary; GER, gastoesophageal reflux; IPO, intestinal pseudo-obstruction.
based on the compiled data of affected males summarized in [2] (n = 119) [5], (n = 6) [6], (n = 4) [7], (n = 1) [8], (n = 1) [9], (n = 3) [13], (n = 1), and [20] (n = 7).
t(X; A), unbalanced X; autosome translocation; X → A, insertion of Xq into an autosome; dup X, intrachromosomal duplication of Xq; trip X, intrachromosomal triplication of Xq.
3.1. Case reports
Patient 1 is a 10-year old girl, first born to a 24-year old mother and a 25-year old father. Parents are healthy and non-consanguineous. The maternal grandfather developed epilepsy around the age of 40 years, otherwise the family history is unremarkable.
The patient was born at term with a birth weight of 3040 g (−1 SDS). Apgar scores were 8 and 10 at 1 and 5 min, respectively.
It was noted early on that her neuromotor development was slow (rolling over at 10 months of age, standing and walking a few steps (both with support) at the age of 3.5 years). She developed no speech. In the first year of life she made little contact, but this improved gradually and she became a friendly, sociable girl. She disliked changes in daily routine and loved spinning toys.
Her health is severely compromised. She had noisy breathing from birth on and laryngoscopy at the age of 2.5 years showed laryngomalacia. In infancy she was repeatedly admitted because of recurrent infections (at 7 years of age she had had several episodes of pneumonia (13 times), otitis media (five times), and pyelonephritis (twice)). She had surgery for reimplantation of the right urether. Her deciduous teeth did not fall out spontaneously, resulting in a double row of teeth, and had to be removed surgically.
At the age of 8 years and 2 months she was operated because of a luxation of the right hip and a subluxation of the left hip. Afterwards her condition deteriorated, she lost the ability to crawl and to walk with support. At the age of 8.5 years she developed febrile convulsions, followed by seizures and she was diagnosed with Lennox–Gastaut type of epilepsy, with a poor response to medical treatment. She has severe constipation, sleeping problems (refusing to go back to sleep after waking up in the middle of the night), and feeding problems (unable to eat solid food). She had a nasogastric tube for fluid, extra feeding and medication. At the age of 10 years she needed a PEG tube. Placement was complicated and prolonged due to an aberrant position of the liver (ventral to the stomach).
At 8 years of age she was able to communicate using pictures, she has limited understanding of sign language. After the hip surgery and development of epilepsy she lost most of these abilities. Also from that time on, parents noted loss of appetite, loss of energy and reported her to be increasingly lethargic. She showed a delayed reaction to stimuli. She was hypersensitive to loud noise and sunlight.
IQ testing using Kent Infant Development Scale confirmed severe developmental delay and regression (at the ages of 18, 32, 68, and 108 months, her development was conform 7, 8, 12, and 9 months, respectively).
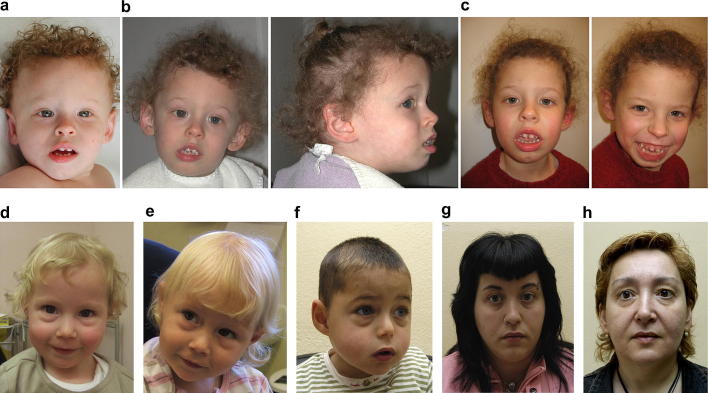
When first assessed at the age of 17 months, her head circumference was 50.5 cm (+2.3 SDS). She showed mild dysmorphic features: prominent broad and high forehead, thin curly hair, telecanthus and epicanthus, thin eyebrows, small nose, full cheeks, open mouth with everted lower lip, broad alveolar ridges, narrow palate, widely spaced teeth, slightly pointed ears, short neck and inverted nipples (Fig. 1a).
Fig. 1.
Phenotypical characteristics of individuals with an Xq28 duplication including MECP2. a-c. patient 1, aged 1 year and 5 months (a), 3 years and 7 months (b) and 7 years (c). Note facial hypotonia, large mouth, and widely spaced teeth. d,e. patient 2, aged 2 years and 8 months (d) and 4 years and 6 months (e). f. Index patient in the described XLMR-family, his mother, patient 4 (g) and grandmother (h). Overall, patients show no common facial features, though patient 2 (c,d) and the index patient in the described XLMR-family (f) show almost identical prominent infraorbital fullness.
On examination at the age of 3 years and 7 months her height was 98 cm (−0.8 SDS), her weight 16.4 kg, (+0.7 SDS for height) and her head circumference 52.5 cm (+1.7 SDS). Most facial features had become more obvious, however her forehead had grown normal and her philtrum was short and prominent (Fig. 1b). She drooled constantly. Neurological examination showed axial and peripheral hypotonia and brisk tendon reflexes. She had no apparent breathing abnormalities.
On follow up at the age of 7 years (Fig. 1c) she could pull herself up to her knees and stand with support. She made stereotypic movements with her hands, with preserved hand function.
On follow up at the age of 9 years her height was 128 cm (−1.5 SDS), her weight was 25 kg (−0.5 SDS for height) and her head circumference 54.5 cm (+1.4 SDS). She had difficulty to sit unsupported. She had no scoliosis. She had a limitation of 20° in the extension of right hip and knee, for which she wears nightly splints.
