Abstract
In the vertebrate head, central and peripheral components of the sensory nervous system have different embryonic origins, the neural plate and sensory placodes. This raises the question of how they develop in register to form functional sense organs and sensory circuits. Here we show that mutual repression between the homeobox transcription factors Gbx2 and Otx2 patterns the placode territory by influencing regional identity and by segregating inner ear and trigeminal progenitors. Activation of Otx2 targets is necessary for anterior olfactory, lens and trigeminal character, while Gbx2 function is required for the formation of the posterior otic placode. Thus, like in the neural plate antagonistic interaction between Otx2 and Gbx2 establishes positional information thus providing a general mechanism for rostro-caudal patterning of the ectoderm. Our findings support the idea that the Otx/Gbx boundary has an ancient evolutionary origin to which different modules were recruited to specify cells of different fates.
Keywords: Chick, Cranial ganglia, Ear, Eye, Fate map, Placodes, Trigeminal, Xenopus
Highlights
► Otx2 and Gbx2 segregate placode progenitors of different fates. ► Otx2 and Gbx2 mutually repress each other. ► Otx2 target activation is required for olfactory, lens and trigeminal specification. ► Gbx2 is required for otic placode specification.
Introduction
In the vertebrate head, placodes give rise to crucial parts of the sensory nervous system including the olfactory epithelium, the lens, the inner ear and the sensory neurons of the cranial ganglia (Baker and Bronner-Fraser, 2001; Streit, 2007; Schlosser, 2010). They form at discrete positions outside of the central nervous system, with which they build complete sense organs and sensory circuits. How are central and peripheral components aligned? Here we explore the possibility that a common molecular mechanism allocates anterior-posterior positional information across the entire ectoderm.
At neurula stages, placode precursors occupy a unique territory, the pre-placodal region (PPR), where cells of different placodal fates are interspersed (Kozlowski et al., 1997; Streit, 2002; Bhattacharyya et al., 2004; Xu et al., 2008; Pieper et al., 2011); their anterior-posterior identity is not fully specified (Henry and Grainger, 1987; Gallagher et al., 1996; Grainger et al., 1997; Baker et al., 1999; Groves and Bronner-Fraser, 2000; Baker and Bronner-Fraser, 2000; Bhattacharyya et al., 2004; Bailey et al., 2006; Bhattacharyya and Bronner-Fraser, 2008). Although some placode inducing signals have been identified (McCabe and Bronner-Fraser, 2009; Ladher et al., 2010; Schlosser, 2010), additional cell intrinsic mechanisms must exist that determine the interpretation of such signals and mediate PPR subdivision. In the neural tube, mutual repression between pairs of transcription factors establishes boundaries to segregate cells of different fates (Broccoli, et al., 1999; Millet, et al., 1999; Katahira, et al., 2000; Li and Joyner, 2001; Kobayashi et al., 2000; Nakamura and Watanabe, 2005). One of the best-studied interactions is that between Otx2 and Gbx2. While Gbx2 is first detected within the posterior neuroectoderm, Otx2 becomes restricted anteriorly (Simeone et al., 1992, 1993; von Bubnoff et al., 1996; Tour et al., 2001). Both factors mutually repress each other to form a sharp border (Millet et al., 1999; Katahira et al., 2000; Tour et al., 2002a; Glavic et al., 2002) and this interaction establishes the midbrain-hindbrain boundary (MHB) (Wassarman et al., 1997; Acampora et al., 1995, 1997, 1998; Rhinn et al., 1998; Broccoli, et al., 1999; Li et al., 2005). However, in the absence of Otx2 or Gbx2 function MHB specific genes remain expressed, but are mislocalized. These observations suggest that the Otx2/Gbx2 interface is primarily important for positioning the MHB (Li and Joyner, 2001; Raible and Brand, 2004).
Does a similar mechanism establish regional identity within the PPR? Some neural plate border derivatives depend on Gbx2 and Otx2 function. Gbx2 is required for neural crest cell formation and transcripts are also found in the PPR (Li et al., 2009). In mice, Gbx2 is necessary for otic vesicle morphogenesis after placode formation (Lin et al., 2005). Anteriorly, Otx2 cooperates with Notch signaling to promote lens fate (Ogino et al., 2008), while loss of Otx2 function in mice results in lens, olfactory and inner ear defects (Acampora et al., 1995). However, due to the severe head phenotypes in these mutants it is difficult to assess the specific requirement of Otx2 in placode formation.
Here, we test the hypothesis that Otx2 and Gbx2 provide a cell intrinsic mechanism to establish anterior-posterior positional information in sensory placode progenitors. We show that they mutually repress each other to form a boundary between prospective otic and trigeminal placodes and mediate cell segregation within the PPR. While Gbx2 is required for otic specification, Otx2 is necessary for the specification of the olfactory, lens and trigeminal placodes. Thus, Otx2 and Gbx2 provide a global mechanism for patterning of the embryonic ectoderm and ensure the coordinated development of the central and peripheral nervous system in the head.
Materials and methods
Embryo techniques
Fertile hens' eggs (Henry Stewart, UK) were incubated at 38 °C for 24–30 h until they had reached the appropriate stage (Hamburger and Hamilton, 1951; HH). Small groups of cells were labeled with DiI (Streit, 2002); the position of labeled cells along the anterior-posterior axis was expressed as a percentage of the distance from the center of Hensen's node to the anterior tip of the prechordal plate (hn-pc distance). The medio-lateral position was determined as a percentage of the distance between the midline and the edge of the neural plate (Fig. 3(A)). Embryos were grown in modified New culture (New, 1955; Stern and Ireland., 1981) until they reached HH12. The fate of labeled cells was determined by morphology or by colocalization with Pax3 for the ophthalmic trigeminal placode. To compare the cell fate to the position of the Gbx2/Otx2 boundary, stage HH7 embryos were processed for double in situ hybridization (ISH) for Otx2 and Gbx2. The gene expression boundary was determined and expressed as a percentage of the hn-pc distance (Fig. 3(A)). Using these measurements, DiI labels were classified as either within the Gbx2 or Otx2 domain.
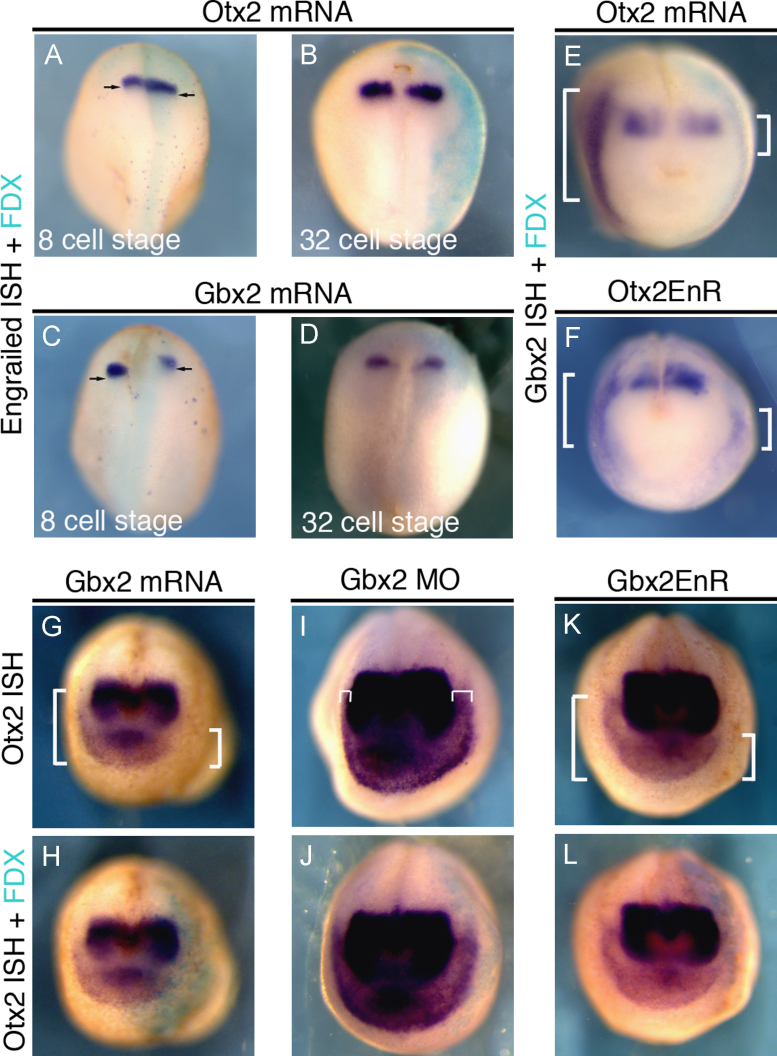
Fig. 3.
Otx2 and Gbx2 mutually repress each other in the PPR. (A) and (B) Injection of Otx2 mRNA into the D1 blastomere of 8-cell stage embryos (A) shifts En-1 posteriorly on the injected side (50%. n=28; FDX: turquoise), while injection into the A3 blastomere of a 32-cell stage embryo has little effect ((B); 5% affected, n=36). (C) and (D) Injection of Gbx2 mRNA into the D1 blastomere at 8-cell stage (C) shifts En-1 anteriorly on the injected side (68%; n=64; FDX: turquoise), while injection into the A3 blastomere at 32-cell stage has no effect ((D); 0% affected, n=31). Dorsal view, anterior to the top. (E) and (F) Injection of Otx2 (E; 68% affected, n=31) or Otx2-EnR ((F); 77% affected, n=17) into A3 at the 32-cell stage inhibits Gbx2 in the PPR. Compare bracket in the injected (FDX: turquoise) and uninjected side. (G) and (H) Injection of Gbx2 into A3 at the 32-cell stage leads to Otx2 repression (68%; n=26); compare brackets (G) on the injected ((H): FDX, turquoise) and uninjected side. (I) and (J) Co-injection of splice and translation blocking Gbx2 morpholinos into A3 at 32-cell stage leads to Otx2 expansion (73%; n=33); compare brackets (I) on the injected ((J) FDX, turquoise) and uninjected side. (K) and (L) Injection of Gbx2-EnR into A3 at the 32-cell stage leads to a loss of Otx2 in 62% of embryos (n=52); compare brackets (K) on the injected ((L) FDX, turquoise) and uninjected side. (E)–(L) Frontal view, dorsal to the top.
