Abstract
Induction of the suppressor of cytokine signalling 3 (SOCS-3) gene is vital to the normal control of inflammatory signalling. In order to understand these processes we investigated the role of the proto-oncogene component of the AP-1 transcription factor complex, c-Jun, in the regulation of SOCS-3 gene induction. We found that cyclic AMP stimulation of HUVECs promoted phosphorylation and activation of JNK MAP kinase and its substrate c-Jun. The JNK responsive element of the human SOCS-3 promoter mapped to a putative AP-1 site within 1000 bp of the transcription start site. The PKC inhibitors, GF-109203X, Gö-6983 and Ro-317549, were all found to inhibit AP-1 transcriptional activity, transcriptional activation of this minimal SOCS-3 promoter and SOCS-3 gene induction in HUVECs. Interestingly, Ro-317549 treatment was also found to promote PKC-dependent activation of ERK and JNK MAP kinases and promote JNK-dependent hyper-phosphorylation of c-Jun, whereas GF-109203X and Gö-6983 had little effect. Despite this, all three PKC inhibitors were found to be effective inhibitors of c-Jun DNA-binding activity. The JNK-dependent hyper-phosphorylation of c-Jun in response to Ro-317549 treatment of HUVECs does therefore not interfere with its ability to inhibit c-Jun activity and acts as an effective inhibitor of c-Jun-dependent SOCS-3 gene induction.
Abbreviations: Cyclic AMP, 3′, 5′ cyclic adenosine monophosphate; C/EBP, CCAAT/enhancer binding protein; HUVEC, human umbilical vein endothelial cell; SOCS-3, suppressor of cytokine signalling 3; SEM, standard error of mean; EPAC, exchange protein activated by cyclic AMP; AP-1, activator protein 1; ERK, extracellular signal regulated kinase; JNK, c-Jun N-terminal kinase; MAP kinase, microtubule associated kinase
Keywords: MAP kinases, Cyclic AMP, SOCS-3, Transcription, c-Jun, Protein kinase C
Highlights
► Ro-317549 triggers hyper-phosphorylation of c-Jun in HUVECs. ► Elevations in intracellular cyclic AMP also induce JNK-dependent, c-Jun activation. ► c-Jun is required for the full transcriptional activation of the human SOCS-3 gene. ► Ro-317549, GF-109203X and Gö 6983 inhibit c-Jun and SOCS-3 gene induction.
1. Introduction
The suppressor of cytokine signalling (SOCS) protein family consists of eight closely related members, cytokine inducible Src homology 2 protein (CIS) and SOCS-1 to 7 [1]. The basic structure of SOCS proteins consists of a central SH-2 and a C-terminal SOCS box domain [1]. SOCS-3, in particular, has been studied extensively and is known to play a vital role in the regulation of inflammatory processes [1,2]. For example, levels of SOCS-3 protein are increased at sights of inflammation [3] and conditional deletion of the SOCS-3 gene in hematopoietic and endothelial cells causes mice to die from severe inflammatory lesions [4]. Pro-inflammatory cytokines, such as interleukin 6 (IL-6), activate the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, leading to the induction of the SOCS-3 gene [2]. SOCS-3 protein inhibits the JAK-STAT pathway, forming part of a negative feedback loop [1]. SOCS-3 can down-regulate the JAK-STAT signalling through several mechanisms, including targeting SH-2 bound proteins for ubiquitination and proteosomal degradation, through the recruitment of an E2 ubiquitin transferase [5], competitively inhibiting JAK proteins binding to the receptor and inhibiting STAT activation through its kinase inhibitory region (KIR) [1].
It has been demonstrated that recombinant cell-penetrating forms of SOCS-3 protein can serve as an effective therapy against pathogen-derived acute inflammation [6]. Clearly, therefore, small molecule regulators of SOCS-3 gene activity could also have a similar effect in combating acute and chronic inflammation [7]. In this respect we have aimed investigations into unravelling the molecular control of SOCS-3 gene activity and have found that induction of SOCS-3 by cyclic AMP has an anti-inflammatory effect in vascular endothelial cells [8,9]. Here, elevations in intracellular cyclic AMP lead to SOCS-3 gene induction through the mobilisation of C/EBP transcription factors β and δ through the concomitant activation of exchange protein activated by cAMP 1 (EPAC1) and the ERK MAP kinase pathway [10–12]. Further work in COS1 cells highlighted a potential role for protein kinase C isoforms α and δ, acting downstream of EPAC1 in the pathway leading to SOCS-3 induction [13]. In the current work we aim to further delineate the signalling mechanisms underlying cyclic AMP-regulated SOCS-3 induction in VECs in order to define future targets for therapeutic intervention. To this end we have investigated the mechanisms of action of the bisindolemaleimide PKC inhibitors, RO-318220 [14] Gö-6983 [15] and GF-109203X [16], which we previously determined to be effective inhibitors of cyclic AMP-induced SOCS-3 induction in COS1 cells [10]. Our results demonstrate a number of “off-target” effects of RO-318220 that, nevertheless, allowed us to identify the transcription factor c-Jun as a key regulator of cyclic AMP-induced SOCS-3 gene induction in VECs.
2. Materials and methods
2.1. Materials
Primary antibodies to anti-total ERK, anti-phospho-ERK (Thr202/Tyr204), anti-total c-Jun, anti-phospho-c-Jun (Ser63), anti-total JNK, anti-phospho-JNK, pan-PKC and anti-β‐tubulin were purchased from New England Biolabs. Anti-SOCS-3 antibody was from Santa Cruz Biotechnology. Secondary antibodies anti-rabbit, anti-goat and anti-mouse IgG conjugated with HRP were purchased from GE Healthcare. Forskolin, rolipram, 12-myristate 13-acetate (PMA), MG132, U0126, SB 202190, JNK inhibitor III, GF-109203X, GÖ-6983 and Ro-317549 were purchased from Merck/Calbiochem. The AP-1 reporter construct was provided by Professor Walter Kolch, University College, Dublin.
2.2. Cell culture and transfections
COS-1 cells were grown in 75 cm2 tissue culture flasks in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% (v/v) foetal bovine serum (Sigma-Aldrich UK), 2 mM glutamine and 2% (v/v) penicillin/streptomycin (Sigma-Aldrich UK) at 37 °C in a humidified 5% (v/v) CO2 atmosphere. Human umbilical vein endothelial cells (HUVECs) were grown in human endothelial cell growth medium 2 (PromoCell Heidelberg, Germany) at 37 °C in humidified 5% (v/v) CO2. Cultures of 80%–90% confluent COS-1 cells grown on 12-well culture clusters were transfected with 0.125 μg Renilla Luciferase reporter construct (pGL4.74) plus 1.125 μg of human SOCS3-Luc promoter constructs. Plasmids were diluted in a total volume of 12.5 μl Hanks balanced salt solution (HBS; Sigma-Aldrich UK) before being added to 25 μl transfection agent 30% (v/v) DOTAP (Roche, UK) in HBS. Transfected cells were then incubated overnight at 37 °C and experiments carried out the next day.
