Abstract
We sought both to evaluate the clinical value of transesophageal echocardiography in minimally invasive surgical closure of ventricular septal defects and to evaluate the feasibility, safety, and efficacy of the surgical occlusion procedure.
We selected 49 pediatric patients who had perimembranous ventricular septal defects as determined by preoperative transthoracic echocardiographic examination. After the patients were under general anesthesia, we used transesophageal echocardiography to determine the number of defects and their positions, shapes, and sizes, these last in order to choose the appropriate occluder. Under transesophageal echocardiographic monitoring and guidance, we introduced and deployed the occluder. The evaluation of therapy was performed by means of transesophageal echocardiography immediately after occluder release. All patients underwent follow-up transthoracic echocardiography within 2 to 5 postoperative days.
Satisfactory occluder deployment was achieved in 38 patients. No death occurred. No occluder displacement or valve dysfunction was observed during the last transesophageal echocardiographic study. In addition, follow-up by transthoracic echocardiography showed improvement of left ventricular dimensions and ejection fractions.
Our initial experience has been encouraging. Transesophageal echocardiography plays a crucial role in performing minimally invasive surgical closure of cardiac defects. It enables the feasible, safe, and effective closure of ventricular septal defects. However, larger sample sizes and longer-term follow-up are necessary for the accurate evaluation of this procedure's safety and effectiveness as an alternative to cardiopulmonary bypass surgery and transcatheter closure of congenital cardiac defects.
Key words: Child; echocardiography/methods; echocardiography, transesophageal; heart defects, congenital/surgery/ultrasonography; heart septal defects, ventricular/surgery/ultrasonography; infant; prostheses and implants; surgical procedures, minimally invasive; treatment outcome
Over the past 30 years, the use of transcatheter devices for ventricular septal defect (VSD) closure has become a widely accepted alternative to surgical closure,1,2 but this technique has its limitations. In young children, the sheath size is large relative to vascular size, and, in patients of all ages, interventional closure of large defects and of some specific types of defects can be problematic. Minimally invasive surgical closure of VSD is a new technique derived from percutaneous occlusion.3,4 In this report, we describe our initial experience in using transesophageal echocardiography (TEE) for minimally invasive surgical closure of VSD.
Patients and Methods
Patients
From March 2006 through September 2009, we attempted minimally invasive surgical closure with TEE assistance in 49 pediatric patients who had VSD, all of them having perimembranous VSD (PMVSD). The ages of these patients ranged from 7 months to 43 months. One case was associated with persistent left superior vena cava, 5 with minor patent ductus arteriosus, and 25 with mild tricuspid regurgitation. The maximal dimensions of the defects ranged from 3 mm to 13 mm. In accordance with the shape of the defects and the surrounding fibrous tissue, we divided the PMVSD group into 4 subtypes: funnel-shaped, tuberculated, irregular, and tubular.5
Equipment and Devices
For echocardiography, we used the Philips HP Sonos 4500® echocardiographic system* with a biplanar pediatric TEE transducer. We used several types of VSD occluders (Shanghai Shape Memory Alloy Co. Ltd.; Shanghai, PRC), which included symmetric membranous occluders, asymmetric membranous occluders, and muscle occluders. The sizes of the VSD occluders varied from 6 mm to 14 mm. All occluders were made of woven mesh nitinol wire, with 3 to 5 layers of polyester mesh inside.
Procedural Protocol
First, transthoracic echocardiographic (TTE) examination was performed on all patients to determine which ones had appropriate indications for the procedure. Our selection criteria for minimally invasive closure of PMVSD were evidence of heart failure indicating early intervention and age younger than 3 years or body weight less than 15 kg. This study was approved by our hospital's ethics committee.
