Abstract
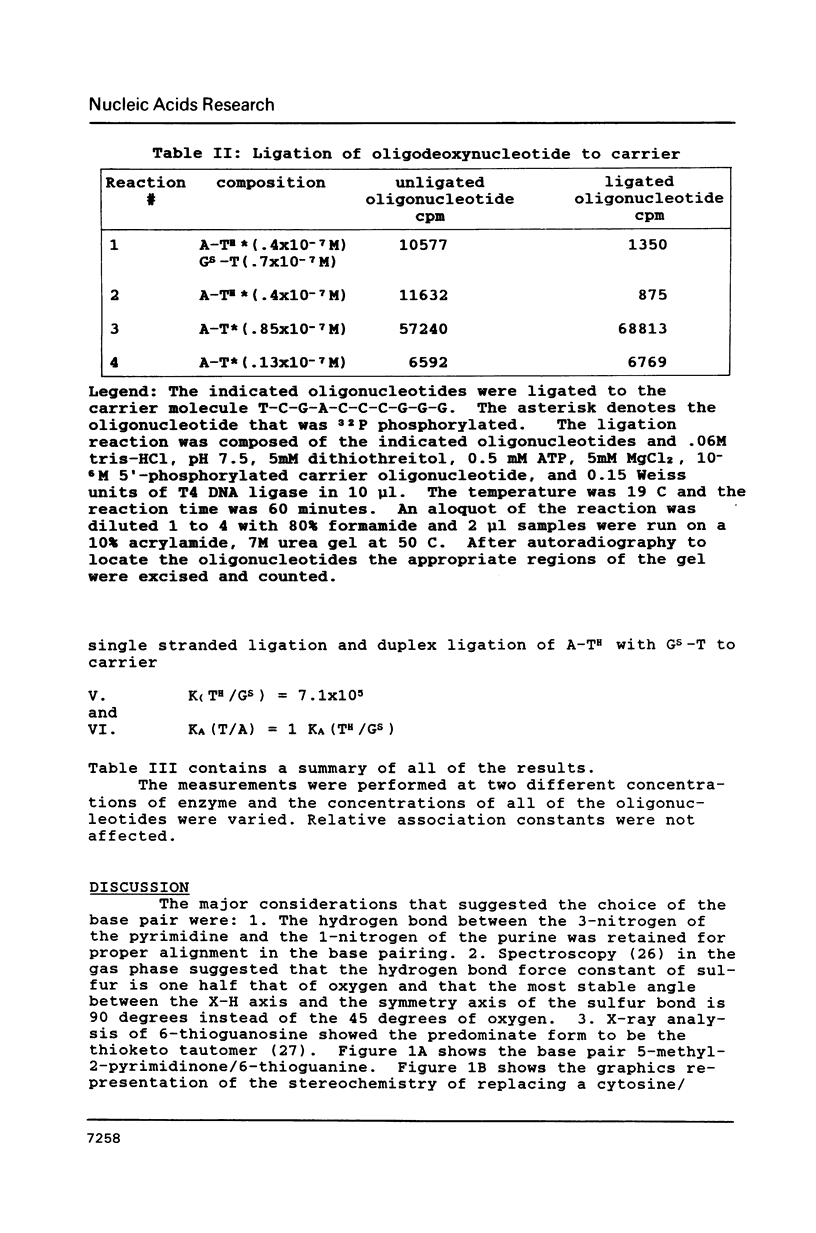
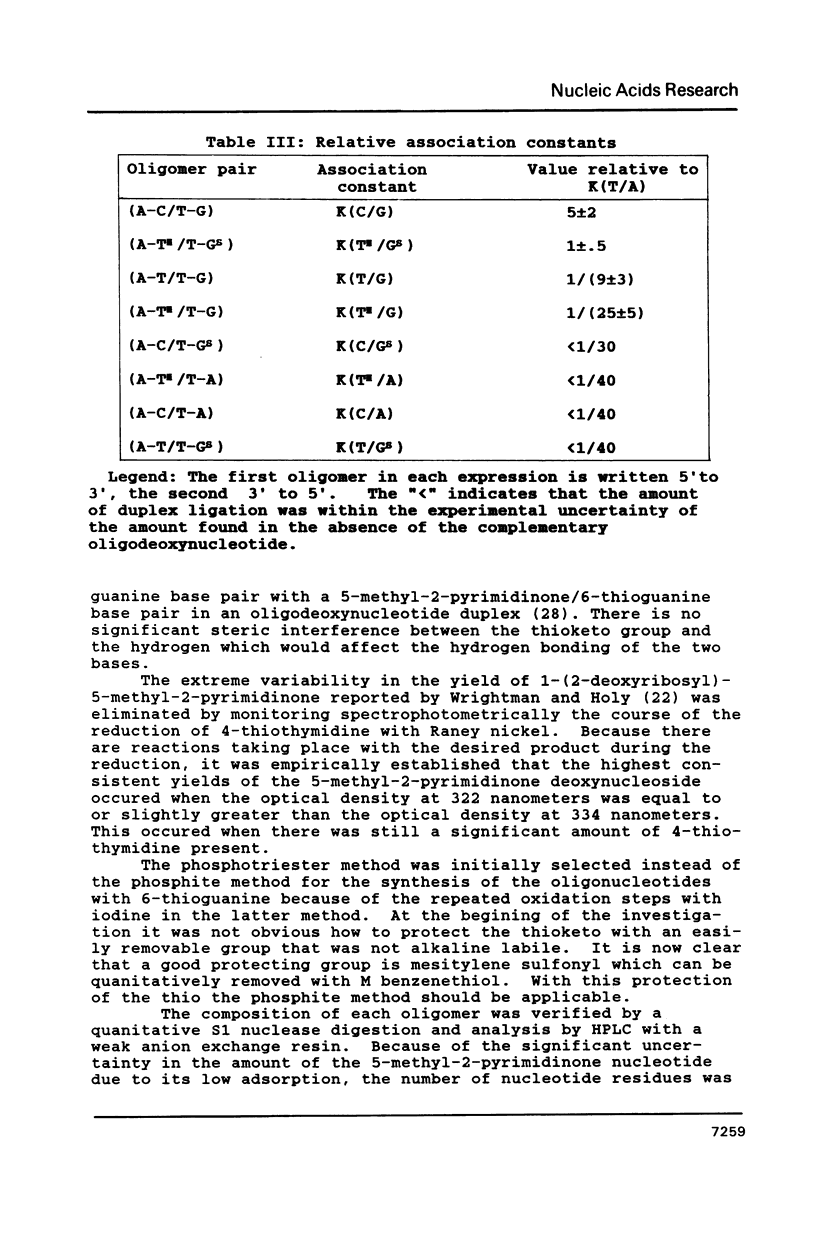
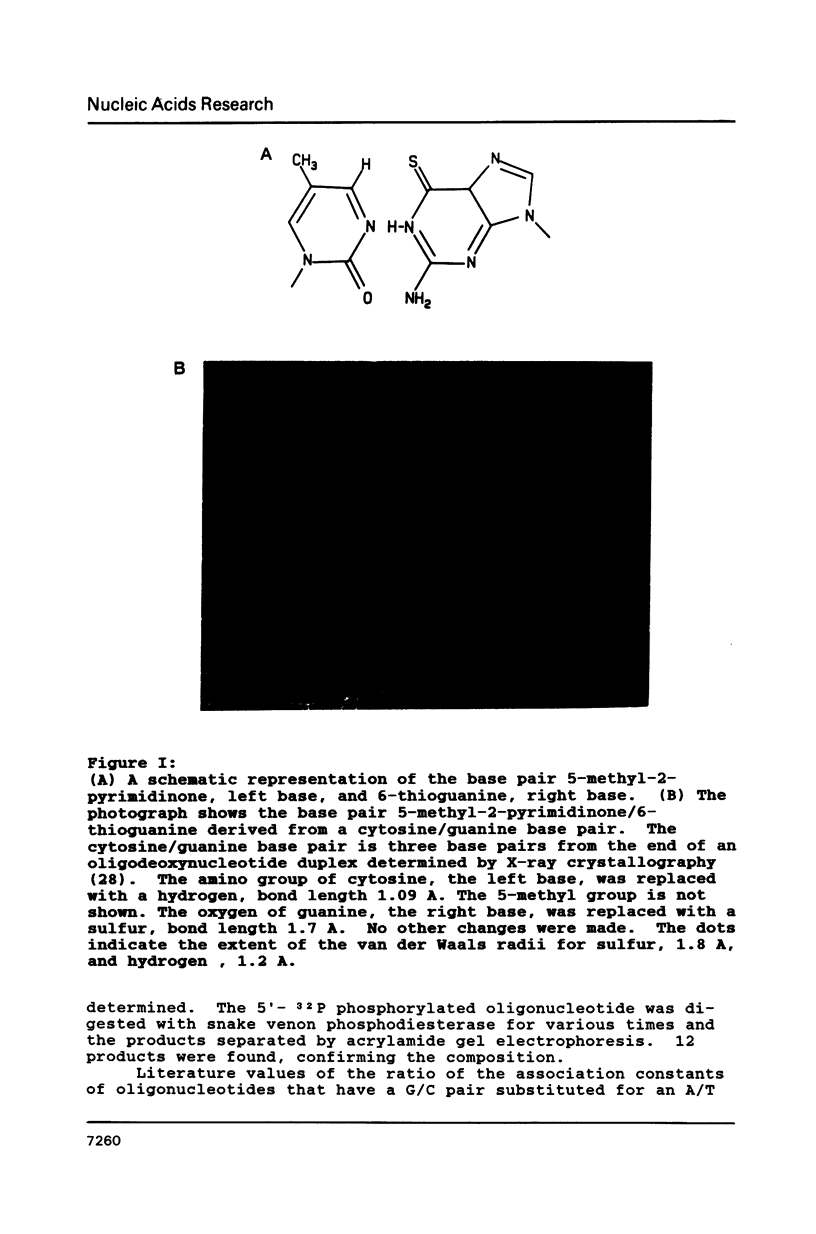
As part of a program to determine the physical possibility of expanding the number of types of base pairs in DNA, the pairing stabilities of the analog bases 6-thioguanine (GS) and 5-methyl-2-pyrimidinone (TH) in oligodeoxynucleotides were measured. Procedures were developed to synthesize oligodeoxynucleotides with the analog bases. The sequences of the synthesized oligomers were T-C-G-A-C-G-G-X-Y-C-C-G. An enzymatic procedure was developed to measure relative association constants of oligomer pairs with the self complementary reference oligomer, X = A and Y = T, K(T/A) = K. The results were K(C/G) = (5 +/- .5)K, K(TH/GS) = K/(1 +/- .5), K(T/G) = K/(9 +/- 3), K(TH/G) = K/(25 +/- 5), K(C/GS) less than K/30, K(TH/A) less than K/40, K(T/GS) less than K/40, K(C/A) less than K/40. The results with the standard bases are consistent with other methods of measurement. The stability of the base pair GS/TH is approximately the same as the standard base pair A/T.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Gumport R. I. T4 RNA ligase catalyzed synthesis of base analogue-containing oligodeoxyribonucleotides and a characterization of their thermal stabilities. Nucleic Acids Res. 1985 Dec 20;13(24):8665–8684. doi: 10.1093/nar/13.24.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Kennard O., Kneale G., Rabinovich D. High-resolution structure of a DNA helix containing mismatched base pairs. Nature. 1985 Jun 13;315(6020):604–606. doi: 10.1038/315604a0. [DOI] [PubMed] [Google Scholar]