A brain MRI at the age of 10 months showed mildly enlarged ventricles and prominent sulci, suggestive of mild brain atrophy. MRI was repeated at 4 years of age and showed a Dandy-–Walker malformation and demyelination.
MLPA analysis of the MECP2 gene showed a duplication of this gene. Subsequent SNP array analysis identified a 279 kb duplication on chromosome Xq28 and a 207 kb duplication on chromosome 3q25.33q26.1. The duplication on Xq28 encompasses eight protein coding genes including MECP2, OPN1LW, TEX28P2, OPN1NW, TEX28P1, TEX28, OPN1NW2 and TKTL1. Parental analysis showed that both rearrangements occurred de novo. X-inactivation analysis unravelled a random-methylation (60:40). Additional FISH analysis showed that the Xq28 duplication has been inserted into chromosome 3, adjacent to the 3q25.33q26.1 duplication (Fig. 2a).
Fig. 2.
Illustration of Xq duplication of the MECP2 region. a. FISH analysis in patient 1. The Xq duplication (RP11-333O06; red) is inserted in the long arm of chromosome 3 (black arrow), coinciding with the 3q duplication (RP11-67F24; green). Control probes: centromere chromosome 3 (a-sat 3, Cytocell; green) and centromere chromosome X (pBamX5; red). b. Array-CGH ratio profile in patient 3. Chromosome X array CGH ratio profile using DNA from the patient and a reference DNA from a normal female. On the left, the chromosome X ideogram. On the right, the log2 ratio of the chromosome X probes plotted as a function of chromosomal position. Each dot represents a single probe (oligo) spotted on the array. Oligos with a value of zero represent equal fluorescence intensity ratio between sample and reference. c. MLPA profiles showing an Xq duplication in family 4. Graphical representation of MLPA data (left panel, index patient; right panel, maternal grandmother), showing the bars of the duplicated Xq region in red (from SLC6A8 to OPN1MW including all MECP2 exons). In the index, peak valuesreach 1.50, suggesting a duplication as normal dose is normalized to 0.75. In the grandmother, peak values of the duplication (bars in red) reach 1.25, as normal dose corresponds to two X chromosomes.
Patient 2 is a currently 7-year old girl, born at term after an uneventful pregnancy with a birth weight of 2940 g (−1 SDS). Postnatally, she was diagnosed with laryngomalacia at 3 days of age, which improved with time.
She presented at the age of 2 years and 8 months with failure to thrive despite a good appetite (height 81 cm, −3.5 SDS; weight 10.6 kg, −2.4 SDS, −0.2 SDS for height), microcephaly (OFC 45.5 cm, −2.2 SDS), and developmental delay. She has had recurrent urinary tract infections, with no underlying abnormalities.
Her development was moderately delayed, with sitting at the age of 14 months and crawling at 22 months. She had no speech, although her level of understanding was thought to be better than her expressed speech. She had some repetitive behaviour (hair pulling, hand flapping, biting herself). She had sleeping problems (refused to go back to sleep after waking up in the middle of the night).
On examination she was microcephalic (head circumference 45.6 cm (−2.1 SDS). She had no apparent dysmorphic features, apart from prominent infraorbital fullness (the appearance is of very prominent ‘bags’ under her eyes) (Fig. 1d). She had repeated chest infections and a persistent cough.
She was seen for follow up at the age of 4 years and 6 months (Fig. 1e). She had started to walk at the age of 3 years and 9 months, she walked with a wide-based gait. She was smiling a lot and she might use ‘mama, dada, and bye–bye’ appropriately, but had no other words. Her repetitive behaviour was getting less as her communicating skills had increased. Her sleeping problems had not responded to melatonin. She was about to start at a normal school, with full time personal help.
Apart from a marginally elevated TSH, additional investigations were normal, including an EEG and a brain MRI.
Array CGH analysis showed a 1.69 Mb duplication on chromosome Xq28 and a 1.54 Mb deletion on chromosome 21q22.3. Confirmatory FISH experiments using tiling path BAC clones, showed that BAC clones RP11-119A22, RP11-103M23, and RP11-402H20 from the Xq28 region have been translocated to the distal long arm of one of the chromosomes 21 homologues. FISH analysis with specific clones from the distal 21q region, RP11-162F19 and RP11-71A7, confirmed the telomeric deletion of one of the chromosome 21 homologues. Consequently, this patient carries a cryptic unbalanced translocation between chromosomes X and 21, der(21)t(X; 21)(q28; q22.3). Studies of parental chromosomes showed that the rearrangement is de novo and occurred on the paternal chromosomes. X-inactivation analysis showed random-methylation (45:55).
Patient 3 is a 6.5-year old girl, first child of non-consanguineous and healthy Caucasian parents. She was born at term after an uneventful pregnancy by caesarian section, due to breech presentation. At birth, weight was 2320 g (−1.5 SDS), length 47 cm (−1.0 SDS) and head circumference 33.5 cm (−0.5 SDS). Apgar scores were 8 and 9 at 1 and 5 min, respectively.
Her development was delayed: sitting at the age of 10 months, walking with ataxic gait at 24 months, babbling at 4 years. She had feeding difficulties and growth deficit. In infancy, she suffered from constipation. She had several episodes of upper airway infections (bronchitis, pneumonia, and tonsillitis) since the age of 7 months, as well as nocturnal apnea.
She was evaluated at the age of 5 years. She was moderately interactive and she had a sociable behaviour. On follow up at the age 6 years and 5 months she showed little interest in people. She had developed dystonic movements. On physical examination her height was 112 cm (−1.0 SDS) and weight 23 kg (+0.7 SDS). She was microcephalic (head circumference 48.1 cm, −2.0 SDS), with a round face, up-slanting palpebral fissures, severe bilateral ptosis of eyelids (also noted in her paternal grandmother), bilateral epicanthic folds, flat and wide nasal bridge, small and widely spaced teeth, normal hand and foot length, bilateral hallux valgus, and cutis marmorata. She walked with ataxic gait, did not speak and she made repetitive movements with her head (stretching upward).
