Abstract
The Wnt/β-catenin signaling pathway, one of the most conserved intercellular signaling cascade, is a known regulator of cellular functions related to tumor initiation and progression, cell proliferation, differentiation, survival and adhesion. Because aberrant Wnt/β-catenin signaling has been observed in a variety of human cancers including a majority of colorectal cancers, about half of prostate cancers and a third of melanomas, inhibitors of its complex signaling pathways are being investigated for therapy as well as chemoprevention of these cancers. During the last decade, several naturally occurring dietary agents have been shown to target intermediates in the Wnt/β-catenin signaling pathway. In this review, we highlight the current understanding of the Wnt/β-catenin signaling pathway and present an analysis of the key findings from laboratory studies on the effects of a panel of dietary agents against a variety of cancers. Promise of these agents for treating and preventing human cancer is then discussed.
Introduction
The term ‘Wnt’ was coined by the amalgamation of Drosophila segment polarity gene Wingless (1) and the mouse proto-oncogene Int-1 (2,3). Wnt signaling pathway is known as a key regulator of a variety of cellular processes (4,5). It is also known that aberrant Wnt signaling pathway occurs in a variety of cancers, including a third of melanomas and a majority of colorectal cancers (5–7). Many studies have repeatedly demonstrated that bioactive food components present in fruits, vegetables and spices exhibit cancer chemopreventive effects in variety of preclinical models (8–16) and thus, these have drawn a great deal of attention owing to their ability to suppress cancers (17). Accumulating research evidence suggests that many dietary factors may be used alone or in combination with traditional chemotherapeutic agents to prevent the occurrence of cancer, their metastatic spread or even to treat cancer. Because Wnt is considered to play an important role in development and progression of cancer(s), which in turn are recognized to be moderated by dietary agents, in this review, we will discuss various dietary agents that have been shown to serve as antagonists of the Wnt/β-catenin signaling pathway.
Wnt/β-catenin signaling: an overview
The Wnt genes encode a large family of secreted protein growth factors that have been identified in animals from hydra to humans (18). In humans, 19 Wnt genes have been identified and the chromosomal location of each is known (18). During development, Wnt(s) have diverse roles in governing cell fate, proliferation, migration, polarity and death. β-Catenin alternatively is a 781 amino acid protein that is encoded by CTNNB1 gene in humans. β-Catenin together with α- and γ-catenin was originally isolated as a protein associated with the cytoplasmic region of E-cadherin, a transmembrane protein involved in homotypic cell–cell contact (19,20). Cytoplasmic β-catenin levels are normally kept low through continuous proteosome-mediated degradation by a ‘destructive complex’ of adenomatous polyposis coli (APC)/glycogen synthase kinase-3β (GSK-3β)/Axin (Figure 1). When cells receive Wnt signals, the degradation of β-catenin is inhibited and levels of β-catenin build up in the cytoplasm and nucleus (Figure 1) (21). Nuclear β-catenin interacts with transcription factors, such as T-cell factor/lymphoid enhancer-binding factor (Tcf/Lef) where it serves as a transcription regulator for several genes that, in part, regulates tumor formation and progression (Table I) (21). Once in the nucleus, β-catenin is thought to convert the Tcf repressor complex into a transcriptional activator complex. This may occur through displacement of Groucho from Tcf/Lef and recruitment of the histone acetylase cyclic adenosine monophosphate response element-binding protein/p300. Cyclic adenosine monophosphate response element-binding protein may bind to the β-catenin/Tcf complex as a coactivator (47,48). The oncogenic role of β-catenin was prominent by the discovery in which activating mutations in β-catenin was detected in half of the colorectal cancers that exhibit wild-type APC (20,49,50).
Fig. 1.
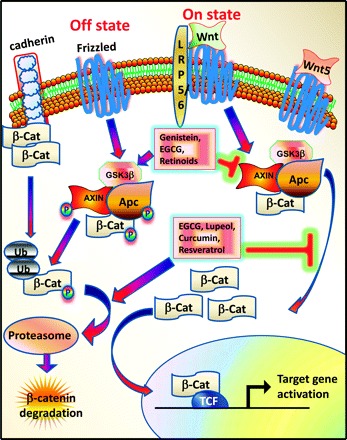
Wnt/β-catenin signaling pathway.
Table I.
Target genes of the Wnt/β-catenin signaling pathway
| Gene | Condition/disease | Reference |
| WNT5a | Leukemia | (22) |
| DSH/DVL | Lung cancer | (23) |
| MITF | Melanomas | (24–27) |
| Tcf-1 | Human colon cancer | (28) |
| Lef-1 | Human colon cancer | (29,30) |
| CTLA-4 | T cells, melanoma | (27) |
| APC | CRC | (31) |
| AXIN | Several cancers | (5) |
| MMP 2/9 | T cells | (32,33) |
| Tiam1 | Colon tumors | (34) |
| Endothelin 1 | Human colon cancer | (35) |
| VEGF | Human colon cancer | (36) |
| Cyclin D1 | Human colon cancer | (37,38) |
| c-myc | Human colon cancer | (39) |
| Axin2 | Human cancer, tooth agenesis | (40–43) |
| Osteocalcin | Mouse | (44) |
| BMP4 | Xenopus | (45) |
| p16ink4A | Melanomas | (46) |
CTLA-4, Cytotoxic T-lymphocyte antigen-4; VEGF, Vascular endothelial growth factor; BMP4, Bone morphogenetic protein 4.
Wnt signaling can promote the expression of various components of Wnt pathway indicating that the feedback control is a key feature of the regulation of Wnt/β-catenin signaling (Table II). One class of targets that respond to Wnt signaling is Frizzled (Fz). Two cytoplasmic antagonists are also induced by Wnt signaling (51–53). The naked cuticle (naked) gene binds directly to Dsh and inhibits Wnt signaling in drosophila (48) and vertebrates (55). The Axin2 gene is also another negative regulator that is a direct target of Wnt signaling.
Table II.
Targets of Wnt signaling that serve as components of the Wnt signaling pathway
| Target gene | Interact with | Reference |
| Fz (Frizzled) | Wnt | (51–53) |
| Dfz2 | Wnt | (51) |
| Arrow/LRP | Wnt | (54) |
| naked | Dsh | (55,56) |
| Axin2 | β-catenin | (41) |
| β-TrCp | β-catenin | (57) |
| Tcf-1 | Tcf | (28,58) |
| LEF1 | β-catenin | (30) |
LRP, LDL receptor related protein.