Xenopus embryos were obtained as described previously (Gomez-Skarmeta et al., 1998) and staged according to Nieuwkoop and Faber (1967). D1 blastomeres of 8-cell or A3 blastomeres of 32-cell embryos were injected with RNA and fluorescein or rhodamine dextran (FDX; RDX) or with FDX alone (Aybar et al., 2003). Plasmids were linearized; RNA transcribed using SP6 or T7 RNA polymerases, and the GTP cap analog (Harland and Weintraub, 1985). Purified RNA was resuspended in DEPC-water and mixed with FDX to label the injected side. Full length nuclear GFP, nuclear RFP, Otx2 and Gbx2 mRNA, or Gbx2 morpholinos were used (Li et al., 2009). To repress or activate Gbx2 and Otx2 downstream targets, constructs in which their homeodomain was fused to the repressor domain of engrailed (Otx2-EnR and Gbx2-EnR) or the activator E1A were injected; fusion of these constructs to the glucocorticoid receptor (Otx2-EnR-GR, Gbx2-EnR-GR; Glavic et al., 2002) allows temporally controlled activation upon addition of dexamethasone (DEX; 10 μM). Embryos were then grown to the desired stage and processed for ISH and antibody staining. Embryos with the lineage tracer outside of the PPR or inside the neural plate were discarded unless otherwise stated.
In situ hybridization and immunohistochemistry
For ISH, antisense digoxigenin (DIG) or fluorescein labeled RNA probes were used. Xenopus embryos were prepared, hybridized and stained as previously described (Harland, 1991), and NBT/BCIP or BCIP alone were used to reveal the signal. The genes analyzed were Otx2 (Blitz and Cho, 1995), Gbx2 (von Bubnoff et al., 1996), Eya1 (David et al., 2001), Pax8 (Heller and Brändli, 1999), Pax2 (Heller and Brändli, 1999), Pax3 (Bang et al., 1997), Dmrt4 (Huang et al., 2005), Pax6 (Hirsch and Harris, 1997), Runx3 (Park and Saint-Jeannet, 2010) and FoxE3 (Kenyon et al., 1999). FDX was visualized with an alkaline phosphatase conjugated anti-fluorescein antibody (AP-anti-FLU; Roche). Whole-mount ISH in chick (Streit et al., 1997) was performed with DIG labeled anti-sense probes for Otx2 (Bally-Cuif et al., 1995) and fluorescein-labeled probes for Gbx2 (Shamim and Mason, 1998). For double ISH, embryos were hybridized with both probes followed by consecutive antibody staining with alkaline phosphatase coupled-anti-DIG and anti-fluorescein antibodies (Roche) using fast red and NBT/BCIP for color development, respectively. Immunostaining on cryosections was performed (Bailey et al., 2006) using antibodies against Otx2 (Abcam; 1:50) and Pax3 (Developmental Hybridoma Bank; 1:10) and appropriate secondary antibodies (Invitrogen; 1:1000). Sections were imaged on a Leica TCS SP5 confocal microscope.
Results
Otx2 and Gbx2 form a boundary within the sensory placode territory
In the neural tube Otx2 and Gbx2 expression initially overlaps but then resolves to form a boundary at the MHB (Simeone et al., 1993; von Bubnoff et al., 1996; Millet et al., 1999; Tour et al., 2002a; Glavic et al., 2002). In chick Eya2 expression identifies the PPR (Fig. 1(A); McLarren et al., 2003; Streit, 2007). While Otx2 and Gbx2 expression overlap in this territory at HH5 (Fig. 1(E)–(G)), both domains abut later (Fig. 1(B)–(D), (H)–(J)) with Gbx2 restricted to the posterior PPR. A similar boundary is observed in the Xenopus PPR (Fig. 1(K)–(P)). At stage 11.5, Otx2 encompasses both the anterior neural plate and its border (Fig. 1(K)), while Gbx2 is present posteriorly but overlapping with Otx2 (Fig. 1(L) and (M); red bracket). At neural plate stages, Eya1 demarcates the PPR (Fig. 1(N); black bracket); Otx2 and Gbx2 expression has resolved into a neural plate domain dorsally and a PPR domain laterally (Fig. 1(O) and (P); black bracket). In both regions, Otx2 and Gbx2 expression does not overlap (Fig. 1(Q)). Thus, like in the neural plate, Gbx2 and Otx2 form a gene expression boundary within the PPR in Xenopus and chick.
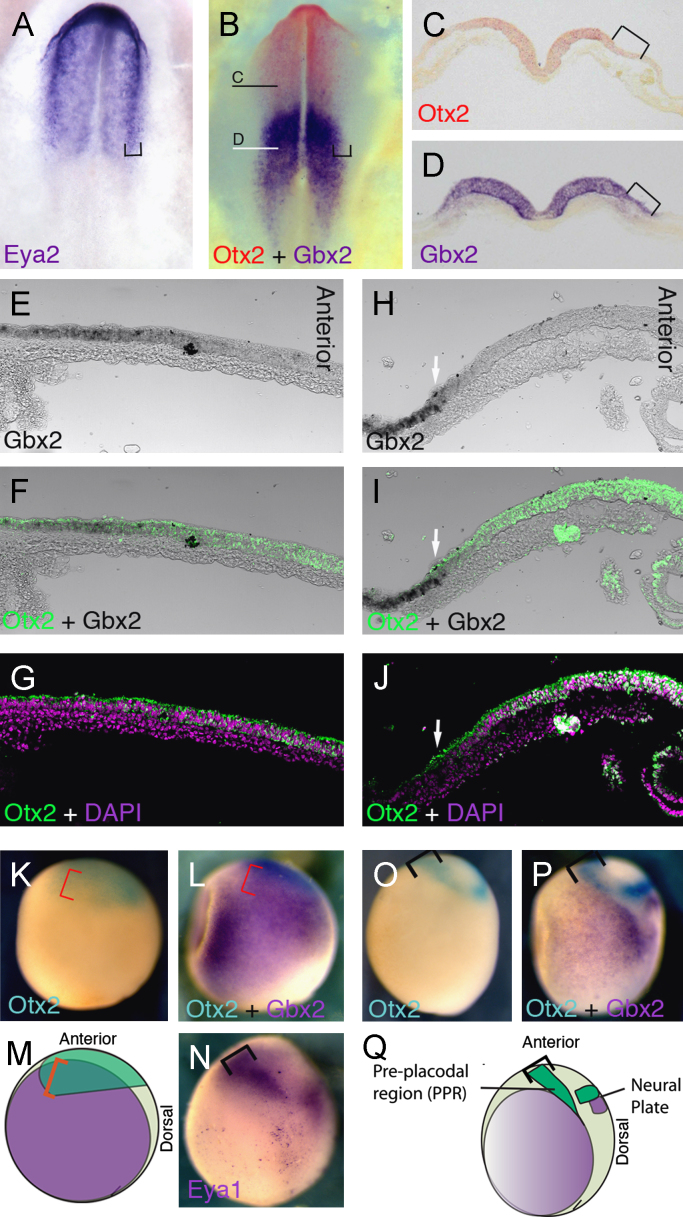
Fig. 1.
Otx2 and Gbx2 form a boundary within the PPR. (A) Expression of Eya2 marks the PPR (bracket). (B)–(D) Double ISH for Otx2 (red) and Gbx2 (blue) at HH7. Lines indicate the level of sections shown in (C) and (D). (E)–(J) Parasagittal sections of stage HH5 (E)–(G) and HH7 (H)–(J) chick embryos after Gbx2 ISH (black (E), (F), (H), (I)) and Otx2 immunostaining (green; (F), (G), (I), (J)); DAPI labels nuclei ((G), (J); magenta). Arrows in (H)–(J) indicate anterior limit of Gbx2. (K) and (L) Double ISH in Xenopus for Otx2 ((K), (L); turquoise) and Gbx2 ((L); blue) at stage 12, dorsal to right anterior to the top. Red brackets indicate overlapping expression. (M) Diagram summarising expression of Otx2 and Gbx2 at stage 12 in Xenopus; red bracket: PPR. (N) Eya1 at stage 13 labels the PPR (bracket). (O) and (P) Double ISH for Otx2 ((O), (P); turquoise) and Gbx2 ((P); blue) at stage 13, dorsal to the right. Black brackets indicate the PPR. (Q) Diagram summarising neural plate and PPR expression of Otx2 and Gbx2 at stage 13 in Xenopus.
Otx2 and Gbx2 segregate otic and trigeminal fates
Otx2 and Gbx2 have previously been implicated in the maintenance of compartment boundaries (Zervas et al., 2004; Sunmonu et al., 2011). Do they segregate progenitors of different fates in the PPR? At HH 7 in chick, the Otx2/Gbx2 boundary lies on average at 35% of the distance from Hensen's node to the anterior tip of the prechordal plate (Fig. 2(A) and (A'); 35±7%; most anterior: 42%, most posterior: 24.5%) roughly corresponding to the most anterior location of otic progenitors (Streit, 2002). DiI labeling shows that cells near the boundary contribute to two placodes: the otic and the trigeminal. The majority of labels that gave rise to both placodes are found close to this boundary, with the exception of two injections at around 20% i.e., within the Gbx2 territory. Anterior to the boundary cells mainly contribute to the maxillomandibular trigeminal placode (mmV) and to the ophthalmic Pax3+ (opV) trigeminal territory (Fig. 2(B), (F)–(H)). In contrast, posterior to the Otx2/Gbx2 border, cells mainly localize to the otic placode (Fig. 2(B)–(D)), but are occasionally found in the mmV (Fig. 2(B); Xu et al., 2008). However, except the two injections mentioned above their original location lies well within the most posterior position of the Otx2/Gbx2 boundary measured suggesting that these cells may indeed arise from the Gbx2 territory. It is therefore possible that due to differences in individual embryos, fate maps overestimate cell mixing (see Pieper et al., 2011 for discussion). In general, our current findings agree with previously published fate maps (Fig. 2(B); Streit, 2002; Xu et al., 2008). Thus, although no strict cell fate segregation is observed, the vast majority of trigeminal precursors come from the Otx2 region, while otic cells largely arise from Gbx2+ cells suggesting that in the PPR the Otx2/Gbx2 boundary roughly separates trigeminal and otic territories.
