Abstract
In type 1 diabetes, the impairment of the glucagon response to hypoglycemia increases both its severity and duration. In nondiabetic individuals, hypoglycemia activates the autonomic nervous system, which in turn mediates the majority of the glucagon response to moderate and marked hypoglycemia. The first goal of this minireview is therefore to illustrate and document these autonomic mechanisms. Specifically we describe the hypoglycemic thresholds for activating the three autonomic inputs to the islet (parasympathetic nerves, sympathetic nerves, and adrenal medullary epinephrine) and their magnitudes of activation as glucose falls from euglycemia to near fatal levels. The implication is that their relative contributions to this glucagon response depend on the severity of hypoglycemia. The second goal of this minireview is to discuss known and suspected down-regulation or damage to these mechanisms in diabetes. We address defects in the central nervous system, the peripheral nervous system, and in the islet itself. They are categorized as either functional defects caused by glucose dysregulation or structural defects caused by the autoimmune attack of the islet. In the last section of the minireview, we outline approaches for reversing these defects. Such reversal has both scientific and clinical benefit. Scientifically, one could determine the contribution of these defects to the impairment of glucagon response seen early in type 1 diabetes. Clinically, restoring this glucagon response would allow more aggressive treatment of the chronic hyperglycemia that is linked to the debilitating long-term complications of this disease.
One of the primary physiologic functions of insulin is to store ingested glucose for later use. The insulin released during carbohydrate ingestion does so by potentiating the effect of glucose to increase both liver and muscle glucose uptake. The ingested glucose is stored primarily as glycogen, which is released as glucose from the liver during fasting and used by the muscle during exercise. Patients with diabetes have either an absolute or relative insulin deficiency, which prevents optimal storage of ingested glucose leading to both prandial and fasting hyperglycemia. Treatment of this diabetic hyperglycemia with exogenous insulin is a delicate balance. Inadequate treatment allows the chronic hyperglycemia that is a major contributor to several of the long-term complications of this disease. On the other hand, mistimed or excessive insulin treatment causes acute hypoglycemia that is very aversive, leading diabetic patients to be nonadherent to the demands of the intensive insulin therapy shown to prevent certain long-term complications. This problem of treatment-related hypoglycemia is exacerbated by a deficient glucagon response to insulin-induced hypoglycemia in those diabetic patients with severe β-cell loss. In the absence of diabetes this glucagon response limits the severity and duration of the hypoglycemia produced by exogenous insulin. Thus, a defective glucagon response in diabetic patients predisposes them to more severe and more prolonged hypoglycemia (1). For this reason, considerable effort has been expended by many groups to understand both the normal mechanisms by which glucagon secretion increases during insulin-induced hypoglycemia as well as the defects that impair this glucagon response in diabetic patients.
Normal mechanisms
Three major mechanisms have been proposed to mediate the glucagon response to hypoglycemia. The first is the withdrawal of the direct inhibitory effect of glucose on the glucagon-secreting islet α-cell. However, data from isolated, purified α-cells argue against such a direct inhibitory effect of glucose. The second is the withdrawal of the inhibitory paracrine effects of the islet β-cell on the islet α-cell. The evidence supporting such a role for the islet β-cell is summarized in a companion minireview in this issue of Endocrinology (2). The third major mechanism is direct stimulation of the α-cell by the three autonomic inputs to the islet, which are activated by the brain during hypoglycemia (3). Because the first two mechanisms remove a passive restraint whereas the third mechanism produces active stimulation, one might expect, on a theoretical basis, a larger glucagon response from the later. For this reason and because it is the author's area of interest, this minireview will focus primarily on the role of the autonomic nervous system in mediating the glucagon response to insulin-induced hypoglycemia, with some comments related to islet β-cells. Because citations for a minireview are severely restricted, only critical primary sources and reviews are referenced. Those interested in a version with expanded citations should contact the authors.
