Abstract
Protein kinase Cη (PKCη) is a member of the novel PKC subfamily, which also includes δ, ε, and θ isoforms. Compared to the other novel PKCs, the function of PKCη in the immune system is largely unknown. Several studies have started to reveal the role of PKCη, particularly in T cells. PKCη is highly expressed in T cells, and is upregulated during thymocyte positive selection. Interestingly, like the θ isoform, PKCη is also recruited to the immunological synapse that is formed between a T cell and an antigen-presenting cell. However, unlike PKCθ, which becomes concentrated to the central region of the synapse, PKCη remains in a diffuse pattern over the whole area of the synapse, suggesting distinctive roles of these two isoforms in signal transduction. Although PKCη is dispensable for thymocyte development, further analysis of PKCη- or PKCθ-deficient and double-knockout mice revealed the redundancy of these two isoforms in thymocyte development. In contrast, PKCη rather than PKCθ, plays an important role for T cell homeostatic proliferation, which requires recognition of self-antigen. Another piece of evidence demonstrating that PKCη and PKCθ have isoform-specific as well as redundant roles come from the analysis of CD4 to CD8 T cell ratios in the periphery of these knockout mice. Deficiency in PKCη or PKCθ had opposing effects as PKCη knockout mice had a higher ratio of CD4 to CD8 T cells compared to that of wild-type mice, whereas PKCθ-deficient mice had a lower ratio. Biochemical studies showed that calcium flux and NFκB translocation is impaired in PKCη-deficient T cells upon TCR crosslinking stimulation, a character shared with PKCθ-deficient T cells. However, unlike the case with PKCθ, the mechanistic study of PKCη is at early stage and the signaling pathways involving PKCη, at least in T cells, are essentially unknown. In this review, we will cover the topics mentioned above as well as provide some perspectives for further investigations regarding PKCη.
Keywords: development, homeostatic proliferation, immune synapse, immunological synapse, protein kinase C, signaling, T cell, T cell activation
Introduction
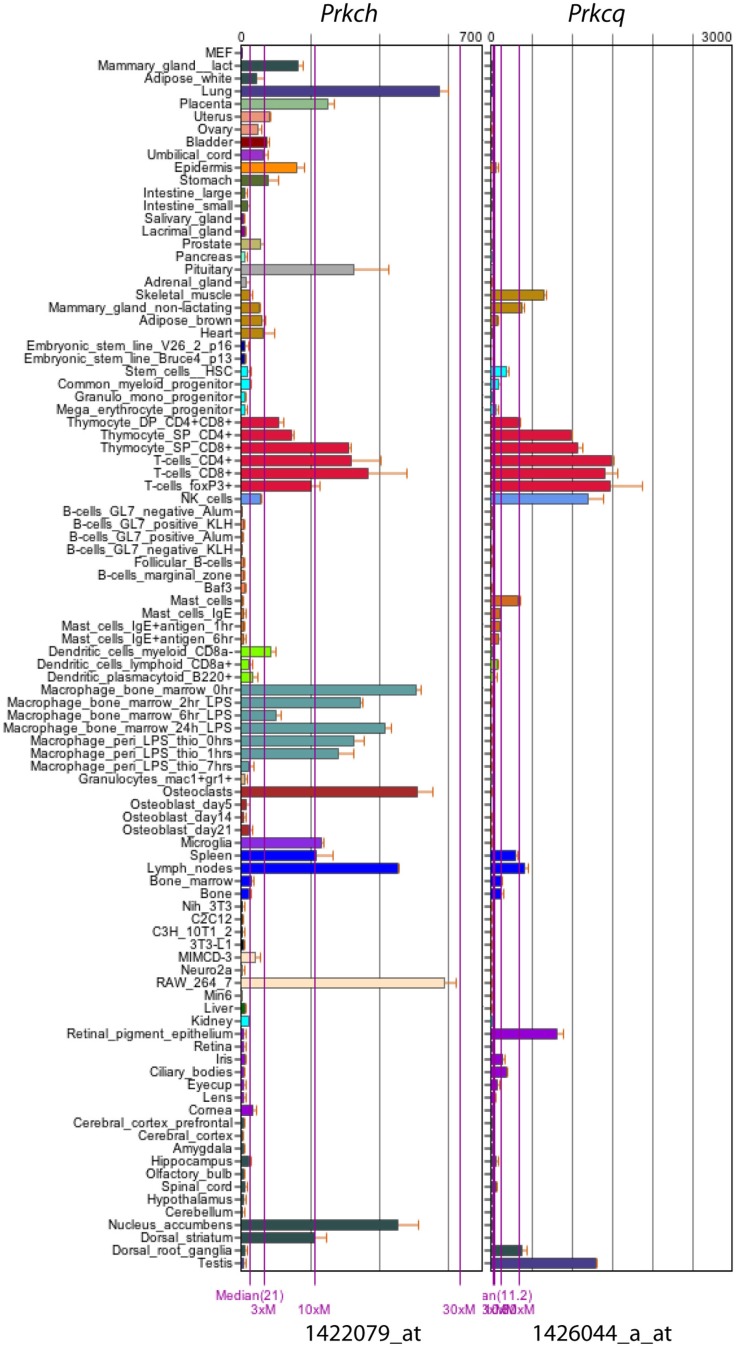
Protein kinase C (PKC) is a large family of serine/threonine kinases that can be divided into three subfamilies based on their structural homology and requirement of cofactors for activation (Baier, 2003). The conventional PKC subfamily contains α, βI, βII, and γ, requiring calcium and diacylglycerol (DAG) for activation. The novel PKC subfamily contains δ, ε, θ, and η, and requires DAG but not calcium for activation. In contrast, the atypical PKC subfamily (i.e., ζ and λ/℩) requires neither DAG nor calcium for their activation (Pfeifhofer et al., 2003). Studies using PKC isoform-specific knockout mice have shown differential roles of each isoform in T cell development and function (Sun et al., 2000; Thuille et al., 2004; Gruber et al., 2005a,b; Pfeifhofer et al., 2006). For example, PKCα-deficient mice have a normal T cell development phenotype. In peripheral T cells, PKCα is dispensable for normal T cell activation and IL2 production, but it is required for proliferation and IFN-γ production (Pfeifhofer et al., 2006). PKCβ is dispensable for normal T cell development and function (Thuille et al., 2004), although it was found to be important for LFA-1-mediated T cell locomotion in a PKCβ-deficient cell line (Volkov et al., 2001). PKCδ is a negative regulator of T cell activation, as PKCδ-deficient T cells are hyperproliferative and produce more IL2 cytokine upon stimulation (Gruber et al., 2005a). This negative regulatory role is also reflected in PKCδ-deficient B cells (Mecklenbrauker et al., 2002; Miyamoto et al., 2002). In striking contrast, PKCθ-deficient T cells completely lose the ability to proliferate or to produce IL2 after stimulation through the T cell receptor (TCR) in in vitro assays (Sun et al., 2000; Pfeifhofer et al., 2003), even though both δ and θ have the closest identity (60%) within the novel PKC subfamily (Kong et al., 2011; Quann et al., 2011). PKCε was dispensable for T cell development and activation (Gruber et al., 2005b). In PKCζ-deficient mice, there is no overt defect in T cell development (Leitges et al., 2001), but these mice showed impaired Th2 cell differentiation (Martin et al., 2005). Interestingly, although discovered more than two decades ago (Osada et al., 1990), and like PKCθ, highly expressed in T cells (Baier, 2003; Figure 1: data from www.biogps.org (Su et al., 2004; Wu et al., 2009) the