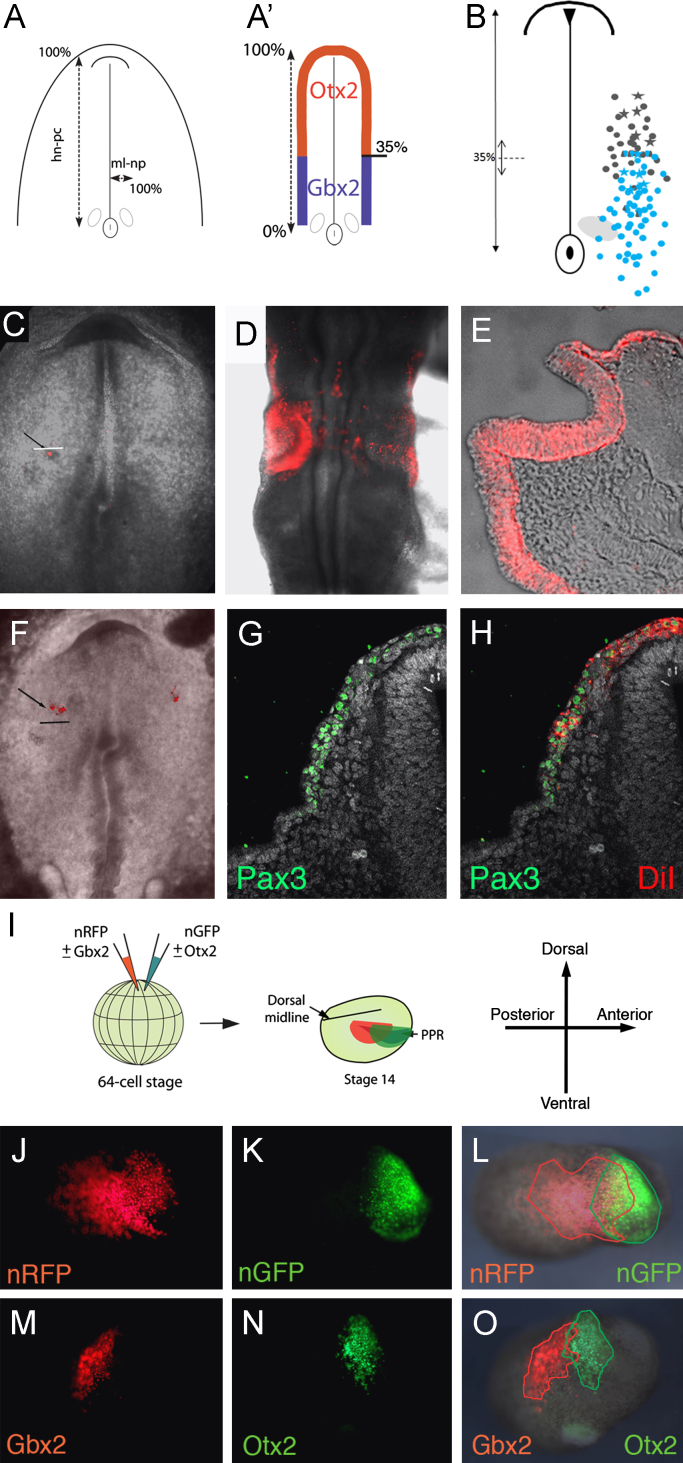
Fig. 2.
The Otx2/Gbx2 boundary separates otic and trigeminal precursors. (A) Diagram showing HH 7 stage chick embryo: the distance from the center of Hensen's node to the anterior tip of prechordal plate (hn-pc) and from the midline to the edge of the neural plate (ml-np) were set to 100%, respectively. The position of DiI label was measured and expressed as percentage of each distance. (A′). The position of the Gbx2/Otx2 boundary was measured using the same landmarks. In total 9 embryos were measured with the boundary on average at 35±7%; most anterior position measured: 42%, most posterior position: 24.5%. (B) Diagram combining labels from this study and published fate maps (Streit, 2002; Xu et al., 2008); gray: labels contributing to the trigeminal placode; blue: labels contributing to the otic placode. Circles: labels from published fate maps; squares: labels with dual fate from the current study; stars: labels from the current study. 35% indicates the average position of the Otx2/Gbx2 boundary (dotted line)±standard deviation (small arrow); note: mixed trigeminal and otic fates mostly locate near this boundary. (C) HH7 embryo with DiI labeled cells posterior to the average position of the Otx2/Gbx2 boundary (white line). (D) and (E) At HH12 their descendants contribute to the otic placode as shown in whole mount (D) and in transverse sections (E) and (F). HH7 embryo with DiI label anterior to the average position of the Otx2/Gbx2 boundary (black line). (G) and (H) At HH11 their descendants overlap with Pax3 protein (green) in the trigeminal placode. In total, 21 labels were placed into the Otx2+ and 12 into the Gbx2+ domain. (I) Diagram showing the experimental design: blastomeres were injected at the 64-cell stage in Xenopus and their position scored at stage 14. Arrows show the orientation of all embryos. (J)–(L) Neighboring blastomeres were injected with nGFP and nRFP and grown until stage 14. Descendants from injected cells are intermingled as indicated by red and green outlines in L (100%, n=10). (M)–(O) When injected with nGFP/Otx2 and nRFP/Gbx2 descendants from adjacent blastomeres do not mix (boundary in 79% of embryos, n=14). Red and green outlines in O show the distribution of cells.
Can Otx2 and Gbx2 sort cells in the PPR? Using Xenopus we compared the behavior of control-injected cells with those carrying exogenous Gbx2 and Otx2. Descendents of the A3 blastomere, which gives rise to placodes, were injected at the 64-cell stage with mRNA encoding nuclear-GFP and nuclear-RFP alone or in combination with Otx2 and Gbx2, respectively (Fig. 2(I)). Significant overlap between GFP and RFP expressing cells is observed at stage 14 in controls (Fig. 2(J)–(L)). In contrast, cells in the PPR and future epidermis overexpressing Gbx2 form a boundary with cells expressing exogenous Otx2 with only some cells intermingling (Fig. 2(M)–(O)). Together these results show that Otx2 and Gbx2 control cell sorting and are part of the molecular mechanism that segregates sensory progenitors to different placodes.
The Otx2/Gbx2 boundary in the PPR forms by a cross-repressive mechanism
In the neural plate, Otx2 and Gbx2 mutually repress each other transcriptionally to form a sharp boundary (Millet et al., 1999; Katahira et al., 2000; Tour et al., 2002a; Glavic et al., 2002). To confirm this we injected Otx2 or Gbx2 mRNA into the D1 blastomere at the 8-cell stage targeting the neural plate and its border. As expected Otx2 misexpression shifts the MHB marker En-1 posteriorly (Fig. 3(A)), whereas misexpression of Gbx2 leads to an anterior shift (Fig. 3(C); see also: Tour et al., 2002b). In contrast, when Otx2 or Gbx2 mRNA is targeted to the PPR (A3 blastomere injection at 32-cell stage) changes in neural En-1 expression are rarely observed (Fig. 3(B) and (D)). This approach therefore allows us to analyze the role of these transcription factors specifically in placode progenitors, without interfering with neural patterning.
In the PPR, misexpression of Otx2 mRNA results in a loss of Gbx2 expression (Fig. 3(E)). To ask whether Otx2 acts as a transcriptional repressor in this context we used a constitutive repressor form Otx2-EnR. Like full-length Otx2, misexpression of Otx2-EnR leads to Gbx2 reduction in the PPR (Fig. 3(F)). Misexpression of Gbx2 (Fig. 3(G) and (H)) or the constitutive repressor Gbx2-EnR (Fig. 3(K) and (L)) reduces Otx2 expression in the PPR, while Gbx2 morpholino knock-down (Li et al., 2009) expands its expression (Fig. 3(I) and (J)). Together, these results demonstrate that in the PPR Otx2 and Gbx2 act as transcriptional repressors and mutually repress each other suggesting that this interaction generates the Gbx2/Otx2 expression boundary.
Dual Gbx2 function in otic placode specification
The otic placode forms within the Gbx2+ territory; is Gbx2 required for its specification? Gbx2 knock-down by splice- and translation-blocking morpholinos prevents the expression of the otic markers Pax8 and Pax2 (Fig. 4(A) and (B)). When analyzed at later stages, the size of the otic vesicle in embryos injected with both morpholinos is severely reduced (Fig. 4(D) – (F)). Although Gbx2 is normally expressed prior to the pre-placodal marker Eya1 (Li et al., 2009), Eya1 expression is unaffected in Gbx2 morphants (Fig. 4(C)). Thus, Gbx2 is required for otic, but not for PPR specification.