- Chu Y. G., Tinoco I., Jr Temperature-jump kinetics of the dC-G-T-G-A-A-T-T-C-G-C-G double helix containing a G . T base pair and the dC-G-C-A-G-A-A-T-T-C-G-C-G double helix containing an extra adenine. Biopolymers. 1983 Apr;22(4):1235–1246. doi: 10.1002/bip.360220415. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Dohet C., Wagner R., Radman M. Repair of defined single base-pair mismatches in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jan;82(2):503–505. doi: 10.1073/pnas.82.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eritja R., Kaplan B. E., Mhaskar D., Sowers L. C., Petruska J., Goodman M. F. Synthesis and properties of defined DNA oligomers containing base mispairs involving 2-aminopurine. Nucleic Acids Res. 1986 Jul 25;14(14):5869–5884. doi: 10.1093/nar/14.14.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. H., Morgan A. R. Characterization of imperfect DNA duplexes containing unpaired bases and non-Watson-Crick base pairs. Nucleic Acids Res. 1986 May 27;14(10):4267–4280. doi: 10.1093/nar/14.10.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough G. R., Singleton C. K., Weith H. L., Gilham P. T. Protected deoxyribonucleoside-3' aryl phosphodiesters as key intermediates in polynucleotide synthesis. Construction of an icosanucleotide analogous to the sequence at the ends of Rous sarcoma virus 35S RNA. Nucleic Acids Res. 1979 Apr;6(4):1557–1570. doi: 10.1093/nar/6.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer B., Köster H. Enzymatic synthesis, ligation, and restriction of DNA containing deoxy-4-thiothymidine. Nucleic Acids Res. 1981 Feb 25;9(4):753–767. doi: 10.1093/nar/9.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. N., Brown T., Anand N. N., Kennard O. Structure of an adenine-cytosine base pair in DNA and its implications for mismatch repair. Nature. 1986 Apr 10;320(6062):552–555. doi: 10.1038/320552a0. [DOI] [PubMed] [Google Scholar]
