Abstract
Thyroid hormone is critical for central nervous system development. Fetal hypothyroidism leads to reduced cognitive performance in offspring as well as other effects on neural development in both humans and experimental animals. The nature of these impairments suggests that thyroid hormone may exert its effects via dysregulation of the neurotrophin brain-derived neurotrophic factor (BDNF), which is critical to normal development of the central nervous system and has been implicated in neurodevelopmental disorders. The only evidence of BDNF dysregulation in early development, however, comes from experimental models in which severe prenatal hypothyroidism occurred. By contrast, milder prenatal hypothyroidism has been shown to alter BDNF levels and BDNF-dependent functions only much later in life. We hypothesized that mild experimental prenatal hypothyroidism might lead to dysregulation of BDNF in the early postnatal period. BDNF levels were measured by ELISA at 3 or 7 d after birth in different regions of the brains of rats exposed to propylthiouracil (PTU) in the drinking water. The dose of PTU that was used induced mild maternal thyroid hormone insufficiency. Pups, but not the parents, exhibited alterations in tissue BDNF levels. Hippocampal BDNF levels were reduced at both d 3 and 7, but no significant reductions were observed in either the cerebellum or brain stem. Unexpectedly, more males than females were born to PTU-treated dams, suggesting an effect of PTU on sex determination. These results support the hypothesis that reduced hippocampal BDNF levels during early development may contribute to the adverse neurodevelopmental effects of mild thyroid hormone insufficiency during pregnancy.
Thyroid hormones (T3 and T4) are crucial for normal central nervous system (CNS) development. Before the onset of fetal thyroid function in humans and rats, the maternal thyroid gland is the only source of T4 for the fetus, in which it is deiodinated to the biologically more potent T3 (1).
Maternal T4 insufficiency has serious adverse consequences for the neurodevelopment of the offspring, including irreversible cognitive deficits (2–4). Even modest reductions in maternal T4 levels can result in impaired neurological development in children (5). Moreover, impaired neurodevelopment may occur after reduced maternal T4, even if circulating maternal T3 and TSH levels are normal (3). Infants may exhibit impaired cognitive development and other debilitating conditions such as cerebral palsy, even when maternal T4 is decreased only temporarily, as in transient hypothyroxinemia of prematurity (6, 7).
In rats, feeding iodine-deficient diets to create hypothyroidism results in neurodevelopmental defects (8–10). Cortical (9), hippocampal (10), and cerebellar (8) development are all abnormal in pups born to iodine-deficient dams. These studies, and observations in humans, have led to the suggestion that neurodevelopmental defects in children living in areas with severe iodine deficiency could be caused by fetal thyroid hormone insufficiency (11–14).
The mechanisms underlying the effects of hypothyroidism on the developing brain are not completely understood. Growing evidence suggests a role for brain-derived neurotrophic factor (BDNF), a neurotrophin that is critical to normal development of the CNS and also regulates synaptic transmission, dendritic structure, and synaptic plasticity in adulthood (15). In rats, maternal thyroidectomy significantly reduces BDNF expression in the brains of developing rat pups (16). The effects of milder hypothyroidism, however, are uncertain. Understanding the effects of subclinical hypothyroidism is important, not only because of the neurodevelopmental concerns (3, 16) but also because many environmental chemicals interfere with thyroid hormone action (17–19). Rising rates of neurodevelopmental disorders such as autism may reflect the prevalence of these chemicals, making the issue a potential public health concern (19).
Mild thyroid hormone insufficiency in rats does not alter BDNF levels in the hippocampus at postnatal d 14 (20). The present study was performed to test the hypothesis that there might be BDNF deficits earlier, during the first postnatal week, which is important because this time period is critical for the developing CNS. To impair thyroid function in a manner comparable with earlier studies (20), we administered propylthiouracil (PTU) to dams. PTU inhibits both the synthesis of thyroid hormones in the thyroid gland and the conversion of T4 to its active form, T3 (21). In development, PTU has been shown to reduce growth in the offspring and induce long-term structural and functional brain deficits (22–28), even at doses that produce only a relatively mild, transient hypothyroidism in the mothers (22, 29).