2.3. Generation of human SOCS-3 promoter constructs
A 1.7 kbp fragment of the human SOCS-3 promoter cloned into pGL3-Basic (hSOCS3-1.7 kbp) was generously provided by Dr. Jason Mathews, University of Toronto [17]. Consecutive promoter truncates were generated with the QuikChange II Site-Directed Mutagenesis Kit (Agilent) using this promoter fragment as an initial template. The primers used were hSOCS3-1.1 kbp (forward 5′-GCCGAGGCTGGGTAGCCCCTGCTCGCGGCC-3′ and reverse 5′‐GGCCGCGAGCAGGGGCTACCCAGCCTCGGC-3′), hSOCS3-1.1-T1 (forward 5′-GCCGAGGCTGGGTAGTTTCTCTGCTGCG-3′ and reverse 5′-CGCAGCAGAGAAACTACCCAGCCTCGGC-3′), hSOCS3-1.1-T2 (forward 5′‐GCCGAGGCTGGGTAGGCCGGCCGCGCAGTTCC-3′ and reverse 5′-GGAACTGCGCGGCCGGCCTACCCAGCCTCGGC-3′) and hSOCS3-1.1-T3 (forward 5′-GCCGAGGCTGGGTAGGCGGGGCGCGGCGGC-3′ and reverse 5′‐GCCGCCGCGCCCCGCCTACCCAGCCTCGGC-3′). The putative AP-1 site in pGL3-Basic-hSOCS3-1.1-T1 was disrupted (GTGACTAA to AAGCTTAA, generating T1-delta AP1) using QuikChange mutagenesis and the primers, forward 5′-GCTGCGAGTAAAGCTTAAACATTACAAGAAGGCCGGCCGCGC-3′ and reverse 5′‐GCGCGGCCGGCCTTCTTGTAATGTTTAAGCTTTACTCGCAGC-3′.
2.4. Dual Luciferase Reporter Assays
COS-1 cells transfected with human SOCS3-Luc promoter constructs were incubated for 16 h in the presence or absence of 10 μM or 0.1 μM phorbol 12-myristate 13-acetate (PMA) (Merck, UK). In some experiments cells were co-incubated with 10 μM SB600125, 10 μM JNK inhibitor III, 10 μM SB202190, 10 μM U0126, 5 μM RO-317549, 25 μM Gö-6983, 25 μM GF-109203X. After incubation the medium was removed and the cells washed with PBS. Cells were then lysed with 250 μl of 1× passive lysis buffer (Promega, UK) and placed on a rocking platform for 20 min at room temperature. Cell lysates were collected and 20 μl samples were assayed in triplicate for luciferase activity using the Promega Dual Luciferase Reporter Assay System according to the manufacturers' protocols. Luciferase activities were measured using a BMG Labtech luminometer.
2.5. RT-PCR
HUVECs were incubated with the indicated drugs, washed with PBS, harvested by scraping into 350 μl RLT buffer (Qiagen) and then lysed with 10 passes through a 21-gauge needle attached to a 1 ml plastic syringe. RNA was then extracted from cell extracts using the Qiagen RNeasy Mini kit according to the manufacturer's protocols. RNA samples were then diluted with water to a final concentration of 5 ng/μl RNA and the RT-PCR reaction was carried out using the Qiagen One-Step RT-PCR Kit, using 0.4 mM dNTPs and 0.6 μM of each primer, according to published protocols. The primers used were, hSOCS3-Forward, 5′-CACATGGCACAAGCACAAGA-3′, hSOCS3-Reverse, 5′-AAGTGTCCCCTGTTTGGAGG-3′, actin-Forward, 5′-CTGGCACCCAGCACAATG-3′ and actin-Reverse, 5′-GCCGATCCACACGGAGTACT-3′. The RT-PCR programme consisted of 30 min at 50 °C, 15 min at 95 °C and then 30 cycles of 30 s at 94 °C, 30 s at 50 °C and 72 °C followed by 10 min at 72 °C. The RT-PCR products were resolved on 1.5% (w/v) agarose gels for 1 h at 80 V.
2.6. Intracellular Ca2+ measurements
HUVECs were grown overnight and then loaded with 1 mM FURA-2. Cells were then stimulated with the indicated treatments and changes in intracellular Ca2+ concentration were determined as previously described [18].
2.7. Immunoblotting
Cells lysates were prepared in sample buffer (50 mM Tris–HCl, pH6.8, 2% (w/v) SDS, 10% (v/v) glycerol, 1% (v/v) β-Mercaptoethanol, 12.5 mM EDTA, 0.02% (w/v) bromophenol blue, 100 mM DTT). Protein samples were then separated by SDS-PAGE on 10% (w/v) gels, transferred to nitrocellulose, blocked for 1 h at room temperature in 5% (w/v) BSA, immunoblotted with antibodies specific for JNK, phospho-JNK, ERK, phospho-ERK, c-Jun, phospho-c-Jun or PKC and then developed using ECL chemiluminescence (GE Healthcare).
2.8. c-Jun activation assay
A TransAM™ AP-1/c-Jun activation kit was purchased from Active Motif. Following stimulation nuclear extracts were prepared from HUVEC cells using an Active Motif nuclear extract kit, according to the manufacturers' instructions. Equal amounts of nuclear extract were then added to ELISA plates coated with oligonucleotides corresponding to the AP-1 consensus sequence and developed with an anti-c-Jun antibody. The extent of c-Jun binding to AP-1 oligonucleotides was determined colourimetrically using a spectrophotometer set at 495 nm.
2.9. Statistics
Data was analysed using one-way analysis of variance (ANOVA) with a Tukey–Kramer post test.