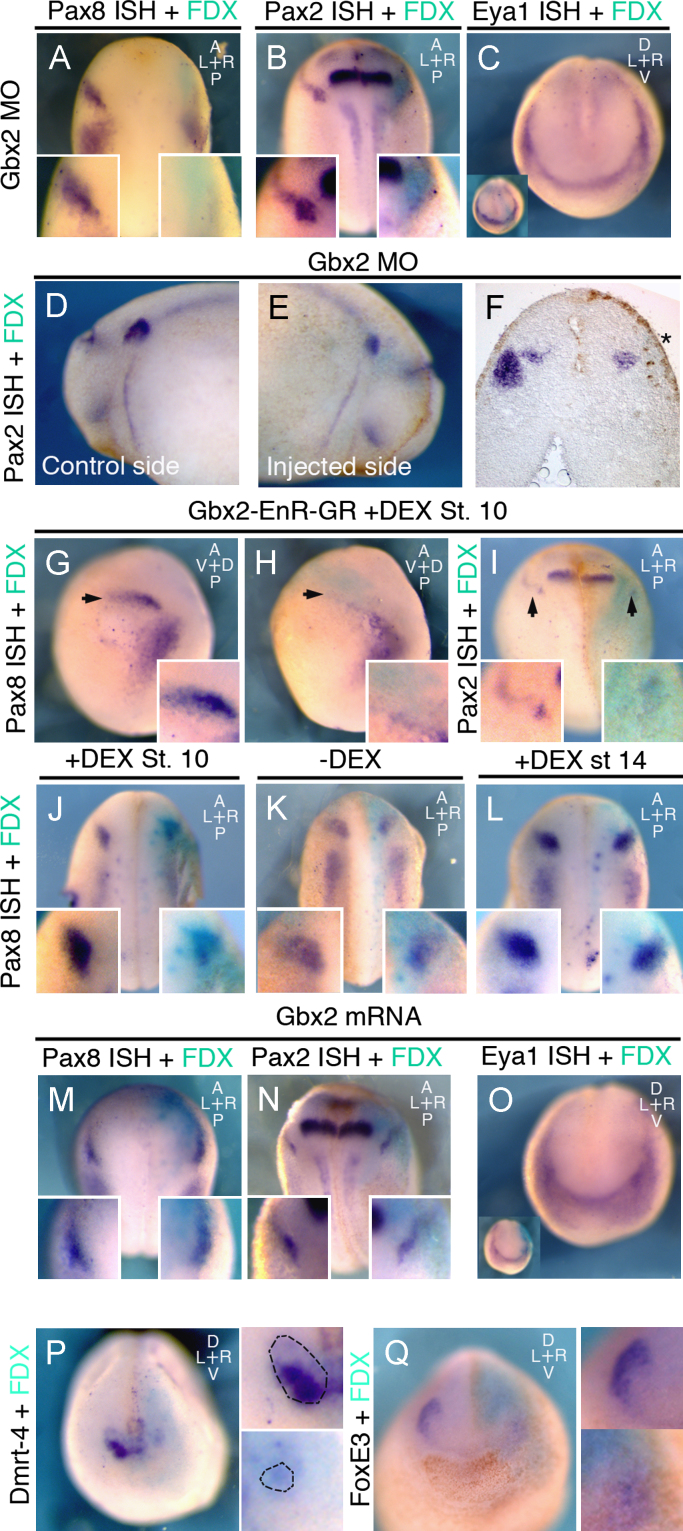
Fig. 4.
Gbx2 is required for otic specification. (A)–(C) Injection of splice and translation blocking Gbx2 morpholinos inhibits otic Pax8 (A; 54%, n=28) and otic Pax2 ((B); 55%, n=131; Splice MO: 66% affected, n=29; ATG MO: 49% affected, n=79). There is no effect on Eya1 (0% affected, n=30. (C): blue). (D)–(F) At stage 25, Pax2 expression is reduced and the otic vesicle is small (asterisk in transverse section F) after injection of splice and translation blocking Gbx2 morpholinos ((E) 59% affected, n=66; splice MO: 44% affected, n=25) when compared to the uninjected side (D). (G)–(I) Injection of inducible Gbx2-EnR-GR: activation at stage 10 reduces otic Pax8 (arrow) at stage 13 ((H); 49%, n=43) compared to uninjected side (G) and Pax2 at stage 16 ((I); arrow; 46% n=18). (J)–(L) Activation of inducible Gbx2-EnR-GR at stage 10 (J) reduces Pax8; no change is observed in absence of DEX ((K); 0% affected, n=17) or when DEX is added at stage 14 ((L) 0% affected, n=110). (M)–(O) Gbx2 mRNA does not expand Pax8 ((M); 0% affected, n=35), Pax2 ((N); 0% affected, n=22), and Eya1 ((O) 0% affected, n=22). (P), (Q) Overexpression of Gbx2 reduces Dmrt-4 ((P) 66%, n=29) and FoxE3 ((Q) 92%, n=13). Small panels in (P) and (Q) show higher magnification of the control (top) and injected (bottom) side. All embryos were injected into the A3 blastomere at the 32-cell stage; inserts in (A), (B), (G)–(N) high magnification of the otic region. In (C) and (O) turquoise staining reveals FDX. Crosses indicate the orientation of embryos; a: anterior, l: left, p: posterior, r: right, d: dorsal, v: ventral.
Is this function of Gbx2 simply due to its Otx2-repressing activity or does it also regulate otic-specific genes? To test this we used the inducible Gbx2-EnR-GR, which constitutively represses all Gbx2 targets including Otx2 (Fig. 3(K) and (L)). When this construct is activated at the beginning of gastrulation (stage 10), the earliest expression of Pax8 (stage 13) and Pax2 (stage 16) is reduced (Fig. 4(G)–(I)); Pax8 remains absent when embryos are grown to stage 18 (Fig. 4(J)) similar to Gbx2 morphants. In contrast, without activation (Fig. 4(K)) no effect is observed. Thus, even in the absence of Otx2, Gbx2-EnR prevents the expression of otic genes suggesting that the loss of otic markers in Gbx2 morphants is not solely a consequence of ectopic Otx2 expression. To assess whether Gbx2 function is required after initial otic specification, we activated Gbx2-EnR-GR later at neural plate stages (Fig. 4(L)): otic genes continue to be expressed normally suggesting that Gbx2 function is not required for the maintenance of otic fate.
Finally, we tested whether Gbx2 is sufficient to impart otic character to cells in the anterior PPR. Gbx2 misexpression does not lead to expansion or ectopic expression of Pax8 (Fig. 4(M)) or Pax2 (Fig. 4(N)), nor does it affect the general PPR marker Eya1 (Fig. 4(O)). However, ectopic Gbx2 expression does repress anterior cell fates as demonstrated by the loss of the olfactory marker Dmrt4 (Huang et al., 2005; Fig. 4(P)) and the lens marker FoxE3 (Fig. 4(Q)). Thus, while Gbx2 is not sufficient to impart otic identity to non-otic cells, it plays a dual role during otic specification: it restricts Otx2 (which otherwise inhibits posterior fate; see below) and provides a positive input for otic specifiers. However, once induced maintenance of otic identity is independent of Gbx2 function.
Otx2 is required for trigeminal placode specification
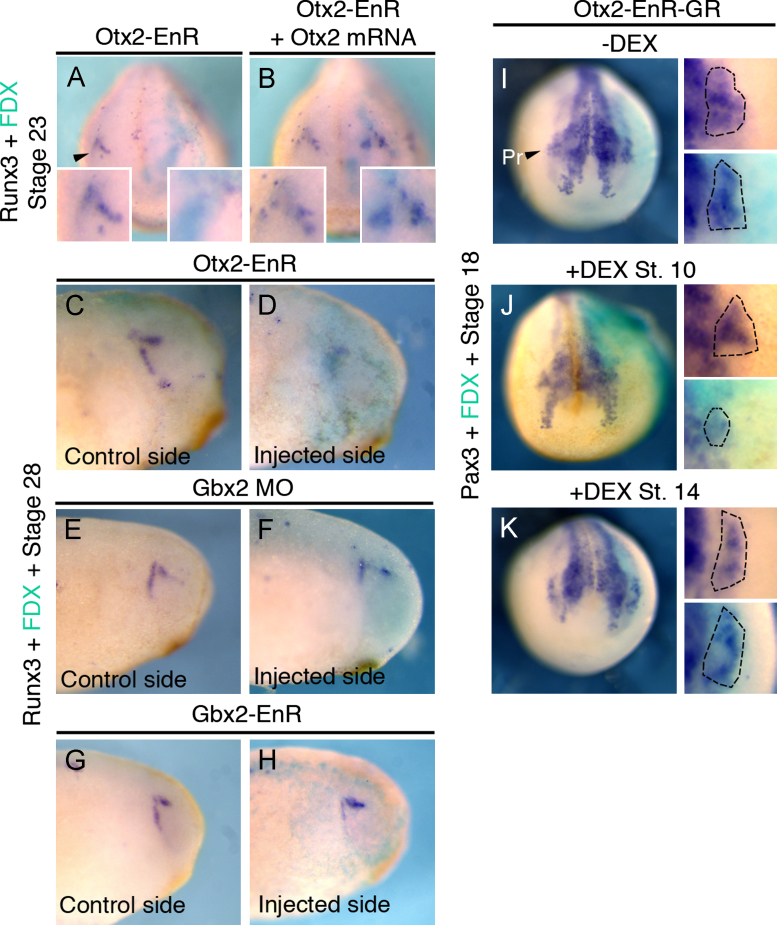
Future trigeminal cells initially lie within the Otx2 domain (Fig. 2(H) and (B)). Is the activation of Otx2 target genes required for trigeminal cell specification? Targeting the PPR with RNA encoding the constitutive repressor form Otx2-EnR leads to a loss of Runx3 labeling trigeminal/profundal precursors (Park and Saint-Jeannet, 2010; Fig. 5(A)) at stage 23, while co-injection with full length Otx2 mRNA restores its expression (Fig. 5(B)). To analyze whether activation of Otx2 targets is required for the profundal and trigeminal placode (opV and mmV in amniotes), Runx3 expression was assessed at stage 28 when both can be distinguished: after Otx2-EnR injection both placodes are absent (Fig. 5(C) and (D)). Our results suggest that Otx2 and Gbx2 separate prospective otic and trigeminal territories (Fig. 2(B)) predicting that profundal and trigeminal specification should be independent of Gbx2. Indeed, injection of Gbx2 morpholinos (Fig. 5(E) and (F)) or of Gbx2-EnR (Fig. 5(G) and (H)) does not alter Runx3 expression to the same extent as Otx2-EnR, and Pax3 expression is normal after Gbx2 knock down (Supplementary Fig. 1D).