Our results indicate that such treatments alter BDNF levels in the early postnatal brain in a region-specific manner: hippocampal BDNF is reduced, whereas BDNF concentrations in the cerebellum and brain stem remain unchanged. These findings suggest that even mild maternal thyroid hormone insufficiency could affect early CNS development via dysregulation of BDNF.
Materials and Methods
Animals
Male and female Sprague Dawley rats were purchased from Charles River (Kingston, NY), bred in-house, weaned at 23 d, and housed with one to two others until use. Animals were maintained on a 12-h light, 12-h dark cycle (lights on at 0700 h), with food (Purina 5001; WF Fisher, Somerville, NJ) and water ad libitum. Animal care and use were in accordance with procedures outlined in the National Institutes of Health Guidelines. Food and water intake of parents were calculated as the mean intake per day from measurements taken Monday, Wednesday, and Friday throughout the mating and gestation periods.
Thyroid hormone measurements
Trunk blood was centrifuged (2000 RPM, 10 min, 23 C) after 1–2 h at room temperature. Serum was stored at −80 C for later analyses by RIA. Serum concentrations of TSH, free T3, free T4, and total T4 were assayed according to kit instructions (Siemens Healthcare Diagnostics, Deerfield, IL). All samples were run in duplicate. The intraassay coefficients of variation for the TSH, free T3, free T4, and total T4 assays were 6, 7, 5, and 4%, respectively. The corresponding interassay coefficients of variation were 9, 9, 9, and 14.5%, respectively. Based on a 95% specific binding criterion, the sensitivity of the RIA for TSH, free T3, free T4, and total T4 was 0.15 μIU/ml, 0.66 pg/ml, 0.08 ng/ml, and 0.82 μg/ml, respectively. Results below these limits were recorded as the lower limit of detection for statistical analysis.
PTU and BDNF
Virgin males and females were housed together throughout mating, pregnancy, and postpartum. PTU (4 parts per million; Sigma-Aldrich, St. Louis, MO) was made every 1–2 wk, dissolved in distilled H2O, and stored at room temperature in the dark. Control animals received distilled H2O. PTU treatment started at the time the dam and stud were first placed together and continued until P (postnatal day) 3 (P0 = day of birth) or 7 d after birth (P7). At approximately the same time of day (1000–1400 h), parents and pups were rapidly (<45 sec) anesthetized by isoflurane inhalation and decapitated. Trunk blood was collected first, and the brain was immediately microdissected afterward (Supplemental Methods and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Dissections were conducted in the same order (first stud, then dam, then pups). Brain regions were placed in Eppendorf tubes, immersed immediately in liquid N2, and stored at −80 C. BDNF levels were measured by ELISA (R&D Systems, Minneapolis, MN; see Supplemental Information for details). Assays were conducted blinded to the sex of the pups. Pup sex determination, conducted blinded to treatment, was based on PCR amplification of the male-specific sex determining region of the Y chromosome Sry (30) using small samples of subcortical tissue that were frozen immediately after the other brain regions.
Statistical analyses
All values are expressed as mean ± sem. The α-level for all analyses was set at 0.05. Student's t test was two tailed and evaluated using Microsoft Excel (Microsoft Corp., Redmond, WA). A χ2 analysis used Quantitative Skills software (Hilversum, The Netherlands). ANOVA was conducted using Statview (SAS Institute, Cary, NC). Before ANOVA, Bartlett's test (31) was used to test for homoscedasticity of variance. To minimize the effects of departure from homoscedasticity (in the hormone assay data) data were log transformed before ANOVA.