role of PKCη had never been thoroughly examined in T cells until the recent study from our group (Fu et al., 2011). This despite the fact that PKCη-deficient mice have existed for almost 10 years (Chida et al., 2003). Meanwhile, although discovered only a little later than PKCη, PKCθ is considered paramount in T cell function. Our recent work on PKCη has significantly filled this gap by showing both isoform-specific and redundant (with PKCθ) roles of PKCη in T cell development and function (Fu et al., 2011; Fu and Gascoigne, 2012). In this article, we will first briefly review some earlier studies on PKCη, then mainly focus on four subjects currently under study: (1) the recruitment of PKCη to the immunological synapse; (2) its role in T cell development; (3) its role in T cell function; (4) its role in TCR signaling. Finally, we would like to share some of our thoughts with the readers about future investigations regarding PKCη.
Figure 1.
mRNA expression profile of mouse PKCη (Prkch) and PKCθ (Prkcq). Data were obtained from www.biogps.org using GeneAtlas MOE430, gcrma (Su et al., 2004; Wu et al., 2009), with probes 1422079_at (Prkch) and 1426044_a_at (Prkcq).
Comparison of PKCη and PKCθ Molecules
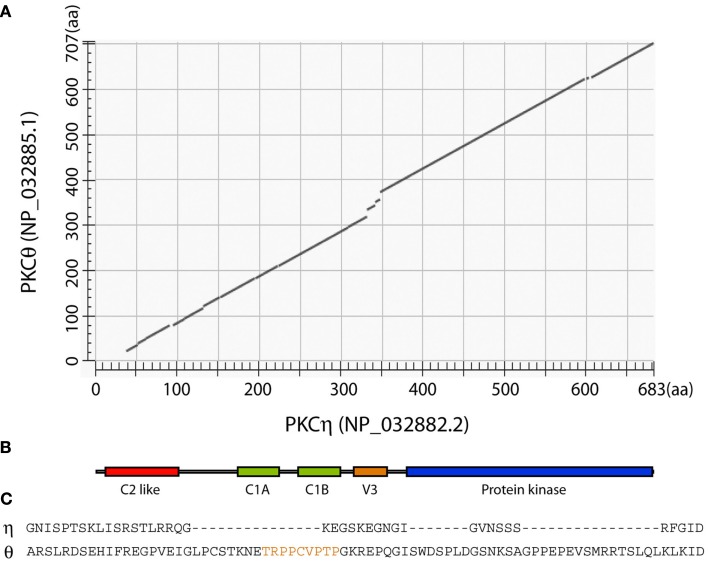
In the novel PKC subfamily, PKCδ and PKCθ are closely related (60% identity), as are PKCε and PKCη (also 60% identity; Baier, 2003; Quann et al., 2011). A cross comparison between PKCη and PKCθ reveals that these two isoforms bear 42% identity (Figure 2A). The overall domain structure of PKCη and PKCθ proteins shows a high degree of similarity. This domain architecture is shown in Figure 2B. In both isoforms, there is a “C2-like” domain near the amino-terminal of the protein, which cannot bind calcium, unlike the C2 domains in conventional PKC isoforms (Baier, 2003). Following the C2-like domain, there are tandem repeats of two DAG binding C1 domains and the V3 hinge region. This is the most different region between PKCη and PKCθ (Figure 2C). In PKCθ, V3 is important in association of the kinase with CD28 and as a result is required to mediate PKCþeta’s localization in the central synapse (Kong et al., 2011). The motif within PKCθV3 domain that is required for CD28 interaction, including the conserved PXXP sequence (Kong et al., 2011), is missing in PKCη (Figure 2C). The C2-like, C1, and V3 domains together form a regulatory region, which likely performs the isoform-specific functions, as the carboxyl-terminal serine/threonine kinase domain is rather conserved across all PKC isoforms. The difference between the V3 domains of PKCθ and PKCη suggests that this may be responsible for their different localization in the immunological synapse.
Figure 2.
Comparison of mouse PKCη and PKCθ proteins. (A) Alignment of mouse PKCη and PKCθ was performed using NCBI BLAST program. (B) Cartoon showing the arrangement of known conserved domains that applies to both PKCη and PKCθ proteins. (C) Alignment of PKCη and PKCθ V3 domains. The region identified as important for PKCθ interaction with CD28 (Kong et al., 2011) is highlighted in orange.
A Brief History of PKCη Studies
PKCη was originally identified from a mouse epidermis cDNA library and found to be highly expressed in mouse tissues such as skin, lung, and heart (Osada et al., 1990). Because of this tissue-specific expression pattern, most studies regarding PKCη were historically focused on keratinocyte proliferation and differentiation (Ohba et al., 1998; Cabodi et al., 2000). However, development of skin was normal in PKCη-deficient mice in steady state (Chida et al., 2003). In contrast, under challenging conditions, these PKCη-deficient mice were susceptible to skin tumor induction and showed impaired wound healing (Chida et al., 2003). In immune cells, PKCη is highly expressed in mouse macrophages and T cells, but not B cells (Figure 1). However, interestingly, potential roles of PKCη in B cells were suggested in a number of studies (Morrow et al., 1999; Oda et al., 2008). For example, PKCη was shown to be specifically transcribed in pro-B but not pre-B cells, and a pro-apoptotic role of PKCη in B cells was suggested (Morrow et al., 1999). In another study, PKCη was shown to direct IRF4 expression and Igκ gene rearrangement in pre-BCR signaling (Oda et al., 2008). Surprisingly, nothing was known about the specific role of PKCη in T cells until quite recent work from our group and others (Singleton et al., 2009; Fu et al., 2011; Quann et al., 2011; Sewald et al., 2011), which is the topic we address below.