Wnt/β-catenin signaling in cancer
Mutations that result in Wnt/β-catenin signaling pathway being constitutively active lead to cancer. Axin2 also known as axin-like protein (Axil) or axis inhibition protein 2 (Axin2) or conductin is a protein that in humans is encoded by the Axin2 gene. Axin2 apparently plays an important role in the regulation of the stability of β-catenin in the Wnt signaling pathway and is known to display a predisposition to colon cancer (40). Familial adenomatous polyposis is an inherited disorder in which patients display numerous polyps in the colon and rectum. Truncations in APC, which promotes aberrant activation of Wnt/β-catenin signaling pathway leading to adenomatous lesions, are the most frequent cause of familial adenomatous polyposis (59–62). Mutations in β-catenin and APC have been observed in a variety of other tumors (4,5,63), suggesting that deregulation of Wnt/β-catenin signaling is an important event in the genesis of many cancers. In fact, aberrant Wnt/β-catenin signaling following the loss of APC is thought to initiate colon adenoma formation (64). Considerable evidence for this model has come from mouse models of APC truncation where nuclear β-catenin was found to be detectable soon after loss of APC (64). Several studies observed that stabilizing mutations in β-catenin in the absence of mutations in APC in ∼7% of sporadic human colon carcinomas and thus, these studies provide additional genetic evidence linking APC loss and β-catenin activation (49,64–66). Furthermore, transgenic mice with stabilized mutant β-catenin develop numerous intestinal adenomas (64,67). Taken together, these studies suggest that dysregulation of β-catenin is a key oncogenic event that follows loss of APC.
β-Catenin mutations and interstitial deletions have been reported in both primary hepatocellular carcinomas (HCC) and hepatoma cell lines (68–70). Ser 37 mutation was rarely observed in HCC but alterations affecting the putative phosphorylation residues were common. Studies suggest that 50% of HCC that develop in transgenic mice expressing c-myc or H-ras in the liver contain β-catenin mutations, indicating that β-catenin activation can cooperate with ras or myc in progression of HCC (69,70). Forty-eight percent of sporadic hepatoblastomas, a type of childhood malignant liver tumors, exhibit β-catenin mutations and exon 3 deletions (71). Wilms tumor, a common childhood renal cancer, has been shown to harbor mutations in β-catenin. An interesting finding in these studies was that 90% of these tumors have β-catenin mutation at codon 45 (72–74).
Wnt/β-catenin signaling in melanomas.
A single amino acid substitution at the N-terminus of β-catenin was identified as a melanoma-specific antigen (75) and this sequence is part of a sequence recognized by a melanoma-specific tumor-infiltrating lymphocyte. This study was the first to suggest a mutation in β-catenin could result in cancer. Abnormally high levels of β-catenin have been observed in a subset of melanomas and are usually associated with mutation in CTNNB1 (50). β-catenin mutations were reported to occur at Ser 37, a residue rarely reported in colon cancer (50,76). However, similar to colon cancer, overexpression of APC reduced the levels of β-catenin in these lines (50). Studies suggests that deregulation of Wnt signaling may ultimately lead to deregulation of microphthalmia-associated transcription factor-M (MITF-M) expression resulting in improper cell functions (differentiation, proliferation and survival) (6,77). MITF is essential for melanocyte differentiation and its heterozygous mutations cause auditory–pigmentary syndromes. Functional cooperation of MITF with Lef1 results in synergistic transactivation of an early melanoblast marker dopachrome tautomerase gene promoter (24). Lef1 also interacts with PAX3 and Groucho-related corepressor Grg4 in melanocytes. During active Wnt/β-catenin signaling, Grg4 and Pax3 are removed from the dopachrome tautomerase gene promoter and no longer interact with Lef1 thus resulting in a loss of Pax3-mediated repression (78). Another factor that has been implicated in the control of proliferation and strongly upregulated in melanomas is the POU domain transcription factor Brn-2 (79–81). In melanoma cells, downregulation of Brn-2 is associated with changes in morphology and loss of melanocytic and neural crest markers, like MITF and tyrosinase (81). A decrease in the production of Brn-2 is associated with a loss of tumorigenicity of these cells in nude mice (79). An active Lef/Tcf-binding site has been identified in the Brn-2 promoter (81), implying that Wnt/β-catenin signaling can induce BRN-2 protein, which is involved in cell proliferation and another protein (MITF) that is involved in cell differentiation.
Wnt/β-catenin signaling in colorectal cancer.
Colorectal cancer (CRC) is the third most common cause of cancer death in the USA (64,82). One of the central players, in the series of molecule changes resulting in CRC, is APC which was identified as the responsible germline mutation in familial adenomatous polyposis patients (62,83). Eighty percent of non-inherited CRC cases carry mutations in APC, an important component in the Wnt/β-catenin signaling pathway. Since mutations in CRC occur at the early stages of colorectal carcinogenesis, it means the functional loss of APC is a prerequisite for further progression toward malignancy (84). Reintroduction of wild-type APC results in decreased levels of β-catenin. Studies suggest that transfection of APC in colon cancer cells reduces the abnormally high Tcf reporter plasmid activity (85). Interestingly, rare cell lines without APC mutations also displayed β-catenin/Tcf transcriptional activity as measured by luciferase reporter assay (49). Sequencing the β-catenin gene (CTNNB1) in two APC+/β-catenin activated cell lines revealed mutations at the GSK-3β consensus phosphorylation site of β-catenin: a deletion of Ser 45 in HCT116 cell line and a Ser 55 to Thr substitution in SW48 cells. Expression of β-catenin stability leads to a dominant effect on Tcf/β-catenin transcription (49). Mutations in β-catenin gene have been observed in ∼50% of CRCs without APC mutations, representing <10% of overall CRCs. These mutations occur mainly in the microsatellite-instable tumors (65,86). Immunolocalization of β-catenin in colon carcinomas showed a strong nuclear enrichment at the invasion front, whereas in large parts of the central tumor area, β-catenin was detected in the cytoplasm and at the membrane, indicating that high levels of nuclear β-catenin play a role in the transition to the invasive state of tumor cells (87–89).
Wnt/β-catenin signaling in prostate cancer.
Prostate cancer is one of the few cancers where the aberrant Wnt/β-catenin signaling appears to be a late event in tumorigenesis. Approximately a third of prostate tumors have aberrant Wnt/β-catenin signaling (90). Mutations in APC, β-catenin and β-TrCP have been detected in prostate cancer (90–92). A study using single-strand conformation polymorphism in a panel of 104 prostate cancers identified five mutations in the regulatory site of β-catenin (91). Four of these sites affected putative phosphorylation sites of β-catenin and one affected a residue adjacent to Ser 33, similar to those seen in colon cancer (65). The expression levels of Wnt ligands (Wnt 1, Wnt 2 and Wnt 5a) were positively correlated with Gleason scores as well as to the cellular level of β-catenin and serum prostate specific antigen levels (93–95). Studies by Truica et al. (96) have demonstrated that β-catenin interacts with androgen receptor and enhances the transcriptional activity for androgen receptor in LNCaP cells suggesting a possible mechanism of cross talk between Wnt and androgen signaling pathways. Several other studies using yeast two-hybrid, in vitro and in vivo protein binding assays, have confirmed the interaction between Wnt and androgen receptor signaling pathways (93,97,98).
