Abstract
Coronary artery fistulae are a rare cardiovascular anomaly. Even less common are multiple fistulae involving more than 1 coronary artery. Herein we report the case of a 47-year-old woman who had fistulae from both the right coronary and left circumflex coronary arteries draining into the coronary sinus. These lesions we attempted to close percutaneously but subsequently closed surgically. We discuss diagnostic imaging approaches, along with closure indications, closure options, and outcomes.
Key words: Arteriovenous fistula/surgery/therapy; coronary artery anomaly; coronary artery fistula/surgery/therapy; coronary computed tomographic angiography; heart defects, congenital
WEBSITE FEATURE
Congenital coronary artery fistula formation is attributed to the persistence of abnormal sinusoidal connections to cardiac structures. Coronary fistulae can involve connection to a cardiac chamber (coronary–cameral fistula) or to a central venous structure (coronary arteriovenous fistula). The most common sites for coronary fistulous connection are the right ventricle, right atrium, and pulmonary artery.1 Most patients who experience symptoms related to a coronary artery fistula present during the 4th through 6th decades of life.2,3 In this report, we describe the case of a middle-aged woman with dyspnea on exertion who underwent successful surgical repair of coronary fistulae from both the right coronary and the left circumflex coronary arteries that drained into the coronary sinus (CS).
Case Report
In June 2007, a 47-year-old woman reported increasing dyspnea with exertion and was noted on physical examination to have a continuous heart murmur. She denied chest discomfort at rest or upon exertion. Occasional, brief episodes of palpitations were reported, but no presyncope or syncope. There was no other notable medical or surgical history and no family history of cardiovascular disease.
To investigate her symptoms and pathologic heart murmur, we began our diagnostic evaluation. Chest radiography revealed cardiomegaly without interstitial edema (Fig. 1). Transthoracic echocardiography (TTE) showed a dilated structure in the atrioventricular groove (Fig. 2). Left and right ventricular function were normal, with enlargement of the right atrium (RA) and left atrium (LA).
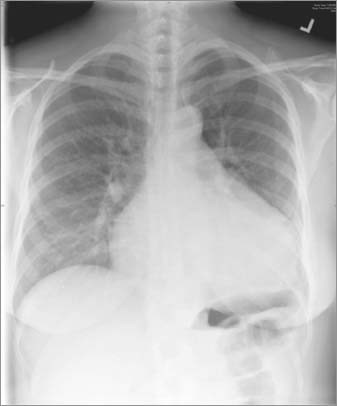
Fig. 1 Posteroanterior chest radiograph shows cardiomegaly.
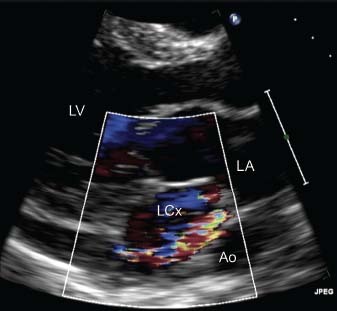
Fig. 2 Transthoracic echocardiography (parasternal long-axis view with color-flow Doppler imaging) shows the turbulent flow of the dilated vessels in the atrioventricular groove. Ao = descending aorta; LA = left atrium; LCx = left circumflex coronary artery; LV = left ventricle Real-time motion image is available at www.texasheart.org/journal.
Cardiovascular magnetic resonance (CMR) showed RA enlargement, normal left ventricular (LV) and right ventricular (RV) systolic function, and aneurysmal dilation of the right coronary artery (RCA) and left circumflex coronary artery (LCx) (Fig. 3). Gadolinium delayed-enhancement imaging did not show any evidence of prior myocardial infarction or scar.