3. Results and discussion
3.1. Effects of Ro-317549 on SOCS-3 gene induction
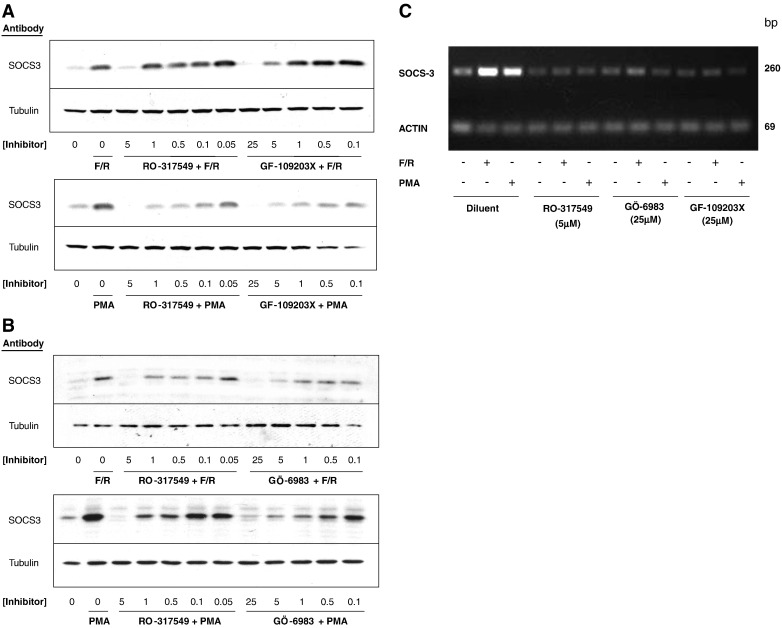
We had previously demonstrated that bisindoylmalemide protein kinase C (PKC) inhibitors effectively inhibited SOCS-3 gene induction in response to cyclic AMP-elevation in COS1 cells [10]. We therefore sought to assess the effect of this class of inhibitor on SOCS-3 protein induction by cyclic AMP in HUVECs, which represents a valid model of the suppressive effects of cyclic AMP on endothelial dysfunction [9]. HUVECs were therefore stimulated with various concentrations of the structurally related PKC inhibitors Ro-31-7459, GÖ-6983 or GF-109203X and either a combination of the adenylate cyclase activator, forskolin, and the type IV, cyclic AMP-specific phosphodiesterase inhibitor, rolipram (F/R) or the phorbol ester activator of novel and conventional PKC isoforms, PMA (Fig. 1). Experiments were carried out in the presence of the proteasome inhibitor, MG132, to prevent SOCS-3 degradation following synthesis so that effects solely on protein induction could be monitored. Immunoblotting cell extracts with anti-SOCS-3 antibodies demonstrated that GÖ-6983 and GF-109203X inhibited SOCS-3 induction in response to both F/R and PMA, with maximal inhibition occurring at around 25 μM for both inhibitors (Fig. 1A and B). In contrast, however, Ro-31-7459 effectively blocked SOCS-3 induction at lower concentrations with maximal inhibition occurring at 5 μM (Fig. 1A and B), indicating that perhaps this inhibitor was acting through mechanisms distinct from GÖ-6983 and GF-109203X.
Fig. 1.
Protein kinase C inhibitors block human SOCS-3 gene induction in HUVECs. A). HUVECs were stimulated for 5 h with MG132 (10 μM) in the presence or absence of either a combination of 10 μM forskolin plus 10 μM rolipram (F/R; upper panel) or 10 μM PMA (lower panel) plus the indicated concentrations of the protein kinase C (PKC) inhibitors Ro-31-7549 or GF-109203X. Cell extracts were then prepared and immunoblotted with antibodies to SOCS-3 or β-tubulin as indicated. B). HUVECs were stimulated for 5 h with MG132 (10 μM) in the presence or absence of either F/R (upper panel) or 10 μM PMA (lower panel) plus the indicated concentrations of the PKC inhibitors Ro-31-7549 or Gö-6983. Cell extracts were then prepared and immunoblotted with antibodies to SOCS-3 or β-tubulin as indicated. C). HUVECs were stimulated for 5 h in the presence or absence of F/R (upper panel) or 10 μM PMA (lower panel) plus Ro-31-7549 (5 μM), Gö-6983 (25 μM) or GF-109203X (25 μM). Total RNA was then extracted from cells and subjected to one-step RT-PCR, with specific primers towards SOCS-3 or actin, as described in Materials and methods. Amplified DNA fragments were visualised by agarose gel electrophoresis.
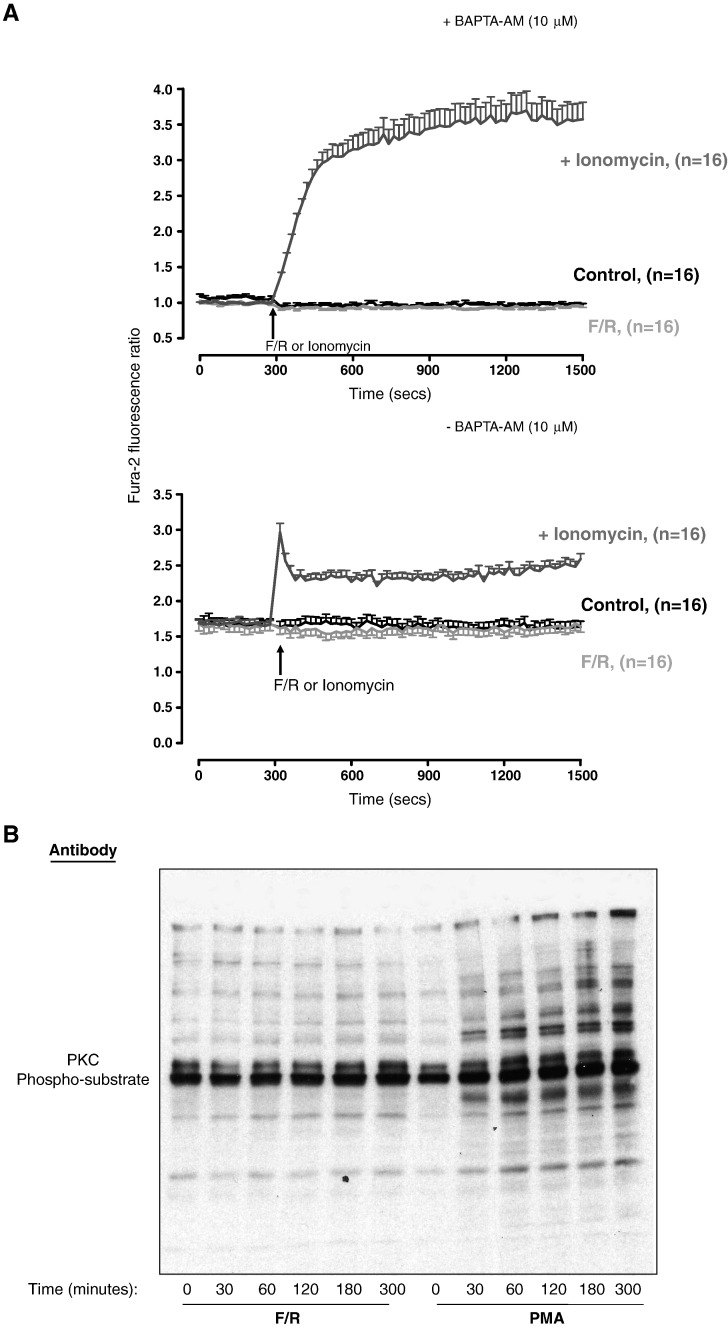
To determine whether any of the PKC inhibitors was acting at the level of SOCS-3 gene induction, RT-PCR reactions were carried out on F/R- and PMA-stimulated cell extracts using specific primers to SOCS-3 mRNA. As can be seen in Fig. 1C, all three PKC inhibitors blocked SOCS-3 mRNA production in response to PMA and F/R treatment, and that Ro-31-7459 was equally effective at 5 μM. These experiments show that Ro-31-7459 is an effective inhibitor of SOCS-3 gene expression in HUVECs in response to PKC activation or elevations in intracellular cyclic AMP as induced following F/R treatment. The fact that PKC inhibitors effectively block cyclic AMP-stimulated SOCS-3 gene induction implies that PKC conventional and novel PKC isoforms may lie downstream of cyclic AMP production in the pathway leading to gene activation. While there is some evidence that cyclic AMP can regulate PKC activity in certain cell contexts, perhaps through the activation of Rap1-dependent phospholipase Cε [10], we have so far been unable to detect activation of PKC isoforms or mobilisation of intracellular calcium, following cyclic AMP stimulation of HUVECs, as detected by FURA2-labelling of cells and immunoblotting for phosphorylated PKC-substrates (Fig. 2). The fact that the PKC inhibitors are acting at the level of the SOCS-3 gene (Fig. 1C) suggests that they may be modifying the basal activity of one or more transcription factors linked to SOCS-3 induction.