Fig. 5.
Activation of Otx2 target genes is required for trigeminal placode specification. (A), (B) Otx2-EnR inhibits Runx3 at stage 23 ((A); 59%, n=26; arrowhead: trigeminal placode on uninjected side). This is rescued by co-injection of Otx2 ((B) inhibition reduced to 17%, n=36). Inserts show higher magnification of the trigeminal region. (C), (D) At stage 28 the profundal and trigeminal placodes can be distinguished; both are reduced after Otx2-EnR injection (78%, n=18). Compare control (C) and injected side (D). (E), (F) Injection of Gbx2 splice and translation blocking morpholinos ((F) 0% affected, n=25) does not affect Runx3; compare uninjected (E) and injected side (F). (G), (H). Injection of Gbx2-EnR does not affect Runx3 ((H); 3% affected, n=31); compare control (G) and injected side (H). (I)–(K) Activation of Otx2-EnR-GR at stage 10 inhibits Pax3 the profundal placode ((J) 49%, n=33); no change is observed without DEX ((I) 5% affected, n=61; PR: profundal placode) or when added at stage 14 ((K) 5% affected, n=42). Magnifications show profundal region (dotted outline) on the uninjected (top) and injected side (bottom; FDX: turquoise). All embryos were injected into the A3 blastomere at the 32-cell stage.
To test when the activation of Otx2 target genes is required for trigeminal development, we used inducible Otx2-EnR-GR. In the absence of DEX, profundal Pax3 is normal (Fig. 5(I)); activation at gastrulation stage (stage 10) reduces Pax3 (Fig. 5(J)), while activation at stage 14 has no effect (Fig. 5(K)). Otx2 or Otx2E1A (not shown) expression in the trigeminal territory has no effect on Pax3 expression (Supplementary Fig. 1A); thus the constitutive repressor form of Otx2 does not mimic misexpression of wild type Otx2 suggesting that Otx2 acts as a transcriptional activator in trigeminal precursors. In addition, ectopic Otx2 expression in the posterior PPR is not sufficient to expand Pax3 transcripts (Supplementary Fig. 1A). Thus, activation of Otx2 target genes is required early for trigeminal specification, but not late for the maintenance of trigeminal fate.
An early requirement for Otx2 in lens and olfactory placode specification
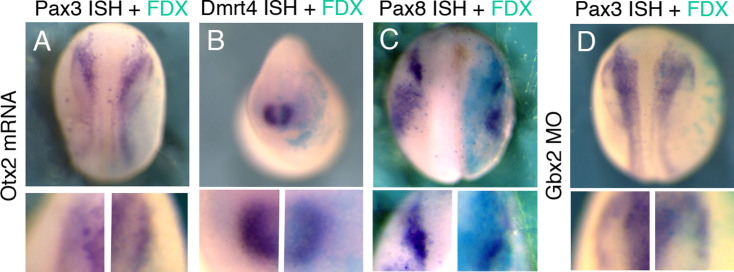
Otx2 transcripts are present in the anterior PPR including in lens and olfactory precursors and remain expressed once the placodes have formed. While a role for Otx2 has been demonstrated for late lens development (Ogino et al., 2008), its early requirement for either placode has not been investigated. When mRNA encoding the repressor form Otx2-EnR is injected into the A3 blastomere at the 32-cell stage the early lens marker Pax6 (Fig. 6(A)) and the olfactory marker Dmrt4 (Fig. 7(A)) are strongly reduced. The loss of both markers is rescued by co-injection with full length Otx2 mRNA (Pax6: Fig. 6(B); Dmrt4: Fig. 7(B)). Activation of inducible Otx2-EnR-GR at the beginning of gastrulation results in a reduction or loss of both Pax6 (Fig. 6(D)) and Dmrt4 (Fig. 7(D)), while no effect is observed without DEX (Pax6: Fig. 6(C); Dmrt4: Fig. 7(C)). Activation of Otx2 target genes is also required later at placode stages: Otx2-EnR-GR activation at stage 18 results in a complete absence of Dmrt4 in the olfactory region (Fig. 7(F)) and down-regulation of FoxE3 in the lens (Ogino et al., 2008; Fig. 6(F)) while their expression is normal without DEX (FoxE3: Fig. 6(E); Dmrt4: Fig. 7(E)). Thus, activation of Otx2 target genes is required for early specification and maintenance of lens and olfactory fates.
Fig. 6.
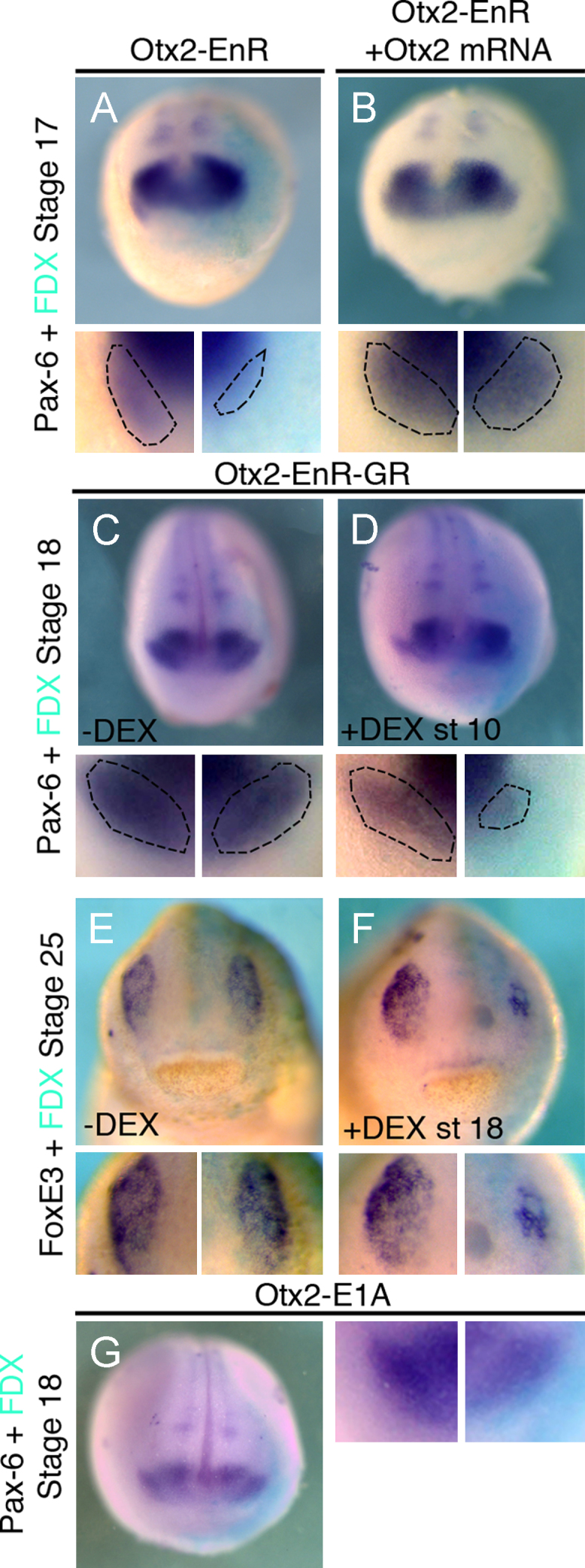
Activation of Otx2 target genes is required at early and late stages of lens placode formation. (A), (B) Otx2-EnR inhibits Pax6 in the lens at stage 17 (50%, n=26). This is rescued by co-injection of Otx2 ((B); inhibition reduced to 15%, n=20). (C)–(F) Activation of Otx2-EnR-GR at stage 10 reduces lens-specific Pax6 at stage 18 ((D) 52%, n=25); without DEX Pax6 expression is normal ((C) 0% affected, n=23). FoxE3 at stage 25 is normal without DEX ((E) 0% affected, n=30), while addition of DEX at stage 18 leads to reduction ((F) 64%, n=22). (G) Otx2-E1A activates Otx2 targets but does not affect lens Pax6 (0% affected, n=25). All embryos were injected into the A3 blastomere at the 32-cell stage and are shown in frontal view with dorsal to the top. High magnifications of the lens region are shown below each panel; dotted lines demarcate placodal Pax6. Turquoise staining reveals the lineage tracer FDX.
Fig. 7.