Results
PTU-treated parents gave birth to more males than females
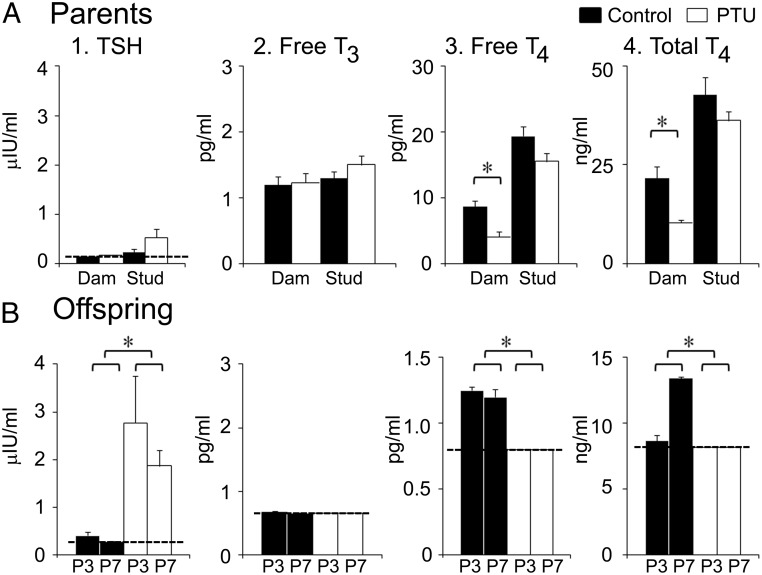
To evaluate the ratio of females to males in litters of control vs. PTU-treated parents, only those litters were used where all pups were sexed (12–13 pups/litter; control: n = 6; PTU: n = 5). Fewer females were born to the PTU-treated parents (control females: 64 of 111 pups, or 57.6%; PTU: 19 of 86, or 22.1%; χ2, P < 0.000001).
Dams and studs used for the control and PTU groups were not different in either age at the time of mating or the time to mate and deliver, and there were no differences in litter size (Supplemental Table 1). To ensure that differences in food and water intake of control and PTU-treated parents did not influence the results, measurements were made in a subset of the parents (three breeding pairs per group), and there were no significant differences (food intake in grams per day, t-test, P = 0.4558; water in milliliters per day, t-test, P = 0.0642; Supplemental Table 1). Weight gain was also not significantly different in these control and PTU-treated parents (Supplemental Table 1).
Serum thyroid hormone levels
Parents
Three-way ANOVA revealed no significant effect of PTU on either TSH or free T3, but there was a statistically significant reduction in both free and total T4 (Fig. 1A and Supplemental Table 2A). In the dams, both free and total T4 were reduced by greater than 50% after PTU treatment (Fig. 1A and Supplemental Table 2A). Significant sex differences were observed in free and total T4 because of the higher overall values observed in males (Supplemental Table 2A).
Fig. 1.
Thyroid hormone levels in PTU-treated parents and their offspring. A, Parental levels of four hormones were assayed from serum after control (black columns) or PTU treatment: 1) TSH, 2) free T3, 3) free T4, and 4) total T4. There were six dams and six studs (from three litters that were euthanized at P3 and another three litters at P7). There were significant effects of PTU treatment on maternal free T4 (two-way ANOVA, F = 14.423; df 1,20; P = 0.0011 followed by a t-test of control vs. PTU-treated dams, P = 0.004539) and total T4 (F = 10.144; df 1,20; P = 0.0047 followed by a t-test, control vs. PTU-treated dams, P = 0.009917; Supplemental Table 2A). For TSH and free T3, there was no effect of PTU (Supplemental Table 2A). B, Thyroid hormone levels of pups born to control and PTU-treated parents. For assays at P3 or P7, sera from six pups were pooled for each litter (P3, n = 6 litters; P7, n = 3). Two-way ANOVA showed significant effects of PTU on TSH (F = 48.817; df 1,14; P < 0.0001), free T4, (F = 9.663; df 1,12; P = 0.0090), and total T4, (F = 61.959; df 1,14; P < 0.0001; Supplemental Table 2B). A dotted line designates the lower limit of detection of the assays. Asterisks indicate significance (P < 0.05).
Offspring
Hormone levels of offspring were assayed using serum pooled from six pups per litter at P3 (n = 6 litters) or P7 (n = 3 litters). Two-way ANOVA revealed that PTU treatment significantly increased circulating TSH at both P3 and P7 (Fig. 1B and Supplemental Table 2B). Values for free T3, free T4, and total T4 were often below the lower limits of detection of the assays; this was rare in control litters (Fig. 1B). The PTU effect was not statistically significant for free T3 but highly significant for both free and total T4 (Fig. 1B and Supplemental Table 2B).
Effects of PTU on BDNF levels in hippocampus
Preliminary one-way ANOVA established that there were no significant litter-dependent effects on BDNF levels in any of the treatment groups. Therefore, data from different litters were combined for subsequent analysis.