The autonomic inputs to the pancreatic islet are traditionally divided into the sympathetic and parasympathetic branches. However, this discussion will also include the sympathoadrenal system because the preganglionic sympathetic nerves innervating the adrenal medulla are activated during hypoglycemia, release acetylcholine to activate the nicotinic acetylcholine receptor (nAChR) on the chromaffin cells, and stimulate epinephrine secretion, which in turn stimulates glucagon secretion from the islet α-cell. The two classical autonomic inputs, the islet sympathetic and parasympathetic nerves, are also activated by insulin-induced hypoglycemia (4, 5), and both stimulate glucagon secretion from the α-cell. Their preganglionic nerve terminals also release acetylcholine to activate the nAChR on their postganglionic neurons whose axons project to the islet (6). At the islet, postganglionic nerve terminals release the classical sympathetic and parasympathetic neurotransmitters, norepinephrine and acetylcholine, respectively, as well as the sympathetic and parasympathetic neuropeptides, neuropeptide tyrosine, and vasoactive intestinal polypeptide, respectively (7). Thus, in a broad sense, there are three autonomic inputs to the islet that are activated by the central nervous system (CNS), which could mediate the glucagon response during insulin-induced hypoglycemia (IIH) (see Fig. 1). This realization led us to propose (6) that blockade or ablation of all three autonomic inputs to the islet would be required to determine their net contribution of autonomic activation to the glucagon response to insulin-induced hypoglycemia. In a decade long series of studies, Dr. Peter Havel, in collaboration with us and Dr. Bo Ahrén, tested this hypothesis by blocking the ganglionic nAChR in all three autonomic pathways in mice, rats, dogs, monkeys, and humans (8). In each case we found that ganglionic blockade eliminated 75–90% of the glucagon response to insulin-induced hypoglycemia. Surgical ablation of all three autonomic inputs produces a similar result. These studies established in species from mice to men that autonomic activation mediates the majority of the glucagon response to moderate or marked hypoglycemia. Interestingly, blocking the receptors for the classical neurotransmitters does not reduce the glucagon response in humans at all (9), yet blocking the nAChR in people nearly abolishes this response (8). One explanation is that autonomic neuropeptides are important mediators of the glucagon response to insulin-induced hypoglycemia in humans.
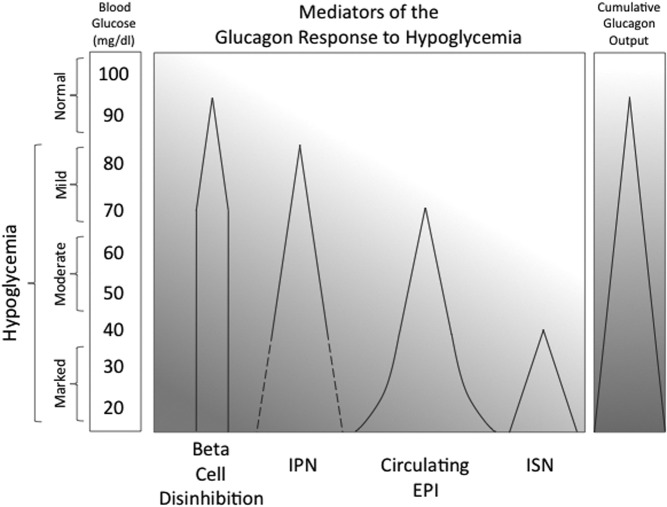
Fig. 1.
A schematic illustrating the threshold (apex of triangle) and magnitude (width of triangle) for four factors that cumulatively mediate the glucagon response to hypoglycemia. IPN, Islet parasympathetic nerves; EPI, epinephrine; ISN, islet sympathetic nerves; dashed line, unconfirmed.
Thresholds for activation
In this 2012 minireview, we summarize more recent information that shows differences in the glucose thresholds at which each of the three autonomic inputs to the islet begin to activate (see Fig. 1). Most of them activate before the clinical symptoms of hypoglycemia appear (nominally at 50 mg/dl), consistent with physiological mechanisms designed to prevent hypoglycemia. The data detailed below show that there is progressive recruitment of autonomic inputs as the glucose levels fall from a fasting level (nominally 100 mg/dl in Fig. 1) all the way to a near lethal level of 15 mg/dl. The implication is that their relative contributions to the glucagon response depend on the severity of the hypoglycemia (see Fig. 1). Although there is a known influence of prior hyperglycemia on the thresholds for activation, it is not considered below. Furthermore, early studies established that for acute hypoglycemia, it is the glucose nadir achieved, rather than the rate of glucose fall, that triggers autonomic activation (10).
The parasympathetic nervous system is the first autonomic input to the islet activated because its threshold of activation occurs at a glucose level between 85 and 75 mg/dl (11) (see Fig. 1), which is very mild hypoglycemia by any standard. It is important to recognize that this parasympathetic activity is measured indirectly via the surrogate of the pancreatic polypeptide response of the islet F cell, which is under strong cholinergic control (5). An alternative measure, the spillover of parasympathetic neurotransmitter from pancreatic nerves, is not experimentally feasible due to the extremely rapid degradation of acetylcholine by endogenous acetylcholinesterases.
In contrast, it is possible to use the neurotransmitter spillover technique to measure the activity of sympathetic nerves during hypoglycemia, as we and others have reported. Measurements of pancreatic norepinephrine spillover suggest that more marked hypoglycemia is required to activate pancreatic sympathetic nerves since such activation is first detected around 35 mg/dl (12) (see Fig. 1).