Recruitment of PKCη to the Immunological Synapse
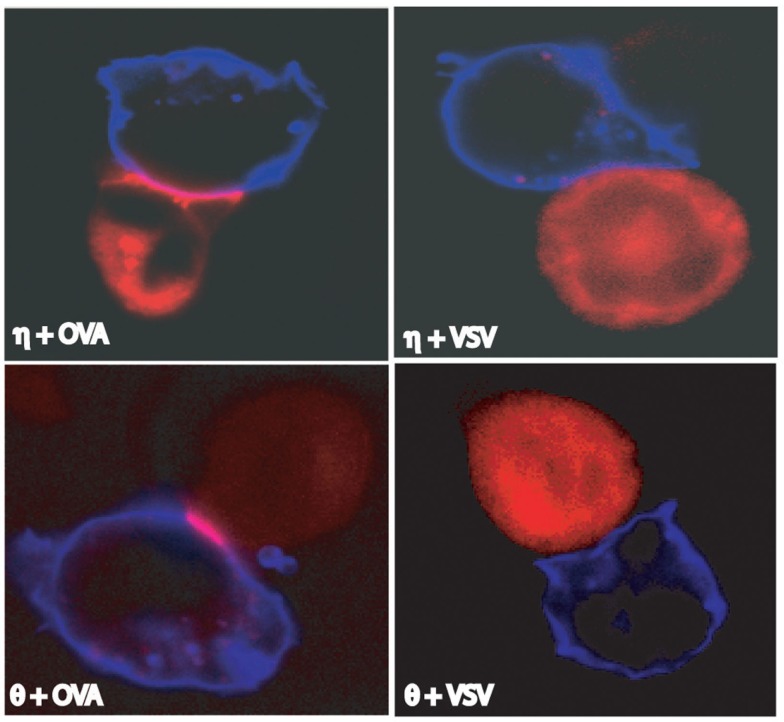
The immunological synapse or supramolecular activation cluster (SMAC) forms at the interface between a T cell and an antigen-presenting cell (APC; or a surrogate), and is the site at which early signaling events occur (Grakoui et al., 1999). The widely accepted importance of PKCθ in T cells is largely due to its identification as the only PKC isoform recruited to the immunological synapse (Monks et al., 1997), and particularly to the central synapse region (cSMAC), along with TCR and other molecules (Monks et al., 1998). Since then, PKCθ has served as a landmark for defining the immunological synapse. However, studies from our group and others challenged the view that only PKCθ is recruited to the synapse (Singleton et al., 2009; Fu et al., 2011; Quann et al., 2011). PKCη is recruited to the immunological synapse upon T cell recognition of its cognate antigenic peptide-MHC (pMHC), but not non-stimulatory pMHC, presented by APCs (Figure 3; Fu et al., 2011). More interestingly, PKCη and PKCθ showed different recruitment patterns, as PKCη forms a diffuse pattern at the immunological synapse, whereas PKCθ concentrates into the central region (Figure 3; Singleton et al., 2009; Fu et al., 2011), suggesting different functions in time and space of these two PKC isoforms. In addition to PKCη and PKCθ, PKCε is also recruited to the immunological synapse (Quann et al., 2011). In this study, polarization of the T cell microtubule-organizing center (MTOC) is directed by diacylglycerol (DAG) at the immunological synapse via three PKC isoforms, in two sequential steps. Initially, PKCε and PKCη accumulate in a broad region of the interface between T cell and APC, followed by PKCθ concentrating in a smaller, central, zone (Quann et al., 2011). It seems that in different cell types, recruitment of PKC isoforms could also be different. For example, it has been shown that, in contrast to the immunological synapse-localization in effector T cells, PKCθ is sequestered away from the immunological synapse in regulatory T cells (Treg), and thus mediates negative feedback on Treg cell function (Zanin-Zhorov et al., 2010). This intriguing observation may be also worth examination for PKCε and PKCη.
Figure 3.
Recruitment of PKCη and PKCθ to the immunological synapse. Both PKCη and PKCθ were recruited to the immunological synapse by antigenic stimulation (i.e., with stimulatory peptide OVA) but not by non-antigenic stimulation (i.e., with non-stimulatory peptide VSV). Blue are EL4 cells used as antigen-presenting-cells. Red are OT-I T hybridoma cells transfected with PKCη- or PKCθ-RFP as indicated. Adapted from Fu et al. (2011).
