Abstract
Better preventive strategies are required to reduce ultraviolet (UV)-caused photodamage, the primary etiological factor for non-melanoma skin cancer (NMSC). Accordingly, here we examined the preventive efficacy of silibinin against UVB-induced photodamage using mouse epidermal JB6 cells and SKH1 hairless mouse epidermis. In JB6 cells, silibinin pretreatment protected against apoptosis and accelerated the repair of cyclobutane pyrimidine dimers (CPD) induced by moderate dose of UVB (50 mJ/cm2), which we are at risk of daily exposure. Silibinin also reversed UVB-induced S phase arrest, reducing both active DNA synthesizing and inactive S phase populations. In mechanistic studies, UVB-irradiated cells showed a transient upregulation of both phosphorylated (Ser-15 and Ser-392) and total p53, whereas silibinin pretreatment led to a more sustained upregulation and stronger nuclear localization of p53. Silibinin also caused a marked upregulation of GADD45α, a downstream target of p53, implicated in DNA repair and cell cycle regulation. Importantly, under p53 and GADD45α knockdown conditions, cells were more susceptible to UVB-induced apoptosis without any significant S phase arrest, and protective effects of silibinin were compromised. Similar to the in vitro results, topical application of silibinin prior to or immediately after UVB irradiation resulted in sustained increase in p53 and GADD45α levels and accelerated CPD removal in the epidermis of SKH1 hairless mice. Together, our results show for the first time that p53-mediated GADD45α upregulation is the key mechanism by which silibinin protects against UVB-induced photodamage and provides a strong rationale to investigate silibinin in reducing the risk and/or preventing early onset of NMSC.
Introduction
Non-melanoma skin cancer (NMSC) has the highest incidence in the USA (1). Solar ultraviolet (UV) B is the major etiologic factor (2) causing DNA lesions namely cyclobutane pyrimidine dimers (CPD) and 6–4 photoproducts, which are formed between adjacent pyrimidine residues in the DNA strand and considered ‘hot spots’ for UV-induced mutations (3,4). Cellular surveillance machinery recognizes and removes these lesions via nucleotide excision repair; however, if not efficiently removed, they can cause C to T and CC to TT mutations eventually leading to NMSC (3). Sunscreens offer only partial protection against the deleterious effects of solar UV, suggesting that more efforts are needed to prevent NMSC. In this regard, strategies that target occurrence and/or progression of preneoplastic lesions through natural or synthetic agents carry translational potential in controlling NMSC (5–8).
Silibinin, isolated from milk thistle seeds, is widely consumed as a dietary supplement for its anti-hepatotoxic efficacy. Extensive studies in the past have established its anticancer efficacy against various epithelial cancers, and currently, silibinin is being evaluated clinically for its usefulness against human pathological conditions (9). Importantly, it is extremely well tolerated and doses up to 1% w/w in diet or 750 mg/kg body wt fed to mice show no adverse effects (10,11). Recently, we have reported the chemopreventive efficacy of silibinin against UVB-induced skin carcinogenesis (12,13); however, the critical targets of silibinin, mediating its protective response against UVB-induced cellular damages, are not yet identified.
The preservation of genomic stability is critical for cell survival, and UVB-induced mutagenic lesions are the major threat to genomic integrity of human skin cells (4,14). Following genotoxic stress, several cellular responses are activated depending on the damage intensity. For example, cell cycle checkpoints and DNA repair machinery are upregulated to restrain and/or remove lesions, whereas apoptosis is induced following severe damage (3). Tumor suppressor p53, the most important cellular transcriptional factor for preserving genomic stability, regulates cell cycle, DNA repair enzymes as well as apoptosis and plays a major protective role against UVB-induced photodamage (15–19). p53 also activates other transcriptional factors including GADD45α (growth arrest and DNA damage-inducible protein alpha) (20), which also has pleiotropic functions; it could facilitate DNA repair through enhancing accessibility of the lesion for repair proteins or through directly binding with DNA repair protein proliferating cell nuclear antigen (21,22). GADD45α could also induce growth arrest by interacting with p21/Cip1 and cyclin-Cdk complex (23,24). Moreover, depending on cell type and extent of stress induced, GADD45α could stimulate or inhibit UVB-mediated apoptosis (25–27). Thus in light of the above discussion, here for the first time, we examined the effects of silibinin treatment on the molecular events involved in DNA damage repair following exposure to UVB and studied the critical role of p53 and GADD45α therein.
Materials and methods
Reagents
p53 and GADD45α antibodies, goat serum, p53-small interfering RNA (siRNA), fluorescein isothiocyanate (FITC)-conjugated secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA), BrdU-FITC antibody was from Becton Dickinson (Franklin Lakes, NJ), BrdU and actin antibody were from Sigma (St Louis, MO), phosphorylated p53 (Ser-15 and Ser-392), cleaved Caspase 3, cleaved PARP and total PARP antibodies were from Cell Signaling (Beverly, MA). Genomic DNA purification kit was from Promega (Madison, WI), GADD45α-siRNA was from Dharmacon (Lafayette, CO) and CPD antibody was from MBL International (Woburn, MA).
Cell culture and UVB irradiation
JB6 cells were maintained under standard conditions of the cell culture in modified Eagle's medium containing 5% fetal bovine serum and 25 μg/ml gentamicin. Cells were grown to 80% confluency and treated with 100 μM silibinin for 12 h followed by UVB exposure as detailed earlier (28). The 100 μM dose of silibinin was selected based upon our earlier published results in JB6 cells (28,29) and the rationale that this concentration of silibinin could be achieved in human plasma (30).