Fig. 2.
Elevation of intracellular cyclic AMP in HUVECs does not promote mobilisation of intracellular calcium or activation of protein kinase C. A). HUVECs were loaded with FURA-2 in Hepes–Ca2+ buffer and then stimulated with either F/R or 10 μM ionomycin in the presence (upper panel) or absence (lower panel) of 10 μM BAPTA-AM. Fluorescence was measured at 340 nm and 380 nm for 5 min prior to the addition of reagents and then continued for a further 20 min after addition of reagents. B). HUVECs were stimulated for the indicated times with either F/R or PMA. Cell extracts were then prepared and immunoblotted with antibodies that specifically detects multiple protein substrates that are phosporylated by PKC.
3.2. Effects of PKC inhibitors on c-Jun phosphorylation
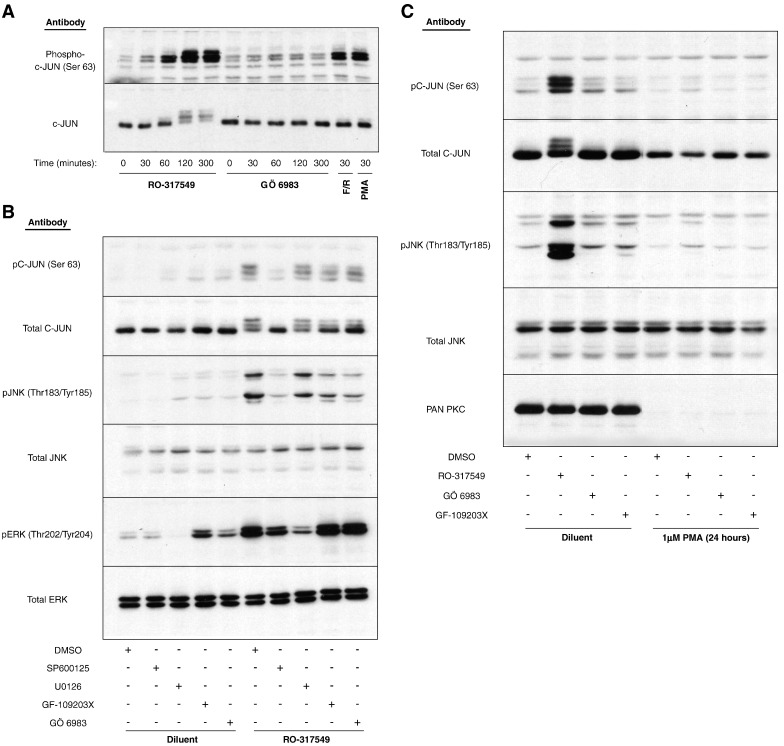
Given that the structurally related PKC inhibitor, Ro-31-8220, has been shown to induce the activation of the MAP kinase, JNK [19], and that PMA has been shown to promote the phosphorylation and activation of the JNK substrate, the transcription factor c-Jun [20], we decided to test whether c-Jun activation is effected by PKC inhibitors. HUVECs were therefore stimulated for 30 min with either F/R or PMA, or for various times with either 5 μM Ro-31-7459 or 25 μM GÖ-6983. Cell extracts were then prepared and the phosphorylation status of c-Jun was assessed by immunoblotting with antibodies that recognise one of the JNK-dependent, activating phosphorylations of c-Jun, at Ser 63 (Fig. 3A). Both F/R and PMA were found to promote a robust phosphorylation of C-JUN on Ser 63 following 30 min stimulation, which was mirrored by a small upward shift in electrophoretic mobility of the protein as detected by a total c-Jun protein antibody (Fig. 3A). Surprisingly, however, incubation of cells with Ro-31-7459 alone, but not GÖ-6983, also led to phosphorylation of Ser 63 by 60 min, which increased further by 120 min coincident with a dramatic decrease in electrophoretic mobility as detected by the total c-Jun antibody (Fig. 3A). This indicates that Ro-31-7459 triggers multiple phosphorylations of c-Jun in HUVECs and that these may be non-specific effects since they are not observed when cells are stimulated with GÖ-6983.
Fig. 3.
Treatment of HUVECs with Ro-317549, but not Gö-6983 or GF-109203X, triggers hyperphosphorylation of c-Jun. A). HUVECs were stimulated for the indicated times with 10 μM Ro-31-7549, 10 μM Gö-6983, F/R or PMA. Cell extracts were then prepared and immunoblotted with specific antibodies to total c-Jun protein (lower panel) or c-Jun phosphorylated on Ser-63 (upper panel). B). HUVECs were pre-treated for 30 min with 10 μM of the JNK inhibitor, SP600125, 10 μM of the ERK inhibitor, U0126, or 10 μM of the PKC inhibitors, Gö-6983 or GF-109203X. Cells were then stimulated with 10 μM Ro-317549 for 120 min. Cell extracts were then prepared and immunoblotted with the indicated antibodies. C). HUVECs were pre-treated for 24 h in the presence or absence of 1 μM PMA, to down-regulate endogenous conventional and novel PKC isoforms as detected with a pan-specific, anti-PKC antibody (lower panel). Cells were then stimulated for a further 120 min in the presence or absence of 10 μM Ro-31-7549, Gö-6983 (25 μM) or GF-109203X (25 μM). Cell extracts were prepared and immunoblotted with the indicated antibodies.