PKCη in T Cell Development
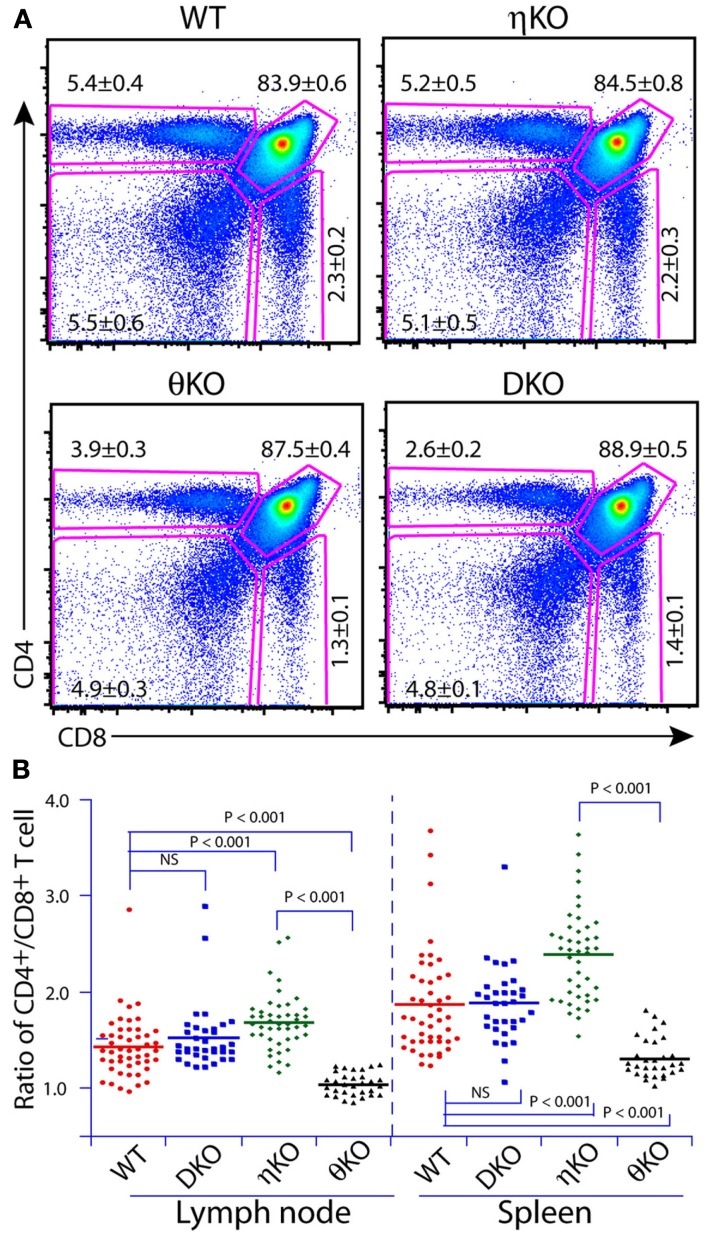
Our initial speculation that PKCη may play a role in T cell development was based on the finding that PKCη mRNA expression was upregulated during thymocyte positive selection (Mick et al., 2004; Niederberger et al., 2005). These observations were surprising given the established important role of PKCθ in T cell biology, but intriguing because PKCθ-deficient mice have only a very minor defect in thymocyte development. Initial phenotyping of PKCθ-deficient mice did not identify any defects in thymocyte development (Sun et al., 2000; Pfeifhofer et al., 2003), although later studies did find a mild thymocyte development defect in such mice (Morley et al., 2008; Fu et al., 2011). However, phenotyping of PKCη-deficient mice showed rather normal thymocyte development. This was not completely unexpected given the multiple novel PKC isoforms co-expressed in T cells, and redundancy could play a role to compensate for the absence of any particular isoform. We also noted that induction of PKCη mRNA is much higher and earlier in PKCθ-deficient mice than in wild-type mice (i.e., induction during positive selection in wild-type mice, but induction before positive selection in PKCθ-deficient mice), suggesting a compensatory effect due to redundancy of function between PKCη and PKCθ (Fu et al., 2011). In accord with this notion, PKCη is recruited to the immunological synapse in immature CD4+CD8+ (DP) thymocytes in the PKCθ–/– mice, as is PKCθ in the PKCθ-sufficient DP cells. In PKCθ-sufficient cells, PKCη is only recruited to the synapse in mature CD4+ or CD8+ (SP) thymocytes. These results are only suggestive of redundant function, but clear redundancy between PKCη and PKCθ in thymocyte development was confirmed when we phenotyped PKCη–/–θ–/– mice. Positive selection of thymocytes in these double-knockout mice was more severely impaired than either single PKC-knockout mice. However, the blockade of thymocyte development in PKCη–/–θ–/– mice was not complete, as SP cell numbers were only reduced by about 50% (Figure 4A; Fu et al., 2011). Therefore, it is possible that other PKC isoforms than PKCη and θ can still compensate for their deficiency, perhaps most likely those members within the same subfamily (e.g., PKCε).
Figure 4.
Redundancy and isoform-specific role of PKCη and PKCθ in T cell development. (A) Redundant role of PKCη and PKCθ is revealed by phenotyping T cell development in the thymus of double-knockout mice. (B) Isoform-specific role of PKCη and PKCθ is revealed by the CD4/CD8 T cell ratio in the periphery. Adapted from Fu et al. (2011).
It is natural to speculate that in a multimember protein family, there are some overlapping functions between individual members (i.e., redundancy), as well as isoform-specific functions. In our study, we found that PKCη and PKCθ had opposite effects on the CD4 to CD8 T cell ratios in the secondary lymphoid organs (Figure 4B). PKCη-deficient mice had a higher CD4/CD8 ratio than wild-type mice, whereas PKCθ-deficient mice had a lower ratio, indicating an isoform-specific role of these PKCs in balancing CD4 and CD8 T cell homeostasis. Interestingly, these effects are “neutralized” by each other in that PKCη–/–θ–/– mice exhibited normal CD4/CD8 ratios. Multiple factors can affect the CD4/CD8 T cell ratio during thymocyte development (Corbella et al., 1994; Suzuki et al., 1995;Sim et al., 1998a,b). The SP thymocytes from these knockout mice did not show altered CD4/CD8 ratios, indicating that the effects on the CD4/CD8 ratio occur post-thymically (Fu et al., 2011). Another intriguing observation is that PKCη-deficient mice have an irregular distribution of T cells between spleen and peripheral lymph nodes. The total T cell numbers are increased in the lymph nodes of PKCη-deficient mice, which mirrored the phenomenon that the lymph nodes are much larger in size in PKCη-deficient mice compared to wild-type mice. In contrast, the total T cell numbers are reduced in the spleen in PKCη-deficient mice compared to wild-type mice. Enlarged lymph nodes (i.e., lymphadenopathy) were also observed in PKCδ-deficient mice, which was mainly attributed to the increased B cell numbers (Mecklenbrauker et al., 2002). Currently, it is not clear what causes this biased T cell distribution in PKCη-deficient mice. We speculate that altered lymphocyte homing and/or homeostasis could be one of the reasons.