The hypoglycemic threshold for activation of the sympathoadrenal system falls between those of pancreatic parasympathetic and sympathetic nerves. Thus, there is a modest epinephrine response to insulin-induced hypoglycemia that begins between 75 and 65 mg/dl (11) (see Fig. 1).
In summary, in the nondiabetic state, all three autonomic inputs to the islet are activated by hypoglycemia, but there is a progressive recruitment of these inputs as the glucose level falls in the order of parasympathetic nerves, sympathoadrenal system, and sympathetic nerves, respectively (see Fig. 1). Thus, at a level of 50 mg/dl, at which the clinical symptoms of hypoglycemia first appear, two of the three autonomic inputs are already activated.
The concept of hypoglycemic threshold also applies to the effect of hypoglycemia to inhibit the islet β-cell (or islet δ-cell) and to thereby disinhibit the islet α-cell. Plasma insulin and C-peptide levels are sensitive indices of β-cell secretion, which in turn is very sensitive to the fasting glucose level. The inhibition of the islet β-cell starts at a glucose level between 95 and 85 mg/dl (11) (see Fig. 1). Thus, the inhibition of the β-cells starts before the activation of any of the three autonomic inputs to the islet. Recognition of the different thresholds required for both autonomic activation and β-cell disinhibition may explain some of the discrepancies in the literature regarding the mechanisms proposed for the glucagon response to hypoglycemia.
Magnitude of activation
Once the threshold for activation of an autonomic input is reached, the magnitude of activation usually increases as glucose falls further. This phenomenon is most clearly illustrated by the response of the adrenal medulla to progressive hypoglycemia: plasma epinephrine increases progressively as glucose falls from its glycemic threshold in the mild hypoglycemic range into the moderate hypoglycemic range (11) (see Fig. 1). When the glucose level enters the marked hypoglycemic range, there is an exponential increase in epinephrine secretion.
Available data also suggest an increased magnitude of activation of parasympathetic nerves with progressive hypoglycemia. For example, the pancreatic polypeptide response to hypoglycemia increases until glucose falls to the upper end of the marked hypoglycemic range (13) (see Fig. 1). It is not known, however, whether the pancreatic polypeptide response increases further when glucose enters the lower end of the marked hypoglycemic range because the pancreatic polypeptide responses to this level of hypoglycemia have not been reported.
The available data also show that as hypoglycemia progresses from the upper (12) to the lower end (4) of the marked hypoglycemic range, there is increased activation of pancreatic sympathetic nerves (see Fig. 1). Although it was necessary to produce marked hypoglycemia to detect a significant increase of norepinephrine spillover, it should be noted that this technique may underestimate sympathetic activation. Such an interpretation is supported by data from another metabolic organ influenced by sympathetic nerves, the liver. For example, low frequencies of sympathetic nerve stimulation decrease hepatic arterial blood flow (14) without increasing hepatic norepinephrine spillover. These data suggest that moderate sympathetic activation releases enough norepinephrine to cause a biological effect but not enough to escape avid local reuptake mechanisms. In contrast, high-frequency sympathetic nerve stimulation does increase hepatic norepinephrine spillover. Such data raise the possibility that both the magnitude of pancreatic sympathetic activation, and the hypoglycemic threshold at which it first occurs, may be underestimated by pancreatic norepinephrine spillover.
Finally, with regard to nonautonomic mediation of the glucagon response, the inhibition of the islet β-cell and the resultant disinhibition of the islet α-cell also increase but only until the glucose level falls to 65–70 mg/dl (11). At this lower end of mild hypoglycemic range, the inhibition of the β-cell is nearly complete (see Fig. 1). Thus, it is likely that the contribution of disinhibition to the glucagon response to insulin-induced hypoglycemia is near maximal by 70 mg/dl. The implication is that the larger increases of glucagon secretion seen when glucose levels fall less than 70 mg/dl are due to different, likely autonomic, mechanisms.
In conclusion, because the four main mediators of the glucagon response to hypoglycemia differ in their thresholds and magnitudes of activation throughout various degrees of hypoglycemia, their relative contributions to the resultant glucagon response depends on the level of hypoglycemia (see Fig. 1). Disinhibition is likely the sole mediator of the small glucagon response seen as the upper end of the mild hypoglycemic range (15, 16), whereas it is likely that both disinhibition and parasympathetic activation contribute at the lower end of this range. Within the moderate hypoglycemic range, it is likely that parasympathetic nerves and the adrenal medulla overtake disinhibition as the major mediators of the glucagon response. Finally, in the marked hypoglycemic range, it is likely that all three autonomic inputs mediate the large glucagon response, perhaps in redundant fashion (17), as we originally proposed (6).