A cerebral CT recorded at the age of 7 months showed a cyst of the septum pellucidum and cavum vergae. A brain MRI at 2 years and 7 months of age showed an immature aspect of cortical white matter, mainly in the frontal lobes. An EEG, recorded at the age of 4 years and 10 months showed slowing down of background rhythm. Endocrinological evaluation performed for her growth deficit showed hypothyroidism. CD4/CD8 ratio and the study of T-lymphocyte subpopulations were normal.
Array CGH analysis identified a chromosome X subtelomeric duplication of about 2.10 Mb (Fig. 2b). Confirmatory FISH experiments showed that the Xq28 region has been translocated to the short arm of one of the chromosomes 22 homologues. Studies of parental chromosomes showed that the rearrangement is de novo. The karyotype is 46,XX.ish der(22),t(X; 22)(q28; p13)(VAMP7+)dn.
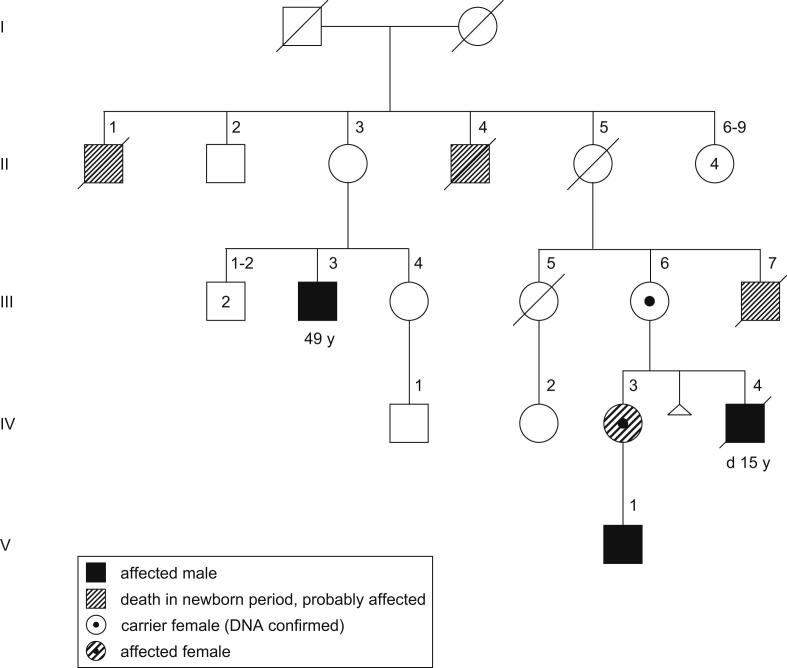
Patient 4 is a member of an X-linked mental retardation family. The index patient in this family (Fig. 3V-1; Fig. 1f) was included in a Spanish collaborative XLMR study. The duplication was identified by an X-chromosome array-CGH and previously published (case 5, pedigree E in [30]). Subsequent attempts to study the complete family were in vain. The family consists of three known affected males in three generations (Fig. 3). Three other males died a few days after birth and were probably also affected. Only one branch of the family could be studied (III-6; IV-2; IV-3 and the index patient V-1). The aforementioned paper included few clinical details [30]. A detailed description of the index is provided in the Supplementary data, clinical details on his mother and grandmother are presented here. Clinical data from other family members are scarce, but confirm X-linked inheritance of severe mental retardation.
Fig. 3.
Pedigree of X-linked mental retardation family (previously published as pedigree E in Madrigal et al., 2007[30]). 49 y, age at last examination; d 15 y, died at the age of 15 years. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Patient 4, mother of the index patient (IV-3, Fig. 3) was born to healthy non-consanguineous parents. Pregnancy and delivery were uneventful. She was born at 39 weeks of gestation, birth weight 2850 g (−1.1 SDS), length 48 cm (−1.2 SDS), and OFC 32 cm (−1.7 SDS). During early childhood she did not show signs of developmental delay, speech development was normal. She had autistic features, interaction both with her mother and others was poor. She presented with learning difficulties at school and she could not finish primary school. Before having her son, psychiatric symptoms had become apparent (depression, compulsions). She is currently rather impulsive and has a strong and difficult character when she is upset. Her cognitive level is below average (IQ 84) and below the level in her family (see Suppl. Table 1 for Wechsler Adult Intelligence Scale (WAIS-III) results). She shows little interest in other people and she is unemployed. She lives with her mother.
On physical examination at 26 years of age her height was 162 cm (−0.3 SDS), weight was 68 kg (+1.2 SDS), and OFC 56.5 cm (+0.7 SDS). Apart from fifth finger clinodactyly she had no apparent dysmorphic features (Fig. 1g).
Patient 4's mother, grandmother of the index case (III-6) is healthy, with a cognitive level within the normal range (see Suppl. Table 1 for the results of formal testing). She takes care of her daughter and grandson. She reported not to be very skillful with her hands and to suffer from a panic disorder. On physical examination weight and height were within normal limits, OFC is 56.5 cm (+0.7 SDS). She had large ears. Apart from clinodactyly of fifth fingers and hyperextensible interphalangeal joints, no dysmorphic features were present (Fig. 1h).
Her son (IV-4) suffered from meningitis when he was 9 days of age. Subsequently he presented with severe mental retardation, he never developed speech although he was able to understand simple tasks. He walked independently at the age of 3 years despite ataxic gait. He presented progressive epilepsy at 11 years of age, had recurrent episodes of severe pneumonia and died when he was 15 years old due to a complicated respiratory infection.