PKCη in Peripheral T Cell Homeostasis and Response to Antigen
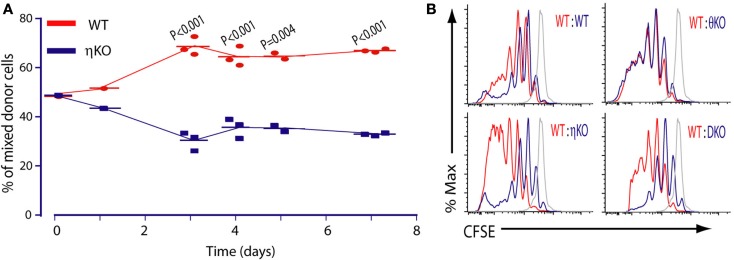
For the sake of simplicity, we focused on CD8 T cells for most functional studies on PKCη–/– mice (Fu et al., 2011). PKCη-deficient CD8 T cells showed a mild proliferation defect compared to wild-type T cells upon anti-CD3 antibody stimulation. In contrast, under the same conditions, PKCθ-deficient CD8 T cells were completely non-proliferative, as previously reported (Sun et al., 2000; Pfeifhofer et al., 2003). However, this striking difference between PKCη–/– and PKCθ–/– CD8 T cells was blurred under more physiological conditions. For example, when we used APCs pulsed with antigenic peptide to stimulate these PKC-deficient CD8 T cells, both PKCη–/– and PKCθ–/– CD8 T cells still proliferated less well than wild-type cells, but the relative difference between PKCη–/– and PKCθ–/– CD8 T cells is much more subtle than with anti-CD3 crosslinking (Fu et al., 2011). In general, we observed that antigen-specific proliferation of PKCη-deficient T cells was more severely reduced compared to wild-type cells than was anti-CD3 antibody induced proliferation. It may be that this is because the anti-CD3 stimulation does not involve the formation of the immunological synapse, whereas the synapse is important in the antigen-specific responses. The proliferation defect of PKCη–/– CD8 T cells was also confirmed in in vivo experiments, where wild-type and PKCη–/– CD8 T cells were co-transferred into recipient mice and stimulated by antigenic peptide (Figure 5A; Fu et al., 2011).
Figure 5.
Requirement of PKCη in T cell proliferation. (A) PKCη is required for efficient antigen-specific T cell proliferation in vivo. (B) PKCη but not PKCθ is required for T cell homeostatic proliferation in vivo. Adapted from Fu et al. (2011).
Therefore the proliferation defect of PKCη–/– CD8 T cells is consistent both in vitro and in vivo. However, in the case of PKCθ–/– CD8 T cells, in vivo reductions in responses were much less severe than those observed in vitro. For instance, the absence of PKCθ does not impair antigen-specific proliferation (Barouch-Bentov et al., 2005) or antiviral immune responses, in which PKCθ–/– CD8 T cells were found to proliferate normally (Berg-Brown et al., 2004; Marsland et al., 2005). The role of PKCθ in the Listeria infection model is controversial, with one group showing PKCθ is not important (Valenzuela et al., 2009) and another group claiming the opposite (Sakowicz-Burkiewicz et al., 2008). These conflicting results may be due to the different bacterial infection doses used between these two groups. One common explanation of PKCþeta’s dispensable role in these infection models is that in vivo innate signals can compensate for the absence of PKCθ (Marsland et al., 2005; Valenzuela et al., 2009), however it is also possible that PKCη functions in place of PKCθ in these cases.
In stark contrast, in an experiment to measure T cell homeostatic proliferation, we found that PKCη, but not PKCθ, is required (Figure 5B; Fu et al., 2011). In these experiments, no matter whether we used polyclonal T cells or monoclonal TCR transgenic T cells as donor cells, only PKCη–/– CD8 T cells showed impaired proliferation in lymphopenic animals, whereas PKCθ–/– CD8 T cells showed normal homeostatic proliferation. The non-essential role of PKCθ in T cell homeostatic proliferation was also independently reported by others (Valenzuela et al., 2009). This was indeed an unexpected result: one would have assumed that defective homeostatic proliferation might occur in PKCθ–/– T cells, at least as a reflection of strong deficiency in in vitro proliferation. Both TCR mediated signaling and the cytokines IL7 and IL15 are required to support normal homeostatic proliferation (Jameson, 2002; Surh and Sprent, 2005). However, we think altered responsiveness to these cytokines is unlikely to contribute to the defective homeostatic proliferation in PKCη-deficient T cells, because the amounts of IL7Rα (CD127) and IL15R (CD122) on the PKCη-deficient T cells were the same as those of wild-type T cells (Fu et al., 2011). We were also unable to find any difference in the numbers of apoptotic cells between PKCη-deficient and -sufficient mice, suggesting that the requirement for PKCη for homeostatic proliferation is not due to differential cell survival. PKCθ has been found to be a survival factor for CD8 T cells. In contrast to antigen-specific T cell proliferation, which is the clonal expansion of particular T cells recognizing their cognate antigen, homeostatic proliferation is the response of T cells to self-MHCp complexes for survival. Therefore the strength of TCR signaling is different in these two scenarios. It is possible that PKCη and PKCθ play dominant roles in homeostatic and antigen-specific proliferation respectively. PKCθ may be more important in antigen-specific activation because of its reported role in breaking the “symmetry” of the synapse (Sims et al., 2007). This is required for T cell movement, such as during scanning over the surface of an APC.
PKCη in T Cell Receptor Signaling
Compared to the very well characterized mechanisms regarding PKCθ in the molecular signaling machinery in T cells (Egawa et al., 2003; Wang et al., 2004; Roose et al., 2005; Manicassamy et al., 2006), similar studies of PKCη are at a very early stage. In our study, we showed that Ca2+ flux and NFκB nuclear translocation were impaired in PKCη–/– T cells, but that TCR-proximal signaling pathways were intact. These signaling defects are similar to those defects reported in PKCθ–/– T cells (Sun et al., 2000; Pfeifhofer et al., 2003). Thus two questions remain: First, if the signaling defects are the same in PKCη- and PKCθ-deficient T cells, why are the defects in PKCη-deficient T cells not as strong as PKCθ-deficient T cells, at least in vitro? One possibility is that more signaling pathways are interrupted by PKCθ-deficiency compared to PKCη-deficiency, in addition to NFκB (Sun et al., 2000) and NFAT (i.e., Ca2+ signaling-related) defects (Pfeifhofer et al., 2003). For example, it was recently shown that PKCθ can bind to CD28 and thus mediates a co-stimulation-driven signaling pathway from the immunological synapse (Yokosuka et al., 2008; Kong et al., 2011). More importantly, are there non-overlapping or distinct pathways between PKCη and PKCθ? The answer is likely yes. First of all, as shown in our study, PKCη and PKCθ have distinct roles in homeostatic proliferation, with η being required but θ being dispensable (Fu et al., 2011). Second, the different spatio-temporal localization of PKCη and PKCθ in the immunological synapse, with η showing an earlier and more diffuse pattern and θ showing a later and more concentrated pattern in the central region of the synapse (Singleton et al., 2009; Fu et al., 2011; Quann et al., 2011). Finally, there is a study showing PKCη and PKCθ having differential downstream functions in EL4 thymoma cells (Resnick et al., 1998). Collectively, these results strongly indicate the existence of an at least partially independent signaling pathway involving PKCη.
