Abstract
Objective:
The objective of this study was to evaluate the safety of using tegafur–uracil (UFT) in colorectal cancer patients with partial dihydropyrimidine dehydrogenase (DPD) deficiency.
Patients and Methods:
The study included five colorectal cancer patients who presented with acute toxicity (grades 3 and 4) after being given the first cycle of chemotherapy using 5-fluorouracil. The DPD deficiency was confirmed by gene sequencing. After a full recovery from all side effects, we changed the regimen to UFT (300 mg/m2/day) associated with leucovorin (90 mg/day) for 21 days, with an empirical dose reduction of at least 10% in the first cycle.
Results:
We prospectively analysed 22 UFT cycles in 5 patients. We did not observe any episodes of grade 3 or 4 toxicity. The predominant toxicities were of grades 1 and 2 (nausea, vomiting and diarrhoea).
Conclusion:
Here, we demonstrate a complete absence of severe toxicity in all patients and cycles analysed. We believe that UFT is a safe alternative for the treatment of patients with partial DPD deficiency.
Keywords: colorectal neoplasms, dihydropyrimidine dehydrogenase deficiency, drug toxicity, fluorouracil, tegafur, uracil
Introduction
Fluoropyrimidines are still the cornerstone of chemotherapeutic regimens for the treatment of most gastrointestinal tract tumours. After these drugs were introduced in the mid-1960s, they were, for decades, the only active drug category for colorectal cancer, doubling the average survival rate of metastatic disease patients [Scheithauer et al. 1993; Simmonds, 2000]. Since their introduction, every standard chemotherapy regimen for colorectal cancer, adjuvant or first-line palliative treatment, has included a fluoropyrimidine [Venook, 2005; Schrag, 2004].
Fluoropyrimidines are usually well tolerated; most patients only experience slight myelotoxicity (in bolus endovenous injections) or light-to-moderate gastrointestinal toxicity, such as mucositis and diarrhoea as well as hand and foot syndrome, especially when using protracted techniques of administration [Meta-Analysis Group In Cancer, 1998a, 1998b; Fraile et al. 1980].
The metabolism of these drugs depends on an enzyme called dihydropyrimidine dehydrogenase (DPD). The quantitative and qualitative deficiency of this enzyme is responsible for at least 50% of severe toxicity cases (grades 3 and 4) that are attributed to fluoropyrimidines [van Kuilenburg et al. 2000].
The prevalence of partial DPD deficiency in the general population is approximately 3–5% [Etienne et al. 1994; Lu et al. 1993; van Kuilenburg et al. 2003], reaching up to 12.3% in at-risk groups, such as African-American women [Mattison et al. 2006]. There is no difference by gender in the incidence of DPD deficiency in the White population [Mattison et al. 2006]. A more severe or complete deficiency of this enzyme is less frequent, occurring in approximately 0.2% of individuals [Etienne et al. 1994; Lu et al. 1993; van Kuilenburg et al. 2003]. In these patients, these drugs may cause severe toxicity or may even be lethal (in some patients with complete DPD deficiency).
DPD deficiency may be identified through radioimmunoassay techniques that evaluate the activity of this enzyme in peripheral lymphocytes [Johnson et al. 1997], or by gene sequencing of its coding region to identify mutations and polymorphisms [Hisamuddin et al. 2007; Morel et al. 2006]. However, even individuals with normal DPD level and no detectable mutations may present with acute toxicity to fluoropyrimidines. This finding is probably due to the epigenetic mechanisms that control the expression or activity of this enzyme [Ezzeldin et al. 2005].
Another way to indirectly determine the status of DPD deficiency is to measure the concentration of uracil in serum, urine or in the exhaled air, since this nitrogenated base is metabolized by the same enzyme [Gamelin et al. 1999; Mattison et al. 2004]. However, tests to evaluate the DPD status of patients are not commercially available and, in clinical practice, these patients are only identified after the first cycle of chemotherapy with fluoropyrimidines, which is often the result of acute and ‘unexpected’ toxicity. From that moment on, many physicians stop using fluoropyrimidines, while others recommend replacing these drugs with raltitrexed, a folate-analogue antimetabolite, which inhibits thymidilate synthase and blocks purine synthesis. Importantly, few studies have been conducted to investigate this medication in this setting [Wilson et al. 2007].
For patients diagnosed with metastatic diseases, a regimen that omits fluoropyrimidines, such as the so-called IROX (irinotecan + oxaliplatin) regimen may be an option. However, this strategy represents greater toxicity for elderly patients and has been proven to be inferior to standard treatment (FOLFOX: folinic acid, fluorouracil, oxaliplatin) [Ashley et al. 2007]. Another well-tolerated and safe alternative to regimens with fluoropyrimidines is TOMOX (raltitrexed–oxaliplatin) [Cascinu et al. 2002].