MLPA confirmed the Xq duplication in the index patient (Fig. 2c) and identified the same duplication in his mother (patient 4, IV-3) and grandmother (III-6, Fig. 2c). X-inactivation studies in the mother, patient 4, showed random X-inactivation (63.3:36.7), whereas it showed skewed X-inactivation in the grandmother (88.2:11.8).
Patient 5 is an 8-year old girl, first born to 29-year old parents. Maternal hypertension developed at 36 weeks of gestation. The girl was born at 38 weeks of gestation, weighing 3430 g (0 SDS), length 53.3 cm (+1.7 SDS) and with normal Apgar scores.
Apart from eczema, no health problems were noted in the neonatal period. At 6 months of age hypotonia and developmental delay became apparent. Neurological evaluation at the age of 18 months left this unexplained. Brain MRI and EEG were normal.
Her motor development was delayed (walking around 22 months of age), as was her speech development (first words around 3 years of age). At the age of 7 years she could speak in 5-word sentences, though she was at times difficult to understand. She was in a special needs class. She had difficulties with social interaction and with changes in her environment or routine. Formal assessment for autism could not diagnose autism spectrum disorder.
When first assessed in a genetics clinic at the age of 22 months, she had a history of recurrent otitis media. On physical examination, length was 82.2 cm (−0.5 SDS), weight 10.8 kg (−1.0 SDS), and head circumference 47.7 cm (+0.5 SDS). Apart from slight epicanthal folds, there were no dysmorphic features. The skin revealed scattered patches of eczema. There was muscular hypotonia.
On follow up at the age of 7 years, she had a history of chronic constipation and chronic ear infections. On examination, her height was 121.8 cm (−0.5 SDS), her weight was 23 kg (−0.2 SDS), and her head circumference was 53.3 cm (+1.0 SDS). She had no apparent dysmorphic features.
Family history is unremarkable, her younger sister is doing well without concern for any delays.
SNP array analysis detected a 107.5 kb duplication on Xq28 and an 824.5 kb duplication on chromosome 2q23.1–q23.2. FISH experiments with BAC clone RP11-119A22 confirmed the duplication on the X chromosome. X inactivation studies showed skewing, though not complete (84:16). Parental analyses showed that the phenotypically normal father carried the 2q23 duplication, the Xq28 rearrangement is de novo.
In summary, all females described in this study had mental retardation or learning difficulties (Table 1). Accompanying features were mixed, but speech delay and recurrent infections were frequent symptoms. Notably, patient 1 had the most features as usually seen in affected boys.
Array CGH data are summarized in Table 2 and Fig. 4.
Table 2.
Summary of array results in patients with Xq28 duplications, including MECP2.
| Case ID (DECIPHER code) | Platform | Chr | Starting probe | Ending probe | Start position | End position | Confirmation | Status of inheritance |
|---|---|---|---|---|---|---|---|---|
| 1 (LEI249115) | Affymetrix 6.0 | X | SNP_A-8289707 | CN_919279 | 152944369 | 153229444 | FISH | de novo |
| 3, dup | SNP_A-8679270 | CN_1009211 | 161179692 | 161387072 | FISH | de novo | ||
| 2 (WSX002126) | Agilent 44K | X | A_14_P139931 | A_14_P111123 | 152850692 | 154494649 | FISH | de novo |
| 21, del | A_14_P106169 | A_14_P100394 | 45354820 | 46892352 | FISH | de novo | ||
| 3(SIE004469) | Agilent 44K | X | A_14_P104261 | A_14_111123 | 152417785 | 154494590 | FISH | de novo |
| 4 | X-chromosome tiling-path array | X | RP11-54I20 | CTD-2238E23 | 152359267 | 153108683 | MLPA | X-linked |
| 5 | Affymetrix 6.0 | X | unknown | unknown | 152914248 | 153021750 | FISH | de novo |
| 2, dup | unknown | unknown | 148878326 | 149702817 | paternal |
Mapping positions are according to the hg18 assembly of the UCSC genome browser. Del, deletion; dup, duplication.
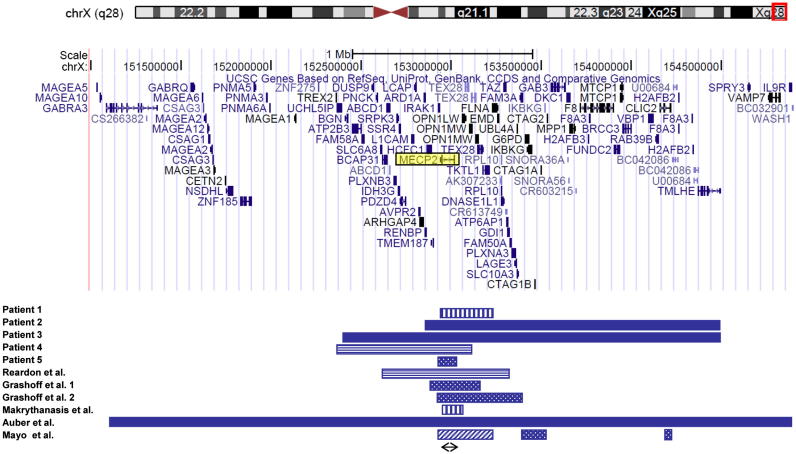
Fig. 4.
Schematic representation of part of the Xq28 region. The location of the duplications of our five patients and five previously reported females is depicted. Due to missing data, the patient described by Kirk et al. [15] could not be included. Pattern codes: vertical stripes, insertion of Xq into an autosome; solid bar, unbalanced X-autosome translocation; horizontal stripes, familial intrachromosomal Xq duplication; dotted, de novo intrachromosomal duplication of Xq, diagonal stripes, de novo intrachromosomal triplication of Xq. Arrow, minimal critical region. Gene content of the region is shown from the UCSC Genome Browser version Human March 2006 (NCBI36/hg18).