Wnt/β-catenin signaling in other diseases
Misregulation of Wnt/β-catenin signaling pathway leads to a variety of abnormalities. A mutation in LRP receptor related protein has been identified that leads to increased bone density at defined locations, such as the jaw and palate (99,100). A single amino acid substitution makes LRP5 insensitive to the inhibition of Wnt signaling (99,101). Frame shift and missense mutations in LRP5 resulted in loss of function in LRP5 ultimately leading to decreased bone mass (102). Mutations in LRP5 and Fz4 receptor (FZD4) result in defective vasculogenesis in the peripheral retina (osteoporosis–pseudoglioma syndrome and familial exudative vitreopathy) (58,103–105). Mutations in other components of Wnt signaling pathway produce dramatic defects, such as disturbances in skeletal bone mass or cancer (reviewed in detail by Clevers in ref. 63). Germline mutations in the Wnt pathway cause several hereditary diseases, and somatic mutations are associated with cancer of the intestine and a variety of other tissues (63). Mutation in Axin2 results in oligodontia (severe tooth agenesis), a condition in which multiple permanent teeth are missing (40).
Role of Wnt signaling in cancer stem cells
Wnt signaling, besides performing vital role in embryonic development, is also thought to play key role in the biology of cancer stem cells (CSCs) and acquisition of epithelial to mesenchymal transition phenotype of the cells (106–109). A study demonstrated the importance of canonical Wnt signaling in mediating development of early hematoendothelial progenitors during human development (106). In this study, several complementary methods were used to demonstrate that canonical Wnt signaling is important for development of human embryonic stem cells-derived cells with both hematopoietic and endothelial potential. Wnt/β-catenin signaling is known to be required for neural differentiation of embryonic stem cells (110) and outcome decision in neural crest stem cells (111). Canonical Wnt signaling is believed to play an important role in the maintenance of hematopoietic progenitors and also in the lineage commitment of progenitors during hematopoiesis (107). Survivin, which is a Wnt/cyclic adenosine monophosphate response element-binding protein/β-catenin-regulated gene, is important during hematopoiesis and is prominently upregulated in CD34+ hematopoietic stem and progenitor cells upon growth factor treatment.
Recent studies are beginning to show the use of chemopreventive agents in targeting CSCs. Sulforaphane (SFN), a natural agent found in cruciferous vegetables such as broccoli, has been reported to inhibit the growth of breast CSCs in vitro and in vivo through inhibition of Wnt-regulated self-renewal (112). Preclinical and epidemiological data suggest the role of vitamin D in exerting antiproliferative and prodifferentiating effects on a variety of stem and progenitor cells (113,114). Genistein has been reported to act synergistically with a vitamin D derivative to inhibit the growth of prostate cells and regulate genes that are involved in stem cells renewal (115–117). A recent study by Kakarla et al. (118) showed that curcumin inhibits Wnt/β-catenin signaling in mammary stem cells by inhibiting their self-renewal capability. Another study by Yu et al. (119) showed that curcumin in combination with the therapeutic cocktail of leucovorin, 5-fluorouracil and oxaliplatin (FOLFOX) eliminated colon CSC population suggesting that curcumin by itself or together with the conventional chemotherapeutic could be an effective treatment strategy for preventing the emergence of chemoresistant colon cancer cells by reducing/eliminating CSCs.
Naturally occurring compounds targeting Wnt/β-catenin signaling
Naturally occurring compounds represent attractive candidates for development as chemopreventive agents. Naturally occurring dietary agents circumvent the need to introduce foreign compounds into healthy asymptomatic individuals. Dietary agents are also in general less toxic, more accessible and less expensive than synthetic agents. In the past few years, several laboratories have identified numerous phytochemicals that have useful biological properties; however, only few groups have directly evaluated the ability of these agents to disrupt β-catenin-mediated Wnt signaling. Flavanoids (genistein), curcumin, epigallocatechin-3-gallate (EGCG), resveratrol, lupeol, retinoids, lycopene, deguelin and SFN are been described here.
Flavonoids
The flavonoids comprise a large class of low-molecular weight natural products of plant origin ubiquitously distributed in foods. They are recognized as dietary constituents having a variety of biological effects among which there are some that have been associated with protective activity in the development of degenerative diseases, such as atherosclerosis or cancer (120–123). Several studies suggest that an intake of flavonoids results in reduced cancer risk (17,124,125). Genistein, an important isoflavone, is found in a number of plants, such as soybeans and soy products like tofu. Kaempferol and isorhamnetin are also important flavones found in broccoli. Studies by Park et al. (120) indicate that β-catenin/Tcf-driven transcription was suppressed strongly by flavanone in AGS gastric cancer cells dose dependently. Studies by Sarkar et al. (126) suggest that isoflavone (notably genistein) upregulated the expression of GSK-3β, enhanced GSK-3β binding to β-catenin and increased β-catenin phosphorylation, suggesting that genistein could inactivate Wnt/β-catenin signaling thereby inhibiting prostate cancer growth (126,127). Genistein also diminished Wnt 1-induced proliferation and decreased the expression of Wnt targets viz. c-myc and cyclin D1 (127–129).
Curcumin
Curcumin or diferuloylmethane [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], a yellow pigment, is a major component of turmeric and a member of the ginger Curcuma longa (Zingiberaceae). Turmeric has been used in ayurvedic medicine in India for several thousands of years (130). The therapeutic benefits of curcumin have been reported as an analgesic, anticoagulant, antibacterial, antiviral, antiparasitic, antioxidant, antiarthritic, antihypercholesterolemic and antihypertensive (131–133). Its anti-inflammatory activity is used in the treatment of a variety of diseases ranging from asthma (by downregulating hyaluronidase activity) to Alzheimer’s disease (reducing expression of β-amyloid) and human immunodeficiency virus (17,134–136). Dietary curcumin has been shown to prevent cancer in the skin, forestomach, duodenum and colon in mice and the tongue, colon, mammary and sebaceous glands in rats (137,138). Furthermore, curcumin has also been associated with regression of established malignancy in humans (17,127,139–141). Curcumin specifically inhibits the expression of cyclooxygenase-2, which plays an important role in colon carcinogenesis (130,142). Studies suggest that curcumin is not toxic to humans up to a dose of 8000 mg/day when taken orally for 3 months, suggesting a biological effect in the area of chemoprevention (130). Curcumin treatment inhibits the messenger RNA expression of Wnt target genes c-myc, c-fos, c-jun and iNOS in a variety of cancer cells (143,144). Studies suggest that curcumin treatment resulted in a dose-dependent decrease in β-catenin transcriptional activity in a variety of CRC cell lines (145,146). Curcumin treatment (40 μM dose) resulted in the increased degradation of cytoplasmic β-catenin and decreased nuclear β-catenin levels (146). Studies by Ryu et al. (146) also demonstrate a dose-dependent decrease in expression of nuclear p300 coactivator upon curcumin treatment. This is especially significant since nuclear β-catenin forms a complex with Tcf4 and p300 coactivator to generate a transcriptional active complex (147).