Future Directions
As mentioned earlier, the study of PKCη in T cell biology and the immune system in general, is far behind the state of knowledge we have on its cousin PKCθ (Fu and Gascoigne, 2012). Several recent studies have finally brought PKCη under the spotlight (Singleton et al., 2009; Suzuki et al., 2009; Fu et al., 2011; Quann et al., 2011; Sewald et al., 2011). In Table 1, we summarize the available results regarding PKCη in comparison with PKCθ. However, much more work needs to be done before we have a comprehensive understanding of the role of PKCη. First, what molecular machinery is involved in PKCη signaling? Does PKCη share the same signaling complex with PKCθ, such as the CAMA1/MALT1/Bcl10 complex? Second, what drives PKCη to the immunological synapse and what is the importance of differential localization of PKCη compared to PKCθ in the synapse? A recent study shows that the V3 domain is required for PKCθ recruitment to the immunological synapse (Kong et al., 2011). Is this also true for PKCη, considering their generally similar structures, or is the diffuse synapse-localization of PKCη due to the lack of the relevant motif in the V3 domain? Since PKCθ interacts with CD28 through a V3 motif, does PKCη also interact with CD28, or if not, is it due to the different V3 sequences? Does PKCη interact with other co-stimulatory molecules? Third, what roles does PKCη have in other T cell subsets or in other immune cells? In mice, it has been shown that PKCθ-deficiency impairs regulatory T cell development (Schmidt-Supprian et al., 2004), and in humans it has been shown that PKCθ plays a negative feedback role in regulatory T cell function, which is in contrast to its positive feedback role in naïve conventional T cells (Zanin-Zhorov et al., 2010). Thus it may be informative to check the role of PKCη in Treg cell development and function. PKCθ-deficiency has been shown to specifically impair Th2 cell responses but not Th1 responses, and thus has various effects in anti-pathogen immune responses (Berg-Brown et al., 2004;Marsland et al., 2004, 2005; Sakowicz-Burkiewicz et al., 2008). Could PKCη play an opposing role in these cases or a redundant role? What effects may PKCη have on CD4 T-helper cell subset differentiation? The role of PKCη in infection models and autoimmune diseases is another area that clearly needs attention. A simple but very informative study would be to directly compare the immune responses in η-, θ-, or ηθ-double deficient mice to the same viral and bacterial pathogens to get a full picture of the role of these two PKC isoforms in immunity. All these questions deserve more systematic studies in the future.
Table 1.
Comparison of PKCη and PKCθ in T cell biology.
| PKCθ | PKCη | |
|---|---|---|
| T cell development in KO mice | Mildly impaired1 | Normal2 |
| MATURE T CELLS IN KO MICE | ||
| CD4/CD8 ratio | Lower than WT2 | Higher than WT2 |
| Proliferation | ||
| to αCD3 in vitro | Severely impaired3,4 | Mildly impaired2 |
| to PMA/ionomycin | Normal4 or Impaired3 | Normal2 |
| to antigen in vivo | Normal5–8 or Impaired9 | Impaired2 |
| to antigen in vitro | Impaired1,3,4 | Impaired2 |
| Homeostatic proliferation | ||
| Non-tg CD8 T cells | Normal2,7 | Impaired2 |
| OT-I tg CD8 T cells | Normal2 | Impaired2 |
| SIGNALING EVENTS IN KO CELLS | ||
| Calcium flux | Impaired4 | Impaired2 |
| NFκB | Impaired3,4 | Impaired2 |
| NFAT | Normal3 or Impaired4 | Not available |
| AP-1 | Impaired3,4 | Not available |
| IMMUNOLOGICAL SYNAPSE (IS) | ||
| In effector T cells | Recruited to IS10,12,15 | Recruited to IS2,12 |
| Spatial pattern | Central region11,12 | Diffuse pattern2,12 |
| Temporal kinetic | Late, after η13 | Early, before θ13 |
| Domain(s) required | V3 domain14 | Not available |
| In regulatory T cells | Not recruited to IS15 | Not available |
1Morley et al. (2008), 2Fu et al. (2011), 3Sun et al. (2000), 4Pfeifhofer et al. (2003), 5Berg-Brown et al. (2004), 6Barouch-Bentov et al. (2005), 7Valenzuela et al. (2009), 8Marsland et al. (2005), 9Marsland et al. (2004), 10Monks et al. (1997), 11Monks et al. (1998), 12Singleton et al. (2009), 13Quann et al. (2011), 14Kong et al. (2011), 15Zanin-Zhorov et al. (2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH grants GM065230 and AI074074 to Nicholas R. J. Gascoigne. This is manuscript 21769 from The Scripps Research Institute.