Parents
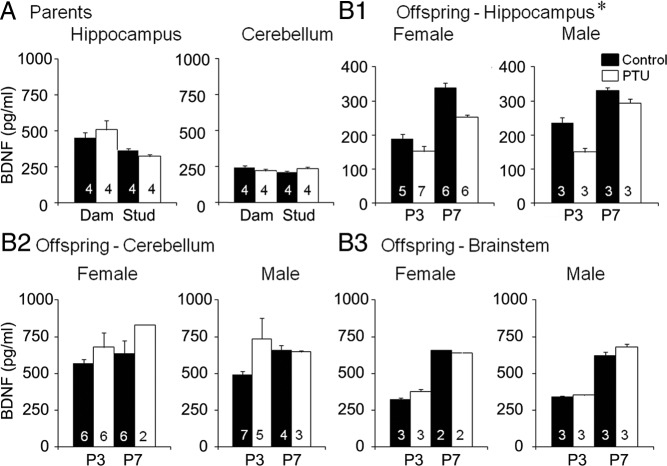
PTU did not significantly affect hippocampal BDNF levels of parents (Fig. 2A and Supplemental Table 3A). A sex difference was noted, reflecting higher hippocampal BDNF levels in the dams (Fig. 2A and Supplemental Table 3A).
Fig. 2.
Lower hippocampal BDNF protein levels in offspring born to PTU-treated parents. A, Hippocampal BDNF levels of parents measured by ELISA (control, black columns; PTU-treated, white columns) showed that there were no effects of PTU on BDNF levels, but fathers had lower BDNF levels than mothers (two-way ANOVA; F = 12.447; df 1,12; P = 0.0042 followed by a t-test, all dams vs. all studs, P = 0.006735; Supplemental Table 3A). For A and B, the number at the base of the bar reflects the number of litters. There were no effects of PTU on cerebellar BDNF levels of parents (Supplemental Table 3A). B1, Hippocampal BDNF levels of pups showed that there was an effect of prenatal PTU (three-way ANOVA; F = 27.828; df 1,74 P < 0.0001; Supplemental Table 3B). Offspring of parents that were treated with PTU had lower BDNF levels than controls at P3 (t-test, P = 0.00001353) and P7 (t-test, P = 0.001094). There were no significant effects of sex (Supplemental Table 3B). B2 and B3, There were no detectable effects of PTU on the BDNF levels in the cerebellum or brain stem (Supplemental Table 3B).
Offspring
Hippocampal BDNF levels of offspring were lower in PTU-treated litters compared with controls at both P3 and P7 (Fig. 2B1 and Supplemental Table 3B). There were no statistically significant sex differences, although the small number of females in PTU-treated litters limits the statistical power of the sex comparison.
Additional analysis of PTU effects
Parents
Other brain regions and serum were sampled in the parents to confirm that their BDNF levels were relatively unaffected by PTU. The pituitary, frontal regions of the brain, and serum showed no significant effect of PTU treatment (Supplemental Table 4).
Offspring
Frontal regions, pituitary, and serum were not possible to assay in offspring, but the cerebellum and brain stem were. Cerebellum BDNF levels tended to be higher at P7 and after PTU treatment, but these trends were not statistically significant (Fig. 2B2 and Supplemental Table 3B). There were no significant sex differences (Fig. 2B2 and Supplemental Table 3B). Mean BDNF levels in brainstem stem increased almost 2-fold between P3 and P7, but there were no significant effects of PTU (Fig. 2B3 and Table 3B).
Discussion
These results demonstrate that PTU exposure of adult rats, at a dose inducing modest reductions in circulating T4 concentrations and no significant effect on brain BDNF, significantly altered the thyroid hormones and hippocampal BDNF levels in the offspring at 3 and 7 d after birth.
Although these effects are consistent with our initial hypothesis, the data must be interpreted cautiously. A decrease in hippocampal BDNF does not necessarily imply a decrease in BDNF-dependent function because receptors (e.g. trkB, p75) may increase to compensate. Other factors to consider include potential changes in the precursor to BDNF, proBDNF, which plays an important role in development, and members of the sortilin family, which modulate effects of neurotrophins (32). Changes in offspring born to PTU-treated dams may be due not only to direct effects of hypothyroidism but also indirect effects of the PTU treatment in dams, transmitted to the offspring. Although maternal BDNF is unlikely to cross the placenta, other factors do so and could affect fetal development.