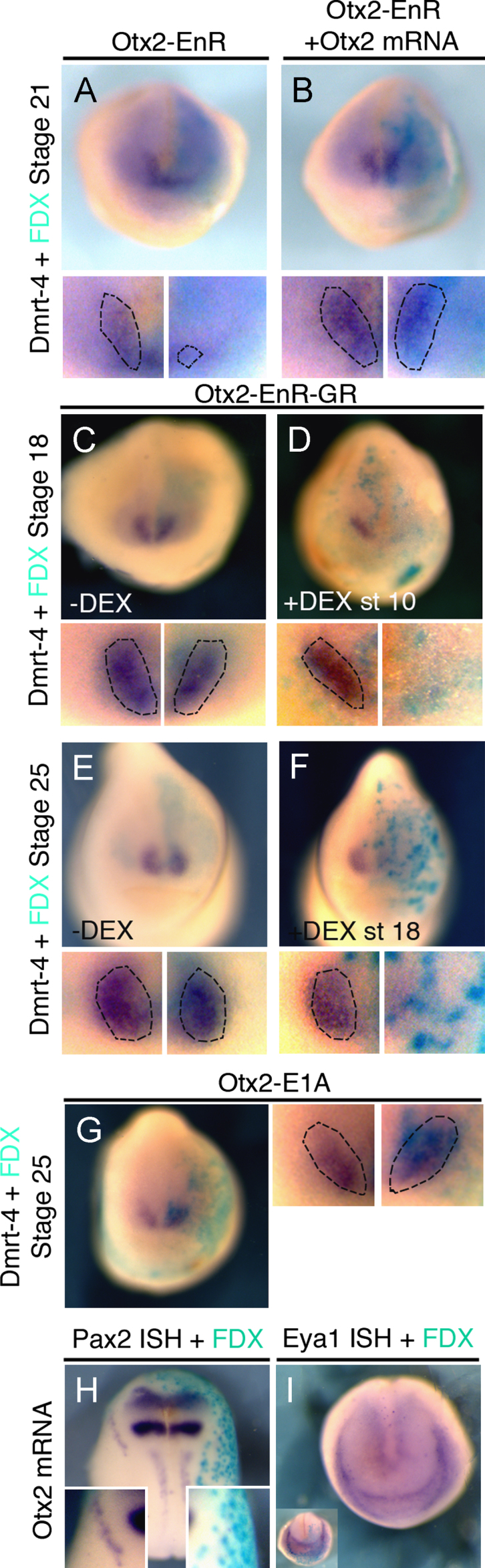
Activation of Otx2 target genes is required at early and late stages of olfactory placode formation. (A), (B) Otx2-EnR inhibits the olfactory placode marker Dmrt4 at stage 21 (59%, n=29). This is rescued by co-injection of Otx2 ((B) inhibition reduced to 12%, n=33). (C–F) Otx2-EnR-GR injections: in the absence of DEX Dmrt4 expression is normal ((C) 5% affected, n=20); when DEX is added at stage 10 Dmrt4 expression is lost at stage 18 ((D) 59%, n=44). At stage 25 Dmrt4 is normal in absence of DEX ((E) 8% affected, n=26), while activation at stage 18 strongly reduces Dmrt4 ((F); 63%, n=24). (G) Otx2-E1A has no effect on Dmrt4 (0% affected, n=14). (H)–(I) Otx2 mRNA reduces Pax2 ((H) 72%, n=25), but does not change Eya1 ((I) 0% affected, n=44). All embryos were injected into the A3 blastomere at the 32-cell stage and are shown in frontal view with dorsal to the top. High magnifications of the olfactory region are shown in small panels; dotted lines demarcate placodal Dmrt4. Turquoise staining reveals the lineage tracer FDX.
Otx2 alone cannot induce ectopic lenses (Ogino et al., 2008); we confirm this using the constitutive activator form of Otx2-E1A (Fig. 6(G)). The same holds true for the olfactory placode: misexpression of Otx2 (Supplementary Fig. 1B) or Otx2-E1A does not expand Dmrt4 expression (Fig. 7(G)). However, when misexpressed in the posterior placode territory Otx2 mRNA represses otic fates as indicated by the absence of both Pax8 (Supplementary Fig. 1C) and Pax2 (Fig. 7(H)) while the general PPR marker Eya1 remains unaffected (Fig. 7(I)). Thus, while ectopic Otx2 represses posterior placodes and activation of its targets is required for olfactory and lens development, alone it cannot impart anterior fate to posterior the PPR.
Discussion
Otx2 and Gbx2 in global ectodermal patterning
To form a functional nervous system its peripheral and central components must develop in register. In the head, the olfactory bulb, the retina and the targets and proximal parts of the sensory ganglia are derived from the central nervous system, while the olfactory epithelium, the lens, inner ear and distal cranial ganglia arise in the non-neural ectoderm from specialized structures, the sensory placodes. How is anterior-posterior patterning between both territories integrated? During development sensory placode precursors originate in the pre-placodal region, where cells of different fates are initially intermingled (Kozlowski et al., 1997; Streit, 2002; Bhattacharyya et al., 2004; Xu et al., 2008; Pieper et al., 2011; for review: Streit, 2007; Schlosser, 2010). Over time, they acquire distinct rostro-caudal identity leading to the alignment with their central counterparts suggesting that a global patterning mechanism imparts positional information to the entire ectoderm (see also: Wada et al., 2006; Patthey et al., 2008). Here we show that the transcription factors Otx2 and Gbx2 are important components of such a mechanism. In the PPR, they segregate otic and trigeminal progenitors (this study), while they establish a compartment boundary at the MHB in the neural plate and prevent mixing of cells with different fates (Millet et al., 1999; Katahira et al., 2000; Tour et al., 2002a; Glavic et al., 2002; Zervas et al., 2004; Sunmonu et al., 2011). In both regions, Otx2 and Gbx2 seem to play a dual role: they repress each other to endow cells with unique identities and to suppress the alternative fate (trigeminal vs otic; midbrain vs rhombomere1), while simultaneously mediating sorting. Initially, both genes partially overlap and mutual repression at the transcriptional level is likely to form a gene expression the boundary. Subsequently, cell sorting ensures compartmentalization to restrict cells of the same fate to a contiguous domain. Accordingly, in the brain, Otx2 deficient cells segregate from wild type neighbors as do cells expressing exogenous Otx2 in rhombomere (Rhinn et al., 1998; Sunmonu et al., 2011). Likewise, our results show that Otx2 and Gbx2 expressing cells sort out in the non-neural ectoderm. The degree of cell mixing in the placode territory is still under debate with more cell mixing observed in chick than in Xenopus (Streit, 2002; Bhattacharyya et al., 2004; Xu et al., 2008; Pieper et al., 2011). As fate maps may introduce some error due to variability between different embryos, ultimately live imaging over long time periods will be required to resolve this question. Nevertheless, together with previous studies on neural and neural crest cells (Wassarman et al., 1997; Acampora et al., 1995, 1997, 1998; Rhinn et al., 1998; Broccoli, et al., 1999; Li et al., 2005; Li et al., 2009; Sunmonu et al., 2011) our findings establish cross-regulatory interactions between Otx2 and Gbx2 as key components for global ectodermal patterning. Both factors establish anterior-posterior identity across the embryonic ectoderm and mediate cell sorting to segregate cells of different fates.
These observations also suggest that signals that establish anterior-posterior identity (for review: Wilson and Houart, 2004) not only pattern the neural plate, but the entire ectoderm with transcription factors like Otx2 and Gbx2 as a read-out. Among these Fgfs, Wnts, Retinoic Acid, Nodals and BMPs provide posteriorizing factors, while their antagonists protect anterior identity. Indeed, elevated Wnt activity in zebrafish leads to an expansion of posterior neural and placodal fates (Kim et al., 2000; Heisenberg et al., 2001). Wnt signaling also promotes derivatives of the posterior neural plate border, neural crest cells, and Gbx2, a direct Wnt target, mediates its activity (Chang, Hemmati-Brivanlou, 1998; Bang et al., 1999; Villanueva et al., 2002; García-Castro et al., 2002; Bastidas et al., 2004; Basch et al., 2006; Patthey et al., 2008; Steventon et al., 2009; Li et al., 2009). In addition to such global patterning mechanisms local signaling and downstream transcriptional networks subsequently fine tune allocation of different cell fates.
Patterning the placode territory
In the PPR, the Otx2/Gbx2 boundary roughly separates prospective otic and trigeminal fates suggesting that olfactory, lens and trigeminal precursors receive different transcriptional inputs from otic progenitors. The transcriptional regulation of the PPR marker Six1 supports this idea. Although Six1 is expressed in a contiguous domain containing all sensory progenitors, different enhancers control its expression along the anterior-posterior axis (Sato et al., 2010). Cells from the anterior Six1 domain contribute to the olfactory, lens and trigeminal placodes, but not to the otic. These findings suggest that one of the first subdivisions of the placode territory occurs between trigeminal and otic precursors clearly grouping trigeminal precursors together with other anterior placodes unlike an earlier suggestion to group profundal and trigeminal placodes in Xenopus with posterior progenitors (Schlosser, 2006). Shortly thereafter, the PPR begins to express other transcription factors in nested domains to subdivide this territory further (for review: Schlosser, 2006).
Otx2 and Gbx2 are already expressed at gastrula stages (Simeone et al., 1992, 1993; von Bubnoff et al., 1996; Tour et al., 2001) and act early during placode specification. Gbx2 is required for the onset of otic-specific genes, where it appears to act as transcriptional activator: the constitutive repressor Gbx2-EnR mimics MO-mediated knock-down. This is in contrast to its earlier role as repressor during boundary formation (see above) suggesting that the availability of cofactors determines the final outcome as observed for other homeobox factors (Brugmann et al., 2004; Anderson et al., 2012). After initial specification, otic development is Gbx2 independent, although it is later involved in ear morphogenesis (Lin et al., 2005). The lack of an early ear phenotype in Gbx2 mutant mice (Lin et al., 2005) is likely due to functional redundancy with Gbx1. In contrast, Otx2 is necessary for both formation and maintenance of lens and olfactory identity (Ogino et al., 2008; this work) consistent with its continued expression in both placodes. In the trigeminal placode, Otx2 is downregulated shortly after its specification probably due to repression by Pax3 (unpublished observations), which also inhibits Pax6 in this territory (Wakamatsu, 2011). Like Gbx2, Otx2 switches from a transcriptional repressor at early stages to an activator later. In summary, like in the neural plate, in the PPR Otx2 and Gbx2 are among the earliest factors that subdivide a contiguous territory along the anterior-posterior axis.
Although Otx2 and Gbx2 are required for early placode specification, neither factor alone is sufficient to endow cells with new regional character or to induce ectopic placodes. This appears to differ considerably from their activity in the neural tube, where ectopic expression of either factor respecifies anterior-posterior identity (Glavic et al., 2002; Tour et al., 2002a). However, here Otx2 and Gbx2 mainly function to position the MHB (Li and Joyner, 2001; Raible and Brand, 2004), an organizer region that itself patterns the brain. Thus, changes in regional identity are likely to be a consequence of MHB induction. Whether a similar organizing center forms at the Otx2/Gbx2 boundary in the PPR remains to be established, however, so far our results argue against this notion. The finding that neither Gbx2 nor Otx2 is sufficient to induce ectopic placodes suggests that additional factors cooperate to control the expression of placode-specific downstream targets. This is indeed the case in the lens, where Otx2 directly binds to the lens-specific FoxE3 enhancer and together with intracellular effectors of Notch signaling activates its transcription (Ogino et al., 2008).