Patients that are in no condition to receive polychemotherapy may be subjected to the sequential use of these drugs [Volk et al. 2001].
UFT (tegafur–uracil) could be an alternative for individuals with partial DPD deficiency. Some specialists empirically recommend its use, even though there are no prospective studies to corroborate such practices [Niederhuber et al. 2004].
The rationale for this indication is the fact that UFT is an anticancer medication that is a combination of tegafur (a prodrug of 5-fluorouracil) and uracil (a DPD inhibitor). When uracil is administered orally to allow for the intact absorption of fluoropyrimidine (tegafur), an artificial state of DPD deficiency is created. This means that both individuals with normal DPD levels as well as those with partial DPD deficiency may present in a similar situation, i.e. their DPD is partially depleted. Nevertheless, the dose of tegafur is already calculated for this situation, which means that an overdose is avoided. Certainly, this strategy is not valid for individuals with complete DPD deficiency, since even small doses of fluoropyrimidine can be very toxic in these patients. This paper presents the results of a pilot study that evaluated the safety of using UFT in colorectal cancer patients with partial DPD deficiency.
Patients and methods
This study included colorectal cancer patients with a good performance index (Karnofsky performance status 90% or 100%) who presented with acute (grades 3 and 4) and unexpected toxicity after being given the first cycle of chemotherapy using 5-fluorouracil. After a full recovery from all side effects, we changed the regimen to UFT (300 mg/m2/day of tegafur and uracil, divided in three doses) associated with leucovorin (30 mg by oral route, every 8 hours) for 21 days, followed by a 7-day resting period.
All patients completed the first UFT cycle with an empirical dose reduction of at least 10%, which was adjusted after each cycle, according to their tolerance. Since tablets contain 100 mg of tegafur, UFT doses were rounded down to the nearest total dose. The first evaluation was scheduled for 1 week after the beginning of the new therapy. At that time, we evaluated patients clinically and looked at their blood counts for early signs of severe toxicity.
DPD deficiency was evaluated by gene sequencing of peripheral blood leukocytes. DNA extraction from peripheral blood leukocytes was performed following the GFX− Kit protocol (Amersham-Pharmacia). Identification of DPD-gene polymorphisms was performed as recommended by Seo and colleagues [Seo et al. 2009].
This study was conducted in accordance with the Declaration of Helsinki 1975, revised Hong Kong 1989.
Results
We included the first five consecutive patients undergoing colorectal neoplasia treatment that presented with acute and unexpected severe toxicity (grades 3 and 4) after the first cycle of chemotherapy with 5-fluorouracil. Table 1 lists the main characteristics of each patient. Four of the five patients had a DPD-gene mutation on chromosome 1p22 (IVS14+1G>A).
Table 1.
Included patients’ main characteristics.
| Patient number | Age (years) | Gender | Race | KPS | Diagnosis | Comorbidities | DPD gene |
|---|---|---|---|---|---|---|---|
| 1 | 67 | ♀ | White | 90% | Colon adenocarcinoma pT3N2M1 | None | Heterozygote |
| 2 | 70 | ♀ | White | 100% | Adenocarcinoma of the rectum pT3N1M1 | None | Wild type |
| 3 | 58 | ♀ | White | 100% | Colon adenocarcinoma pT3N1M0 | Systemic hypertension | Heterozygote |
| 4 | 51 | ♀ | White | 100% | Sigmoid adenocarcinoma pT3N1M0 | None | Heterozygote |
| 5 | 73 | ♀ | White | 100% | Adenocarcinoma of the rectum pT3N1M1 | None | Heterozygote |
DPD, dihydropyrimidine dehydrogenase; KPS, Karnofsky performance status.
Most of the patients had received the first cycle of chemotherapy according to the Mayo Clinic regimen, and their treatment had been changed to UFT and leucovorin after a full recovery from side effects. One of the patients received two cycles of raltitrexed after two initial cycles of UFT. This change was a consequence of a temporary lack of UFT in the Brazilian market (Table 2).
Table 2.
Chemotherapy regimen applied during each cycle.
| Patient number | Initial chemotherapy | Cycle 1 (Dose) | Cycle 2 (Dose) | Cycle 3 (Dose) | Cycle 4 (Dose) | Cycle 5 (Dose) |
|---|---|---|---|---|---|---|
| 1 | Mayo Clinic | UFT (60%) | UFT (80%) | UFT (100%) | UFT (90%) | UFT (90%) |
| 2 | FOLFOX6 | UFT (75%) | UFT (75%) | Raltitrexed (85%) | Raltitrexed (85%) | |
| 3 | Mayo Clinic | UFT (90%) | UFT ( 90%) | UFT (90%) | UFT (90%) | UFT (90%) |
| 4 | Mayo Clinic | UFT (90%) | UFT (90%) | UFT (90%) | UFT (90%) | UFT (90%) |
| 5 | Mayo Clinic | UFT (90%) | UFT (90%) | UFT (90%) | UFT (90%) | UFT (90%) |
Mayo Clinic, 5-fluorouracil [5-FU] (425 mg/m2) + leucovorin (20 mg/m2) IV bolus day1 and 5; FOLFOX6, oxaliplatin (85 mg/m2) + leucovorin (200 mg/m2) + 5-FU bolus (400 mg/m2) + 5-FU infusion for 46 hours (2400 mg/m2); UFT, tegafur (300 mg/m2/day) in three doses + leucovorin 30 mg VO every 8 hours for 21 days every 28 days; raltitrexed, 3 mg/m2.