Alteration in BDNF levels in early life could contribute to the adverse neurodevelopmental effects that occur after mild prenatal hypothyroidism. This conclusion is consistent with results of previous studies of more severe maternal hypothyroidism (16, 33, 34). For example, Liu et al. (16) found that BDNF levels were reduced in the hippocampus of pups born to thyroidectomized mothers implanted with minipumps containing T4. In the T4-replaced dams, circulating TSH concentrations remained more than 15-fold higher than those of nonthyroidectomized controls, 1 d after birth, suggesting that the mothers remained severely hypothyroid despite T4 infusion (16). In the present study, by contrast, there were only modest changes in thyroid status in the PTU-treated dams. In the pups, by contrast, circulating TSH was markedly elevated compared with the offspring of control parents, whereas hippocampal BDNF levels were reduced. The PTU effect may have been more pronounced in the pups because the rat thyroid gland does not start to develop until shortly before birth, so the fetus is critically dependent on maternal T4 (1, 35, 36).
A recent study using low-dose prenatal PTU in rats found no detectable effect of PTU on hippocampal BDNF, 14 d after birth (20). Those results are not discordant with the present findings because a transient PTU-dependent reduction in hippocampal BDNF in early postnatal life of rats may be followed by a period of relatively normal BDNF levels. Hormonal effects during early development often remain latent until later in life, when they may reemerge as changes in hormone sensitivity (37, 38). The results from the present study are consistent with the hypothesis that prenatal PTU exposure may alter BDNF regulation in the brain at a critical stage of early development, the effects becoming fully apparent only later in life. The data of Lasley and Gilbert (20) fit this model: even though in their study BDNF levels in the second and third postnatal weeks were unaffected by fetal hypothyroidism, BDNF protein levels and hippocampal physiology were impaired in adulthood (20, 22).
Similar considerations may apply to the cerebellum. The development of the cerebellum is highly dependent on thyroid hormone and neurotrophins, including BDNF (24, 39, 40). Prenatal hypothyroidism does not produce dramatic changes in cerebellar BDNF expression, however, until well after birth (24, 39, 40). In the present study, cerebellar BDNF was slightly, but not significantly, elevated in the pups. Significant PTU-induced deficits in cerebellar BDNF might not be apparent until much later in development.
A major difference in the experimental design between the present and most previous studies is that the latter have frequently initiated treatments to induce hypothyroidism only toward the end of the first week of pregnancy or later (2, 20). Our animals were treated during mating and conception and for the entire duration of pregnancy. This is likely to have been the reason for the unexpected differences we observed in sex ratios of offspring because dams and studs were exposed to PTU when the sex of the offspring was being determined. Interestingly, other recent studies also suggest that thyroid hormone may influence sex ratios. In amphibia, thyroid hormone affects gonadal sexual differentiation (41), whereas in bovine embryos developing in vitro, the addition of T3 and T4 increases the number of females reaching the blastocyst stage of development (F.A. Ashkar and W.A. King, personal communication).
In summary, these results support the view that low doses of PTU throughout pregnancy represent a valuable model to evaluate the effects of fetal hypothyroidism and suggest that measurements of neurotrophin levels as well as neurotrophin-mediated responses could be useful in assessing the potential effects of prenatal exposure to drugs and chemicals that interfere with thyroid function. Such exposure may not be reflected in detectable changes in maternal indices of thyroid function and, moreover, may be apparent only transiently in the pups, even under conditions in which there may be lasting developmental effects on the brain (20).
Supplementary Material
Acknowledgments
We thank Tom Cooper for his help with hormone assays, Menachem Miodovnik for his support, and Mary Gilbert for helpful comments.
This work was supported by Grants HD047890 (to H.E.S. and J.G.U.) and NS37562 and the New York State Office of Mental Health (to H.E.S.), Grant UL1RR031975 (to J.G.U.), and a grant from the Natural Sciences and Engineering Research Council of Canada (to N.J.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BDNF
- Brain-derived neurotrophic factor
- CNS
- central nervous system
- P
- postnatal day
- PTU
- propylthiouracil.