EGCG and green tea polyphenols
Tea, derived from the plant Camellia sinesis, is the most popular beverage consumed by two-thirds of the world’s population (17). Green, black and oolong tea are the main commercial types and these differ in how they are processed and fermented. Green tea contains epigallocatechin-3-gallate (EGCG), epigallocatechin, epicatechin-3-gallate and epicatechin. These compounds grouped as catechins are strong radical scavengers and metal chelators in a number of in vitro and chemical-based assays (148). An increasing number of studies have also demonstrated these antioxidative effects in vivo. Studies have shown that tea treatment can induce Phase II metabolism and antioxidant enzymes in both animal models and humans (85,149–151). Several studies have shown that the effect of EGCG and other tea polyphenols on key cell signaling pathways may be dependent on polyphenol-mediated reactive oxygen species. Green tea was found to inhibit the formation of 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine-induced colonic aberrant crypts in the rat as well as intestinal polyps in the APC-Min mouse (152,153). Decrease in tumor burden was associated with downregulation of β-catenin expression in the intestine as well as markedly lowered expression of β-catenin/Tcf target genes (c-jun and cyclin D1). These findings are important because they suggest the possibility for intervention by tea against the early stages of human CRC, involving the β-catenin/APC pathway and the alteration of Wnt target genes (65,85,151). Studies suggest that β-catenin/Tcf4 transcriptional activity was inhibited in a concentration-dependent fashion upon treatment with EGCG (154) in HEK293 cells induced with β-catenin/Tcf4 (154). Wnt/β-catenin signaling was found to be inhibited by EGCG in a dose-dependent manner in breast cancer cells. Treatment with EGCG resulted in upregulation of HMG-box containing protein 1, an important antagonist of Wnt signaling (127,155). EGCG reduced both proliferation and invasiveness of breast cancer cells through induction of HMG-box containing protein 1 and the subsequent downregulation of Wnt/β-catenin signaling.
Resveratrol
Resveratrol, a dietary polyphenol, that has been shown to possess potent antioxidant as well as anti-inflammatory properties, mediates induction of antioxidant enzymes and modulates lipid metabolism while attenuating hepatic lipid peroxidation (156–158). It has been shown that resveratrol increases hepatic glutathione content, scavenges free radicals and induces enzymes of phase II hepatic metabolism. It also inhibits the transcription factor nuclear factor-kappa B (NF-κB), which induces the inflammatory cascade (158–160). Resveratrol has been shown to reduce the nuclear translocation of NF-κB (161) as well as interfere with its transcription (162). Additionally, resveratrol also produces a significant reduction in the expression of several proinflammatory cytokines (156,162). Studies suggest that resveratrol inhibits skin tumorigenesis through the regulation of phosphatidylinositol-3-kinase and Akt proteins, which have been implicated in development and progression of cancer (127,163). Resveratrol has shown to significantly decrease the level of β-catenin in the nucleus of colon cancer cells. The decreased nuclear localization of β-catenin by resveratrol treatment could be due to reduced expression of lgs and pygoI, which are regulators of β-catenin localization (164). In addition to the solid tumor, resveratrol also inhibits proliferation and induced cell cycle arrest and apoptosis in Waldenstrom’s macroglobulinemia cells. These effects of resveratrol were found to be mediated via the downregulation of Akt, mitogen-activated protein kinase and Wnt signaling pathways (165). Several studies suggest that resveratrol-induced apoptosis is associated with the activation of the p53 in a dose- and time-dependent manner (127,165). Resveratrol also modulates DNA double-strand break repair pathways in p53-dependent manner (166), suggesting the regulatory effect of resveratrol on p53 signaling.
Lupeol
Lupeol is a well-studied dietary triterpene. Lupeol (C30H50O) has a melting point of 215–216°C and a molecular weight of 426. It is found in several fruits (olives, figs, mangoes, strawberries and grapes), vegetables (green peppers, white cabbage and tomato) and medicinal plants (American ginseng, Shea butter plant, Tamarindus indica, Crataeva nurvala and Bombax ceiba). Lupeol was used extensively by native tribes of North America, China, Africa and the Caribbean islands (167). Lupeol has been shown to exhibit various pharmacological activities under in vitro and in vivo conditions. These include its beneficial activity against inflammation, cancer, arthritis, diabetes, heart diseases, renal toxicity and hepatic toxicity (reviewed in ref. 167–185). It is noteworthy to mention that lupeol exhibits strong antimutagenic activity under in vitro and in vivo systems (179,186). Studies by Nigam et al. (179) have demonstrated that application of lupeol (200 μg per mouse) prevents 7,14-dimethylbenz(a) anthracene-induced DNA damage (DNA strand breaks) in mouse skin. Recently, lupeol was shown to inhibit benzo-a-pyrene genotoxicity in a mouse model (180). In benzo-a-pyrene-treated animals, a significant induction of chromosomal aberration and micronuclei was recorded, with a decrease in mitotic index, and significant decrease in benzo-a-pyrene-induced clastogenicity was recorded upon lupeol administration (180). It has demonstrated that lupeol inhibits tumor promotion in a murine mouse model of the skin (181). Topical administration of lupeol (40 mg per animal per thrice a week) was shown to significantly decrease tumor burden in mice (181). The antitumor effects of lupeol were observed to be associated with its potential to modulate NF-κB and phosphatidylinositol-3-kinase/Akt pathway which play an important role in tumor progression. Our laboratory has studied the beneficial effects of lupeol in a variety of cancers, such as prostate, pancreas and melanoma (182–184,187,188). A recent study suggested that lupeol caused inhibition in the growth and proliferation of human prostate cancer cells irrespective of their androgen status (187). Recent studies from our laboratory have suggested the inhibition of human metastatic melanoma cells in vitro and in a xenograft mouse model (182). Our laboratory has also demonstrated that lupeol has a significant growth inhibitory potential on melanoma cells that exhibit constitutive Wnt/β-catenin signaling (189). We have also demonstrated that lupeol inhibits the localization of β-catenin into the nucleus and decreases the phosphorylation status of β-catenin at important serine sites (ser 552 and ser 675). Recent studies suggest that phosphorylation of β-catenin at Ser-552 or Ser-675 abets β-catenin to localize to the nucleus and induce transcription of various downstream targets (190–192).
Retinoids
Vitamin A is converted in vivo to a number of different metabolites collectively referred to as retinoids. Retinoids are potent regulators of cell proliferation and differentiation that are applicable to cancer therapy and prevention (193,194). The use of retinoids as an important chemopreventive agent has been highlighted by several in vitro studies: preclinical animal model experiments, epidemiologic evidence and clinical trials (195). Retinoids have been shown to inhibit the function of the oncogenic AP-1 and Wnt/β-catenin signaling pathways as well as stabilize components of adherens junctions (196). GSK-3β, an important component of the Wnt/β-catenin signaling pathway, plays a key role in retinoid-induced degradation of cyclin D1. In human bronchial epitherial cells, mutation of Thr286 made cyclin D1 resistant to retinoid treatment and pharmacological inhibitors of GSK-3β antagonized the effect of retinoids (193,197).