It should be noted, however, that these conclusions apply to nondiabetic individuals. The relative contributions of each of these mechanisms to the glucagon response to hypoglycemia in diabetic individuals is likely to be different because diabetes can cause both functional and structural defects in these mechanisms, as detailed below.
Defective mechanisms in diabetes
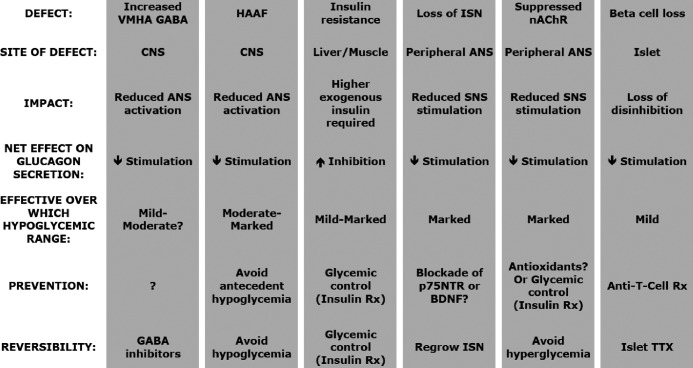
Several of the mechanisms that mediate the glucagon response to insulin-induced hypoglycemia in nondiabetic individuals can either be down-regulated or damaged in diabetes (see Table 1). Such mechanistic defects can either impair the glucagon response to insulin-induced hypoglycemia or, if the glucagon response is redundantly mediated at that level of hypoglycemia, increase its reliance on the remaining intact mechanisms. Most of these defects result in decreased stimulation of the islet α-cell, but one defect (insulin resistance) increases inhibition of the islet α-cell (see Table 1 and text below).
Table 1.
Defects in autoimmune diabetes that inhibit the glucagon response to IIH
ISN, Islet sympathetic nerves; ANS, autonomic nervous system; SNS, sympathetic nervous system; Rx, treatment; BDNF, brain derived neurotrophic factor; TTX, transplantation.
Decreased stimulation
Central nervous system
Because the CNS controls the autonomic outflow to the pancreatic islet during hypoglycemia, it is logical to look for diabetes-related CNS defects, which might account for the impaired glucagon response to insulin-induced hypoglycemia seen in diabetes. Although there have been many excellent mechanistic studies of CNS factors that influence both the adrenal medullary and glucagon responses to insulin-induced hypoglycemia in nondiabetic animals (18), there have been few analogous studies in diabetic animals. One such study (19) points to ventromedial hypothalamic (VMH) levels of γ-aminobutyric acid (GABA) as contributing to the impaired glucagon and epinephrine responses to insulin-induced hypoglycemia seen in streptozotocin diabetic and biobreeder diabetic rats (see Table 1). In brief, basal VMH levels of this inhibitory neurotransmitter are elevated in both diabetic models and do not decrease with hypoglycemia as they do in nondiabetic animals (19). More importantly, partial knockdown of glutamic acid decarboxylase, a synthetic enzyme in the GABA pathway, reduces the VMH levels of GABA to those of nondiabetic rats and normalizes both the glucagon and epinephrine responses insulin-induced hypoglycemia, despite marked β-cell loss.
Prior episodes of hypoglycemia can result in reduced autonomic outflow from the CNS to a subsequent episode of equivalent hypoglycemia (20). This phenomenon has been named hypoglycemia-associated autonomic failure (HAAF). If the reduction of autonomic responses is large enough, then the glucagon response to a subsequent episode of equivalent hypoglycemia is also reduced (21) (see Table 1). The most widely documented autonomic impairment is that of adrenal medullary activation: the epinephrine response to subsequent hypoglycemia is clearly impaired by prior hypoglycemia (20, 21). Because prior hypoglycemia also impairs the pancreatic polypeptide response to subsequent hypoglycemia (20, 21), this autonomic failure also includes the parasympathetic input to the islet. Although the impact of HAAF on the activation of the sympathetic input to the islet has not been measured, the hypoglycemia-induced increase of muscle sympathetic nerve activity is impaired by prior hypoglycemia (21), suggesting that the sympathetic activation of the islet may be as well. Thus, all three autonomic inputs to the islet may be impaired by prior hypoglycemia. A direct link between reduced autonomic activation and the reduced glucagon response to insulin-induced hypoglycemia has not been established in HAAF. However, because a marked reduction in the activation of all three of the autonomic inputs does markedly reduce the glucagon response to moderate or marked hypoglycemia (3), a direct link is likely.