Evolutionary conservation of anterior-posterior patterning by Otx2 and Gbx2
The development of cranial sensory placodes and neural crest is considered to be a key step in the evolution of the vertebrate head (Northcutt and Gans, 1983). Like in vertebrates Gbx and Otx form a boundary within the Amphioxus ectoderm (Williams and Holland, 1996, 1998; Castro et al., 2006; Benito-Gutiérrez, 2006) raising the question whether, at an early stage of their evolution, neural crest and placodes co-opted an already existing gene expression boundary to position themselves along the anterior-posterior axis. Despite Gbx2/Otx2 apposition in Amphioxus, MHB specific genes such as En, Wnt1, FGF8/17/18 and Pax2/5/8 are not restricted to this boundary (Holland et al., 1997, 2000; Meulemans and Bronner-Fraser, 2007), indicating that MHB organizer genes were recruited to the Otx/Gbx border in early vertebrates (Castro et al., 2006; Holland and Short, 2008; Holland, 2009). A Gbx/Otx boundary appears to have been present in the early bilaterian ancestor as Unpg/Gbx and Otd/Otx also negatively regulate one another to form a boundary that positions En and Pax2/5/8 in Drosophila (Hirth et al., 2003). In addition, Gbx2 and Otx2 form a boundary in the annelid Platynereis dumerilii that corresponds to a band of En expression (Arendt et al., 2001; Steinmetz et al., 2007, 2011). Together these findings raise the possibility that Otx2 and Gbx2 form an ancient boundary of gene expression responsible for anterior-posterior patterning of both the neural plate and neural plate border. However, this boundary has been utilized differently in each territory: to position an organizing region at the MHB, and to specify placodal fates in the PPR.
Acknowledgments
We would like to thank Claudio Stern and Jeremy Green for critical comments on the manuscript, Ivor Mason, Thomas Hollemann, Andre Brandli, Milan Jamrich and Jean-Pierre Saint-Jeannet for cDNA clones and Susmitha Rao and Ewa Kolano for excellent technical support. This work was funded by a Wellcome Trust project grant to AS and RM, and by a Medical Research Council grant to RM.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.ydbio.2012.04.025.
Appendix A. Supplementary materials
Fig. S1.
References
- Acampora D., Mazan S., Lallemand Y., Avantaggiato V., Maury M., Simeone A., Brulet P. Forebrain and midbrain regions are deleted in Otx2 −/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121:3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- Acampora D., Avantaggiato V., Tuorto F., Briata P., Corte G., Simeone A. Visceral endoderm-restricted translation of Otx1 mediates recovery of Otx2 requirements for specification of anterior neural plate and normal gastrulation. Development. 1998;125:5091–5104. doi: 10.1242/dev.125.24.5091. [DOI] [PubMed] [Google Scholar]
- Acampora D., Avantaggiato V., Tuorto F., Simeone A. Genetic control of brain morphogenesis through Otx gene dosage requirement. Development. 1997;124:3639–3650. doi: 10.1242/dev.124.18.3639. [DOI] [PubMed] [Google Scholar]
- Arendt D., Technau U., Wittbrodt J. Evolution of the bilaterian larval foregut. Nature. 2001;409:81–85. doi: 10.1038/35051075. [DOI] [PubMed] [Google Scholar]
- Anderson A.M., Weasner B.M., Weasner B.P., Kumar J.P. Dual transcriptional activities of SIX proteins define their roles in normal and ectopic eye development. Development. 2012;139:991–1000. doi: 10.1242/dev.077255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar M.J., Nieto M.A., Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483–494. doi: 10.1242/dev.00238. [DOI] [PubMed] [Google Scholar]
- Bailey A.P., Bhattacharyya S., Bronner-Fraser M., Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev. Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Baker C.V., Stark M.R., Marcelle C., Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–156. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Baker C.V., Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker C.V.H., Bronner-Fraser M. Vertebrate Cranial Placodes I. Embryonic Induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Bally-Cuif L., Gulisano M., Broccoli V., Bonicelli E. C-otx2 is expressed in two different phases of gastrulation and is sensitive to retinoic acid treatment in chick embryo. Mech. Dev. 1995;49:49–63. doi: 10.1016/0925-4773(94)00301-3. [DOI] [PubMed] [Google Scholar]
- Bang A.G., Papalopulu N., Kintner C., Goulding M.D. Expression of Pax-3 is initiated in the early neural plate by posteriorizing signals produced by the organizer and by posterior non-axial mesoderm. Development. 1997;124:2075–2085. doi: 10.1242/dev.124.10.2075. [DOI] [PubMed] [Google Scholar]
- Bang A.G., Papalopulu N., Goulding M.D., Kintner C. Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev. Biol. 1999;212:366–380. doi: 10.1006/dbio.1999.9319. [DOI] [PubMed] [Google Scholar]
- Benito-Gutiérrez È. A gene catalogue of the amphioxus nervous system. Int. J. Biol. Sci. 2006;2:149–160. doi: 10.7150/ijbs.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch M., Bronner-Fraser M., García-Castro M. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;41:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Bastidas F., De Calisto J., Mayor R. Identification of neural crest competence territory: role of Wnt signaling. Dev. Dyn. 2004;229:109–117. doi: 10.1002/dvdy.10486. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Bailey A.P., Bronner-Fraser M., Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev. Biol. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135:4165–4177. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- Blitz I.L., Cho K.W. Anterior neurectoderm is progressively induced during gastrulation: the role of the Xenopus homeobox gene orthodenticle. Development. 1995;121:993–1004. doi: 10.1242/dev.121.4.993. [DOI] [PubMed] [Google Scholar]
- Broccoli V., Boncinelli E., Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401:164–168. doi: 10.1038/43670. [DOI] [PubMed] [Google Scholar]
- Brugmann S.A., Pandur P.D., Kenyon K.L., Pignoni F., Moody S.A. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Castro L.F.C., Rasmussen S.L.K., Holland P.W.H., Holland N.D., Holland L.Z. A Gbx homeobox gene in amphioxus: Insights into ancestry of the ANTP class and evolution of the midbrain/hindbrain boundary. Dev.Biol. 2006;295:40–51. doi: 10.1016/j.ydbio.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Chang C., Hemmati-Brivanlou A. Neural crest induction by Xwnt7B in Xenopus. Dev. Biol. 1998;194:129–134. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- David R., Ahrens K., Wedlich D., Schlosser G. Xenopus Eya1 demarcates all neurogenic placodes as well as migrating hypaxial muscle precursors. Mech. Dev. 2001;103:189–192. doi: 10.1016/s0925-4773(01)00355-0. [DOI] [PubMed] [Google Scholar]
- Gallagher B.C., Henry J.J., Grainger R.M. Inductive processes leading to inner ear formation during Xenopus development. Dev. Biol. 1996;175:95–107. doi: 10.1006/dbio.1996.0098. [DOI] [PubMed] [Google Scholar]
- García-Castro, Marcelle C., Bronner-Fraser M. Ectodermal Wnt functions as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Glavic A., Gómez-Skarmeta J.L., Mayor R. The homeoprotein Xiro1 is required for midbrain-hindbrain boundary formation. Development. 2002;129:1609–1621. doi: 10.1242/dev.129.7.1609. [DOI] [PubMed] [Google Scholar]
- Gomez-Skarmeta J.L., Glavic A., de la Calle-Mustienes E., Modolell J., Mayor R. Xiro, a Xenopus homolog of Drosophila Iroquois complex genes, controls development at the neural plate. EMBO J. 1998;17:181–190. doi: 10.1093/emboj/17.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger R.M., Mannion J.E., Cook T.L., Zygar C.A. Defining intermediate stages in cell determination: acquisition of a lens-forming bias in head ectoderm during lens determination. Dev. Genet. 1997;20:246–257. doi: 10.1002/(SICI)1520-6408(1997)20:3<246::AID-DVG7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Groves A.K., Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Harland R.M., Weintraub H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. Cell Biol. 1985;10:1511–1514. doi: 10.1083/jcb.101.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R.M. In situ hybridization: An improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Heisenberg C.-P., Houart C., Take-uchi M., Rauch G., Young N., Coutinho P., Masai I., Caneparo L., Concha M.L., Geisler R. A mutation in the Gsk3β-binding domain of zebrafish masterblind/axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes. Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller N., Brändli A.W. Xenopus Pax-2/5/8 orthologues: Novel insights into Pax gene evolution and identification of Pax-8 as the earliest marker for otic and pronephric cell lineages. Dev. Genet. 1999;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Henry J.J., Grainger R.M. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev. Biol. 1987;124:200–214. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- Hirth F., Kammermeier L., Frei E., Walldorf U., Noll M., Reichert H. An urbilaterian origin of the tripartite brain: developmental genetici nsights from Drosophila. Development. 2003;130 doi: 10.1242/dev.00438. 2365-237. [DOI] [PubMed] [Google Scholar]
- Hirsch N., Harris W.A. Xenopus Pax-6 and retinal development. J. Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- Holland L.Z., Kene M., Williams N.A., Holland N.D. Sequence and embryonic expression of the amphioxus engrailed gene (AmphiEn): the metameric pattern of transcription resembles that of its segment-polarity homolog in Drosophila. Development. 1997;124:1723–1732. doi: 10.1242/dev.124.9.1723. [DOI] [PubMed] [Google Scholar]
- Holland L.Z., Holland N.D., Schubert M. Developmental expression of AmphiWnt1, an amphioxus gene in the Wnt1/wingless subfamily. Dev. Genes. Evol. 2000;210:522–524. doi: 10.1007/s004270000089. [DOI] [PubMed] [Google Scholar]