Other natural products
Lycopene is a bioactive red-colored pigment naturally occurring in plants. Interest in lycopene is increasing due to increasing evidence proving its preventive properties toward numerous diseases. In vitro, in vivo and ex vivo studies have demonstrated that lycopene-rich foods are inversely associated to diseases, such as cancers, cardiovascular diseases, diabetes and others (198–200). Experimental studies have shown that lycopene inhibits cell growth in breast, prostate and endometrial cancer cells with regulation of cell cycle-related genes (201,202). The inhibition of NF-κB DNA-binding activity by lycopene was mediated through the downregulation of IκB phosphorylation, NF-κB expression and NF-κB p65 subunit translocation from cytosol to nucleus (203). Studies suggest that the consumption of lycopene is inversely related to human prostate cancer risk (127). Lycopene reduced localization of β-catenin in the nucleus perhaps by attenuating the effects of GSK-3β phosphorylation (109).
Deguelin is a rotenoid from Mundulea sericea (Family: Leguminosae). This agent has shown great potential as a chemopreventive and therapeutic agent against several types of cancers, including colon, lung and breast (204–209). Deguelin induces apoptotic death by downregulating cell survival pathways, including Akt, mitogen-activated protein kinase and surviving in a variety of human breast cancer cells (206). Research studies suggest that deguelin has been shown to suppress hypoxia-inducible factor-1α/heat shock protein 90 interaction and expression in radioresistant lung cancer cells (204,210).
SFN is an isothiocyanate that is abundantly present in cruciferous vegetables like broccoli and cauliflower. SFN has been found to inhibit carcinogen-induced mammary gland tumorigenesis (211), colonic aberrant crypt foci (212,213), stomach tumors (214) and lung cancer (215) in rats/mice. Several animal model studies suggest that SFN can inhibit carcinogenesis (211,212,216). A study by Hu et al. (217) suggests that ApcMin/+ mice fed with SFN-supplemented diet developed significantly fewer and smaller polyps.
Summary
Aberrant Wnt/β-catenin signaling is observed in a variety of cancers and plays an important role in cancer progression. The data from in vitro, in vivo and preclinical human studies highlight the importance of natural products that exert their inhibitory effects on carcinogenesis and cancer progression by downregulating Wnt/β-catenin signaling pathway. We believe that these natural products that are non-toxic in nature could be used either alone or in combination with conventional therapeutics for the prevention and/or treatment of a variety of human malignancies. Appropriate and relevant animal model studies along with novel clinical trials are needed to fully appreciate the value of these and other natural products for improving the quality of human health.
Funding
The original work in this review was supported by U.S. Public Health Service Grants (RO1 CA 160867). I.A.S. was supported by a postdoctoral fellowship by U.S. PHS Grant (T32AR055893) and in part by Mentored Research Scholar Grant (MRSG-11-019-01-CNE) from the American Cancer Society.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
adenomatous polyposis coli
- CRC
Colorectal cancer
- CSC
cancer stem cell
- EGCG
epigallocatechin-3-gallate
- GSK-3β
glycogen synthase kinase-3β
- HCC
hepatocellular carcinoma
- Lef
lymphoid enhancer-binding factor
- LRP
LDL receptor related protein
- MITF
microphthalmia-associated transcription factor
- NF-κB
nuclear factor-kappa B
- SFN
Sulforaphane
- Tcf
T-cell factor
References
- 1.Sharma RP, et al. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev. Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- 2.Luu HH, et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr. Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 3.Nusse R, et al. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 4.Chien AJ, et al. A Wnt survival guide: from flies to human disease. J. Invest. Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giles RH, et al. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 6.Larue L, et al. The WNT/Beta-catenin pathway in melanoma. Front. Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 7.Behrens J. The role of the Wnt signalling pathway in colorectal tumorigenesis. Biochem. Soc. Trans. 2005;33:672–675. doi: 10.1042/BST0330672. [DOI] [PubMed] [Google Scholar]
- 8.Pezzuto JM. Plant-derived anticancer agents. Biochem. Pharmacol. 1997;53:121–133. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 9.Cragg G, et al. Metabolism of plant-derived anticancer agents. Pharmacol. Ther. 1988;37:425–461. doi: 10.1016/0163-7258(88)90006-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang HK. Plant-derived anticancer agents currently in clinical use or in clinical trials. IDrugs. 1998;1:92–102. [PubMed] [Google Scholar]
- 11.Dragsted LO, et al. Cancer-protective factors in fruits and vegetables: biochemical and biological background. Pharmacol. Toxicol. 1993;72(suppl. 1):116–135. doi: 10.1111/j.1600-0773.1993.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 12.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 13.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 14.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007;74:533–544. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Khan N, et al. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adhami VM, et al. Molecular targets for green tea in prostate cancer prevention. J. Nutr. 2003;133:2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 17.Khan N, et al. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid. Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 18.Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozawa M, et al. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Moon RT. Wnt/beta-catenin pathway. Sci. STKE. 2005;2005:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 23.Uematsu K, et al. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 24.Yasumoto K, et al. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002;21:2703–2714. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito H, et al. Melanocyte-specific microphthalmia-associated transcription factor isoform activates its own gene promoter through physical interaction with lymphoid-enhancing factor 1. J. Biol. Chem. 2002;277:28787–28794. doi: 10.1074/jbc.M203719200. [DOI] [PubMed] [Google Scholar]
- 26.Dorsky RI, et al. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14:158–162. [PMC free article] [PubMed] [Google Scholar]
- 27.Shah KV, et al. CTLA-4 is a direct target of Wnt/beta-catenin signaling and is expressed in human melanoma tumors. J. Invest. Dermatol. 2008;128:2870–2879. doi: 10.1038/jid.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roose J, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 29.Filali M, et al. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J. Biol. Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- 30.Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 31.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 32.Wu B, et al. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc. Natl Acad. Sci. USA. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malliri A, et al. The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J. Biol. Chem. 2006;281:543–548. doi: 10.1074/jbc.M507582200. [DOI] [PubMed] [Google Scholar]
- 35.Kim TH, et al. beta-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005;24:597–604. doi: 10.1038/sj.onc.1208237. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, et al. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- 37.Tetsu O, et al. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 38.Shtutman M, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl Acad. Sci. USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 40.Lammi L, et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am. J. Hum. Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan D, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc. Natl Acad. Sci. USA. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahler RA, et al. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J. Biol. Chem. 2003;278:11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- 45.Baker JC, et al. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delmas V, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hecht A, et al. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takemaru KI, et al. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J. Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 50.Rubinfeld B, et al. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 51.Cadigan KM, et al. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- 52.Muller HA, et al. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development. 1999;126:577–586. doi: 10.1242/dev.126.3.577. [DOI] [PubMed] [Google Scholar]