Cell cycle synchronization assays and western blotting
For cell synchronization, 50–60% confluent JB6 cells were incubated with 0.5% fetal bovine serum-containing media for 12 h and then incubated in serum-free media for 24 h and subsequently released in 5% fetal bovine serum-containing media with or without silibinin. Cells were then sham- or UVB irradiated after 8 or 16 h when they were maximally at G1 or S phase, respectively, and then harvested at various time points for cell cycle distribution and immunoblot analyses as detailed earlier (28). The immunoblots shown are representative of at least two independent experiments, and protein loading was monitored by stripping and reprobing the membranes with β-actin antibody. The densitometry values presented below the bands are ‘fold change’ compared with appropriate control after loading control normalization.
BrdU incorporation
After desired treatment, cells were labeled with BrdU for 1 h, fixed with ice cold ethanol and subsequently incubated with BrdU-FITC antibody. Cells were resuspended in Propidium iodide solution and BrdU incorporation was analyzed by FACS.
siRNA transfection
JB6 cells were transfected with specific siRNAs for p53 or GADD45α along with control siRNA using Trans-IT TKO transfection reagent as per manufacturer's protocol (Mirus Bio LLC, Madison, WI). Transfected cells were subsequently treated with dimethyl sulfoxide or 100 μM silibinin for 12 h and then UVB irradiated.
Immunofluorescence
p53 expression and localization were also studied by immunofluorescence as described earlier (31). Images were captured at ×1000 magnification on a Nikon inverted confocal microscope using 488/402 nm laser wavelengths to detect FITC (green) and 4′,6-diamidino-2-phenylindole (blue) emissions, respectively.
Animal treatment protocol
Five-week-old SKH1 hairless mice (Charles River Laboratories, Wilmington, MA) were divided into following treatment groups: (i) unexposed and untreated, (ii) topically applied with 9 mg silibinin in 200 μl acetone per mouse, (iii) irradiated once with 180 mJ/cm2 UVB dose, (iv) topically applied with 9 mg silibinin in 200 μl acetone per mouse 30 min prior to UVB and (v) topically applied with 9 mg silibinin in 200 μl acetone per mouse immediately after UVB. The dose of silibinin was chosen based on our earlier dose–response studies where the same dose showed significant protection against UVB-induced DNA damage and photocarcinogenesis without any adverse effects on body weight or food intake (32–36). The UVB exposure details have been reported earlier (33,34,36). Experiment was terminated at desired time points, animals killed, dorsal skin epidermis collected and immunoblotting performed (33,34,36).
Southwestern slot blot
For slot blot assay, genomic DNA was isolated from JB6 cells or SKH1 mouse epidermis using genomic DNA purification kit from Promega and transferred to positively charged nitrocellulose membrane by vacuum slot blotting (Bio-Dot Apparatus; Bio-Rad, Hercules, CA). The membranes were baked at 80°C, blocked overnight with 5% non-fat milk and incubated with anti-CPD antibody followed by secondary antibody, and bands detected by chemiluminescence. DNA loading was verified by methylene blue staining.
Statistical analysis
Statistical analysis was performed using SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data were analyzed using analysis of variance and a statistically significant difference was considered at P < 0.05.
Results
Silibinin inhibits UVB-induced apoptosis and enhances DNA repair in JB6 cells
We first quantified the extent of apoptosis induced by 30–100 mJ/cm2 doses of UVB and found that 50 mJ/cm2 dose induces moderate levels, whereas 75–100 mJ/cm2 doses induce high level of apoptosis in JB6 cells (data not shown). Since the aim of present study was to study the protective efficacy of silibinin against the moderate damage caused by physiologically relevant dose of UVB, we selected 50 mJ/cm2 UVB dose for subsequent experiments. Next, we assessed the effect of silibinin (100 μM) pretreatment (for 12 h) and posttreatment (immediately after irradiation) on UVB-induced apoptosis. We found that 50 mJ/cm2 UVB induces 18% apoptosis and silibinin pretreatment reduced the apoptotic population to 11% (P < 0.001; Figure 1A). Silibinin pretreatment also reversed the PARP and caspase 3 cleavage caused by UVB in JB6 cells (Figure 1A). Silibinin posttreatment, however, did not show any significant effect on UVB-induced apoptosis under these conditions (data not shown), and therefore, only silibinin pretreatment regimen was followed in subsequent cell culture experiments.
Fig. 1.
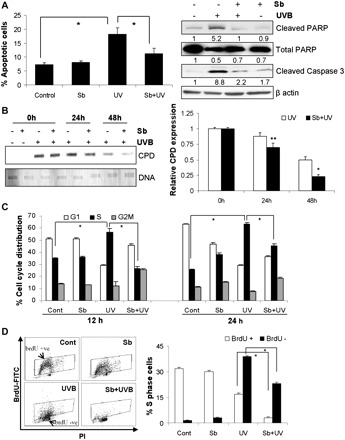
Silibinin inhibits UVB-induced apoptosis, accelerates CPD repair and reverses UVB-induced accumulation of cells in S phase. JB6 cells were pretreated with dimethyl sulfoxide or 100 μM silibinin for 12 h and then sham irradiated or irradiated with 50 mJ/cm2 UVB, and (A) after 24 h, apoptosis was measured by Yo-pro/Propidium iodide and by western blotting for cleaved PARP, total PARP and cleaved caspase 3. Densitometric values for cleaved PARP bands were normalized with respective total PARP values, and cleaved caspase 3 densitometric values were normalized with respective β-actin values. (B) CPD expression was analyzed by Southwestern slot blot assay after 24 and 48 h of UVB irradiation. The densitometric values for CPD bands were normalized with respective DNA loading on the membrane. (C and D) JB6 cells were treated as described in Materials and methods and thereafter analyzed for cell cycle distribution and BrdU-FITC-positive cells by Fluorescence activated cell sorting. The quantitative data shown are mean ± SEM of two independent experiments done in triplicates. Sb, silibinin; Cont, control; *P < 0.001; **P < 0.05.