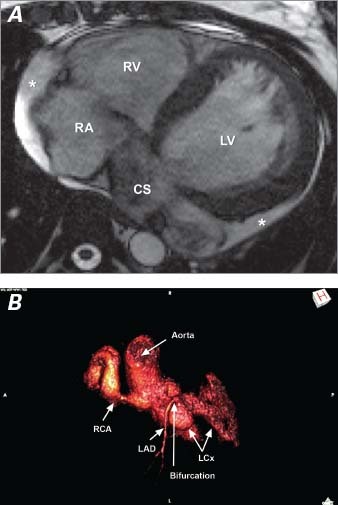
Fig. 3 Cardiovascular magnetic resonance shows A) left and right ventricular enlargement, and the dilated coronary sinus; and B) the origins of the fistulae from the dilated left circumflex and right coronary arteries, along with the uninvolved, normal-diameter left anterior descending coronary artery. * = small pericardial effusion; CS = coronary sinus; LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; LV = left ventricle; RA = right atrium; RCA = right coronary artery; RV = right ventricle
Gated cardiac computed tomography with contrast agent revealed dilation of the RCA (diameter, 18 mm) and the LCx (diameter, 21 mm) (Fig. 4). Both the RCA and the LCx were serpiginous and appeared to drain into the CS just before the CS entered the RA. We noted a prominent, dilated thebesian valve that restricted CS flow into the RA.
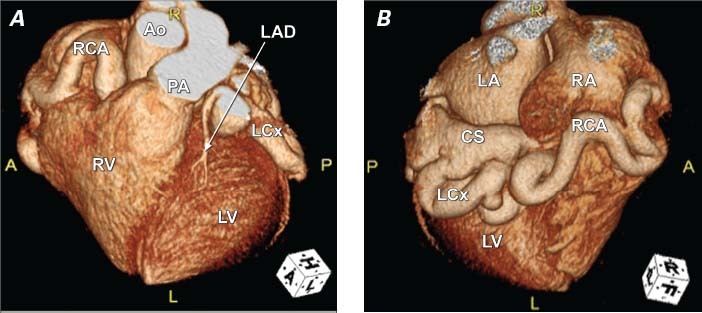
Fig. 4 Cardiac computed tomographic angiography with 3-dimensional image reconstruction of the A) anterior and B) posterior surfaces of the heart shows the dilated left circumflex and right coronary arteries with fistulous connections to the coronary sinus. Ao = ascending aorta; CS = coronary sinus; LA = left atrium; LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; LV = left ventricle; PA = main pulmonary artery; RA = right atrium; RCA = right coronary artery; RV = right ventricle Real-time motion image is available at www.texasheart.org/journal.
In anticipation of percutaneous closure, we performed coronary angiography, which showed the dilated RCA and LCx vessels, along with late filling of the RA on cine angiography (Fig. 5). Hemodynamic and oximetric evaluation revealed a large left-to-right shunt (Qp: Qs ratio, 2.5:1) due to the fistulae, and mild pulmonary hypertension (mean pulmonary artery pressure, 30 mmHg) in the presence of normal pulmonary vascular resistance (1.1 Wood units).
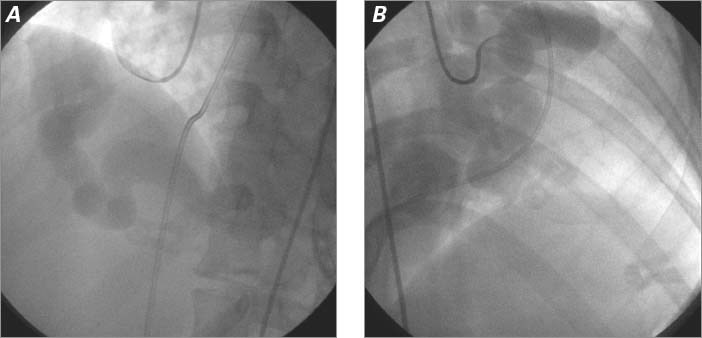
Fig. 5 A) Selective right coronary angiogram (left anterior oblique projection) shows a dilated right coronary artery. B) Selective left main coronary angiogram (right anterior oblique projection) shows a dilated left circumflex coronary artery without visualization of the left anterior descending coronary artery. Real-time motion images are available at www.texasheart.org/journal.