- 53.Willert J, et al. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev. Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wehrli M, et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 55.Wharton KA, Jr., et al. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev. Biol. 2001;234:93–106. doi: 10.1006/dbio.2001.0238. [DOI] [PubMed] [Google Scholar]
- 56.Rousset R, et al. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 2001;15:658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spiegelman VS, et al. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol. Cell. 2000;5:877–882. doi: 10.1016/s1097-2765(00)80327-5. [DOI] [PubMed] [Google Scholar]
- 58.Toomes C, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am. J. Hum. Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishisho I, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 60.Michils G, et al. Large deletions of the APC gene in 15% of mutation-negative patients with classical polyposis (FAP): a Belgian study. Hum. Mutat. 2005;25:125–134. doi: 10.1002/humu.20122. [DOI] [PubMed] [Google Scholar]
- 61.Kinzler KW, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 62.Kinzler KW, et al. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 63.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Phelps RA, et al. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 65.Sparks AB, et al. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 66.Iwao K, et al. Activation of the beta-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res. 1998;58:1021–1026. [PubMed] [Google Scholar]
- 67.Romagnolo B, et al. Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res. 1999;59:3875–3879. [PubMed] [Google Scholar]
- 68.Miyoshi Y, et al. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res. 1998;58:2524–2527. [PubMed] [Google Scholar]
- 69.Murata M, et al. Accumulation of beta-catenin in the cytoplasm and the nuclei during the early hepatic tumorigenesis. Hepatol. Res. 2001;21:126–135. doi: 10.1016/s1386-6346(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 70.de La Coste A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl Acad. Sci. USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koch A, et al. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59:269–273. [PubMed] [Google Scholar]
- 72.Koesters R, et al. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res. 1999;59:3880–3882. [PubMed] [Google Scholar]
- 73.Kusafuka T, et al. Codon 45 of the beta-catenin gene, a specific mutational target site of Wilms' tumor. Int. J. Mol. Med. 2002;10:395–399. [PubMed] [Google Scholar]
- 74.Maiti S, et al. Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res. 2000;60:6288–6292. [PubMed] [Google Scholar]
- 75.Robbins PF, et al. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 1996;183:1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rimm DL, et al. Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am. J. Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larue L, et al. Beta-catenin in the melanocyte lineage. Pigment Cell Res. 2003;16:312–317. doi: 10.1034/j.1600-0749.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 78.Lang D, et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- 79.Thomson JA, et al. The brn-2 gene regulates the melanocytic phenotype and tumorigenic potential of human melanoma cells. Oncogene. 1995;11:691–700. [PubMed] [Google Scholar]
- 80.Eisen T, et al. The POU domain transcription factor Brn-2: elevated expression in malignant melanoma and regulation of melanocyte-specific gene expression. Oncogene. 1995;11:2157–2164. [PubMed] [Google Scholar]
- 81.Goodall J, et al. Brn-2 expression controls melanoma proliferation and is directly regulated by beta-catenin. Mol. Cell. Biol. 2004;24:2915–2922. doi: 10.1128/MCB.24.7.2915-2922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jemal A, et al. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 83.Fearon ER, et al. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 84.Munemitsu S, et al. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl Acad. Sci. USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 86.Kitaeva MN, et al. Mutations in beta-catenin are uncommon in colorectal cancer occurring in occasional replication error-positive tumors. Cancer Res. 1997;57:4478–4481. [PubMed] [Google Scholar]
- 87.Brabletz T, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl Acad. Sci. USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brabletz T, et al. [beta-Catenin induces invasive growth by activating matrix metalloproteinases in colorectal carcinoma] Verh. Dtsch. Ges. Pathol. 2000;84:175–181. [PubMed] [Google Scholar]
- 89.Hlubek F, et al. Heterogeneous expression of Wnt/beta-catenin target genes within colorectal cancer. Int. J. Cancer. 2007;121:1941–1948. doi: 10.1002/ijc.22916. [DOI] [PubMed] [Google Scholar]
- 90.Chesire DR, et al. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45:323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 91.Voeller HJ, et al. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–2523. [PubMed] [Google Scholar]
- 92.Gerstein AV, et al. APC/CTNNB1 (beta-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer. 2002;34:9–16. doi: 10.1002/gcc.10037. [DOI] [PubMed] [Google Scholar]
- 93.Yang F, et al. Linking beta-catenin to androgen-signaling pathway. J. Biol. Chem. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 94.Iozzo RV, et al. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995;55:3495–3499. [PubMed] [Google Scholar]
- 95.Verras M, et al. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Truica CI, et al. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- 97.Mulholland DJ, et al. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J. Biol. Chem. 2002;277:17933–17943. doi: 10.1074/jbc.M200135200. [DOI] [PubMed] [Google Scholar]
- 98.Pawlowski JE, et al. Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. J. Biol. Chem. 2002;277:20702–20710. doi: 10.1074/jbc.M200545200. [DOI] [PubMed] [Google Scholar]
- 99.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 100.Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Little JW. Melanoma: etiology, treatment, and dental implications. Gen. Dent. 2006;54:61–66. quiz, 67. [PubMed] [Google Scholar]
- 102.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 103.Robitaille J, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- 104.Nikopoulos K, et al. Overview of the mutation spectrum in familial exudative vitreoretinopathy and Norrie disease with identification of 21 novel variants in FZD4, LRP5, and NDP. Hum. Mutat. 2010;31:656–666. doi: 10.1002/humu.21250. [DOI] [PubMed] [Google Scholar]
- 105.Xu Q, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 106.Woll PS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi-Yanaga F, et al. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 108.Malizia AP, et al. CUX1/Wnt signaling regulates epithelial mesenchymal transition in EBV infected epithelial cells. Exp. Cell Res. 2009;315:1819–1831. doi: 10.1016/j.yexcr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 109.Sarkar FH, et al. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–394. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zechner D, et al. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 111.Hari L, et al. Lineage-specific requirements of beta-catenin in neural crest development. J. Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Y, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maund SL, et al. The tissue-specific stem cell as a target for chemoprevention. Stem Cell Rev. 2011;7:307–314. doi: 10.1007/s12015-010-9205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lehmann B, et al. Conversion of vitamin D3 to hormonally active 1alpha,25-dihydroxyvitamin D3 in cultured keratinocytes: relevance to cell growth and differentiation. J. Steroid Biochem. Mol. Biol. 2010;121:322–323. doi: 10.1016/j.jsbmb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Slusarz A, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 116.Regenbrecht CR, et al. The molecular basis of genistein-induced mitotic arrest and exit of self-renewal in embryonal carcinoma and primary cancer cell lines. BMC Med. Genomics. 2008;1:49. doi: 10.1186/1755-8794-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rao A, et al. Genistein and vitamin D synergistically inhibit human prostatic epithelial cell growth. J. Nutr. 2002;132:3191–3194. doi: 10.1093/jn/131.10.3191. [DOI] [PubMed] [Google Scholar]