To investigate whether inhibition of UVB-caused apoptosis by silibinin correlates with reduction of DNA lesions, we next analyzed CPD expression. UVB-irradiated cells showed a gradual time-dependent disappearance of CPD expression, whereas silibinin pretreatment significantly accelerated this response (Figure 1B). After 48 h, about 77% reduction (P < 0.001 versus reduction in UVB alone-irradiated cells) was observed in silibinin pretreated cells, while UVB alone-irradiated cells showed 50% reduction in CPD expression compared with 0 h time point (Figure 1B). Since comparable CPD levels were observed in both treatment groups immediately after irradiation (i.e. at 0 h), it could be concluded that the greater reduction in CPD expression with silibinin pretreatment is due to an enhanced DNA repair and not lesser damage to begin with. In other words, silibinin pretreatment did not exhibit any ‘sunscreen’ effect under experimental conditions in cell culture and actually accelerates the repair of UVB-damaged DNA.
Silibinin reverses UVB-induced accumulation of JB6 cells in S phase
Next, we examined the effect of UVB exposure on cell cycle progression and the influence of silibinin pretreatment therein. Fluorescence activated cell sorting analyses showed that UVB causes a strong accumulation of cells in the S phase of cell cycle 12–24 h following irradiation, which was strongly inhibited by silibinin pretreatment (Figure 1C and Supplementary Figure 1 is available at Carcinogenesis Online). To understand the status of DNA synthesis in S phase cells following UVB irradiation, we next measured BrdU incorporation and found that while 56% cells accumulated in S phase at 12 h after UVB irradiation, only 17% were actively synthesizing DNA and silibinin pretreatment decreased both active DNA synthesizing (BrdU positive) and inactive (BrdU negative) S phase population (Figure 1D). These results suggest that UVB exposure induces a replication-dependent S phase arrest in JB6 cells and silibinin pretreatment reverses this by reducing the overall S phase population, especially with stronger effect on the replicating population, thereby ensuring that DNA damage is not carried over to the next generation.
Silibinin significantly delays the cell cycle progression of UVB-irradiated JB6 cells
One of the cellular defense mechanisms against UVB-induced DNA damage is cell cycle arrest providing damaged cells additional time to repair the lesions and thereby avoiding transfer of lesions to the progeny. Since silibinin pretreatment significantly reversed S phase accumulation of cells following UVB exposure, we next addressed the question whether this effect is due to delay in the G1 to S phase transit of damaged cells. To do so, we synchronized the cells at G1 (84% cells in G1 at 0 h) and then UVB irradiated in the presence or absence of silibinin, followed by cell cycle progression analysis (Figure 2A). Unirradiated cells readily came out of G1 phase (85% cells in S phase within 6 h) and UVB-irradiated cells arrested in G1 phase only transiently (∼6 h) and then progressed through the cell cycle (the G1 population reduced to 33% and 14% in 12 and 24 h, respectively) (Figure 2B and Supplementary Figure 2 is available at Carcinogenesis Online). However, silibinin pretreated cells showed a more sustained and prolonged G1 arrest and ∼73% (P < 0.001 versus UVB) and 32% (P < 0.001 versus UVB) cells remained in G1 phase in 12 and 24 h, respectively (Figure 2B and Supplementary Figure 2 is available at Carcinogenesis Online). Next, as UVB irradiation resulted in significant increase in S phase population of asynchronous cells, we also synchronized the cells in S phase (80% at 0 h) and exposed them to UVB in the presence or absence of silibinin, followed by cell cycle progression analysis. As shown in Figure 2C and Supplementary Figure 3 (available at Carcinogenesis Online), UVB exposure caused a stronger retention of cells in S phase and silibinin pretreatment further prolonged their stay in S phase (58% cells in S phase compared with 38% in UVB alone group after 24 h). Together, these results suggest that silibinin treatment causes a generalized delay in cell cycle progression in UVB-irradiated cells.
Fig. 2.
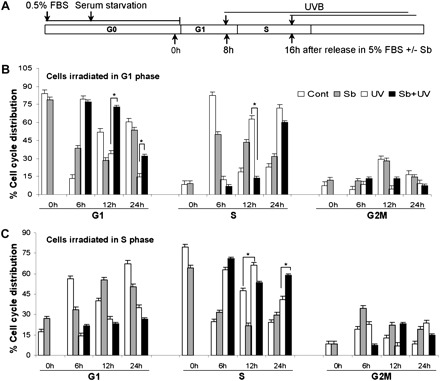
Silibinin delays cell cycle progression of synchronized JB6 cells after UVB irradiation. (A) Schematic representation of the treatment protocol for cell synchronization. (B and C) Cells were synchronized as described in Materials and methods. Briefly, cells were synchronized in G0/G1 phase by incubating in 0.5% fetal bovine serum-containing media for 12 h followed by serum-free media for 24 h. The cells were subsequently released in 5% fetal bovine serum-containing media with or without silibinin and irradiated with UVB after 8 h and after 16 h when cells were maximally in G1 phase and S phase, respectively. Cells were harvested 6, 12 and 24 h after UVB irradiation and analyzed for cell cycle distribution through Fluorescence activated cell sorting. The quantitative data shown are mean ± SEM of two independent experiments done in triplicates. Sb, silibinin; Cont, control; *P < 0.001.