4. Discussion
We describe a series of five females carrying a submicroscopic Xq28 duplication involving MECP2. Whereas the phenotypic effect of a MECP2 duplication is already well documented in males, it is a comparatively rare cause of mental retardation in females. In a recent paper, only two cases out of 1000 unselected patients with mental retardation were identified [26]. Though females with large Xq28 duplications have been documented (as the results of X-autosome translocations, summarized in [21]), only after introduction of array techniques were females with small de novo duplications including MECP2 reported [25–27]. In addition, two affected carrier females in X-linked families have been reported [15,20]. Our series is compared with published cases in Table 1, array data (where available) are summarized in Fig. 4. As can be deduced from Fig. 4, the minimal critical region in this series of females contains only the MECP2 gene, confirming its role in mental retardation in females.
Previously, it has been stated that females with a (de novo) MECP2 duplication lack the typical symptoms of affected boys, such as seizures, poor speech development, and recurrent severe infections ([26], Table 1). Several females in our series however, do have poor speech development and patient 1 has recurrent severe infections. Furthermore, as patient 1 in our series only developed seizures after the age of 8 years, the younger patients with a de novo duplication may still be at risk of developing this feature. Compared to the compiled data in affected males with a MECP2 duplication (Table 1), the course of the disease in patient 1 is strikingly similar, with regression after the start of seizures. Follow-up of patient 3 indicated also regression. Based on our data, we conclude that the associated phenotype in females with a MECP2 duplication may be as severe as seen in affected males.
In our series, several mechanisms were found that resulted in functional disomy for region Xq28: i.e. X-autosome translocation, duplication (either familial or de novo) on the X-chromosome combined with random X-inactivation, and insertion duplication into an autosome.
4.1. X-autosome translocations
In unbalanced X-autosome translocations, the translocated segments will escape X-inactivation and cause functional disomy for genes contained within the translocated segments. In our series, patients 2 and 3 carry an unbalanced X-autosome translocation, as does the case described by Auber et al.[25]. Previously reported X-autosome translocations have been excluded from the comparison as duplicated regions were either very large (∼16 Mb) or undefined [21]. Patients in this group show various phenotypes, ranging from apparently mild mental retardation in patient 2 to a more severe clinical course in patient 3, with regression, loss of attention and emerging dystonic and repetitive movements, to a clinical picture that is comparable with the male Xq28 duplication syndrome in the patient described by Auber et al. [25]. As the concurrent imbalance in these X-autosome patients is most likely of minor significance (see below), the phenotype is most likely determined by the Xq duplication. In this small series, the size of the Xq duplication appears to correlate with the severity of the clinical course.
4.2. Intrachromosomal Xq duplications; familial and de novo
Our series contains one female carrying a familial Xq duplication including MECP2. Patient 4 is a mildly affected obligate carrier from an X-linked mental retardation (XLMR) family. Previously reported families demonstrated asymptomatic carriers with extreme or complete skewing of X-chromosome inactivation. Unlike previously reported carrier females however, patient 4 showed random X-inactivation. Taking only her IQ score into account, she is not mentally retarded, yet she has notable learning difficulties and a striking difference in IQ compared to her mother. We hypothesize that this difference in performance is caused by the difference in XCI in mother and daughter (88:12 versus 63:37) and that the clinical features in patient 4 are caused by random X-inactivation. Previous papers lend support to this view. Recently, Reardon et al. [20] reported a manifesting carrier with skewed X-inactivation in the ratio of 70:30. She had non-specific developmental delay, without seizures, speech problems, or recurrent infections. Furthermore, Kirk et al. [15] describe a manifesting carrier with mild learning disability who had a mosaic X-inactivation pattern: in blood she showed complete skewing, however in hair roots XCI was random (74:26). It has been suggested that a 70% skewing or less will lead to manifestation of the disease in female carriers of a familial Xq duplication [20]. Data on our patient support this hypothesis and indicate that the resulting phenotype is probably mild.
On the other hand, in a recent study focused on clinical and neuropsychiatric phenotype of MECP2 duplication carriers, three out of nine female carriers of a familial MECP2 duplication had IQs in the low normal range [19]. Female carriers also had psychiatric symptoms, including anxiety, depression, and compulsion, despite 100% skewing of X-inactivation in blood. In comparison, the phenotype of patient 4 may fit into the clinical spectrum depicted in that paper, indicating that low normal IQ and psychiatric symptoms may be part of the clinical spectrum in female carriers, regardless of their XCI status. Alternatively, as X-inactivation pattern has only been tested in blood, the females with low scores in Ramocki's series may in fact have mosaic XCI, leading to tissue-specific dosage alterations, and probably to poor performance. A final conclusion awaits further studies.
A separate group is formed by patients with a de novo intrachromosomal MECP2 duplication. It has been suggested previously that in these cases random XCI may be causative for the phenotype [20]. To date, two females with a de novo Xq duplication of this type and random XCI have been reported, revealing moderate mental retardation in childhood and development of neurological features in the second decade [26]. Patient 5 in our series also belongs to this category. As she is symptomatic, we assumed random XCI in her. Analysis in blood however showed skewed X-inactivation, though not complete (84:16). This may indicate that skewing needs to be complete to avoid a phenotypic effect of an Xq duplication. The XCI in patients 4's mother does not support this presumption, as she is normal functioning despite incomplete skewing (88:12). Alternatively, the phenotypic effect in patient 5 may be caused by an unfavourable skewing pattern, leading to preferential inactivation of the normal X chromosome, or by a mosaic XCI pattern, with random XCI in other tissues.