Peripheral nervous system
Although sympathetic defects are an established part of classical diabetic autonomic neuropathy, this neuropathy usually takes decades to develop in diabetic patients. In contrast, there are two known defects in the sympathetic-islet pathway that occur early in this disease. One involves the sympathetic ganglia, which reside outside the pancreas. The other involves the sympathetic terminals within the pancreatic islet itself. Defects in the sympathetic-islet pathway are not reflected in the systemic plasma of diabetic patients because the pancreas makes a negligible contribution to circulating norepinephrine levels (22). For this reason, the two diabetes-related defects in the sympathetic-islet pathway described below have gone unrecognized in patients with type 1 diabetes.
The diabetes-induced impairment of sympathetic ganglionic neurotransmission has just recently been described (23) (see Table 1). It involves a dysfunction of the specialized subtype of the nAChR found in sympathetic ganglia. The dysfunction is caused by oxidation of the cysteines that reside in the ion channel pore of this receptor. That oxidation is caused by hyperglycemia, which is secondary to diabetes. Although years of chronic hyperglycemia are needed to cause classical diabetic autonomic neuropathy, only 1 wk of untreated diabetic hyperglycemia is needed to cause a major impairment of the activation of the nAChR in sympathetic ganglia. However, the hyperglycemic threshold needed to produce this impairment is unknown. Although this defect has not yet been demonstrated to reduce the sympathetic input to the islet during hypoglycemia, such a reduction is likely because the activation of sympathetic neurons in the celiac ganglia, a subset of which project to the pancreas, is impaired (Taborsky, G.J., and T.O. Mundinger, unpublished observations). Although rapid treatment of diabetic hyperglycemia would be expected to prevent this impairment, it has yet to be demonstrated. Likewise, there are no data on reversibility but potent antioxidants represent one possible treatment.
The second sympathetic defect found early in diabetes is due to a marked loss of sympathetic nerve terminals within the islet (24) when it undergoes autoimmune attack (25) (see Table 1). This neuropathy is found only in the autoimmune form of this disease: in human type 1 diabetes (26) or in immune animal models thereof (24, 25). As expected, the loss of these islet sympathetic nerves impairs sympathetically-mediated glucagon responses (25, 27). However, the contribution of this defect to the impaired glucagon response to insulin-induced hypoglycemia, seen in type 1 diabetes, remains to be demonstrated.
Although the mechanism by which this early sympathetic islet neuropathy (eSIN) occurs has not been definitively established, several pieces of evidence point to a very selective and specialized mechanism, rather than to a generalized nerve loss secondary to nonspecific tissue damage. Neither β-cell loss nor hyperglycemia causes eSIN because islet sympathetic nerves are not lost in chemically induced diabetes (25, 27). Furthermore, this loss is neurally selective because islet parasympathetic nerves are fully retained. Immune mechanisms are involved in eSIN because the loss of islet sympathetic nerves is proportional to invasive insulitis, both in diabetic and nondiabetic nonobese diabetic mice (NOD) (25). However, a T lymphocyte-mediated attack targeting a ubiquitous sympathetic antigen is unlikely because the sympathetic nerves of the adjacent exocrine pancreas are spared (24). Rather, the cause of eSIN may be by the same mechanism that prunes sympathetic axons during the development of target innervation, which involves the p75 neurotrophin receptor (p75NTR). Indeed, our preliminary data show that knocking out the p75NTR prevents eSIN but does not prevent T lymphocyte-mediated diabetes (28).
Currently the only way to prevent eSIN is to block either islet infiltration (25) or the p75NTR (28). Other strategies are likely to emerge once the lymphocytic mediators and the agonist activating the p75NTR become known. Because the parent axons in the surrounding exocrine pancreas appear intact (24), as do their neuronal cell bodies in the celiac ganglia, it should be possible to regrow these islet sympathetic nerves (29). However, data from studies in the diabetic biobreeder rat provide no evidence of recovery of islet sympathetic nerves, even 3 months after the initial nerve loss (24), when reinnervation does occur in nondiabetic animals (29). Therefore, there may be some factor in diabetes that inhibits the expected regrowth of the islet sympathetic nerve terminals.
In contrast to the defects in the sympathetic input to the islet, there are apparently no structural defects in either the parasympathetic or adrenal medullary inputs that contribute to the early impairment of the glucagon response to insulin-induced hypoglycemia because neither the pancreatic polypeptide response nor the epinephrine response is impaired early in type 1 diabetes, if HAAF is avoided.