Silibinin pretreatment enhances p53 and GADD45α levels in UVB-irradiated JB6 cells
As mentioned earlier, p53 plays a major role in response to UVB-caused photodamage; therefore, next we examined the effect of silibinin pretreatment on both phosphorylated and total p53 levels in JB6 cells exposed to UVB. A time kinetics study revealed that UVB causes a strong and early (6 h) induction of phosphorylated (Ser-15 and Ser-392) and total p53, but this effect diminished with time (Figure 3A). On the contrary, silibinin pretreatment caused a lesser increase in both phosphorylated and total p53 levels at earlier time points (up to 12 h) compared with UVB alone; however, at later time point of 24 h, silibinin pretreatment maintained higher levels of both phosphorylated and total p53 (Figure 3A). GADD45α is one of the downstream targets of p53 and is reported to play an important role in the regulation of cell cycle, DNA repair and apoptosis (37). We, therefore, next studied the effect of UVB and silibinin pretreatment on GADD45α levels. UVB exposure alone caused only a moderate increase in GADD45α, whereas silibinin pretreatment followed by UVB exposure strongly upregulated the GADD45α level at all time points studied (Figure 3A).
Fig. 3.
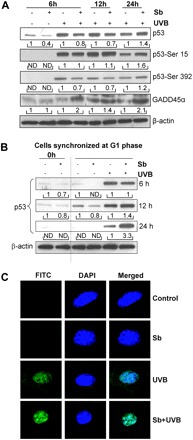
Silibinin enhances p53 and GADD45α levels in UVB-irradiated JB6 cells. (A) JB6 cells were pretreated with silibinin and exposed to UVB, and cell lysates were prepared after 6, 12 and 24 h and immunoblotted for p53, p53-Ser-15, p53-Ser-392 and GADD45α. (B) JB6 cells were synchronized in G1 phase as detailed in Materials and methods and irradiated with UVB in the presence/absence of silibinin, cellular lysates were prepared after 6, 12 and 24 h and immunoblotted for p53. The densitometric values presented below the bands are ‘fold change’ as compared with relevant control (dimethyl sulfoxide or UVB) at each time point after normalization with respective loading control i.e. β-actin. (C) p53 subcellular localization was analyzed by immunofluorescence. Images were captured using Nikon confocal microscope at ×1000 magnification with FITC-green staining for p53 and 4′,6-diamidino-2-phenylindole representing nuclei. Sb, silibinin; ND, not detectable.
Next, we studied the effect of UVB and silibinin pretreatment on G1-synchronized JB6 cells and observed that UVB causes a strong increase in p53 protein level after 6 h (Figure 3B), which also corroborated with the observed maximal accumulation of cells in G1 phase 6 h after UVB exposure (Figure 2B). However, p53 level gradually declined with time as cells started to progress through the cell cycle in UVB alone group (Figure 3B). As shown in Figure 3B with silibinin pretreatment followed by UVB exposure, p53 expression was comparable with UVB alone group after 6 h (where both the groups have comparable G1 population, Figure 2B); however, p53 level remained strongly upregulated up to 24 h with silibinin pretreatment; it should be noted that G1 population was also higher till 24 h in this group (Figure 2B).
Both transcriptional and non-transcriptional activities of p53 are induced in response to DNA damage (38). Nuclear localization of p53 is responsible for its transcriptional activity, whereas its cytosolic and mitochondrial localization have been implicated mainly in apoptotic response (39). We therefore also examined the localization of p53 in different treatment groups via immunofluorescence. At 12 h (data not shown) and 24 h, compared with UVB alone-irradiated JB6 cells showing both nuclear and cytosolic staining for p53, silibinin pretreatment resulted in a marked increase in nuclear accumulation of p53 (Figure 3C). These different subcellular localizations of p53 might account for the differential biological responses observed in with or without silibinin pretreatment groups exposed to UVB.
Silibinin inhibits UVB-induced apoptosis, modulates cell cycle events and regulates GADD45α in a p53-dependent manner
Studies in human fibroblasts have shown that p53 deficient cells have impaired nucleotide excision repair and are more susceptible to apoptosis (40). In order to dissect out the role of p53 in the current experimental milieu, we knocked down p53 expression by RNA interference and then investigated the effects of silibinin on cell cycle and apoptosis. UVB exposure with or without silibinin pretreatment failed to induce GADD45α under p53 knockdown conditions, clearly suggesting that the observed GADD45α upregulation is p53 dependent in these experimental conditions (Figure 4A). Cell cycle analyses revealed that compared with control siRNA-transfected cells, when p53 knockdown cells were UVB irradiated, there was no significant increase in S phase population and silibinin pretreatment only marginally reversed S phase population (Figure 4B). Additionally, knocking down p53 level significantly enhanced the cytotoxic effect of UVB (50% apoptosis in p53-siRNA cells compared with 29% in control-siRNA cells; P < 0.001) (Figure 4C). Moreover, most of the protective effect of silibinin against UVB-induced apoptosis was lost in p53-siRNA cells (Figure 4C). These results suggested that the protective efficacy of silibinin against UVB-induced cellular damage is mainly through modulating p53 level.
Fig. 4.