To test this further, and to check for the PKC-dependency of the effects of Ro-31-7459, HUVECs were stimulated for 60 min with 5 μM Ro-31-7459, in the presence or absence of Gö 6983 and GF-109203X and then immunoblotted for antibodies to total c-Jun or c-Jun phosphorylated on Ser 63 (Fig. 3B). Moreover, since Ser 63 in c-Jun is a known phospho-acceptor site for MAP kinases [21], cells were also treated with Ro-31-7459 in the presence or absence of a JNK inhibitor (SP600125) or an ERK inhibitor (U0126). The relative activities of ERK and JNK were monitored by immunoblotting with phospho-specific antibodies that recognised the active forms of the enzymes. Stimulation with Ro-31-7459, but not Gö 6983 and GF-109203X, was found to promote a robust phosphorylation of JNK and c-Jun, whereas all three inhibitors promoted phosphorylation of ERK (Fig. 3B). The phosphorylation of Ser 63 of C-JUN in response to Ro-31-7459 was effectively inhibited by the JNK inhibitor, but not the ERK inhibitor (Fig. 3B), suggesting that JNK activation by Ro-31-7459, and not ERK activation, is the responsible cause. Ro-31-7459-promoted Ser 63 phosphorylation was not affected by the PKC inhibitors Gö 6983 and GF-109203X, however, probably because they were unable to inhibit JNK activation in this experiment (Fig. 3B). However, long term down-regulation of PKC isoforms, following chronic phorbol ester treatment of cells [19], did effectively block both JNK activation and phosphorylation of c-Jun on Ser-63 following stimulation with Ro-31-7459 (Fig. 3C), suggesting that at least some of the actions of Ro-31-7459 are dependent on PKC. Moreover, although both Gö 6983 and GF-109203X failed to inhibit Ser-63 phosphorylation they both caused an increase in the electrophoretic mobility of total C-JUN protein, indicating that they both inhibit a Ro-31-7459-stimulated, PKC-dependent phosphorylation of c-Jun on a site distinct from Ser-63. These results demonstrate that Ro-31-7459 displays cellular activities distinct from Gö 6983 and GF-109203X in HUVECs that include the PKC-dependent and JNK-dependent phosphorylation of c-Jun on Ser-63 and the PKC-dependent and JNK-independent, phosphorylation of an additional site in c-Jun. This site is highly likely to be one or more of three phosphor-acceptor sites within the DNA-binding domain of c-Jun that have been shown previously to be negatively regulated by PKC [22]. Moreover, although Ro-31-7459, Gö 6983 and GF-109203X all promote a strong activation of ERK in HUVECs, this does not appear to affect the phosphorylation status of c-Jun in these cells, which is in agreement with previous studies indicating that c-Jun in not a bone fide ERK substrate [23].
3.3. Cyclic AMP activates JNK to induce SOCS-3
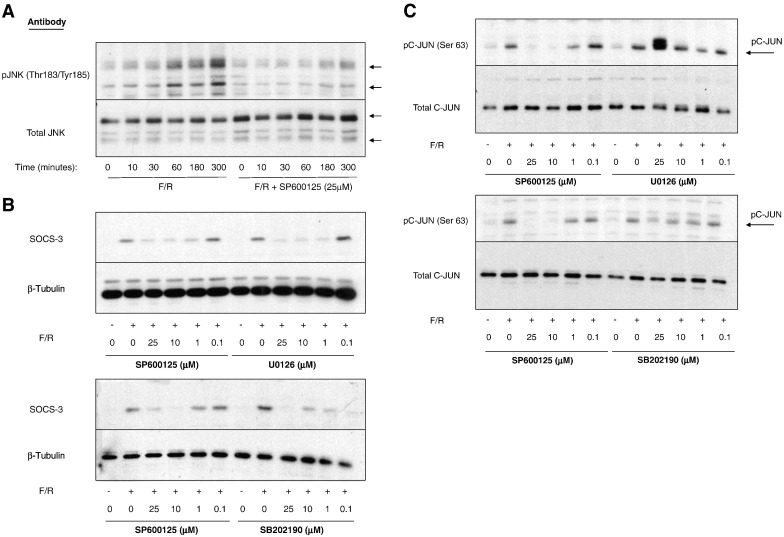
Given that Ro-31-7459 is an effective inhibitor of cyclic AMP-promoted SOCS-3 gene expression (Fig. 1) and a potent activator of JNK MAP kinase signalling to c-Jun (Fig. 3), we next investigated the role of JNK in mediating cyclic AMP-induced SOCS-3 induction. HUVECs were therefore stimulated for various times with F/R in the presence or absence of the broad specificity JNK inhibitor, SP600125 (Fig. 4A). Immunoblotting cell extracts with phospho- and activation-specific antibodies to JNK demonstrated that F/R promoted a sustained activation of JNK between 60 and 300 min, which was completely ablated by incubation with 25 μM SP600125 (Fig. 4A). Moreover, immunoblotting HUVEC extracts from cells stimulated for 5 h with F/R, in the presence or absence of chemical inhibitors of JNK (SP600125), ERK (U0126) and p38 (SB202190) MAP kinases demonstrated that each MAP kinase inhibitor reduced the ability of F/R to induce SOCS-3 expression over a range of concentrations (1–25 μM; Fig. 4B). To investigate a role for c-Jun in cyclic AMP- and MAP kinase-dependent SOCS-3 induction we next immunoblotted cell extracts from cells treated with the same concentration range of MAP kinase inhibitors with anti-phospho-Ser-63 c-Jun antibodies. Results revealed that of the three MAP kinase inhibitors tested, only the JNK inhibitor, SP00125, was able to inhibit F/R-induced c-Jun phosphorylation, with a minimal effective concentration of 10 μM (Fig. 4C).
Fig. 4.
SOCS-3 induction following cyclic AMP elevation is dependent on activation of JNK MAP kinase in HUVECs. A). HUVECs were pre-treated with the JNK inhibitor, 25 μM SP600125, and then stimulated with F/R for the indicated times. Cell extracts were the prepared and immunoblotted with antibodies to total JNK protein and anti-Thr183/Tyr185 phospho-JNK antibodies as indicated. B). HUVECs were pre-incubated in the presence or absence of the indicated concentrations of the JNK inhibitor, SP600125, the ERK inhibitor, U0126, or the p38 MAP kinase inhibitor, SB202190 and then stimulated for a further 3 h in the presence or absence of F/R. Cell extracts were then prepared and immunoblotted with anti-SOCS-3 and anti-β-tubulin antibodies. C). HUVECs were pre-incubated in the presence or absence of the indicated concentrations of SP600125, U0126 or SB202190 and then stimulated for a further 3 h in the presence or absence of F/R. Cell extracts were then prepared and immunoblotted with anti-Ser63 phospho-c-Jun and total-c-Jun antibodies.
Together with the data presented in Fig. 3, these results suggest that JNK activation, in addition to the activation of ERK and p38 MAP kinases, may be an important component of the signalling mechanisms linking cyclic AMP elevation to the induction of SOCS-3 gene expression in HUVECs. However, JNK activation by cyclic AMP, but not ERK or p38 activation, appears to be coupled to the phosphorylation of c-Jun within its transactivation domain (Fig. 4C), which may be a prerequisite for SOCS-3 gene induction by cyclic AMP. Moreover, Ro-31-7459 activates JNK and stimulates Ser-63 phosphorylation of c-Jun (Fig. 3), yet inhibits SOCS-3 induction (Fig. 1). The fact that cyclic AMP also activates JNK, and yet promotes SOCS-3 induction suggests that the inhibitory effects of Ro-31-7459 are not due to effects on JNK-dependent c-Jun phosphorylation but probably through PKC-dependent, phosphorylation of the c-Jun DNA-binding domain [22].