With regard to the mechanism of preferential X-inactivation, it has been suggested that co-duplication of neighbouring genes (i.e. ARD1A or HCFC1) may be responsible for complete skewing of X-inactivation in female carriers of an Xq28 duplication [29]. Consequently, smaller deletions would thus result in random X-inactivation. Again, the XCI patterns in patient 4 and her mother do not support this hypothesis, as the same duplication, including the aforementioned genes, leads to either random XCI or almost extreme skewing in members of the same family.
4.3. Insertion duplication into an autosome
Recently, Makrythanasis et al. [27] reported a patient with an insertion duplication of a small segment of Xq28 into an autosome, causing a short segment on the X-chromosome to escape X-inactivation. Also the additional copy of MECP2 in patient 1 is inserted into an autosome and therefore constitutionally active. Yet the clinical course of both patients is completely different: the patient described by Makrythanasis et al. is mildly affected, whereas the course of the disease in patient 1 is comparable to that in males, with regression after the start of seizures. We have no explanation for this discordance, however a difference in expression level may be postulated. It has been demonstrated in males that a triplicated MECP2 gene resulted in the most severe phenotype [11,33]. Recently, a severely mentally retarded girl with a triplication including MECP2 was described, suggesting a phenotypic effect of copy number [29]. Also, in mice higher MeCP2 protein levels lead to more severe phenotypes [34]. Therefore, it may be speculated that the difference in phenotype between the two insertion/duplication cases is caused by different levels of expression, for instance because the translocated MECP2 gene in patient 1 has been coupled to a strong promotor, thus enhancing the MeCP2 level.
On the other hand, expression studies in males have shown that MECP2 mRNA levels do not correlate with disease severity [19]. Alternatively, under the influence of another promotor, transcription of the translocated MECP2 gene may have been silenced in the patient described by Makrythanasis [27].
As yet, the various mechanisms do not correspond to a distinct clinical phenotype, only familial duplications with random X-inactivation seem to result in a mild phenotype (Table 1).
In addition to the Xq duplication, three patients in our series showed an imbalance of another chromosome region (Table 1). In patient 1, a de novo duplication of 3q on the site of the insertion of Xq in the long arm of chromosome 3 was found. The duplicated region contains one gene, IL12A. To our knowledge no phenotype has been demonstrated for a duplication of this gene, but clinical relevance cannot be excluded. As a result of an unbalanced X-autosome translocation, patient 2 is also monosomic for distal 21q. The contribution of the deleted genes from this region to her phenotype is probably negligible, as larger distal 21q deletions have been described without phenotypic effect [35]. In patient 5, the additional duplication was inherited from her phenotypically normal father, therefore indicating a most probably rare polymorphism. Gene content of additional CNVs in patient 1, 2 and 5 is listed in Suppl. Table 2. Interestingly, second de novo imbalances were also found in previously reported patients ([26,27], see Table 1). In both cases, an association between the additional imbalance and the clinical phenotype was deemed unlikely.
Many authors have stressed the recurrent infections in males with Xq duplications (Table 1). Smyk et al. have suggested that they result from increased IRAK1 dosage [22]. Our data do not support this hypothesis, as in patient 1 IRAK1 is not included in the duplicated fragment, yet she has a typical pattern of recurrent infections as seen in boys with a MECP2 duplication. As none of the other duplicated genes in the region are associated with recurrent infections, this clinical course suggests that this symptom can be attributed to duplication of the MECP2 gene.
In summary, an Xq duplication including MECP2 is described in five females with various grades of developmental delay. With our series of MECP2 duplication carriers we have extended the spectrum of the associated phenotype in females. We conclude that a duplication of MECP2 may be associated with a severe phenotype in both males and females. Especially de novo duplications that escape X-inactivation may be associated with a severe clinical outcome in females. The phenotype of intrachromosomal duplications of this region is more difficult to predict and may be mild. However, the highly variable clinical presentation makes genetic counselling in terms of prognosis difficult, especially in prenatal cases. It has been postulated that carriers of a familial MECP2 duplication may express a phenotype if X-inactivation is not completely skewed. Our data provide additional evidence for this hypothesis. Further studies of more females with a MECP2 duplication are needed to gain better insight in the clinical variability and into the potential pathology associated with rearrangements in this area.
Acknowledgements
We thank the patients and their families for their kind collaboration. Our special thanks go to patient 1's mother, for scrutinizing the internet and thus getting us into contact with patients 2 and 5. Also, we would like to thank prof.dr. Hilde van Esch, for sharing her knowledge about MECP2 duplications in females and bringing patients 3 and 4 to our attention.
Part of this work is supported by Telethon grant GTB07001C to A.R. and a grant from the University of Siena (PAR 2006) to F.M. Part of M.I.T.'s work was supported by grant PI05/1632 (FIS-ISCIII).