Percutaneous closure of the coronary fistulae was attempted via a retrograde approach. The anterograde approach was not pursued due to the marked coronary tortuosity and the distal emptying point of these vessels in the CS. Intracardiac echocardiography revealed a membrane with multiple fenestrations over the CS ostium. A catheter could not be passed across these fenestrations into the CS (Fig. 6). Therefore, the patient was referred for open surgical repair.
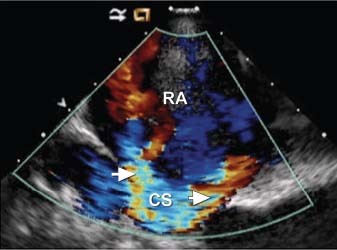
Fig. 6 Intracardiac echocardiography with color-flow Doppler imaging shows multiple turbulent-flow jets entering the right atrium (RA) from fenestrations in the membrane (arrows) covering the coronary sinus (CS) ostium. Real-time motion image is available at www.texasheart.org/journal.
The chest was opened via a midline sternotomy, revealing the enlarged RCA and LCx (Fig. 7). The patient was placed on cardiopulmonary bypass and the heart was opened via a right atriotomy. A thin membrane covering the CS ostium was excised. On exploration of the CS, we saw cardioplegic solution from the 2 fistulae entering the enlarged CS at 2 distinct sites just proximal to its entrance into the RA. These 2 areas of abnormal communication were oversewn (Fig. 8). Because a small amount of cardioplegic flow remained, an atrial septotomy was performed and the CS was opened through the back wall of the LA. No additional coronary artery-to-CS connection could be found. After the patient was weaned from cardiopulmonary bypass, no turbulent flow into the RA was seen on color-flow Doppler transesophageal echocardiography. Intraoperative oximetry measurements revealed no increase in oxygen saturation from the superior vena cava to the pulmonary artery. Eight months after surgery, the patient had returned to an active life with no symptomatic limitation.
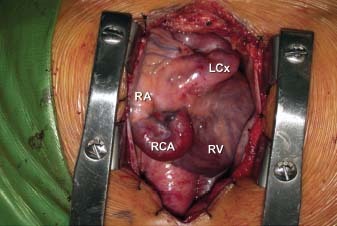
Fig. 7 Intraoperative photograph shows the dilated right coronary and left circumflex coronary arteries. LCx = left circumflex coronary artery; RA = right atrium; RCA = right coronary artery; RV = right ventricle
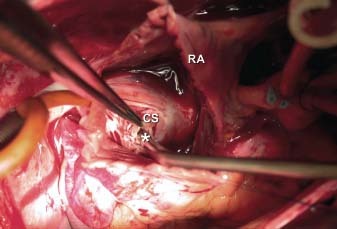
Fig. 8 Intraoperative photograph shows a fistula (asterisk) entering the enlarged coronary sinus (CS) as viewed from the right atriotomy. RA = right atrium
Discussion
Large coronary artery fistulae are uncommon. Small (<1-mm-diameter) connections of coronary arteries, however, are not uncommon. They most frequently originate from the proximal or mid portion of the left anterior descending coronary artery and terminate anteriorly in the pulmonary artery. Such small coronary fistulae are typically of no clinical or hemodynamic consequence.
In a series of 126,595 coronary angiograms over a 28-year period, only 225 coronary fistulae were observed.4 Even less common are fistulae that involve more than 1 coronary artery.4 Medium-to-large coronary artery fistulae can be identified by the associated dilation of the coronary artery (which carries the normal coronary myocardial flow plus the abnormal fistulous flow). This coronary dilation is proximal to the site of fistulous connection into the low-pressure cardiac receiving chamber (coronary–cameral fistula) or central venous structure (coronary arteriovenous fistula).1 The pathophysiology of a fistula is related to the site of abnormal connection: for example, in cases of fistulous insertion into a right-sided heart chamber or systemic venous structure, there is left-to-right shunting and right- and left-sided heart volume overload.1 Left-sided heart volume overload alone occurs when the fistula connects with the LA or a pulmonary vein.1 Moreover, in cases involving a large fistula and marked flow, myocardial ischemia due to a coronary steal phenomenon may occur.1 Other, less common, complications related to coronary artery fistulae—including endarteritis, thrombosis within a fistula, fistula rupture, and pulmonary hypertension—have been reported.1
The management of coronary fistulae must be individualized on the basis of the presence or absence of cardiovascular symptoms, the magnitude of the volume load on the heart, and the presence or absence of myocardial ischemia or ventricular dysfunction. Individual cases and small case series (nonrandomized, observational cohorts)3,5,6 of both percutaneous and surgical correction have shown similar rates of residual fistula flow (20%–30%) during follow-up. High-risk features for subsequent adverse events were found to be more likely in patients with a fistula draining into the CS, regardless of whether they had undergone surgical or percutaneous intervention.6 Even under open surgical inspection, this abnormal coronary connection can be particularly difficult to close completely, consequent to the multiplicity of distal coronary artery-to-CS connections and the location of the connection on the posterior base of the heart.