- Huynh-Dinh T., Duchange N., Zakin M. M., Lemarchand A., Igolen J. Modified oligonucleotides as alternatives to the synthesis of mixed probes for the screening of cDNA libraries. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7510–7514. doi: 10.1073/pnas.82.22.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiricny J., Wood S. G., Martin D., Ubasawa A. Oligonucleotide duplexes containing inosine, 7-deazainosine, tubercidin, nebularine and 7-deazanebularine as substrates for restriction endonucleases HindII, SalI and TaqI. Nucleic Acids Res. 1986 Aug 26;14(16):6579–6590. doi: 10.1093/nar/14.16.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale G., Brown T., Kennard O., Rabinovich D. G . T base-pairs in a DNA helix: the crystal structure of d(G-G-G-G-T-C-C-C). J Mol Biol. 1985 Dec 20;186(4):805–814. doi: 10.1016/0022-2836(85)90398-5. [DOI] [PubMed] [Google Scholar]
- Laland S. G., Serck-Hanssen G. Synthesis of pyrimidin-2-one deoxyribosides and their ability to support the growth of the deoxyriboside-requiring organism Lactobacillus acidophilus R 26. Biochem J. 1964 Jan;90(1):76–81. doi: 10.1042/bj0900076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Castro M. M., Aboul-ela F., Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985 Dec 20;13(24):8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaskar D. N., Goodman M. F. On the molecular basis of transition mutations. Frequency of forming 2-aminopurine-cytosine base mispairs in the G X C----A X T mutational pathway by T4 DNA polymerase in vitro. J Biol Chem. 1984 Oct 10;259(19):11713–11717. [PubMed] [Google Scholar]