β-Cell loss
An obvious major defect in type 1 diabetes is the loss of islet β-cells. At least initially this loss is selective, i.e. islet α-cells are retained (30). In nondiabetic individuals the glucagon response to mild hypoglycemia is likely dependent on β-cell disinhibition of the α-cell because the autonomic inputs to the islet have yet to be activated (see above). Therefore, the impairment of the glucagon response to mild hypoglycemia in diabetic individuals is likely due to the loss of islet β-cells (see Table 1). However, the glucagon response to both moderate and marked hypoglycemia can also be markedly impaired in diabetes, despite normal parasympathetic and adrenal medullary activation. There are two different explanations for this impairment. The first is that the diabetes-induced defects listed above may reduce the sympathetic stimulation of the islet α-cells sufficiently to impair the glucagon response to both moderate and marked hypoglycemia. Because the sympathetic defects induced by diabetes have been discovered recently (23, 24), experiments either correcting these defects in diabetic animals or simulating them in nondiabetic animals have not been done to directly test this hypothesis. However, correcting a CNS defect does restore the glucagon response to IIH in a diabetic animal (19), despite severe β-cell loss. Thus, carefully designed experiments examining diabetes-induced defects in the sympathetic-islet pathway are warranted to determine their contribution to the impairment of glucagon response to moderate and marked hypoglycemia.
The second explanation is that β-cell disinhibition of the islet α-cell may be permissive for the glucagon response, even if autonomic activation is the major mediator of the glucagon response in this hypoglycemic range. There are data from both human and animal studies consistent with this explanation. Studies of nondiabetic humans have shown that stimulation of the β-cell prevents the glucagon response during hypoglycemia, despite normal parasympathetic and adrenal medullary activation (11). Studies in diabetic animals (31) have shown that switching off an intrapancreatic insulin infusion can normalize the glucagon response to hypoglycemia.
Increased inhibition
Insulin resistance
Insulin resistance is a consequence of β-cell loss that can indirectly impair glucagon responses, as outlined below. Insulin-deficient individuals become resistant to the glucose-lowering effects of insulin. This insulin resistance necessitates larger doses of insulin in diabetic individuals to produce a level of hypoglycemia equivalent to that produced by smaller doses of insulin in nondiabetic individuals. The problem is that exogenous insulin itself is a potent inhibitor of glucagon secretion, either directly on the α-cell or via the CNS (see Table 1). This inhibitory effect of exogenous insulin may explain the smaller glucagon response to insulin induced hypoglycemia seen with the advent of the hyperinsulimic euglycemic clamp, especially the stepped hyperinsulemic clamp, which uses increasing insulin doses. Older studies, which usually report larger glucagon responses, used a bolus injection of insulin, which allowed the hypoglycemic nadir to occur during falling rather that sustained levels of exogenous insulin. The inhibitory effect of exogenous insulin on the α-cell is best illustrated by its effect on the glucagon response to mild hypoglycemia. There is a clear glucagon response to mild hypoglycemia when it is induced without insulin (15). However, when the same degree of mild hypoglycemia is achieved by insulin infusion or when insulin is added the glucagon response is abolished (15, 16). Thus, when higher doses of insulin are used in diabetic animals to produce equivalent hypoglycemia, part of the impairment of the glucagon response seen in diabetes is due to additional inhibition of the α-cell by higher levels of circulating insulin. Thus, differences in insulin resistance, necessitating differences in the dose of insulin required to produce equivalent hypoglycemia, may account for the wide spectrum of reported impairments of the glucagon response to insulin-induced hypoglycemia in animals with short-term duration of diabetes. These impairments range from very modest (32) to total loss of the glucagon response (31). It should be pointed out that this analysis assumes that the α-cell itself does not become insulin resistant. Conversely, there is some indirect evidence that α-cells may even become insulin sensitive, exacerbating this experimental problem.
The solution to this experimental confounder is to chronically treat diabetic animals to cure their insulin resistance, before performing acute hypoglycemia experiments. This is difficult to achieve without inducing HAAF, a common consequence of intensive insulin treatment. When such careful treatment has been implemented, there is less impairment of the glucagon response to IIH in diabetic individuals (33).
Future directions
As stated at the outset of this review, the loss of the glucagon response to insulin-induced hypoglycemia in diabetic patients exacerbates the severity and duration of the hypoglycemia occasioned by mistimed or excessive insulin treatment. Despite a significant increase in our understanding of both the mechanisms mediating the glucagon response to insulin-induced hypoglycemia in nondiabetic individuals and the defects potentially causing its impairment in diabetes, this response has proven hard to restore in diabetic subjects, even though their α-cells appear structurally (30) and functionally intact. The functional defects that impair the glucagon response to insulin-induced hypoglycemia are likely reversible by restoring euglycemia with insulin therapy, whereas the structural defects are not. These functional defects include HAAF and insulin resistance, which are reversed by insulin-induced euglycemia, and increased VMH GABA and impaired nAChR in sympathetic ganglia, which may also be. However, normalizing diabetic hyperglycemia is difficult to achieve without producing acute episodes of hypoglycemia. Nonetheless, the potential to reverse the four functional defects above and improve this impaired glucagon response warrants serious effort. Experience gained in treating diabetic hyperglycemia at both the bench and the bedside can be used to achieve this goal. For example, clinical studies support the importance of basal insulin replacement to minimize glucose variability (both hyperglycemia and hypoglycemia). Studies in animals support the importance of the portal route of insulin administration (Taborsky, G.J., and T.O. Mundinger, unpublished data). Finally, both clinical and basic studies support the use of short-acting insulins before meals.