Appendix A. Supplementary material
The following are the Supplementary material related to this article:
References
- 1.Sanlaville D., Schluth-Bolard C., Turleau C. Distal Xq duplication and functional Xq disomy. Orphanet. J. Rare. Dis. 2009;4:4. doi: 10.1186/1750-1172-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramocki M.B., Tavyev Y.J., Peters S.U. The MECP2 duplication syndrome. Am. J. Med. Genet. A. 2010;152A:1079–1088. doi: 10.1002/ajmg.a.33184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pai G.S., Hane B., Joseph M., Nelson R., Hammond L.S., Arena J.F., Lubs H.A., Stevenson R.E., Schwartz C.E. A new X linked recessive syndrome of mental retardation and mild dysmorphism maps to Xq28. J. Med. Genet. 1997;34:529–534. doi: 10.1136/jmg.34.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubs H., Abidi F., Bier J.A., Abuelo D., Ouzts L., Voeller K., Fennell E., Stevenson R.E., Schwartz C.E., Arena F. XLMR syndrome characterized by multiple respiratory infections, hypertelorism, severe CNS deterioration and early death localizes to distal Xq28. Am. J. Med. Genet. 1999;85:243–248. doi: 10.1002/(sici)1096-8628(19990730)85:3<243::aid-ajmg11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Breman A.M., Ramocki M.B., Kang S.H., Williams M., Freedenberg D., Patel A., Bader P.I., Cheung S.W. MECP2 duplications in six patients with complex sex chromosome rearrangements. Eur. J. Hum. Genet. 2011;19:409–415. doi: 10.1038/ejhg.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartsch O., Gebauer K., Lechno S., van Esch H., Froyen G., Bonin M., Seidel J., Thamm-Mucke B., Horn D., Klopocki E., Hertzberg C., Zechner U., Haaf T. Four unrelated patients with Lubs X-linked mental retardation syndrome and different Xq28 duplications. Am. J. Med. Genet. A. 2010;152A:305–312. doi: 10.1002/ajmg.a.33198. [DOI] [PubMed] [Google Scholar]
- 7.Belligni E.F., Palmer R.W., Hennekam R.C. MECP2 duplication in a patient with congenital central hypoventilation. Am. J. Med. Genet. A. 2010;152A:1591–1593. doi: 10.1002/ajmg.a.33311. [DOI] [PubMed] [Google Scholar]
- 8.Budisteanu M., Papuc S.M., Tutulan-Cunita A., Budisteanu B., Arghir A. Novel clinical finding in MECP2 duplication syndrome. Eur. Child. Adolesc. Psychiatry. 2011 doi: 10.1007/s00787-011-0184-2. [DOI] [PubMed] [Google Scholar]
- 9.Campos M., Jr., Churchman S.M., Santos-Reboucas C.B., Ponchel F., Pimentel M.M. High frequency of nonrecurrent MECP2 duplications among Brazilian males with mental retardation. J. Mol. Neurosci. 2010;41:105–109. doi: 10.1007/s12031-009-9296-2. [DOI] [PubMed] [Google Scholar]
- 10.Clayton-Smith J., Walters S., Hobson E., Burkitt-Wright E., Smith R., Toutain A., Amiel J., Lyonnet S., Mansour S., Fitzpatrick D., Ciccone R., Ricca I., Zuffardi O., Donnai D. Xq28 duplication presenting with intestinal and bladder dysfunction and a distinctive facial appearance. Eur. J. Hum. Genet. 2009;17:434–443. doi: 10.1038/ejhg.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Gaudio D., Fang P., Scaglia F., Ward P.A., Craigen W.J., Glaze D.G., Neul J.L., Patel A., Lee J.A., Irons M., Berry S.A., Pursley A.A., Grebe T.A., Freedenberg D., Martin R.A., Hsich G.E., Khera J.R., Friedman N.R., Zoghbi H.Y., Eng C.M., Lupski J.R., Beaudet A.L., Cheung S.W., Roa B.B. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet. Med. 2006;8:784–792. doi: 10.1097/01.gim.0000250502.28516.3c. [DOI] [PubMed] [Google Scholar]
- 12.Echenne B., Roubertie A., Lugtenberg D., Kleefstra T., Hamel B.C.J., van Bokhoven H., Lacombe D., Philippe C., Jonveaux P., de Brouwer A.P.M. Neurologic aspects of MECP2 gene duplication in male patients. Pediatr. Neurol. 2009;41:187–191. doi: 10.1016/j.pediatrneurol.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez R.M., Nunez-Torres R., Gonzalez-Meneses A., Antinolo G., Borrego S. Novel association of severe neonatal encephalopathy and Hirschsprung disease in a male with a duplication at the Xq28 region. BMC. Med. Genet. 2010;11:137. doi: 10.1186/1471-2350-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friez M.J., Jones J.R., Clarkson K., Lubs H., Abuelo D., Bier J.A., Pai S., Simensen R., Williams C., Giampietro P.F., Schwartz C.E., Stevenson R.E. Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics. 2006;118:e1687–e1695. doi: 10.1542/peds.2006-0395. [DOI] [PubMed] [Google Scholar]
- 15.Kirk E.P., Malaty-Brevaud V., Martini N., Lacoste C., Levy N., Maclean K., Davies L., Philip N., Badens C. The clinical variability of the MECP2 duplication syndrome: description of two families with duplications excluding L1CAM and FLNA. Clin. Genet. 2009;75:301–303. doi: 10.1111/j.1399-0004.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 16.Lugtenberg D., Kleefstra T., Oudakker A.R., Nillesen W.M., Yntema H.G., Tzschach A., Raynaud M., Rating D., Journel H., Chelly J., Goizet C., Lacombe D., Pedespan J.M., Echenne B., Tariverdian G., O'Rourke D., King M.D., Green A., van Kogelenberg M., van Esch H., Gecz J., Hamel B.C.J., van Bokhoven H., de Brouwer A.P.M. Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur. J. Hum. Genet. 2009;17:444–453. doi: 10.1038/ejhg.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meins M., Lehmann J., Gerresheim F., Herchenbach J., Hagedorn M., Hameister K., Epplen J.T. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J. Med. Genet. 2005;42:e12. doi: 10.1136/jmg.2004.023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott T.E., Rodningen O.K., Bjornstad A., Stray-Pedersen A. Two brothers with a microduplication including the MECP2 gene: rapid head growth in infancy and resolution of susceptibility to infection. Clin. Dysmorphol. 2009;18:78–82. doi: 10.1097/MCD.0b013e32831e19cd. [DOI] [PubMed] [Google Scholar]