- Millican T. A., Mock G. A., Chauncey M. A., Patel T. P., Eaton M. A., Gunning J., Cutbush S. D., Neidle S., Mann J. Synthesis and biophysical studies of short oligodeoxynucleotides with novel modifications: a possible approach to the problem of mixed base oligodeoxynucleotide synthesis. Nucleic Acids Res. 1984 Oct 11;12(19):7435–7453. doi: 10.1093/nar/12.19.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang S. A., Hsiung H. M., Brousseau R. Improved phosphotriester method for the synthesis of gene fragments. Methods Enzymol. 1979;68:90–98. doi: 10.1016/0076-6879(79)68008-4. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Martin F. H., Tinoco I., Jr DNA and RNA oligomer thermodynamics: the effect of mismatched bases on double-helix stability. Biopolymers. 1981 Dec;20(12):2509–2531. doi: 10.1002/bip.1981.360201204. [DOI] [PubMed] [Google Scholar]
- Tibanyenda N., De Bruin S. H., Haasnoot C. A., van der Marel G. A., van Boom J. H., Hilbers C. W. The effect of single base-pair mismatches on the duplex stability of d(T-A-T-T-A-A-T-A-T-C-A-A-G-T-T-G) . d(C-A-A-C-T-T-G-A-T-A-T-T-A-A-T-A). Eur J Biochem. 1984 Feb 15;139(1):19–27. doi: 10.1111/j.1432-1033.1984.tb07970.x. [DOI] [PubMed] [Google Scholar]
- Werntges H., Steger G., Riesner D., Fritz H. J. Mismatches in DNA double strands: thermodynamic parameters and their correlation to repair efficiencies. Nucleic Acids Res. 1986 May 12;14(9):3773–3790. doi: 10.1093/nar/14.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]