References
- Baier G. (2003). The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol. Rev. 192, 64–79 10.1034/j.1600-065X.2003.00018.x [DOI] [PubMed] [Google Scholar]
- Barouch-Bentov R., Lemmens E. E., Hu J., Janssen E. M., Droin N. M., Song J., Schoenberger S. P., Altman A. (2005). Protein kinase C-θ is an early survival factor required for differentiation of effector CD8+ T cells. J. Immunol. 175, 5126–5134 [DOI] [PubMed] [Google Scholar]
- Berg-Brown N. N., Gronski M. A., Jones R. G., Elford A. R., Deenick E. K., Odermatt B., Littman D. R., Ohashi P. S. (2004). PKCθ signals activation versus tolerance in vivo. J. Exp. Med. 199, 743–752 10.1084/jem.20031022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabodi S., Calautti E., Talora C., Kuroki T., Stein P. L., Dotto G. P. (2000). A PKCη/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol. Cell 6, 1121–1129 10.1016/S1097-2765(00)00110-6 [DOI] [PubMed] [Google Scholar]
- Chida K., Hara T., Hirai T., Konishi C., Nakamura K., Nakao K., Aiba A., Katsuki M., Kuroki T. (2003). Disruption of protein kinase Cη results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res. 63, 2404–2408 [PubMed] [Google Scholar]
- Corbella P., Moskophidis D., Spanopoulou E., Mamalaki C., Tolaini M., Itano A., Lans D., Baltimore D., Robey E., Kioussis D. (1994). Functional commitment to helper T cell lineage precedes positive selection and is independent of T cell receptor MHC specificity. Immunity 1, 269–276 10.1016/1074-7613(94)90078-7 [DOI] [PubMed] [Google Scholar]
- Egawa T., Albrecht B., Favier B., Sunshine M. J., Mirchandani K., O’Brien W., Thome M., Littman D. R. (2003). Requirement for CARMA1 in antigen receptor-induced NF-κB activation and lymphocyte proliferation. Curr. Biol. 13, 1252–1258 10.1016/S0960-9822(03)00491-3 [DOI] [PubMed] [Google Scholar]
- Fu G., Gascoigne N. R. (2012). Protein kinase Cη, an emerging player in T-cell biology. Cell Cycle 11, 837–838 10.4161/cc.11.5.19677 [DOI] [PubMed] [Google Scholar]
- Fu G., Hu J., Niederberger-Magnenat N., Rybakin V., Casas J., Yachi P. P., Feldstein S., Ma B., Hoerter J. A., Ampudia J., Rigaud S., Lambolez F., Gavin A. L., Sauer K., Cheroutre H., Gascoigne N. R. J. (2011). Protein kinase Cη is required for T cell activation and homeostatic proliferation. Sci. Signal. 4, ra84. 10.1126/scisignal.2002058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Bromley S. K., Sumen C., Davis M. M., Shaw A. S., Allen P. M., Dustin M. L. (1999). The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 10.1126/science.285.5425.221 [DOI] [PubMed] [Google Scholar]
- Gruber T., Barsig J., Pfeifhofer C., Ghaffari-Tabrizi N., Tinhofer I., Leitges M., Baier G. (2005a). PKCδ is involved in signal attenuation in CD3+ T cells. Immunol. Lett. 96, 291–293 10.1016/j.imlet.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Gruber T., Thuille N., Hermann-Kleiter N., Leitges M., Baier G. (2005b). Protein kinase Cε is dispensable for TCR/CD3-signaling. Mol. Immunol. 42, 305–310 10.1016/j.molimm.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Jameson S. C. (2002). Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2, 547–556 [DOI] [PubMed] [Google Scholar]
- Kong K. F., Yokosuka T., Canonigo-Balancio A. J., Isakov N., Saito T., Altman A. (2011). A motif in the V3 domain of the kinase PKCθ determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat. Immunol. 12, 1105–1112 10.1038/ni.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M., Sanz L., Martin P., Duran A., Braun U., Garcia J. F., Camacho F., Diaz-Meco M. T., Rennert P. D., Moscat J. (2001). Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol. Cell 8, 771–780 10.1016/S1097-2765(01)00361-6 [DOI] [PubMed] [Google Scholar]
- Manicassamy S., Sadim M., Ye R. D., Sun Z. (2006). Differential roles of PKC-θ in the regulation of intracellular calcium concentration in primary T cells. J. Mol. Biol. 355, 347–359 10.1016/j.jmb.2005.10.043 [DOI] [PubMed] [Google Scholar]
- Marsland B. J., Nembrini C., Schmitz N., Abel B., Krautwald S., Bachmann M. F., Kopf M. (2005). Innate signals compensate for the absence of PKC-θ during in vivo CD8+ T cell effector and memory responses. Proc. Natl. Acad. Sci. U.S.A. 102, 14374–14379 10.1073/pnas.0506250102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland B. J., Soos T. J., Spath G., Littman D. R., Kopf M. (2004). Protein kinase Cθ is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J. Exp. Med. 200, 181–189 10.1084/jem.20032229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Villares R., Rodriguez-Mascarenhas S., Zaballos A., Leitges M., Kovac J., Sizing I., Rennert P., Marquez G., Martinez A. C., Diaz-Meco M. T., Moscat J. (2005). Control of T helper 2 cell function and allergic airway inflammation by PKCζ. Proc. Natl. Acad. Sci. U.S.A. 102, 9866–9871 10.1073/pnas.0501202102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenbrauker I., Saijo K., Zheng N. Y., Leitges M., Tarakhovsky A. (2002). Protein kinase Cδ controls self-antigen-induced B-cell tolerance. Nature 416, 860–865 10.1038/416860a [DOI] [PubMed] [Google Scholar]
- Mick V. E., Starr T. K., McCaughtry T. M., McNeil L. K., Hogquist K. A. (2004). The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J. Immunol. 173, 5434–5444 [DOI] [PubMed] [Google Scholar]
- Miyamoto A., Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., Tsukiyama T., Nagahama H., Ohno S., Hatakeyama S., Nakayama K. I. (2002). Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature 416, 865–869 10.1038/416865a [DOI] [PubMed] [Google Scholar]
- Monks C. R. F., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. (1998). Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 10.1038/25764 [DOI] [PubMed] [Google Scholar]
- Monks C. R. F., Kupfer H., Tamir I., Barlow A., Kupfer A. (1997). Selective modulation of protein kinase C-θ during T-cell activation. Nature 385, 83–86 10.1038/385083a0 [DOI] [PubMed] [Google Scholar]
- Morley S. C., Weber K. S., Kao H., Allen P. M. (2008). Protein kinase C-θ is required for efficient positive selection. J. Immunol. 181, 4696–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow T. A., Muljo S. A., Zhang J., Hardwick J. M., Schlissel M. S. (1999). Pro-B-cell-specific transcription and proapoptotic function of protein kinase Cη. Mol. Cell. Biol. 19, 5608–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger N., Buehler L. K., Ampudia J., Gascoigne N. R. J. (2005). Thymocyte stimulation by anti-TCR-β, but not by anti-TCR-α, leads to induction of developmental transcription program. J. Leukoc. Biol. 77, 830–841 10.1189/jlb.1004608 [DOI] [PubMed] [Google Scholar]