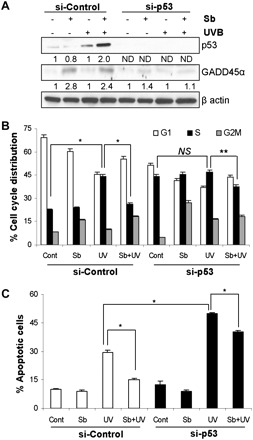
p53 role is critical in silibinin's protective response against UVB-induced cellular damage. JB6 cells were transfected with control siRNA or p53 siRNA, pretreated with or without silibinin for 12 h and then sham or UVB irradiated. The cells were harvested after 24 h and (A) immunoblotted for p53 and GADD45α; and (B and C) analyzed for cell cycle distribution and apoptosis. The quantitative data shown are mean ± SEM of two independent experiments done in triplicates. Sb, silibinin; ND: not detectable; NS, not significant; *P < 0.001; **P < 0.05.
GADD45α plays a key role in silibinin-mediated cell cycle arrest and inhibition of apoptosis in UVB-exposed JB6 cells
Several studies in the past have suggested that GADD45α expression is primarily regulated by p53; however, there are other reports suggesting its regulation independent of p53 (27,37). On the contrary, Jin et al. (41) showed that GADD45α could also regulate p53 through affecting its phosphorylation at Ser-15 site signifying the complexity of GADD45α and p53 interaction. As mentioned above, in our experimental conditions, GADD45α seems to act downstream of p53 (Figure 4A); however, in order to further confirm that we knocked down GADD45α using specific siRNA. As shown in Figure 5A, even under GADD45α knockdown condition, p53 level was induced with UVB in the absence or presence of silibinin pretreatment suggesting that indeed p53 is upstream of GADD45α and not its downstream target.
Fig. 5.
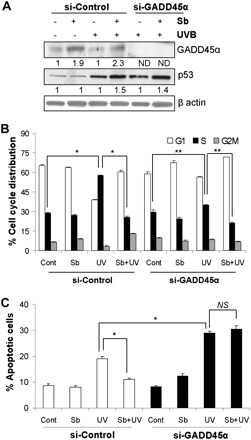
Silibinin mediates growth arrest and protects against apoptosis via GADD45α induction. JB6 cells were transfected with control siRNA or GADD45α siRNA and irradiated with UVB in the presence/absence of silibinin and (A) immunoblotted for GADD45α and p53 and (B and C) analyzed for cell cycle distribution and apoptosis. The quantitative data shown are mean ± SEM of two independent experiments done in triplicates. Sb, silibinin; ND, not detectable; NS, not significant; *P < 0.001; **P < 0.05.
Next, we performed cell cycle analyses using control- and GADD45α siRNA-transfected cells. Unlike p53 knockdown cells, GADD45α reduction itself did not cause a significant increase in S phase population (Figure 5B). In GADD45α knockdown cells, UVB exposure caused only a marginal increase in S phase population, which was moderately inhibited by silibinin pretreatment (Figure 5B). Interestingly, compared with si-control JB6 cells, UVB exposure of si-GADD45α cells resulted in significantly lesser S phase cells, suggesting the importance of GADD45α in UVB-caused increase in S phase population (Figure 5B). We also observed that cells with GADD45α knockdown are more sensitive to apoptosis induction by UVB (29% apoptotic cells in si-GADD45α versus 18% in control-siRNA group; P < 0.001) and silibinin pretreatment completely fails to inhibit UVB-caused apoptosis in GADD45α-reduced cells (Figure 5C); thereby, supporting the importance of GADD45α in the protective efficacy of silibinin against UVB-caused photodamage.
Topical application of silibinin enhances p53 and GADD45α levels and prevents UVB-induced DNA damage in mouse skin epidermis
Based on above cell culture findings and to relate their in vivo significance, first we examined p53 protein levels as a function of time in the skin epidermis obtained from SKH1 hairless mice in different treatment groups. UVB exposure of mice caused a strong upregulation of p53 in 12 h; however, the induced p53 level diminished at 24 h (Figure 6A). In both silibinin pre- and posttreatment groups, p53 induction was moderate and lower compared with UVB alone group at 12 h (Figure 6A); however, at 24 h, it was much stronger and higher in silibinin pre- and posttreatment groups compared with UVB alone (Figure 6A). These results are in keeping with the in vitro studies, where silibinin pretreatment caused a delayed but sustained upregulation of p53 in JB6 cells compared with UVB alone-irradiated cells. Furthermore, similar to in vitro observation, UVB exposure of SKH1 hairless mice caused an induction in GADD45α level in epidermis, and silibinin pre- or posttreatment further enhanced its expression (Figure 6B). We also analyzed CPD expression in epidermis 12 h after UVB irradiation. As shown in Figure 6C, CPD expression was quite high at 12 h post-UVB irradiation; however, both pre- and posttreatment of silibinin dramatically reduced the CPD expression by 60% (P < 0.001) and 43% (P < 0.05), respectively (Figure 6C). Together these results show that similar to cell culture, silibinin exerts its preventive efficacy against UVB-caused photodamage at least in part by upregulation of both p53 and GADD45α levels in mouse skin epidermis.
Fig. 6.