References
- 1. Thorpe-Beeston JG, Nicolaide KH. 1996. Maternal and fetal thyroid function in pregnancy. Canforth, UK: The Parthenon Publishing Group [Google Scholar]
- 2. Opazo MC, Gianini A, Pancetti F, Azkcona G, Alarcón L, Lizana R, Noches V, Gonzalez PA, Marassi MP, Porto M, Mora S, Rosenthal D, Eugenin E, Naranjo D, Bueno SM, Kalergis AM, Riedel CA. 2008. Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology 149:5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morreale de Escobar G, Obregón MJ, Escobar del Rey F. 2000. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 85:3975–3987 [DOI] [PubMed] [Google Scholar]
- 4. Kasatkina EP, Samsonova LN, Ivakhnenko VN, Ibragimova GV, Ryabykh AV, Naumenko LL, Evdokimova YA. 2006. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol 36:619–624 [DOI] [PubMed] [Google Scholar]
- 5. Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. 1999. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155 [DOI] [PubMed] [Google Scholar]
- 6. Hong T, Paneth N. 2008. Maternal and infant thyroid disorders and cerebral palsy. Semin Perinatol 32:438–445 [DOI] [PubMed] [Google Scholar]
- 7. Huang CB, Chen FS, Chung MY. 2002. Transient hypothyroxinemia of prematurity is associated with abnormal cranial ultrasound and illness severity. Am J Perinatol 19:139–147 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Zhong J, Wei W, Gong J, Dong J, Yu F, Wang Y, Chen J. 2011. Developmental iodine deficiency and hypothyroidism impair neural development, upregulate caveolin-1, and downregulate synaptotagmin-1 in the rat cerebellum. Biol Trace Elem Res 144:1039–1049 [DOI] [PubMed] [Google Scholar]
- 9. Lavado-Autric R, Aus ó E, García-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, Morreale de Escobar G. 2003. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 111:1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong J, Dong J, Wang Y, Xu H, Wei W, Zhong J, Liu W, Xi Q, Chen J. 2010. Developmental iodine deficiency and hypothyroidism impair neural development, up-regulate caveolin-1 and down-regulate synaptophysin in rat hippocampus. J Neuroendocrinol 22:129–139 [DOI] [PubMed] [Google Scholar]
- 11. Remer T, Johner SA, Gärtner R, Thamm M, Kriener E. 2010. Iodine deficiency in infancy—a risk for cognitive development. Dtsch Med Wochenschr 135:1551–1556 [DOI] [PubMed] [Google Scholar]
- 12. Delange F. 1994. The disorders induced by iodine deficiency. Thyroid 4:107–128 [DOI] [PubMed] [Google Scholar]
- 13. Vitti P, Aghini-Lombardi F, Antonangeli L, Rago T, Chiovato L, Pinchera A, Marcheschi M, Bargagna S, Bertuccelli B, Ferretti G, et al. 1992. Mild iodine deficiency in fetal/neonatal life and neuropsychological performances. Acta Med Austriaca 19(Suppl 1):57–59 [PubMed] [Google Scholar]
- 14. Li JQ, Wang X, Yan YQ, Wang KW, Qin DK, Xin ZF, Wei J. 1986. The effects on fetal brain development in the rat of a severely iodine deficient diet derived from an endemic area: observations on the first generation. Neuropathol Appl Neurobiol 12:261–276 [DOI] [PubMed] [Google Scholar]
- 15. Binder DK, Scharfman HE. 2004. Brain-derived neurotrophic factor. Growth Factors 22:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu D, Teng W, Shan Z, Yu X, Gao Y, Wang S, Fan C, Wang H, Zhang H. 2010. The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid 20:909–915 [DOI] [PubMed] [Google Scholar]
- 17. Porterfield SP. 1994. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ Health Perspect 102(Suppl 2):125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zoeller TR. 2010. Environmental chemicals targeting thyroid. Hormones (Athens) 9:28–40 [DOI] [PubMed] [Google Scholar]
- 19. Román GC. 2007. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci 262:15–26 [DOI] [PubMed] [Google Scholar]
- 20. Lasley SM, Gilbert ME. 2011. Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicol Teratol 33:464–472 [DOI] [PubMed] [Google Scholar]
- 21. Zoeller RT, Crofton KM. 2005. Mode of action: developmental thyroid hormone insufficiency—neurological abnormalities resulting from exposure to propylthiouracil. Crit Rev Toxicol 35:771–781 [DOI] [PubMed] [Google Scholar]