- Oda A., Ono T., Yamamoto M., Goitsuka R., Kitamura D. (2008). PKCη directs induction of IRF-4 expression and Igκ gene rearrangement in pre-BCR signaling pathway. Int. Immunol. 20, 1417–1426 10.1093/intimm/dxn101 [DOI] [PubMed] [Google Scholar]
- Ohba M., Ishino K., Kashiwagi M., Kawabe S., Chida K., Huh N. H., Kuroki T. (1998). Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the η and δ isoforms of protein kinase C. Mol. Cell. Biol. 18, 5199–5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. (1990). A phorbol ester receptor/protein kinase, nPKCη, a new member of the protein kinase C family predominantly expressed in lung and skin. J. Biol. Chem. 265, 22434–22440 [PubMed] [Google Scholar]
- Pfeifhofer C., Gruber T., Letschka T., Thuille N., Lutz-Nicoladoni C., Hermann-Kleiter N., Braun U., Leitges M., Baier G. (2006). Defective IgG2a/2b class switching in PKCα−/− mice. J. Immunol. 176, 6004–6011 [DOI] [PubMed] [Google Scholar]
- Pfeifhofer C., Kofler K., Gruber T., Tabrizi N. G., Lutz C., Maly K., Leitges M., Baier G. (2003). Protein kinase Cθ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197, 1525–1535 10.1084/jem.20020234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quann E. J., Liu X., Altan-Bonnet G., Huse M. (2011). A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat. Immunol. 12, 647–654 10.1038/ni.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. S., Kang B. S., Luu D., Wickham J. T., Sando J. J., Hahn C. S. (1998). Differential downstream functions of protein kinase Cη and -θ in EL4 mouse thymoma cells. J. Biol. Chem. 273, 27654–27661 10.1074/jbc.273.42.27654 [DOI] [PubMed] [Google Scholar]
- Roose J. P., Mollenauer M., Gupta V. A., Stone J., Weiss A. (2005). A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 25, 4426–4441 10.1128/MCB.25.11.4426-4441.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowicz-Burkiewicz M., Nishanth G., Helmuth U., Drogemuller K., Busch D. H., Utermohlen O., Naumann M., Deckert M., Schluter D. (2008). Protein kinase C-θ critically regulates the proliferation and survival of pathogen-specific T cells in murine listeriosis. J. Immunol. 180, 5601–5612 [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M., Tian J., Grant E. P., Pasparakis M., Maehr R., Ovaa H., Ploegh H. L., Coyle A. J., Rajewsky K. (2004). Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc. Natl. Acad. Sci. U.S.A. 101, 4566–4571 10.1073/pnas.0400885101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewald X., Jimenez-Soto L., Haas R. (2011). PKC-dependent endocytosis of the Helicobacter pylori vacuolating cytotoxin in primary T lymphocytes. Cell. Microbiol. 13, 482–496 10.1111/j.1462-5822.2010.01551.x [DOI] [PubMed] [Google Scholar]
- Sim B.-C., Aftahi N., Reilly C., Bogen B., Schwartz R. H., Gascoigne N. R. J., Lo D. (1998a). Thymic skewing of the CD4/CD8 ratio maps with the T-cell receptor α-chain locus. Curr. Biol. 8, 701–704 10.1016/S0960-9822(98)70276-3 [DOI] [PubMed] [Google Scholar]
- Sim B.-C., Lo D., Gascoigne N. R. J. (1998b). Preferential expression of TCR Vα regions in CD4/CD8 subsets: class discrimination or co-receptor recognition? Immunol. Today 19, 276–282 10.1016/S0167-5699(98)01257-2 [DOI] [PubMed] [Google Scholar]
- Sims T. N., Soos T. J., Xenias H. S., Dubin-Thaler B., Hofman J. M., Waite J. C., Cameron T. O., Thomas V. K., Varma R., Wiggins C. H., Sheetz M. P., Littman D. R., Dustin M. L. (2007). Opposing effects of PKCθ and WASp on symmetry breaking and relocation of the immunological synapse. Cell 129, 773–785 10.1016/j.cell.2007.03.037 [DOI] [PubMed] [Google Scholar]
- Singleton K. L., Roybal K. T., Sun Y., Fu G., Gascoigne N. R. J., van Oers N. S., Wulfing C. (2009). Spatiotemporal patterning during T cell activation is highly diverse. Sci. Signal. 2, ra15. 10.1126/scisignal.2000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B. (2004). A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U.S.A. 101, 6062–6067 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Arendt C. W., Ellmeier W., Schaeffer E. M., Sunshine M. J., Gandhi L., Annes J., Petrzilka D., Kupfer A., Schwartzberg P. L., Littman D. R. (2000). PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404, 402–407 10.1038/35006090 [DOI] [PubMed] [Google Scholar]
- Surh C. D., Sprent J. (2005). Regulation of mature T cell homeostasis. Semin. Immunol. 17, 183–191 10.1016/j.smim.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Punt J. A., Granger L. G., Singer A. (1995). Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity 2, 413–425 10.1016/1074-7613(95)90149-3 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Elias B. C., Seth A., Shen L., Turner J. R., Giorgianni F., Desiderio D., Guntaka R., Rao R. (2009). PKCη regulates occludin phosphorylation and epithelial tight junction integrity. Proc. Natl. Acad. Sci. U.S.A. 106, 61–66 10.1073/pnas.0809819106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuille N., Gruber T., Bock G., Leitges M., Baier G. (2004). Protein kinase Cβ is dispensable for TCR-signaling. Mol. Immunol. 41, 385–390 10.1016/j.molimm.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Valenzuela J. O., Iclozan C., Hossain M. S., Prlic M., Hopewell E., Bronk C. C., Wang J., Celis E., Engelman R. W., Blazar B. R., Bevan M. J., Waller E. K., Yu X. Z., Beg A. A. (2009). PKCθ is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J. Clin. Invest. 119, 3774–3786 10.1172/JCI39692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov Y., Long A., McGrath S., Ni Eidhin D., Kelleher D. (2001). Crucial importance of PKC-β(I) in LFA-1-mediated locomotion of activated T cells. Nat. Immunol. 2, 508–514 10.1038/88700 [DOI] [PubMed] [Google Scholar]
- Wang D., Matsumoto R., You Y., Che T., Lin X. Y., Gaffen S. L., Lin X. (2004). CD3/CD28 costimulation-induced NF-κB activation is mediated by recruitment of protein kinase C-θ, Bcl10, and IκB kinase β to the immunological synapse through CARMA1. Mol. Cell. Biol. 24, 164–171 10.1128/MCB.24.23.10352-10365.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Orozco C., Boyer J., Leglise M., Goodale J., Batalov S., Hodge C. L., Haase J., Janes J., Huss J. W., III, Su A. I. (2009). BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 10, R130. 10.1186/gb-2009-10-11-r130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T., Kobayashi W., Sakata-Sogawa K., Takamatsu M., Hashimoto-Tane A., Dustin M. L., Tokunaga M., Saito T. (2008). Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C θ translocation. Immunity 29, 589–601 10.1016/j.immuni.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin-Zhorov A., Ding Y., Kumari S., Attur M., Hippen K. L., Brown M., Blazar B. R., Abramson S. B., Lafaille J. J., Dustin M. L. (2010). Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328, 372–376 10.1126/science.1186068 [DOI] [PMC free article] [PubMed] [Google Scholar]