Second, TEE was performed with the patients under general anesthesia and hemodynamic monitoring, before the chest and the pericardium were opened (Fig. 1). Using a series of planes (mainly long-axis view, short-axis view, and apical 5-chamber view), we observed the spatial position and shape of each defect. We measured the maximal dimension of the defect and the distance from the defect to the tricuspid and aortic valves in multiple views. Color-flow Doppler imaging was used to observe left-to-right flow through the defect. The size and type of occluder was selected in accordance with these TEE findings.
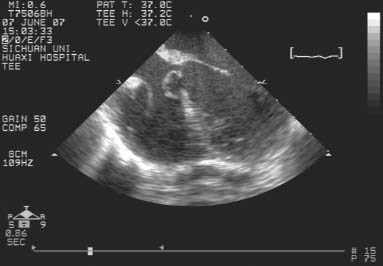
Fig. 1 After the administration of general anesthesia, preoperative transesophageal echocardiography shows the perimembranous ventricular septal defect.
The TEE evaluation was of paramount importance in confirming the presence of a defect and in helping to choose the appropriate occlusion device. It also can help in detecting other cardiovascular anomalies or conditions that would preclude this particular treatment.
Closure with Transesophageal Echocardiographic Assistance
The chest and the pericardium were opened with a 3- to 5-cm incision. Antibiotic prophylaxis and full heparinization (1 mg/kg) were administered routinely. With TEE monitoring and guidance, we punctured the anterior wall of the right ventricle, then inserted a short delivery sheath from the right ventricle to the left ventricle (LV) through the VSD (Fig. 2). When the tip of the sheath was placed in the mid-chamber of the LV, the surgeon deployed the left disk in the LV. After that, the central waist and the right disk were deployed in sequence (Fig. 3). We verified that no part of the atrioventricular valve was impinged upon by the disks. During this period immediately after deployment, we performed TEE to confirm tricuspid and aortic valvular function, observe the stability of the device within the septum, and detect any other occluder-related sequela. If the result was not satisfactory, the occluder would be replaced with another one. This procedure was repeated until a favorable result was achieved.
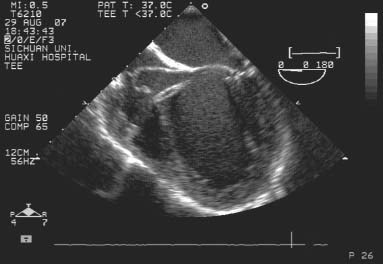
Fig. 2 Intraoperative transesophageal echocardiography is used to guide the long sheath from the right ventricle to the left ventricle, through a short introducing sheath.
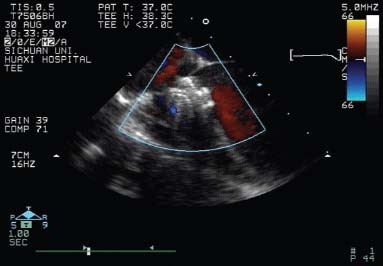
Fig. 3 Intraoperative color-flow transesophageal echocardiography confirms that shunting from the ventricular septal defect is instantly diminished after deployment of the occluder.
Follow-Up
At 3 to 5 postprocedural days, all patients were evaluated by TTE and electrocardiography to determine surgical effectiveness and cardiac function. Dexamethasone was administered for the first 3 days to prevent arrhythmia, and aspirin was administered for the first 6 months to prevent clot formation.
In this group of 49 PMVSD patients, 23 had funnel-shaped defects, 13 had tuberculated defects, 2 had irregular defects, and 11 had tubular defects. We used different types of occluders in accordance with these defect types, in our effort to close the anatomic hole with the most appropriate device for each patient.
Statistical Analysis
Perioperative data were collected and converted to median and range. The SPSS 15.0 software (IBM Corporation; Somers, NY) was used for statistical analysis. Correlation analysis of the various measurements was performed. A value of P <0.05 was regarded as statistically significant. All tests were 2-sided.