Percutaneous closure is often feasible when the fistulous communication departs from the coronary artery proximally. The most common reason for not pursuing transcatheter closure in 1 series was the presence of multiple distal connections.5 The operator must close the fistulous connection distal to the most distal coronary arterial branches that supply myocardium. Provided that this downstream abnormal connection can be reached percutaneously, retrograde closure at the site of connection to the vein or cardiac chamber is also a viable approach. Surgical repair is often preferred when the fistula is large and tortuous, with distal connections to the low-pressure chamber. In the most recent American College of Cardiology/American Heart Association Guidelines for the Management of Adults with Congenital Heart Disease,7 percutaneous or surgical closure is a Class I recommendation for large fistulae regardless of symptoms and for small- to moderate-size fistulae with evidence of myocardial ischemia, arrhythmia, ventricular dysfunction, ventricular enlargement, or endarteritis.
This case illustrates a dramatic example of coronary–arteriovenous fistulae that empty into the CS, and of the difficulty in correcting this particular anomaly. In our patient, the congenital anomaly was not diagnosed until the 5th decade of life, eventuating in cardiomegaly, biventricular volume overload, and symptoms of congestive heart failure.
It is also noteworthy that computed tomographic angiography provided the most accurate imaging for this congenital disorder, more detailed than conventional selective coronary angiography. Conventional angiography is limited by its inability to completely opacify the coronary vasculature due to the turbulent flow in these vessels. Computed tomographic angiography provided excellent definition of these vessels (compare the angiographic images in Figs. 4A and 4B with the open surgical photograph in Fig. 7). Moreover, it provides imaging of the various myocardial branches arising from these dilated, tortuous vessels.
Supplementary Material
Footnotes
Address for reprints: Todd Kiefer, MD, Division of Cardiology, 7th fl., Duke University Medical Center, 2301 Erwin Rd., Durham, NC 27710
E-mail: todd.kiefer@duke.edu
Dr. Jaggers is now at the Division of Pediatric Cardiac Surgery, University of Colorado School of Medicine, Denver, Colorado.
References
- 1.Sommer RJ, Hijazi ZM, Rhodes JF Jr. Pathophysiology of congenital heart disease in the adult: Part I: Shunt lesions. Circulation 2008;117(8):1090–9. [DOI] [PubMed]
- 2.Liberthson RR, Sagar K, Berkoben JP, Weintraub RM, Levine FH. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation 1979;59(5):849–54. [DOI] [PubMed]
- 3.Abdelmoneim SS, Mookadam F, Moustafa S, Zehr KJ, Mookadam M, Maalouf JF, Holmes DR. Coronary artery fistula: single-center experience spanning 17 years. J Interv Cardiol 2007;20(4):265–74. [DOI] [PubMed]
- 4.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21(1):28–40. [DOI] [PubMed]
- 5.Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol 2002;39(6):1026–32. [DOI] [PubMed]
- 6.Valente AM, Lock JE, Gauvreau K, Rodriguez-Huertas E, Joyce C, Armsby L, et al. Predictors of long-term adverse outcomes in patients with congenital coronary artery fistulae. Circ Cardiovasc Interv 2010;3(2):134–9. [DOI] [PubMed]
- 7.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52(23):e143–263. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