3.4. SOCS-3 gene induction requires AP-1 transcription factors
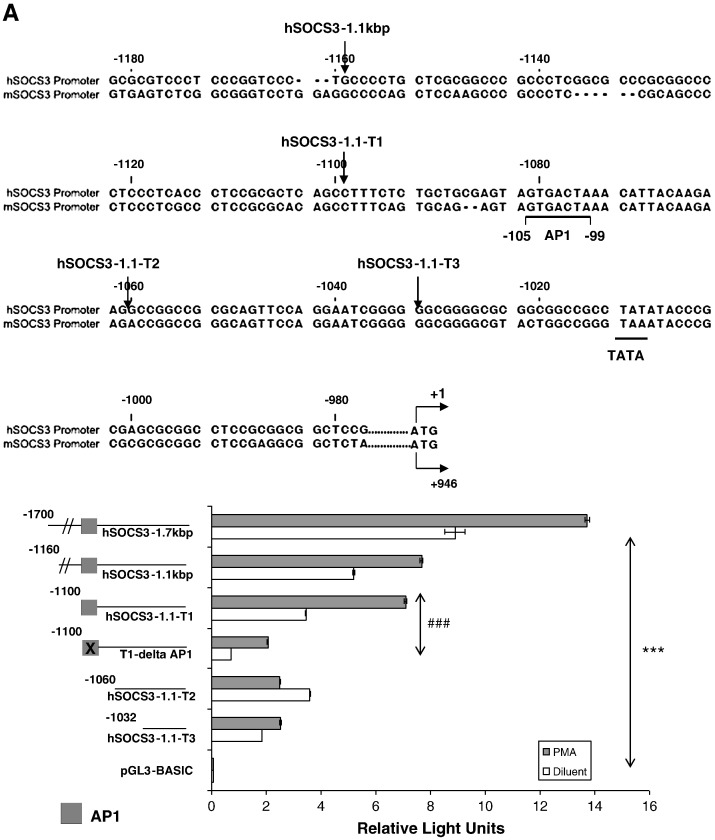
The induction of the SOCS-3 gene in response to elevations in intracellular cyclic AMP in HUVECs is dependent on PKC and JNK activation, both of which lead to the phosphorylation of c-Jun (Figs. 1, 3 and 4). We therefore investigated whether c-Jun is required for the transcriptional activation of the SOCS-3 gene. C-Jun, in combination with c-Fos, is known to form part of the AP-1 transcription factor complex, of which one has been identified within the sequence of the murine SOCS-3 promoter region [24] and this sequence is conserved in the human SOCS-3 promoter (Fig. 5A). To test its importance of this site we generated a deletion series of five human promoter constructs, together with one promoter containing a mutation in the putative AP-1 transcription factor binding site, and cloned them into the promoterless firefly luciferase expression vector, pGL3-basic (Fig. 5A). COS-1 cells were then co-transfected with the promoter constructs together with a Renilla luciferase vector and then stimulated for 16 h in the presence or absence 10 μM PMA, a known activator of AP-1 dependent transcription [20]. Cell lysates were then prepared and luciferase activities measured (Fig. 5A). The activity of firefly luciferase was compared to the activity of Renilla luciferase, which was used as a control for transfection efficiency.
Fig. 5.
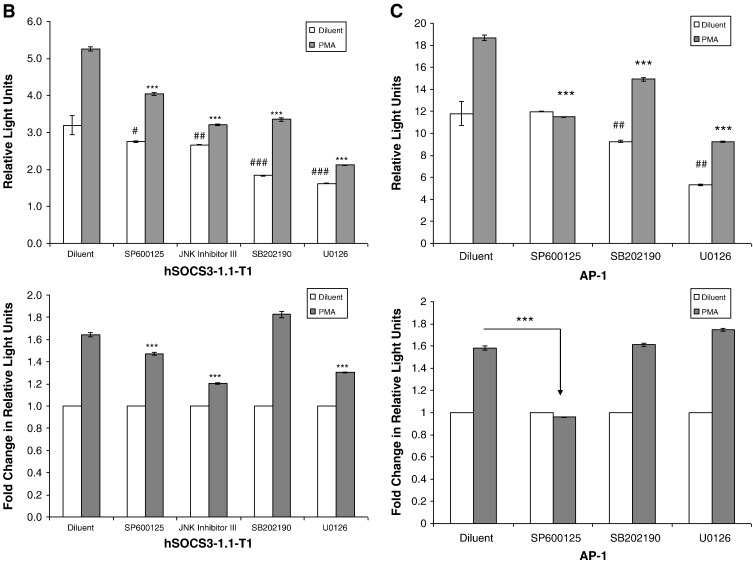
Activation of the human SOCS-3 promoter is dependent on AP-1 transcription factors. A). Comparison of the human and murine SOCS-3 promoter regions indicates the presence of a conserved, putative AP-1 transcription factor binding site (upper panel). Truncations introduced into the human SOCS-3 promoter are indicated by arrows in the upper panel. COS-1 cells were co-transfected with a Renilla luciferase vector and the firefly luciferase reporter constructs hSOCS3-1.7 kbp, hSOCS3-1.1 kbp, hSOCS3-1.1-T1, hSOCS3-1.1-T1-AP-1, hSOCS3-1.1-T2 or hSOCS3-1.1-T3. Cells were then stimulated for 16 h in the presence or absence of 10 μM PMA. Cells were then harvested and the luciferase activities measured. Results were normalised to the activity of Renilla luciferase in cell extracts and results expressed as Relative Light Units. Significant decreases in luciferase activity between the promoter truncates (including mutated promoter hSOCS3-1.1-T1-AP-1) relative to the promoter region hSOCS3-1.7 kbp are indicated, ***p < 0.001, as are the differences between promoter constructs hSOCS3-1.1-T1 and hSOCS1-1.1-T1-AP-1, ###p < 0.001. B). COS-1 cells were co-transfected with the firefly luciferase reporter constructs hSOCS3-1.1-T1 and Renilla luciferase. Cells were treated with 10 μM of the JNK inhibitors, SP202109 and JNK inhibitor IIII, 10 μM of the ERK inhibitor, U0126, or 10 μM of the p38 MAP kinase inhibitor, SB202190, and then stimulated, for 16 h, in the presence or absence of 10 μM PMA. Cells were then harvested and luciferase activities determined. Results are expressed as Relative Light Units (RLUs) in the upper panel and significant reductions in the luciferase activity of PMA stimulated cells relative to diluent-treated cells are indicated, ***p < 0.001, as are significant decreases in basal activity, # (p < 0.05), ## (p < 0.01 and ### (p < 0.001). Results are also expressed in the lower panel as fold change in RLUs relative to diluent treated cells with significant decreases in PMA-promoted luciferase activity following inhibitor treatment indicated, ***p < 0.001. C). COS-1 cells were co-transfected with an AP-1 firefly luciferase reporter construct and Renilla luciferase. Cells were then treated with 10 μM SP202109, 10 μM U0126, or 10 μM SB202190, and then stimulated, for 16 h, in the presence or absence of 10 μM PMA. Cells were then harvested and luciferase activities determined. Results are expressed as Relative Light Units (RLUs) in the upper panel and significant reductions in the luciferase activity of PMA stimulated cells relative to diluent-treated cells are indicated, ***p < 0.001, as are significant decreases in basal activity, ## (p < 0.01 and ### (p < 0.001). Results are also expressed as fold change in RLUs relative to diluent treated cells in the lower panel, with significant decreases in PMA-promoted luciferase activity following inhibitor treatment indicated, ***p < 0.001.