- 22. Gilbert ME, Sui L. 2006. Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res 1069:10–22 [DOI] [PubMed] [Google Scholar]
- 23. Sawin S, Brodish P, Carter CS, Stanton ME, Lau C. 1998. Development of cholinergic neurons in rat brain regions: dose-dependent effects of propylthiouracil-induced hypothyroidism. Neurotoxicol Teratol 20:627–635 [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi K, Tsuji R, Yoshioka T, Mino T, Seki T. 2006. Perinatal exposure to PTU delays switching from NR2b to NR2a subunits of the NMDA receptor in the rat cerebellum. Neurotoxicology 27:284–290 [DOI] [PubMed] [Google Scholar]
- 25. Rabié A, Favre C, Clavel MC, Legrand J. 1979. Sequential effects of thyroxine on the developing cerebellum of rats made hypothyroid by propylthiouracil. Brain Res 161:469–479 [DOI] [PubMed] [Google Scholar]
- 26. Akaike M, Kato N, Ohno H, Kobayashi T. 1991. Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol 13:317–322 [DOI] [PubMed] [Google Scholar]
- 27. Schalock RL, Brown WJ, Smith RL. 1979. Long-term effects of propylthiouracil-induced neonatal hypothyroidism. Dev Psychobiol 12:187–199 [DOI] [PubMed] [Google Scholar]
- 28. Goldey ES, Kehn LS, Rehnberg GL, Crofton KM. 1995. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol 135:67–76 [DOI] [PubMed] [Google Scholar]
- 29. Royland JE, Parker JS, Gilbert ME. 2008. A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 20:1319–1338 [DOI] [PubMed] [Google Scholar]
- 30. Miyajima A, Sunouchi M, Mitsunaga K, Yamakoshi Y, Nakazawa K, Usami M. 2009. Sexing of postimplantation rat embryos in stored two-dimensional electrophoresis (2-DE) samples by polymerase chain reaction (PCR) of an Sry sequence. J Toxicol Sci 34:681–685 [DOI] [PubMed] [Google Scholar]
- 31. Snedecor GW, Cochran WG. 1989. Statistical methods. Ames, IA: Iowa State University Press [Google Scholar]
- 32. Teng KK, Felice S, Kim T, Hempstead BL. 2010. Understanding proneurotrophin actions: recent advances and challenges. Dev Neurobiol 70:350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, Hass U. 2008. Developmental neurotoxicity of propylthiouracil (PTU) in rats: relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicol Appl Pharmacol 232:1–13 [DOI] [PubMed] [Google Scholar]
- 34. Dowling AL, Martz GU, Leonard JL, Zoeller RT. 2000. Acute changes in maternal thyroid hormone induce rapid and transient changes in gene expression in fetal rat brain. J Neurosci 20:2255–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oppenheimer JH, Schwartz HL, Surks MI. 1972. Propylthiouracil inhibits the conversion of l-thyroxine to l-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest 51:2493–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pathak A, Sinha RA, Mohan V, Mitra K, Godbole MM. 2011. Maternal thyroid hormone before the onset of fetal thyroid function regulates reelin and downstream signaling cascade affecting neocortical neuronal migration. Cereb Cortex 21:11–21 [DOI] [PubMed] [Google Scholar]
- 37. MacLusky NJ, Naftolin F. 1981. Sexual differentiation of the central nervous system. Science 211:1294–1302 [DOI] [PubMed] [Google Scholar]
- 38. Matthews SG, Phillips DI. 2010. Minireview: transgenerational inheritance of the stress response: a new frontier in stress research. Endocrinology 151:7–13 [DOI] [PubMed] [Google Scholar]
- 39. Koibuchi N, Yamaoka S, Chin WW. 2001. Effect of altered thyroid status on neurotrophin gene expression during postnatal development of the mouse cerebellum. Thyroid 11:205–210 [DOI] [PubMed] [Google Scholar]
- 40. Neveu I, Arenas E. 1996. Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J Cell Biol 133:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duarte-Guterman P, Trudeau VL. 2011. Transcript profiles and triiodothyronine regulation of sex steroid- and thyroid hormone-related genes in the gonad-mesonephros complex of silurana tropicalis. Mol Cell Endocrinol 331:143–149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.