Reversing these functional defects still leaves a major structural defect in the autonomic nervous system: loss of islet sympathetic nerves. Theoretically one could regrow these nerves in type 1 diabetes because their parent axons in the exocrine pancreas (24) and their neuronal cell bodies in the celiac ganglia appear structurally intact. However, such nerve regrowth may require both reversing the diabetic hyperglycemia that seems to retard reinnervation and replacing local nerve growth factor, which is normally supplied by islet β-cells (34). This method would reconnect islets with their original neurons, thereby maintaining the normal stress-specific activation of sympathetic islet pathway (35). A second approach would be islet transplantation to replace β-cells, both reversing diabetic hyperglycemia and providing an islet source of nerve growth factor. However, newly transplanted islets are sympathetically denervated because the harvest procedure severs the axons that connect the islet nerve terminals to their neuronal cell bodies, which reside outside the pancreas. Fortunately, transplanted islets eventually become reinnervated (29). In both approaches, insulin-induced hypoglycemia can be used before and after reinnervation to determine the contribution of the sympathetic input to the islet to the restoration of a normal glucagon response.
Acknowledgments
Disclosure Summary: No potential conflict of interest exists relative to this article.
Footnotes
- CNS
- Central nervous system
- eSIN
- early sympathetic islet neuropathy
- GABA
- γ-aminobutyric acid
- HAAF
- hypoglycemia-associated autonomic failure
- IIH
- insulin-induced hypoglycemia
- nAChR
- nicotinic acetylcholine receptor
- p75NTR
- p75 neurotrophin receptor
- VMH
- ventromedial hypothalamus.
References
- 1. Cryer PE, Gerich JE. 1983. Relevance of glucose counterregulatory systems to patients with diabetes: critical roles of glucagon and epinephrine. Diabetes Care 6:95–99 [DOI] [PubMed] [Google Scholar]
- 2. Cryer PE. 2012. Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 153:1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taborsky GJ, Jr, Ahrén B, Havel PJ. 1998. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired α-cell responses in type 1 diabetes. Diabetes 47:995–1005 [DOI] [PubMed] [Google Scholar]
- 4. Havel PJ, Mundinger TO, Veith RC, Dunning BE, Taborsky GJ., Jr 1992. Corelease of galanin and NE from pancreatic sympathetic nerves during severe hypoglycemia in dogs. Am J Physiol 263:E8–E16 [DOI] [PubMed] [Google Scholar]
- 5. Schwartz TW. 1983. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 85:1411–1425 [PubMed] [Google Scholar]
- 6. Havel PJ, Taborsky GJ., Jr 1989. The contribution of the autonomic nervous system to changes of glucagon and insulin secretion during hypoglycemic stress. Endocr Rev 10:332–350 [DOI] [PubMed] [Google Scholar]
- 7. Ahrén B, Taborsky GJ, Jr, Porte D., Jr 1986. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 29:827–836 [DOI] [PubMed] [Google Scholar]
- 8. Havel PJ, Ahren B. 1997. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 46:801–807 [DOI] [PubMed] [Google Scholar]
- 9. Towler DA, Havlin CE, Craft S, Cryer P. 1993. Mechanism of awareness of hypoglycemia: perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 42:1791–1798 [DOI] [PubMed] [Google Scholar]
- 10. Amiel SA, Simonson DC, Tamborlane WV, DeFronzo RA, Sherwin RS. 1987. Rate of glucose fall does not affect counterregulatory hormone responses to hypoglycemia in normal and diabetic humans. Diabetes 36:518–522 [DOI] [PubMed] [Google Scholar]
- 11. Banarer S, McGregor VP, Cryer PE. 2002. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 51:958–965 [DOI] [PubMed] [Google Scholar]
- 12. Havel PJ, Mundinger TO, Taborsky GJ., Jr 1996. Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs. Am J Physiol 270:E20–E26 [DOI] [PubMed] [Google Scholar]
- 13. Dunning BE, Scott MF, Neal DW, Cherrington AD. 1997. Direct quantification of norepinephrine spillover and hormone output from the pancreas of the conscious dog. Am J Physiol 272:E746–E755 [DOI] [PubMed] [Google Scholar]
- 14. Mundinger TO, Verchere CB, Baskin DG, Boyle MR, Kowalyk S, Taborsky GJ., Jr 1997. Galanin is localized in sympathetic neurons of the dog liver. Am J Physiol 273:E1194–E1202 [DOI] [PubMed] [Google Scholar]
- 15. Igawa K, Mugavero M, Shiota M, Neal DW, Cherrington AD. 2002. Insulin sensitively controls the glucagon response to mild hypoglycemia in the dog. Diabetes 51:3033–3042 [DOI] [PubMed] [Google Scholar]