Results
Of the 49 PMVSD patients, 38 underwent the entire procedure successfully. In evaluating the correlations between the 2 echocardiographic methods of measurement (TTE and TEE) and occluder size (Table I), we obtained robust results in favor of TEE (r 2=0.585 and r 2=0.839 for TTE and TEE, respectively) (Fig. 4).
TABLE I. Clinical Data for 38 PMVSD Patients Who Underwent Minimally Invasive Surgical Closure
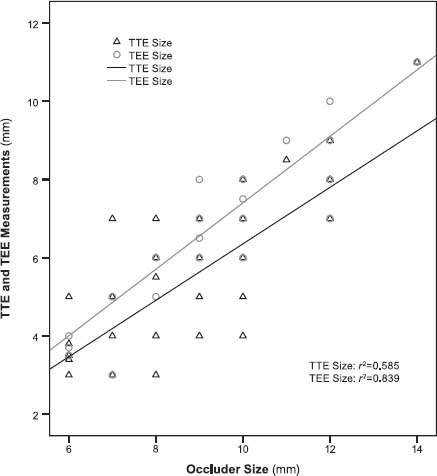
Fig. 4 The correlations between ventricular septal defect dimensions as measured by transthoracic (TTE) and transesophageal echocardiography (TEE), and the corresponding occluder sizes.
The 11 other patients in the study group were converted to cardiopulmonary bypass (CPB) surgery for a variety of reasons. Four patients were converted to surgery due to conditions detected during TEE scanning: 1 had prolapse of the right aortic valve leaflet, and 3 had a defect close to the right aortic cusp (or even overlapping it slightly). The 7 remaining patients were converted to open surgery for severe sequelae to deployment of the occluder: 4 patients had notable residual shunting, 1 had his occluder fall off, 1 had marked tricuspid regurgitation, and 1 had marked aortic regurgitation.
Echocardiographic Observations
In the 38 successful cases, 14 patients underwent operation with the aid of dual echocardiographic guidance (TTE and TEE), which enabled successful closure with asymmetric occluders of juxta-arterial defects (5 patients) and of defects that were only 1 to 2 mm distant from the aortic valve (9 patients). For 2 patients with tubular defects, we used muscle occluders, and for the others we used symmetric occluders. Eighteen patients in the study had a 1- to 3-mm space between the defect and the tricuspid valve. A transient ST-T change on the electrocardiogram was observed in 5 patients, and a transient 1st-degree atrioventricular block occurred in 1 patient. Transesophageal echocardiography confirmed that there was no movement of occluders and no dysfunction of aortic or tricuspid valves; only 2 patients showed slight residual shunts immediately after deployment of the occluder.
Follow-Up
At the 3- to 5-day TTE follow-up, no occluder movement or residual shunt was observed in the 38 successful cases. Tricuspid regurgitation lessened in 10 patients and increased mildly in 2 others; minimal aortic regurgitation developed in 2 patients. No patients needed additional treatment.
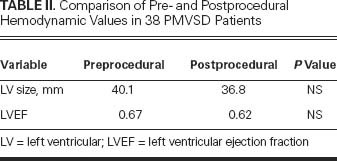
Table II lists the pre-procedural and post-procedural LV diameters and LV ejection fractions (LVEFs). Both LV diameters and LVEFs returned to normal after defect closure, although the difference between “before” and “after” values was not statistically significant.
TABLE II. Comparison of Pre- and Postprocedural Hemodynamic Values in 38 PMVSD Patients
Discussion
Minimally invasive surgical closure of intracardiac defects is a new technique that has expanded the indications for closure. Compared with open-heart surgery, minimally invasive surgery avoids CPB and significantly reduces mortality rates and occluder-related complications. Compared with transcatheter closure, it does not require radiography or contrast agents, and, of more importance, the success rate is higher because the short surgical path tends to facilitate operation of the device.
Our study shows that minimally invasive surgical closure of VSDs under TEE monitoring is feasible, safe, and effective. Although small series have been performed in the operating theatre with a hybrid approach,5,6 no large-series patient study of this technique has been reported in the literature.