- 118.Kakarala M, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu Y, et al. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl. Oncol. 2009;2:321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park CH, et al. Inhibition of beta-catenin-mediated transactivation by flavanone in AGS gastric cancer cells. Biochem. Biophys. Res. Commun. 2005;331:1222–1228. doi: 10.1016/j.bbrc.2005.03.242. [DOI] [PubMed] [Google Scholar]
- 121.Androutsopoulos VP, et al. Dietary flavonoids in cancer therapy and prevention: substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol. Ther. 2010;126:9–20. doi: 10.1016/j.pharmthera.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 122.Yang CS, et al. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 123.Aviram M, et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs Exp. Clin. Res. 2002;28:49–62. [PubMed] [Google Scholar]
- 124.Stoner GD, et al. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. Suppl. 1995;22:169–180. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 125.Amado NG, et al. Flavonoids: potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011;89:545–554. doi: 10.1016/j.lfs.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 126.Li Y, et al. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J. Biol. Chem. 2008;283:27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sarkar FH, et al. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su Y, et al. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–339. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 129.Su Y, et al. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol. Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 130.Narayan S. Curcumin, a multi-functional chemopreventive agent, blocks growth of colon cancer cells by targeting beta-catenin-mediated transactivation and cell-cell adhesion pathways. J. Mol. Histol. 2004;35:301–307. doi: 10.1023/b:hijo.0000032361.98815.bb. [DOI] [PubMed] [Google Scholar]
- 131.Wilken R, et al. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou H, et al. The targets of curcumin. Curr. Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Epstein J, et al. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br. J. Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 134.Aggarwal BB, et al. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 135.Kumar A, et al. Curcumin (diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem. Pharmacol. 1998;55:775–783. doi: 10.1016/s0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 136.Gupta SC, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelloff GJ, et al. Progress in cancer chemoprevention: perspectives on agent selection and short-term clinical intervention trials. Cancer Res. 1994;54:2015s–2024s. [PubMed] [Google Scholar]
- 138.Kelloff GJ, et al. Strategy and planning for chemopreventive drug development: clinical development plans. Chemoprevention Branch and Agent Development Committee. National Cancer Institute. J. Cell. Biochem. Suppl. 1994;20:55–62. doi: 10.1002/jcb.240560906. [DOI] [PubMed] [Google Scholar]
- 139.Thomas SL, et al. Activation of the p38 pathway by a novel monoketone curcumin analog, EF24, suggests a potential combination strategy. Biochem. Pharmacol. 2010;80:1309–1316. doi: 10.1016/j.bcp.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Son M, et al. Ethanol extract of Lycoris radiata induces cell death in B16F10 melanoma via p38-mediated AP-1 activation. Oncol. Rep. 2010;24:473–478. doi: 10.3892/or_00000881. [DOI] [PubMed] [Google Scholar]
- 141.Fu S, et al. Development of curcumin as an epigenetic agent. Cancer. 2010;116:4670–4676. doi: 10.1002/cncr.25414. [DOI] [PubMed] [Google Scholar]
- 142.Goel A, et al. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 143.Prasad CP, et al. Potent growth suppressive activity of curcumin in human breast cancer cells: modulation of Wnt/beta-catenin signaling. Chem. Biol. Interact. 2009;181:263–271. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 144.Lin JK. Molecular targets of curcumin. Adv. Exp. Med. Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 145.Jaiswal AS, et al. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 146.Ryu MJ, et al. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem. Biophys. Res. Commun. 2008;377:1304–1308. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 147.Karim R, et al. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36:120–128. doi: 10.1080/00313020410001671957. [DOI] [PubMed] [Google Scholar]
- 148.Lambert JD, et al. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch. Biochem. Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tulayakul P, et al. The effect of feeding piglets with the diet containing green tea extracts or coumarin on in vitro metabolism of aflatoxin B1 by their tissues. Toxicon. 2007;50:339–348. doi: 10.1016/j.toxicon.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 150.Maliakal PP, et al. Tea consumption modulates hepatic drug metabolizing enzymes in Wistar rats. J. Pharm. Pharmacol. 2001;53:569–577. doi: 10.1211/0022357011775695. [DOI] [PubMed] [Google Scholar]
- 151.Orner GA, et al. Tumor-suppressing effects of antioxidants from tea. J. Nutr. 2004;134:3177S–3178S. doi: 10.1093/jn/134.11.3177S. [DOI] [PubMed] [Google Scholar]
- 152.Carter O, et al. Comparison of white tea, green tea, epigallocatechin-3-gallate, and caffeine as inhibitors of PhIP-induced colonic aberrant crypts. Nutr. Cancer. 2007;58:60–65. doi: 10.1080/01635580701308182. [DOI] [PubMed] [Google Scholar]
- 153.Melgarejo E, et al. Targeting polyamines and biogenic amines by green tea epigallocatechin-3-gallate. Amino Acids. 2010;38:519–523. doi: 10.1007/s00726-009-0411-z. [DOI] [PubMed] [Google Scholar]
- 154.Dashwood WM, et al. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H(2)O(2) at physiologically relevant EGCG concentrations. Biochem. Biophys. Res. Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 155.Kim J, et al. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J. Biol. Chem. 2006;281:10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 156.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 157.Ulrich S, et al. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005;49:452–461. doi: 10.1002/mnfr.200400081. [DOI] [PubMed] [Google Scholar]
- 158.Baur JA, et al. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 159.Muriel P. NF-kappaB in liver diseases: a target for drug therapy. J. Appl. Toxicol. 2009;29:91–100. doi: 10.1002/jat.1393. [DOI] [PubMed] [Google Scholar]
- 160.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev. Res. (Phila). 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 161.Tsai SH, et al. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br. J. Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pendurthi UR, et al. Resveratrol, a polyphenolic compound found in wine, inhibits tissue factor expression in vascular cells: a possible mechanism for the cardiovascular benefits associated with moderate consumption of wine. Arterioscler. Thromb. Vasc. Biol. 1999;19:419–426. doi: 10.1161/01.atv.19.2.419. [DOI] [PubMed] [Google Scholar]
- 163.Roy P, et al. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm. Res. 2009;26:211–217. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 164.Hope C, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol. Nutr. Food Res. 2008;52(suppl. 1):S52–S61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roccaro AM, et al. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom's macroglobulinemia. Clin. Cancer Res. 2008;14:1849–1858. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 166.Gatz SA, et al. Resveratrol modulates DNA double-strand break repair pathways in an ATM/ATR-p53- and -Nbs1-dependent manner. Carcinogenesis. 2008;29:519–527. doi: 10.1093/carcin/bgm283. [DOI] [PubMed] [Google Scholar]