- 16. Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA, Bogan JS, McCrimmon RJ, Sherwin RS. 2010. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes 59:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Havel PJ, Parry SJ, Stern JS, Akpan JO, Gingerich RL, Taborsky GJ, Jr, Curry DL. 1994. Redundant parasympathetic and sympathoadrenal mediation of increased glucagon secretion during insulin-induced hypoglycemia in conscious rats. Metabolism 43:860–866 [DOI] [PubMed] [Google Scholar]
- 18. Sherwin RS. 2008. Bringing light to the dark side of insulin: a journey across the blood-brain barrier. Diabetes 57:2259–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan O, Paranjape S, Czyzyk D, Horblitt A, Zhu W, Ding Y, Fan X, Seashore M, Sherwin R. 2011. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes 60:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heller SR, Cryer PE. 1991. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40:223–226 [DOI] [PubMed] [Google Scholar]
- 21. Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. 1996. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest 98:680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahrén B, Veith RC, Taborsky GJ., Jr 1987. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1) Effects on basal release of insulin and glucagon. Endocrinology 121:323–331 [DOI] [PubMed] [Google Scholar]
- 23. Campanucci V, Krishnaswamy A, Cooper E. 2010. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66:827–834 [DOI] [PubMed] [Google Scholar]
- 24. Mei Q, Mundinger TO, Lernmark A, Taborsky GJ., Jr 2002. Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 51:2997–3002 [DOI] [PubMed] [Google Scholar]
- 25. Taborsky GJ, Jr, Mei Q, Hackney DJ, Figlewicz DP, LeBoeuf R, Mundinger TO. 2009. Loss of islet sympathetic nerves and impairment of glucagon secretion: relationship to invasive insulitis. Diabetologia 52:2602–2611 [DOI] [PubMed] [Google Scholar]
- 26. Mei Q, Foulis AK, Fligner C, Hull R, H. N., Gilliam L, Taborsky GJ., Jr 2006. Selective loss of sympathetic nerves from the islet in human type 1 diabetes: a potential mechanism for impaired glucagon responses to hypoglycemia. Diabetes 55 (Suppl 1):A15 [Google Scholar]
- 27. Mundinger TO, Mei Q, Figlewicz DP, Lernmark A, Taborsky GJ., Jr 2003. Impaired glucagon response to sympathetic nerve stimulation in the BB diabetic rat: effect of early sympathetic islet neuropathy. Am J Physiol Endocrinol Metab 285:E1047–1054 [DOI] [PubMed] [Google Scholar]
- 28. Mei Q, Hackney D, Chang H, Bornfeldt K, Taborsky G., Jr 2011. Evidence that Islet Sympathetic Nerves (ISN) and Islet Beta Cells Are Destroyed via Different Mechanisms in Type 1 Diabetes (T1D). Diabetes 60 (Supplement 1):A136 [Google Scholar]
- 29. Korsgren O, Jansson L, Andersson A, Sundler F. 1993. Reinnervation of transplanted pancreatic islets. Transplantation 56:138–143 [PubMed] [Google Scholar]
- 30. Foulis AK, Stewart JA. 1984. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia 26:456–461 [DOI] [PubMed] [Google Scholar]
- 31. Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. 2004. Regulation of α-cell function by the β-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes 53:1482–1487 [DOI] [PubMed] [Google Scholar]
- 32. Hertelendy ZI, Patel DG, Skau KA. 1992. Progressive and concurrent deterioration of vagus-stimulated and hypoglycemia-induced glucagon secretion in streptozotocin-diabetic rats. Acta Endocrinol (Copenh) 126:80–84 [DOI] [PubMed] [Google Scholar]
- 33. Fanelli CG, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Lepore M, Annibale B, Ciofetta M, Bottini P, Porcellati F, Scionti L, Santeusanio F, Brunetti P, Bolli GB. 1993. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 42:1683–1689 [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum T, Vidaltamayo R, Sánchez-Soto MC, Zentella A, Hiriart M. 1998. Pancreatic B cells synthesize and secrete nerve growth factor. Proc Natl Acad Sci USA 95:7784–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Havel PJ, Veith RC, Dunning BE, Taborsky GJ., Jr 1988. Pancreatic noradrenergic nerves are activated by neuroglucopenia but not hypotension or hypoxia in the dog: evidence for stress-specific and regionally-selective activation of the sympathetic nervous system. J Clin Invest 82:1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]