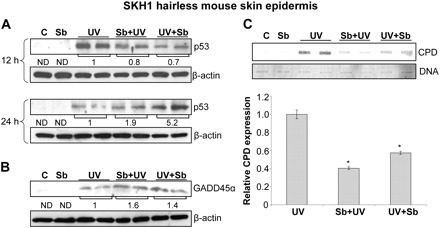
Effect of silibinin topical application on p53, GADD45α and CPD levels in SKH1 hairless mice skin epidermis. Silibinin was applied topically to SKH1 hairless mice pre- or post-UVB exposure as mentioned in Materials and methods. The dorsal skin was collected and analyzed for (A) p53 after 12 and 24 h of UVB irradiation and (B and C) GADD45α and CPD levels after 12 h of UVB exposure. The mean densitometric values after normalization with respective loading control i.e. β-actin is presented below the bands for p53 and GADD45α immunoblots. The densitometric values for CPD bands were normalized with respective DNA loading and presented as mean ± SEM of two independent experiments.
Discussion
UV-caused photodamage is the primary etiological factor for NMSC, the most common malignancy in the USA. UVB primarily causes CPD and 6–4 photoproducts in the epidermis and these lesions, if left unrepaired or mis-repaired, result in cancer initiation (42). Subsequent selection and multiplication of these initiated cells and further accumulation of mutations finally lead to the development of skin tumors (2). Thus for a non-transformed cell, the defense against DNA damages, induced by physiologically relevant UVB dose, includes activation of DNA repair machinery as well as cellular checkpoints to cause cell cycle arrest, thereby, providing the damaged cells sufficient time to repair the lesion and also to ensure that damaged DNA is not replicated and passed onto the next generation (43,44). Therefore, agents that could enhance the DNA repair process as well as prolong cell cycle arrest would be both novel and ideal for protecting the UVB-caused skin photodamage, and thereby, in preventing NMSC. In the present study, we analyzed the protective efficacy of a natural agent silibinin against the photodamage caused by UVB both in vitro and in vivo and studied its effect on the molecular events related to DNA repair and cell cycle progression.
All our previous in vitro studies have been performed with high doses of UVB (100–400 mJ/cm2) where silibinin was found to inhibit cell survival (28,29). In the present study, we used a much lower and physiologically relevant dose of UVB (50 mJ/cm2) and observed that silibinin inhibits UVB-induced apoptosis in JB6 cells, even though it accelerates lesion removal. This dichotomy in silibinin's effect led us to consider that silibinin's biological response is dependent upon the extent of cellular damage caused by UVB. Furthermore, we also observed that silibinin-mediated lesion removal is gradual and relatively more effective at later time points. These results suggest that in cells that have undergone moderate DNA damage, silibinin does not promote apoptosis, rather orchestrates cell cycle checkpoint-mediated growth arrest allowing more time for the cellular repair machinery to remove the damage. Indeed, we observed a significant modulation of cell cycle events with silibinin pretreatment including a decreased level of cyclin A and Cdk2 (data not shown). Indeed, in synchronized cells, silibinin pretreatment clearly mediated a promiscuous delay in cell cycle progression of UVB-irradiated cells, thereby rendering damaged cells more time for DNA repair.
Upon formation of bulky lesions by UV, the cell ‘senses’ the damage by means of phosphoinositide-3-kinase-related kinase family of protein kinases like ataxia telangiectasia mutated, ataxia telangiectasia mutated and Rad-3 related, DNA-dependent protein kinase, which initiate signal transduction cascades involving Chk1 and Chk2 ultimately leading to phosphorylation and stabilization of p53 protein (45). In our earlier study, we have also reported the critical role of phosphoinositide-3-kinase-related kinase family of protein kinases in p53 activation in UVB-exposed JB6 cells (28). We also identified that silibinin promotes the interaction between DNA-dependent protein kinase and p53, and that DNA-dependent protein kinase plays an important role in silibinin-enhanced p53 phosphorylation in UVB-irradiated JB6 cells (28).
The protective role of p53 against diverse genotoxic stress, including UV radiation, is well recognized. Remarkably, of all the hotspot mutation sites identified in the p53 gene, five of them have the dipyrimidine sequence context (5′CCG and 5′CTG), which makes this gene a particularly vulnerable target of UVB (46). p53 knockout mice develop earlier and more aggressive skin tumors compared with their wild-type littermates upon chronic UVB irradiation (47). Though well known for its proapoptotic role, p53 actually has pleiotropic functions and depending on the extent of damage it can induce apoptosis, cell cycle arrest or even senescence. However, the selectivity of p53 response i.e. preference for cell cycle arrest to apoptosis or vice versa is still not clear (20). Speidel et al. showed that moderate expression of p53 protein was associated with cellular senescence in NIH3T3 cells upon exposure to Ionizing Radiation; however, upon ectopic overexpression of p53 in these cells, the same dose of Ionizing Radiation-induced apoptotic response (39). Therefore, the cellular response mediated by p53 could be varied, but the underlying purpose is to preserve genomic integrity, because of which it has been coined ‘the guardian of the genome’. In our study, UVB-caused increase in p53 expression was transient, whereas the p53 level induced by silibinin treatment and then UVB exposure was sustained even at much later time points. This finding suggests that UVB-irradiated cells were committed to apoptosis shortly after irradiation, whereas silibinin plus UVB-treated cells were still arrested in G1/G2M phase via sustained increase in p53 expression, in order to repair DNA damage efficiently.