The truncated promoter constructs hSOCS3-1.7 kbp, hSOCS3-1.1 kbp and hSOCS3-1.1-T1 all contain the putative AP-1 site and show significant increases in promoter activity in response to 10 μM PMA stimulation (Fig. 5A). The truncated promoter constructs, hSOCS-1.1-T2 and hSOCS3-1.1-T3 lack the putative AP-1 site and show a considerable reduction in PMA stimulated promoter activity (Fig. 5A). Moreover, a significant reduction in both basal and stimulated transcriptional activity was noted for the hSOCS-1.1-T1-AP-1 promoter construct, which contains a mutation in AP-1 site and is unable to bind c-Jun [24], compared to the hSOCS3-1.1-T1 promoter (Fig. 5A). Together with previous work on the murine promoter [24], the work presented here strongly suggests that the putative AP-1 site within the human SOCS-3 promoter is important for PMA regulated SOCS-3 transcription.
To confirm that AP-1-depedent SOCS-3 transcriptional activation is dependent on JNK activation, COS1 cells were transfected with the hSOCS3-1.1-T1 minimal promoter construct and stimulated with 10 μM PMA for 16 h, in the presence or absence of inhibitors of JNK (SP600125 and JNK inhibitor III), ERK (U0126) and p38 (SB202190) MAP kinases (Fig. 5B). Results demonstrated that all of the MAP kinase inhibitors caused a significant reduction in the basal levels of SOCS-3 promoter activity (Fig. 5B; upper panel), however only the inhibitors of JNK and ERK MAP kinases caused a significant reduction in PMA-stimulated SOCS-3 promoter activity (Fig. 5B; lower panel). To confirm that JNK and ERK MAP kinases can directly regulate the activity of AP-1 transcriptional activity, COS1 cells were transfected with a luciferase reporter construct containing multiple AP-1 sites and then stimulated with 10 μM PMA for 16 h, in the presence or absence of JNK (SP600125), ERK (U0126) and p38 (SB202190) MAP kinase inhibitors (Fig. 5C). We found that the induction of AP-1 transcriptional activity was absolutely dependent on JNK activation, since the JNK inhibitor, SP600125, completely ablated AP-1 supported transcription (Fig. 5C). Moreover, inhibitors of ERK and p38 MAP kinases had little effect on PMA-induced transcriptional activity (Fig. 5C; lower panel), but rather significantly reduced the basal transcriptional activity of the AP-1 reporter construct (Fig. 5C; upper panel). Together these results suggest that although transcriptional induction of the human SOCS-3 promoter displays a requirement for JNK and AP-1 dependent transcription, this is not absolute and full transcriptional activity probably also requires regulatory input from the ERK MAP kinase pathway, which is probably regulating the transcription factor C/EBPβ, which we have shown to be essential for ERK-dependent SOCS-3 induction in HUVECs and COS1 cells [10,12]. The p38 MAP kinase pathway appears to be required for maintaining basal activity of the human SOCS-3 promoter (Fig. 5B), perhaps through supporting basal activity of AP-1 transcription factors (Fig. 5C); however the mechanisms underlying this effect remain to be determined.
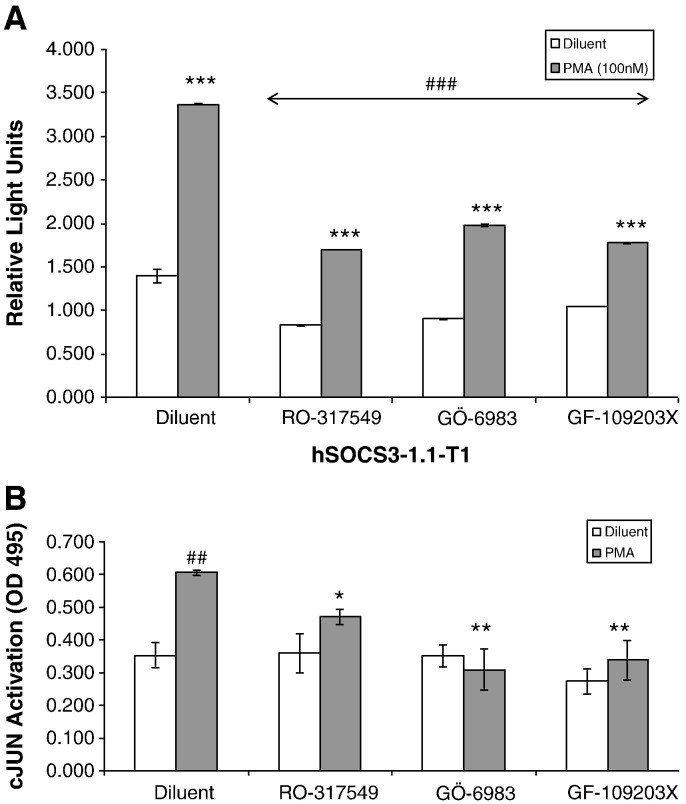
To determine the effect of the PKC inhibitors on RO-317549 (5 μM), Gö-6983 (25 μM), GF-109203x (25 μM) on AP-1-dependent induction of the SOCS-3 gene, COS1 cells transfected with the hSOCS3-1.1-T1 were treated with the PKC inhibitors in the presence or absence of 0.1 μM PMA and luciferase activities determined after 16 h (Fig. 6A). All three inhibitors significantly reduced the PMA stimulated activity of the minimal SOCS-3 promoter (Fig. 6A). To test this further we decided to check whether RO-317549 (5 μM), Gö-6983 (25 μM) or GF-109203x (25 μM) modified the DNA-binding activity of the c-Jun transcription factor itself. HUVECs were therefore stimulated with 0.1 μM PMA, in the presence or absence of RO-317549 (5 μM), Gö-6983 (25 μM) or GF-109203x (25 μM), nuclear extracts were prepared and the DNA-binding activity of C-JUN in cell extracts was assessed by an ELISA-based activation assay which measure c-Jun binding to immobilised AP-1 DNA consensus sites (Fig. 6B). All three PKC inhibitors were found to significantly inhibit PMA-induced c-Jun DNA binding ability indicating that this may underlie the part of the mechanisms by which PKC inhibitors such as RO-317549 effectively block SOCS-3 gene induction. Together with previous results this demonstrates that SOCS-3 gene induction by cyclic AMP or PMA relies on JNK-dependent activation of AP-1 transcription factors and that the PKC inhibitor, RO-317549, promotes the PKC-dependent phosphorylation of the AP-1 transcription factor, c-Jun, which may contribute to its ability to effectively block SOCS-3 gene induction in HUVEC (Fig. 1) and COS1 cells [10].
Fig. 6.