- 167.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bani S, et al. Suppression of T lymphocyte activity by lupeol isolated from Crataeva religiosa. Phytother. Res. 2006;20:279–287. doi: 10.1002/ptr.1852. [DOI] [PubMed] [Google Scholar]
- 169.Bradford PG, et al. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007;51:161–170. doi: 10.1002/mnfr.200600164. [DOI] [PubMed] [Google Scholar]
- 170.Fernandez A, et al. Anti-inflammatory effect of Pimenta racemosa var. ozua and isolation of the triterpene lupeol. Farmaco. 2001;56:335–338. doi: 10.1016/s0014-827x(01)01080-1. [DOI] [PubMed] [Google Scholar]
- 171.Fernandez MA, et al. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001;53:1533–1539. doi: 10.1211/0022357011777909. [DOI] [PubMed] [Google Scholar]
- 172.Geetha T, et al. Anticomplement activity of triterpenes from Crataeva nurvala stem bark in adjuvant arthritis in rats. Gen. Pharmacol. 1999;32:495–497. doi: 10.1016/s0306-3623(98)00212-2. [DOI] [PubMed] [Google Scholar]
- 173.Lima LM, et al. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J. Pharm. Pharmacol. 2007;59:1151–1158. doi: 10.1211/jpp.59.8.0014. [DOI] [PubMed] [Google Scholar]
- 174.Molnar J, et al. Inhibition of multidrug resistance of cancer cells by natural diterpenes, triterpenes and carotenoids. Curr. Pharm. Des. 2006;12:287–311. doi: 10.2174/138161206775201893. [DOI] [PubMed] [Google Scholar]
- 175.Setzer WN, et al. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003;3:540–556. doi: 10.2174/1389557033487854. [DOI] [PubMed] [Google Scholar]
- 176.Sudhahar V, et al. Protective effect of lupeol and lupeol linoleate in hypercholesterolemia associated renal damage. Mol. Cell. Biochem. 2008;317:11–20. doi: 10.1007/s11010-008-9786-5. [DOI] [PubMed] [Google Scholar]
- 177.Vasconcelos JF, et al. The triterpenoid lupeol attenuates allergic airway inflammation in a murine model. Int. Immunopharmacol. 2008;8:1216–1221. doi: 10.1016/j.intimp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 178.Yamashita K, et al. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta. 2002;325:91–96. doi: 10.1016/s0009-8981(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 179.Nigam N, et al. Preventive effects of lupeol on DMBA induced DNA alkylation damage in mouse skin. Food Chem. Toxicol. 2007;45:2331–2335. doi: 10.1016/j.fct.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 180.Prasad S, et al. Protective effects of lupeol against benzo[a]pyrene induced clastogenicity in mouse bone marrow cells. Mol. Nutr. Food Res. 2008;52:1117–1120. doi: 10.1002/mnfr.200700420. [DOI] [PubMed] [Google Scholar]
- 181.Saleem M, et al. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 182.Saleem M, et al. Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin. Cancer Res. 2008;14:2119–2127. doi: 10.1158/1078-0432.CCR-07-4413. [DOI] [PubMed] [Google Scholar]
- 183.Saleem M, et al. Lupeol, a fruit and vegetable based triterpene, induces apoptotic death of human pancreatic adenocarcinoma cells via inhibition of Ras signaling pathway. Carcinogenesis. 2005;26:1956–1964. doi: 10.1093/carcin/bgi157. [DOI] [PubMed] [Google Scholar]
- 184.Saleem M, et al. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–11213. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 185.Lee TK, et al. Lupeol suppresses cisplatin-induced nuclear factor-kappaB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2007;67:8800–8809. doi: 10.1158/0008-5472.CAN-07-0801. [DOI] [PubMed] [Google Scholar]
- 186.Lira Wde M, et al. Modulatory effect of Byrsonima basiloba extracts on the mutagenicity of certain direct and indirect-acting mutagens in Salmonella typhimurium assays. J. Med. Food. 2008;11:111–119. doi: 10.1089/jmf.2007.553. [DOI] [PubMed] [Google Scholar]
- 187.Saleem M, et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis. 2009;30:808–817. doi: 10.1093/carcin/bgp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Saleem M, et al. Lupeol triterpene, a novel diet-based microtubule targeting agent: disrupts survivin/cFLIP activation in prostate cancer cells. Biochem. Biophys. Res. Commun. 2009;388:576–582. doi: 10.1016/j.bbrc.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tarapore RS, et al. Specific targeting of Wnt/beta-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fang D, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hino S, et al. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Taurin S, et al. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 193.Takahashi-Yanaga F, et al. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2008;20:581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 194.Soprano DR, et al. Retinoic acid receptors and cancers. Annu. Rev. Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 195.Freemantle SJ, et al. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 196.Shah S, et al. The role of cadherin, beta-catenin, and AP-1 in retinoid-regulated carcinoma cell differentiation and proliferation. J. Biol. Chem. 2002;277:25313–25322. doi: 10.1074/jbc.M203158200. [DOI] [PubMed] [Google Scholar]
- 197.Ma Y, et al. Retinoid targeting of different D-type cyclins through distinct chemopreventive mechanisms. Cancer Res. 2005;65:6476–6483. doi: 10.1158/0008-5472.CAN-05-0370. [DOI] [PubMed] [Google Scholar]
- 198.Tang FY, et al. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol. Nutr. Food Res. 2008;52:646–654. doi: 10.1002/mnfr.200700272. [DOI] [PubMed] [Google Scholar]
- 199.Kong KW, et al. Revealing the power of the natural red pigment lycopene. Molecules. 2010;15:959–987. doi: 10.3390/molecules15020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Musa-Veloso K, et al. Influence of observational study design on the interpretation of cancer risk reduction by carotenoids. Nutr. Rev. 2009;67:527–545. doi: 10.1111/j.1753-4887.2009.00225.x. [DOI] [PubMed] [Google Scholar]
- 201.Nahum A, et al. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur. J. Nutr. 2006;45:275–282. doi: 10.1007/s00394-006-0595-x. [DOI] [PubMed] [Google Scholar]
- 202.Nahum A, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–3436. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 203.Hung CF, et al. Lycopene inhibits TNF-alpha-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur. J. Pharmacol. 2008;586:275–282. doi: 10.1016/j.ejphar.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 204.Murillo G, et al. Deguelin inhibits growth of breast cancer cells by modulating the expression of key members of the Wnt signaling pathway. Cancer Prev. Res. (Phila). 2009;2:942–950. doi: 10.1158/1940-6207.CAPR-08-0232. [DOI] [PubMed] [Google Scholar]
- 205.Gerhauser C, et al. Rotenoids mediate potent cancer chemopreventive activity through transcriptional regulation of ornithine decarboxylase. Nat. Med. 1995;1:260–266. doi: 10.1038/nm0395-260. [DOI] [PubMed] [Google Scholar]
- 206.Peng XH, et al. Down-regulation of inhibitor of apoptosis proteins by deguelin selectively induces apoptosis in breast cancer cells. Mol. Pharmacol. 2007;71:101–111. doi: 10.1124/mol.106.027367. [DOI] [PubMed] [Google Scholar]
- 207.Lee HY, et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J. Natl Cancer Inst. 2005;97:1695–1699. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 208.Murillo G, et al. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur. J. Cancer. 2002;38:2446–2454. doi: 10.1016/s0959-8049(02)00192-2. [DOI] [PubMed] [Google Scholar]
- 209.Murillo G, et al. Deguelin suppresses the formation of carcinogen-induced aberrant crypt foci in the colon of CF-1 mice. Int. J. Cancer. 2003;104:7–11. doi: 10.1002/ijc.10901. [DOI] [PubMed] [Google Scholar]
- 210.Kim WY, et al. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1alpha. Cancer Res. 2009;69:1624–1632. doi: 10.1158/0008-5472.CAN-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Y, et al. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl Acad. Sci. USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chung FL, et al. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 213.Kassie F, et al. Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis. 2003;24:255–261. doi: 10.1093/carcin/24.2.255. [DOI] [PubMed] [Google Scholar]
- 214.Fahey JW, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl Acad. Sci. USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hecht SS, et al. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 216.Jackson SJ, et al. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004;25:219–227. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- 217.Hu R, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]