Under normoxic conditions, p53 maintains the basal expression of p21 and other cell cycle regulatory proteins necessary for proper control of cell cycle progression, thus, its ablation might compromise the cellular G1 checkpoint (40). This might explain why we observe a spontaneously higher S phase population in p53 siRNA-transfected cells. Moreover, in our studies, p53 ablation caused a marked sensitization of JB6 cells toward apoptosis. This is contrary to the common observation where ablation/mutation of p53 is often shown to confer resistance against apoptosis in response to DNA damage and to enhance carcinogenesis in vivo (48). However, there are also studies in fibroblasts where lack of p53 sensitizes them to UVB-mediated cytotoxicity (40). This effect has been linked to p53-mediated global genomic repair (40,49). In the present study, p53 ablation might be inactivating the G1 checkpoint as well as decreasing the repair capacity of the cells and thereby sensitizing them toward apoptosis. Since p53 knockdown cells were so sensitized to UVB-mediated apoptotic death, we could not estimate the CPD level; however, our in vivo findings do support the role of p53 induction in the protective effect of silibinin against UVB-caused photodamage including CPD levels.
In our previous reports, we have consistently shown that p53 is upregulated with UVB and silibinin (32,33,36); however, the downstream effector molecules involved have not yet been clearly identified. Interestingly, in the present study, p21/Cip1, the most widely studied downstream target of p53, was shown to be downregulated with silibinin pretreatment compared with UVB alone group (data not shown). In fact, p21 ubiquitination is considered necessary for optimal DNA repair in response to low but not high doses of UV (50). Thus, silibinin pretreatment-mediated down regulation of p21 might actually be promoting DNA repair of the damaged cells. However, this observation led us to investigate other p53 downstream molecule that might be preferentially upregulated with silibinin pretreatment. GADD45α, a member of the GADD45 family, was originally identified as a p53 effector gene since it harbors a p53-responsive element (51). GADD45α knockout mice show similar characteristics as p53 knockout mice, such as chromosomal instability and increased radiation-induced carcinogenesis (52). Recent in vivo and in vitro studies have shown a tumor suppressive role of GADD45α in both NMSC and melanoma, and GADD45 has been used as a biomarker to predict the survival of melanoma patients (25,53,54). The present study is the first report on the role of GADD45α in silibinin's protective response and warrants detailed studies to further characterize the relative importance of this molecule in silibinin's skin cancer preventive efficacy.
In conclusion, the present study is novel as it establishes for the first time the preventive effects of silibinin against moderate dose of UVB-mediated DNA damage and apoptosis, which are dependent upon p53 as well as its downstream target GADD45α induction. We propose that silibinin treatment leads to a promiscuous delay in cell cycle progression of irradiated cells via p53 and GADD45α upregulation, which subsequently allows more time for CPD repair. Further mechanistic studies are warranted to understand silibinin's effect on upstream events leading to p53 activation as well as to define its effect on nucleotide excision repair machinery. Also, future studies are needed in p53/GADD45α knockout mice to further support and confirm the role of p53 and GADD45α in the preventive efficacy of silibinin against UVB-induced photodamage. Nevertheless, our results clearly establish the usefulness of silibinin against UVB-induced photodamage and suggest that silibinin use could be an effective strategy to prevent accumulation of mutagenic lesions upon daily exposure to UVB and therefore could be a practical strategy for the control of early stages of NMSC.
Supplementary material
Supplementary Figures 1–3 can be found at http://carcin.oxfordjournals.org/
Funding
United States Public Health Service (RO1 CA140368) from National Cancer Institute.
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CPD
cyclobutane pyrimidine dimer
- FITC
fluorescein isothiocyanate
- NMSC
non-melanoma skin cancer
- siRNA
small interfering RNA
- UV
ultraviolet
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 3.Melnikova VO, et al. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Cleaver JE, et al. UV damage, DNA repair and skin carcinogenesis. Front Biosci. 2002;7:d1024–d1043. doi: 10.2741/A829. [DOI] [PubMed] [Google Scholar]
- 5.Bickers DR, et al. Novel approaches to chemoprevention of skin cancer. J. Dermatol. 2000;27:691–695. doi: 10.1111/j.1346-8138.2000.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 6.Khan N, et al. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid. Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 7.Marquez C, et al. Systemic retinoids for chemoprevention of non-melanoma skin cancer in high-risk patients. J. Drugs Dermatol. 2010;9:753–758. [PubMed] [Google Scholar]
- 8.Dinkova-Kostova AT. Phytochemicals as protectors against ultraviolet radiation: versatility of effects and mechanisms. Planta Med. 2008;74:1548–1559. doi: 10.1055/s-2008-1081296. [DOI] [PubMed] [Google Scholar]
- 9.Deep G, et al. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajamanickam S, et al. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer Res. 2010;70:2368–2378. doi: 10.1158/0008-5472.CAN-09-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raina K, et al. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RP, et al. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur. J. Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Deep G, et al. Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr. Cancer Ther. 2007;6:130–145. doi: 10.1177/1534735407301441. [DOI] [PubMed] [Google Scholar]