PKC-inhibitors block activation of the human SOCS-3 promoter and activation of c-Jun transcription factor. A). COS-1 cells were co transfected with the firefly luciferase reporter construct hSOCS-1.1-T1 and Renilla luciferase. Cells were then pre-treated for 30 min with 5 μM Ro-317549, 25 μM Gö-6983, or 25 μM GF-109203X and then stimulated for 16 h, in the presence or absence of 0.1 μM PMA. Cells were then harvested and the luciferase activities determined. Results expressed in Relative Light Units (RLUs) and significant increases in luciferase activity in PMA stimulated cells relative to diluents cells are indicated, ***p < 0.001. In addition, significant decreases in luciferase activity in PMA-stimulated cells in the presence of Ro-317549, Gö-6983 and GF-109203X, relative to the diluent cells, are also indicated, ###p < 0.001. B). HUVECs were pre-treated for 30 min with 5 μM Ro-317549, 25 μM Gö-6983, or 25 μM GF-109203X and then stimulated for 3 h, in the presence or absence of 0.1 μM PMA. Nuclear extracts were then prepared, normalised for protein concentration. Equal amounts of nuclear extract were then incubated with immoblised AP-1 DNA-binding elements and the relative amounts of c-Jun binding were determined by ELISA using a c-Jun-specific antibody. Results are expressed as relative absorbance units (OD 495 nm) and significant decreases in PMA-induced DNA-binding in the presence of PKC inhibitors are indicated, *p < 0.05 and **p < 0.01. The significant increase in C-JUN activation following PMA treatment is also indicated, ##p < 0.01.
4. Conclusions
The bisindolemaleimide PKC inhibitor Ro-317549 triggers hyper-phosphorylation of c-Jun in HUVECs in a JNK and PKC-dependent manner, whereas structurally related GF-109203X and Gö 6983 have little effect. Despite this Ro-317549, GF-109203X and Gö 6983 all inhibit PKC-dependent c-Jun activation. Using these inhibitors we demonstrate that they all inhibit JNK-dependent c-Jun activation and c-Jun-dependent induction of the SOCS-3 gene in response to elevations in intracellular cyclic AMP. This demonstrates that, despite many off-target effects, bisindolemaleimide PKC inhibitors reveal a central role for the c-Jun transcription factor in controlling the induction of the human SOCS-3 gene by cyclic AMP and PKC-dependent pathways.
Acknowledgements
This work was supported by the British Heart Foundation [Grants PG/10/026/28303 and PG/08/125/26415] awarded to SJY.
References
- 1.Tamiya T., Kashiwagi I., Takahashi R., Yasukawa H., Yoshimura A. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(5):980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- 2.Croker B.A., Kiu H., Pellegrini M., Toe J., Preston S., Metcalf D., O'Donnell J.A., Cengia L.H., McArthur K., Nicola N.A., Alexander W.S., Roberts A.W. Immunology and Cell Biology. 2012;90(1):124–129. doi: 10.1038/icb.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White G.E., Cotterill A., Addley M.R., Soilleux E.J., Greaves D.R. Journal of Molecular Histology. 2011;42(2):137–151. doi: 10.1007/s10735-011-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croker B.A., Metcalf D., Robb L., Wei W., Mifsud S., DiRago L., Cluse L.A., Sutherland K.D., Hartley L., Williams E., Zhang J.G., Hilton D.J., Nicola N.A., Alexander W.S., Roberts A.W. Immunity. 2004;20(2):153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 5.Kamura T., Sato S., Haque D., Liu L., Kaelin W.G., Jr., Conaway R.C., Conaway J.W. Genes & Development. 1998;12(24):3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo D., Liu D., Yao S., Collins R.D., Hawiger J. Nature Medicine. 2005;11(8):892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 7.Parnell E., Smith B.O., Palmer T.M., Terrin A., Zaccolo M., Yarwood S.J. British Journal of Pharmacology. 2012;166(2):434–446. doi: 10.1111/j.1476-5381.2011.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borland G., Smith B.O., Yarwood S.J. British Journal of Pharmacology. 2009;158(1):70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sands W.A., Woolson H.D., Milne G.R., Rutherford C., Palmer T.M. Molecular and Cellular Biology. 2006;26(17):6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borland G., Bird R.J., Palmer T.M., Yarwood S.J. Journal of Biological Chemistry. 2009;284(26):17391–17403. doi: 10.1074/jbc.M109.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarwood S.J., Borland G., Sands W.A., Palmer T.M. Journal of Biological Chemistry. 2008;283(11):6843–6853. doi: 10.1074/jbc.M710342200. [DOI] [PubMed] [Google Scholar]
- 12.Woolson H.D., Thomson V.S., Rutherford C., Yarwood S.J., Palmer T.M. Cellular Signalling. 2009;21(11):1706–1715. doi: 10.1016/j.cellsig.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Baillie G.S., Adams D.R., Bhari N., Houslay T.M., Vadrevu S., Meng D., Li X., Dunlop A., Milligan G., Bolger G.B., Klussmann E., Houslay M.D. Biochemical Journal. 2007;404(1):71–80. doi: 10.1042/BJ20070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieter P., Fitzke E. Biochemical and Biophysical Research Communications. 1991;181(1):396–401. doi: 10.1016/s0006-291x(05)81432-9. [DOI] [PubMed] [Google Scholar]
- 15.Gschwendt M., Dieterich S., Rennecke J., Kittstein W., Mueller H.J., Johannes F.J. FEBS Letters. 1996;392(2):77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 16.Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. Journal of Biological Chemistry. 1991;266(24):15771–15781. [PubMed] [Google Scholar]
- 17.Matthews J., Almlof T., Kietz S., Leers J., Gustafsson J.A. Biochemical and Biophysical Research Communications. 2005;335(1):168–174. doi: 10.1016/j.bbrc.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 18.Liu S., Carrillo J.J., Pediani J.D., Milligan G. Journal of Biological Chemistry. 2002;277(28):25707–25714. doi: 10.1074/jbc.M201015200. (Epub 22002 May 25706) [DOI] [PubMed] [Google Scholar]
- 19.Beltman J., McCormick F., Cook S.J. Journal of Biological Chemistry. 1996;271(43):27018–27024. doi: 10.1074/jbc.271.43.27018. [DOI] [PubMed] [Google Scholar]
- 20.Franklin C.C., Sanchez V., Wagner F., Woodgett J.R., Kraft A.S. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(15):7247–7251. doi: 10.1073/pnas.89.15.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derijard B., Hibi M., Wu I.H., Barrett T., Su B., Deng T., Karin M., Davis R.J. Cell. 1994;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 22.Boyle W.J., Smeal T., Defize L.H., Angel P., Woodgett J.R., Karin M., Hunter T. Cell. 1991;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 23.Minden A., Lin A., Smeal T., Derijard B., Cobb M., Davis R., Karin M. Molecular and Cellular Biology. 1994;14(10):6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barclay J.L., Anderson S.T., Waters M.J., Curlewis J.D. Molecular Endocrinology. 2007;21(10):2516–2528. doi: 10.1210/me.2007-0030. [DOI] [PubMed] [Google Scholar]