- 19.Ramocki M.B., Peters S.U., Tavyev Y.J., Zhang F., Carvalho C.M., Schaaf C.P., Richman R., Fang P., Glaze D.G., Lupski J.R., Zoghbi H.Y. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reardon W., Donoghue V., Murphy A.M., King M.D., Mayne P.D., Horn N., Birk M.L. Progressive cerebellar degenerative changes in the severe mental retardation syndrome caused by duplication of MECP2 and adjacent loci on Xq28. Eur. J. Pediatr. 2010;169:941–949. doi: 10.1007/s00431-010-1144-4. [DOI] [PubMed] [Google Scholar]
- 21.Sanlaville D., Prieur M., de Blois M.C., Genevieve D., Lapierre J.M., Ozilou C., Picq M., Gosset P., Morichon-Delvallez N., Munnich A., Cormier-Daire V., Baujat G., Romana S., Vekemans M., Turleau C. Functional disomy of the Xq28 chromosome region. Eur. J. Hum. Genet. 2005;13:579–585. doi: 10.1038/sj.ejhg.5201384. [DOI] [PubMed] [Google Scholar]
- 22.Smyk M., Obersztyn E., Nowakowska B., Nawara M., Cheung S.W., Mazurczak T., Stankiewicz P., Bocian E. Different-sized duplications of Xq28, including MECP2, in three males with mental retardation, absent or delayed speech, and recurrent infections. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:799–806. doi: 10.1002/ajmg.b.30683. [DOI] [PubMed] [Google Scholar]
- 23.van Esch H., Bauters M., Ignatius J., Jansen M., Raynaud M., Hollanders K., Lugtenberg D., Bienvenu T., Jensen L.R., Gecz J., Moraine C., Marynen P., Fryns J.P., Froyen G. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velinov M., Novelli A., Gu H., Fenko M., Dolzhanskaya N., Bernardini L., Capalbo A., Dallapiccola B., Jenkins E.C., Brown W.T. De-novo 2.15 Mb terminal Xq duplication involving MECP2 but not L1CAM gene in a male patient with mental retardation. Clin. Dysmorphol. 2009;18:9–12. doi: 10.1097/MCD.0b013e3283157cad. [DOI] [PubMed] [Google Scholar]
- 25.Auber B., Burfeind P., Thiels C., Alsat E.A., Shoukier M., Liehr T., Nelle H., Bartels I., Salinas-Riester G., Laccone F. An unbalanced translocation resulting in a duplication of Xq28 causes a Rett syndrome-like phenotype in a female patient. Clin. Genet. 2010;77:593–597. doi: 10.1111/j.1399-0004.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 26.Grasshoff U., Bonin M., Goehring I., Ekici A., Dufke A., Cremer K., Wagner N., Rossier E., Jauch A., Walter M., Bauer C., Bauer P., Horber K., Beck-Woedl S., Wieczorek D. De novo MECP2 duplication in two females with random X-inactivation and moderate mental retardation. Eur. J. Hum. Genet. 2011;19:507–512. doi: 10.1038/ejhg.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makrythanasis P., Moix I., Gimelli S., Fluss J., Aliferis K., Antonarakis S.E., Morris M.A., Bena F., Bottani A. De novo duplication of MECP2 in a girl with mental retardation and no obvious dysmorphic features. Clin. Genet. 2010;78:175–180. doi: 10.1111/j.1399-0004.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 28.Ariani F., Mari F., Pescucci C., Longo I., Bruttini M., Meloni I., Hayek G., Rocchi R., Zappella M., Renieri A. Real-time quantitative PCR as a routine method for screening large rearrangements in Rett syndrome: report of one case of MECP2 deletion and one case of MECP2 duplication. Hum. Mutat. 2004;24:172–177. doi: 10.1002/humu.20065. [DOI] [PubMed] [Google Scholar]
- 29.Mayo S., Monfort S., Rosello M., Orellana C., Oltra S., Armstrong J., Catala V., Martinez F. De novo Interstitial triplication of MECP2 in a girl with neurodevelopmental disorder and random X chromosome inactivation. Cytogenet. Genome Res. 2011 doi: 10.1159/000330917. [DOI] [PubMed] [Google Scholar]
- 30.Madrigal I., Rodriguez-Revenga L., Armengol L., Gonzalez E., Rodriguez B., Badenas C., Sanchez A., Martinez F., Guitart M., Fernandez I., Arranz J.A., Tejada M., Perez-Jurado L.A., Estivill X., Mila M. X-chromosome tiling path array detection of copy number variants in patients with chromosome X-linked mental retardation. BMC. Genomics. 2007;8:443. doi: 10.1186/1471-2164-8-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen R.C., Zoghbi H.Y., Moseley A.B., Rosenblatt H.M., Belmont J.W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am. J. Hum. Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 32.Orstavik K.H. X chromosome inactivation in clinical practice. Hum. Genet. 2009;126:363–373. doi: 10.1007/s00439-009-0670-5. [DOI] [PubMed] [Google Scholar]
- 33.Tang S.S., Fernandez D., Lazarou L.P., Singh R., Fallon P. MECP2 triplication in 3 brothers - a rarely described cause of familial neurological regression in boys. Eur. J. Paediatr. Neurol. 2011 doi: 10.1016/j.ejpn.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Collins A.L., Levenson J.M., Vilaythong A.P., Richman R., Armstrong D.L., Noebels J.L., David S.J., Zoghbi H.Y. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 35.Bertini V., Valetto A., Uccelli A., Tarantino E., Simi P. Ring chromosome 21 and reproductive pattern: a familial case and review of the literature. Fertil. Steril. 2008;90:2004–2005. doi: 10.1016/j.fertnstert.2008.01.087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.