- 14.Black HS, et al. Photocarcinogenesis: an overview. J. Photochem. Photobiol. B. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 15.Ananthaswamy HN, et al. Inhibition of UV-induced p53 mutations and skin cancers by sunscreens: implication for skin cancer prevention. Exp. Dermatol. 2002;11(suppl. 1):40–43. doi: 10.1034/j.1600-0625.11.s.1.10.x. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin CL, et al. p53 and the pathogenesis of skin cancer. Toxicol. Appl. Pharmacol. 2007;224:241–248. doi: 10.1016/j.taap.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouhtit A, et al. Loss of Fas-ligand expression in mouse keratinocytes during UV carcinogenesis. Am. J. Pathol. 2000;157:1975–1981. doi: 10.1016/S0002-9440(10)64836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith ML, et al. p53-mediated protective responses to UV irradiation. Proc. Natl Acad. Sci. U S A. 1997;94:12255–12257. doi: 10.1073/pnas.94.23.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponten F, et al. Ultraviolet light induces expression of p53 and p21 in human skin: effect of sunscreen and constitutive p21 expression in skin appendages. J. Invest. Dermatol. 1995;105:402–406. doi: 10.1111/1523-1747.ep12321071. [DOI] [PubMed] [Google Scholar]
- 20.Carrier F, et al. Characterization of human Gadd45, a p53-regulated protein. J. Biol. Chem. 1994;269:32672–32677. [PubMed] [Google Scholar]
- 21.Hall PA, et al. Characterisation of the interaction between PCNA and Gadd45. Oncogene. 1995;10:2427–2433. [PubMed] [Google Scholar]
- 22.Carrier F, et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol. Cell. Biol. 1999;19:1673–1685. doi: 10.1128/mcb.19.3.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XW, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc. Natl Acad. Sci. U S A. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin S, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21:8696–8704. doi: 10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- 25.Fayolle C, et al. Gadd45a activation protects melanoma cells from ultraviolet B-Induced apoptosis. J. Invest. Dermatol. 2008;128:196–202. doi: 10.1038/sj.jid.5700963. [DOI] [PubMed] [Google Scholar]
- 26.Gupta M, et al. Gadd45a and Gadd45b protect hematopoietic cells from UV-induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J. Biol. Chem. 2006;281:17552–17558. doi: 10.1074/jbc.M600950200. [DOI] [PubMed] [Google Scholar]
- 27.Thyss R, et al. NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 2005;24:128–137. doi: 10.1038/sj.emboj.7600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanalakshmi S, et al. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J. Biol. Chem. 2005;280:20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 29.Singh RP, et al. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol. Cancer Ther. 2006;5:1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 30.Flaig TW, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest. New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 31.Deep G, et al. Isosilybin B causes androgen receptor degradation in human prostate carcinoma cells via PI3K-Akt-Mdm2-mediated pathway. Oncogene. 2008;27:3986–3998. doi: 10.1038/onc.2008.45. [DOI] [PubMed] [Google Scholar]
- 32.Mallikarjuna G, et al. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 33.Dhanalakshmi S, et al. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25:1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 34.Gu M, et al. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67:3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 35.Gu M, et al. Differential effect of silibinin on E2F transcription factors and associated biological events in chronically UVB-exposed skin versus tumors in SKH-1 hairless mice. Mol. Cancer Ther. 2006;5:2121–2129. doi: 10.1158/1535-7163.MCT-06-0052. [DOI] [PubMed] [Google Scholar]
- 36.Gu M, et al. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005;26:1404–1413. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- 37.Maeda T, et al. UV induces GADD45 in a p53-dependent and -independent manner in human keratinocytes. J. Cutan. Med. Surg. 2003;7:119–123. doi: 10.1007/s10227-002-0108-3. [DOI] [PubMed] [Google Scholar]
- 38.Green DR, et al. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speidel D, et al. Dissection of transcriptional and non-transcriptional p53 activities in the response to genotoxic stress. Oncogene. 2006;25:940–953. doi: 10.1038/sj.onc.1209126. [DOI] [PubMed] [Google Scholar]
- 40.Ford JM, et al. Human fibroblasts expressing the human papillomavirus E6 gene are deficient in global genomic nucleotide excision repair and sensitive to ultraviolet irradiation. Cancer Res. 1998;58:599–603. [PubMed] [Google Scholar]
- 41.Jin S, et al. Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene. 2003;22:8536–8540. doi: 10.1038/sj.onc.1206907. [DOI] [PubMed] [Google Scholar]
- 42.Ravanat JL, et al. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B. 2001;63:88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 43.Fotedar R, et al. Role of p21WAF1 in the cellular response to UV. Cell Cycle. 2004;3:134–137. [PubMed] [Google Scholar]
- 44.McGowan CH, et al. The DNA damage response: sensing and signaling. Curr. Opin. Cell Biol. 2004;16:629–633. doi: 10.1016/j.ceb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Latonen L, et al. Cellular UV damage responses—functions of tumor suppressor p53. Biochim. Biophys. Acta. 2005;1755:71–89. doi: 10.1016/j.bbcan.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Pfeifer GP, et al. Mutations induced by ultraviolet light. Mutat. Res. 2005;571:19–31. doi: 10.1016/j.mrfmmm.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 47.van Kranen HJ, et al. Dose-dependent effects of UVB-induced skin carcinogenesis in hairless p53 knockout mice. Mutat. Res. 2005;571:81–90. doi: 10.1016/j.mrfmmm.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Chaturvedi V, et al. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J. Cell. Physiol. 2004;198:100–109. doi: 10.1002/jcp.10392. [DOI] [PubMed] [Google Scholar]
- 49.McKay BC, et al. P53 plays a protective role against UV- and cisplatin-induced apoptosis in transcription-coupled repair proficient fibroblasts. Oncogene. 2001;20:6805–6808. doi: 10.1038/sj.onc.1204901. [DOI] [PubMed] [Google Scholar]
- 50.Bendjennat M, et al. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Kastan MB, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 52.Hollander MC, et al. Genomic instability in Gadd45a-deficient mice. Nat. Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 53.Hildesheim J, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002;62:7305–7315. [PubMed] [Google Scholar]
- 54.Korabiowska M, et al. Differential expression of growth arrest, DNA damage genes and tumour suppressor gene p53 in naevi and malignant melanomas. Anticancer Res. 1997;17:3697–3700. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