Detailed TEE guidance of the entire procedure is important for the success of minimally invasive intracardiac surgery.7 The spatial positions and shapes of defects, and the relationships of those defects with surrounding tissues, should be carefully considered with the aid of TEE before a treatment plan is chosen. Transesophageal echocardiography can also help surgeons to exclude inappropriate patients, judge when and where to release the occluder, and evaluate the effects of closure.
Our early experiences show the following:
A PMVSD with a rim ≥2 mm from the aortic valve responds best.
A PMVSD with a rim <2 mm from the aortic valve requires the use of an asymmetric occluder.
The occluder size chosen is usually 1 to 2 mm larger than the maximal dimension of the defect.
Symmetric or asymmetric occluders are most suitable for funnel-shaped and tuberculated PMVSDs.
For tubular defects, a muscle occluder is best, because its longer central connecting waist adapts better to long sacs.
Symmetric occluders are suitable for blocking the left-sided orifices of irregular-shaped VSDs.
Choosing an occluder of appropriate size and type is important in closing the defect completely and avoiding harmful sequelae. Oversize devices are prone to causing valvular regurgitation and atrioventricular block, while smaller ones tend to cause residual shunting and occluder displacement. Our study shows that TTE measurement usually underestimates the defect's maximal dimension; also, TTE cannot display structures adjacent to the defect as well as TEE can. Therefore, choosing occluder size with the aid of TEE enables more precision. After defect closure, the hemodynamics of the heart are improved, and the LV diameter and LVEF tend to achieve normal values.
Our initial experiences in performing minimally invasive surgical closure of VSD with the assistance of TEE have been encouraging. However, larger sample sizes and longer-term follow-up are necessary for the accurate evaluation of this procedure's safety and effectiveness as an alternative to CPB surgery and transcatheter closure of congenital cardiac defects.
Acknowledgments
We thank Haibo Song and Ke Dian for their help in transferring echocardiographic images and in providing esophageal insertion.
Footnotes
*New units of this model are no longer available.
Address for reprints: Hong Tang, MD, Department of Cardiology, West China Hospital of Sichuan University, Chengdu 610041, PRC
E-mail: hxyyth@gmail.com
This work was supported by China National Institutes of Science/Technology and grant No. 2006BAI01A08.
References
- 1.Carminati M, Butera G, Chessa M, Drago M, Negura D, Piazza L. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol 2005;96(12A):52L–58L. [DOI] [PubMed]
- 2.Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol 2007;50(12):1189–95. [DOI] [PubMed]
- 3.Santoro G, Bigazzi MC, Lacono C, Gaio G, Caputo S, Pisacane C, et al. Transcatheter closure of complex atrial septal defects: feasibility and mid-term results. J Cardiovasc Med (Hagerstown) 2006;7(3):176–81. [DOI] [PubMed]
- 4.Sivakumar K, Krishnan P, Pieris R, Francis E. Hybrid approach to surgical correction of tetralogy of Fallot in all patients with functioning Blalock Taussig shunts. Catheter Cardiovasc Interv 2007;70(2):256–64. [DOI] [PubMed]
- 5.Li J, Zhang J, Shi J, Zhou X, Duan Y, Zhu T, Yao Z. Choice of occluder in different shape, size of membranous ventricular septal defect. Chinese J Med Imaging Technol 2005; 21(5):712–4.
- 6.Amin Z, Gu X, Berry JM, Titus JL, Gidding SS, Rocchini AP. Perventricular [correction of Periventricular] closure of ventricular septal defects without cardiopulmonary bypass [published erratum appears in Ann Thorac Surg 2000;69(2):71]. Ann Thorac Surg 1999;68(1):149–54. [DOI] [PubMed]
- 7.Holzer R, Hijazi ZM. Interventional approach to congenital heart disease. Curr Opin Cardiol 2004;19(2):84–90. [DOI] [PubMed]