- Holland L.Z., Short S. Gene duplication, co-option and recruitment during the origin of the vertebrate brain from the invertebrate chordate brain. Brain Behav. Evol. 2008;72:91–105. doi: 10.1159/000151470. [DOI] [PubMed] [Google Scholar]
- Holland L.Z. Chordate roots of the vertebrate nervous system: expanding the molecular toolkit. Nat. Rev. Neurosci. 2009;10:736–746. doi: 10.1038/nrn2703. [DOI] [PubMed] [Google Scholar]
- Huang X., Hong C.-S., O'Donnell M., Saint-Jeannet J.-P. The doublesex-related gene, XDmrt4, is required for neurogenesis in the olfactory system. Proc. Nat. Acad. Sci. U.S.A. 2005;102:11349–11354. doi: 10.1073/pnas.0505106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira T., Sato T., Sugiyama S., Okafuji T., Araki I., Funahashi J.-I., Nakamura H. Interaction between Otx2 and Gbx2 defines the organizing center for the optic tectum. Mech. Dev. 2000;91:43–52. doi: 10.1016/s0925-4773(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Kenyon K.L., Moody S.A., Jamrich M. A novel fork head gene mediates early steps during Xenopus lens formation. Development. 1999;126:5107–5116. doi: 10.1242/dev.126.22.5107. [DOI] [PubMed] [Google Scholar]
- Kim C.-H., Oda T., Itoh M., Jiang D., Artinger K.B., Chandrasekharappa S.C., Driever W., Chitnis A.B. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Osanai H., Kawakami K., Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech. Dev. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Kozlowski D.J., Murakami T., Ho R.K., Weinberg E.S. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem. Cell Biol. 1997;75:551–562. [PubMed] [Google Scholar]
- Ladher R.K., O'Neill P., Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Li J.Y.H., Joyner A.L. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development. 2001;128:4979–4991. doi: 10.1242/dev.128.24.4979. [DOI] [PubMed] [Google Scholar]
- Li J.Y.H., Lao Z., Joyner A.L. New regulatory interactions and cellular responses in the isthmic organizer region revealed by altering Gbx2 expression. Development. 2005;132:1971–1981. doi: 10.1242/dev.01727. [DOI] [PubMed] [Google Scholar]
- Li B., Kuriyama S., Moreno M., Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009;136:3267–3278. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Cantos R., Patente M., Wu D.K. Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. Development. 2005;132:2309–2318. doi: 10.1242/dev.01804. [DOI] [PubMed] [Google Scholar]
- McCabe K.L., Bronner-Fraser M. Molecular and tissue interactions governing induction of cranial ectodermal placodes. Dev. Biol. 2009;332:189–195. doi: 10.1016/j.ydbio.2009.05.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarren K.W., Litsiou A., Streit A. DLX5 positions the neural crest and pre-placodal region at the border of the neural plate. Dev. Biol. 2003;259:34–47. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Meulemans D., Bronner-Fraser M. Insights from Amphioxus into the evolution of vertebrate cartilage. PLoS ONE. 2007;2:e787. doi: 10.1371/journal.pone.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet S., Campbell K., Epstein D.J., Losos K., Harris E., Joyner A.L. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature. 1999;401:161–164. doi: 10.1038/43664. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Watanabe Y. Isthmus organizer and regionalization of the mesencephalon and metencephalon. Int. J. Dev. Biol. 2005;49:231–235. doi: 10.1387/ijdb.041964hn. [DOI] [PubMed] [Google Scholar]
- New D.A.T. A new technique for the cultivation of the chick embryo in vitro. J. Embryol. Exp. Morphol. 1955;3:326–331. [Google Scholar]
- Nieuwkoop P.D., Faber J. 2nd edn. Daudin; North Holland, Amsterdam: 1967. Normal table of Xenopus laevis. [Google Scholar]
- Northcutt R.G., Gans C. The genesis of neural crest and epidermal placodes: are interpretation of vertebrate origins. Science. 1983;158:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Ogino H., Fisher M., Grainger R.M. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.-Y., Saint-Jeannet J.-P. Expression analysis of Runx3 and other Runx family members during Xenopus development. Gene Expression Pattern. 2010;10:159–166. doi: 10.1016/j.gep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey C.D., Gunhaga L., Edlund T. Early development of the central and peripheral nervous system is coordinated byWnt and BMP signals. PLoS ONE. 2008;3:e1625. doi: 10.1371/journal.pone.0001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper M., Eagleson G.W., Wosniok W., Schlosser G. Origin and segregation of cranial placodes in Xenopus laevis. Dev. Biol. 2011;360:257–275. doi: 10.1016/j.ydbio.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Raible F., Brand M. Divide et impera - the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–734. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rhinn M., Dierich A., Shawlot W., Behringer R.R., Le Meur M., Ang S.L. Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development. 1998;125:845–856. doi: 10.1242/dev.125.5.845. [DOI] [PubMed] [Google Scholar]
- Sato S., Ikeda K., Shioi G., Ochi H., Ogino H., Yajima H., Kawakami K. Conserved expression of mouse Six1 in the pre-placodal region (PPR) and identification of an enhancer for the rostral PPR. Dev. Biol. 2010;344:158–171. doi: 10.1016/j.ydbio.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev. Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making Senses: Development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Shamim H., Mason I. Expression of Gbx-2 during early development of the chick embryo. Mech. Dev. 1998;76:157–159. doi: 10.1016/s0925-4773(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Gulisano M., Stornaiuolo A., Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Mallamaci A., Stornaiuolo A., D'Apice M.R., Nigro V., Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P.R.H., Zelada-Gonzales F., Burgtorf C., Wittbrodt J., Arendt D. Polychaete trunk neuroectoderm converges and extends by mediolateral cell intercalation. Proc. Nat. Acad. Sci. 2007;104:2727–2732. doi: 10.1073/pnas.0606589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P.R.H., Kostyuchenko R.P., Fischer A., Arendt D. The segmental pattern of Otx, Gbx, and Hox genes in the annelid Platynereis dumerilii. Evol. Dev. 2011;13:72–79. doi: 10.1111/j.1525-142X.2010.00457.x. [DOI] [PubMed] [Google Scholar]
- Stern C.D., Ireland. G.W. An integrated experimental study of endoderm formation in avian embryos. Anat. Embryol. 1981;163:245–263. doi: 10.1007/BF00315703. [DOI] [PubMed] [Google Scholar]
- Steventon B., Araya C., Linker C., Kuriyama S., Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev. Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Streit A., Sockanathan S., Perez L., Rex M., Scotting P.J., Sharpe P.T., Lovell-Badge R., Stern C.D. Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development. 1997;124:1191–1202. doi: 10.1242/dev.124.6.1191. [DOI] [PubMed] [Google Scholar]
- Sunmonu N.A., Li K., Guo Q., Li J.Y.H. Gbx2 and Fgf8 are sequentially required for formation of the midbrain-hindbrain compartment boundary. Development. 2011;138:725–734. doi: 10.1242/dev.055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tour E., Pillemer G., Gruenbaum Y., Fainsod A. The two Xenopus Gbx2 genes exhibit similar, but not identical expression patterns and can affect head formation. FEBS Lett. 2001;507:205–209. doi: 10.1016/s0014-5793(01)02963-5. [DOI] [PubMed] [Google Scholar]
- Tour E., Pillemer G., Gruenbaum Y., Fainsod A. Gbx2 interacts with Otx2 and patterns the anterior-posterior axis during gastrulation in Xenopus. Mech. Dev. 2002;112:141–151. doi: 10.1016/s0925-4773(01)00653-0. [DOI] [PubMed] [Google Scholar]
- Tour E., Pillemer G., Gruenbaum Y., Fainsod A. Otx2 can activate the isthmic organizer genetic network in the Xenopus embryo. Mech. Dev. 2002;110:3–13. doi: 10.1016/s0925-4773(01)00591-3. [DOI] [PubMed] [Google Scholar]
- Villanueva S., Glavic A., Ruiz P., Mayor R. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 2002;241:289–301. doi: 10.1006/dbio.2001.0485. [DOI] [PubMed] [Google Scholar]
- von Bubnoff A., Schmidt J.E., Kimelman D. The Xenopus laevis homeobox gene Xgbx-2 is an early marker of anteroposterior patterning in the ectoderm. Mech. Dev. 1996;54:149–160. doi: 10.1016/0925-4773(95)00468-8. [DOI] [PubMed] [Google Scholar]
- Wada H., Escriva H., Zhang S., Laudet V. Conserved RARE localization in amphioxus Hox clusters and implications for hox code evolution in the vertebrate neural crest. Dev. Dyn. 2006;235:1522–1531. doi: 10.1002/dvdy.20730. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y. Mutual repression between Pax3 and Pax6 is involved in the positioning of ophthalmic trigeminal placode in avian embryo. Dev. Growth Differ. 2011;53:994–1003. doi: 10.1111/j.1440-169X.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- Wassarman K.M., Lewandoski M., Campbell K., Joyner A.L., Rubenstein J.L., Martinez S., Martin G.R. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development. 1997;124:2923–2934. doi: 10.1242/dev.124.15.2923. [DOI] [PubMed] [Google Scholar]
- Williams N.A., Holland P.W. Gene and domain duplication in the chordate Otx gene family: insights from amphioxus Otx. Mol. Biol. Evol. 1998;15:600–607. doi: 10.1093/oxfordjournals.molbev.a025961. [DOI] [PubMed] [Google Scholar]
- Williams N., Holland P. Old head on young shoulders. Nature. 1996;383:490. [Google Scholar]
- Wilson S.W., Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Dude C.M., Baker C.V.H. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev. Biol. 2008;317:174–186. doi: 10.1016/j.ydbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Zervas M., Millet S., Ahn S., Joyner A.L. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron. 2004;43:345–357. doi: 10.1016/j.neuron.2004.07.010. [DOI] [PubMed] [Google Scholar]