After the first cycle of chemotherapy with 5-fluorouracil, the main toxicities were myelotoxicity and gastrointestinal toxicity, especially mucositis and diarrhoea (Figure 1A).
Figure 1.
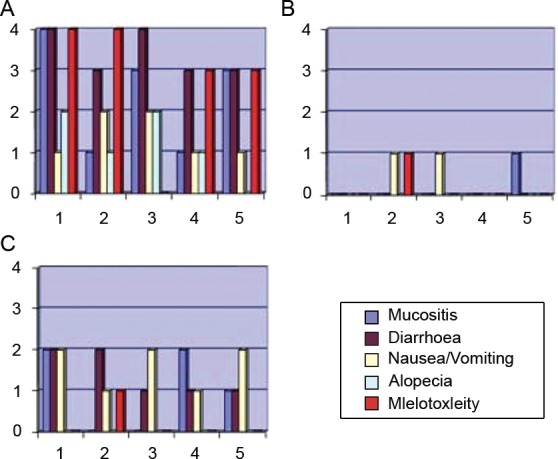
Grade of toxicity after the first cycle of 5-fluorouracil (A), after the first UFT cycle (B) and the worst toxicity presented in all cycles of UFT (C).
The first patient to be included in the protocol received the first cycle of UFT with a 40% dose reduction, the second with a 25% reduction, and the others with a 10% reduction. Given the good tolerance observed in all cycles for the first patient, we opted to quickly escalate the doses for the others. The empirical dose reduction in the first cycle of UFT proved efficient in preventing unexpected toxicities (Figure 1B).
When we prospectively analysed 22 UFT cycles, we did not observe any occurrence of grade 3 or 4 toxicity. The predominant toxicity was of a gastrointestinal nature, including grade 1 and 2 nausea, vomiting and diarrhoea, which were controlled by conventional clinical measures (Figure 1C). Low-intensity (grade 1) myelotoxicity occurred in only one patient, the patient that did not present with any mutations in the DPD gene (wild type).
Three patients received chemotherapy for metastatic disease (patients 1, 2 and 5) and their best response was stable disease. Two patients received adjuvant chemotherapy (patients 3 and 4) and they were disease free 2 years after the end of therapy.
Discussion
The incidence of colorectal cancer in the United States and Europe is 146,970 [Jemal et al. 2009] and 412,900 [Ferlay et al. 2007] new cases per year, respectively. In people with colorectal cancer, the prevalence of partial DPD deficiency is approximately 5%. Considering these facts, we realized that this situation is far from being rare or uncommon. In contrast, DPD deficiency is a problem faced in clinical practice, for which the international literature mentions only few alternatives.
Since tegafur, a component of UFT, is a prodrug of 5-fluorouracil, the use of this medication in patients with partial DPD deficiency should be carefully analysed. In fact, the use of UFT in patients with DPD deficiency has not been studied in depth. In the literature, we find some evidence for its potential application in patients with a partial deficiency of this enzyme [Niederhuber et al. 2004].
Several phase II and III studies comparing UFT with 5-fluorouracil have shown a favourable toxicity profile and a more comfortable administration route, making this drug a valid alternative [Lembersky et al. 2006; Douillard et al. 2002; Carmichael et al. 2002; Borner et al. 2002; Lima and del Giglio, 2005].
In this pilot study, we evaluated the use of chemotherapy with UFT in a restricted but consecutive group of five patients with known toxicity after being exposed to 5-fluorouracil. Four out of five of these patients demonstrated partial DPD deficiency, which was confirmed through gene sequencing.
The150 kb DPYD gene is located on chromosome 1p22 and comprises 23 exons that range in size from 69 to 1404 bps [Johnson et al. 1997]. The DPYD gene is highly polymorphic, and several mutations resulting in a protein with impaired activity have been described [van Kuilenburg et al. 2000]. In Western populations, the most frequent of these mutations is IVS14+1G>A [Paré et al. 2010], as we found at our series. The IVS 14+1G>A is a guanine-to-adenosine point mutation that affects the splice recognition sequence of intron 14 and results in a deletion of 55 amino acids in the native protein [Raida et al. 2001].
Interestingly, all patients experienced good tolerance to the regimen of UFT chemotherapy (Figure 1C). Even the patient that did not present with a mutation in the DPD-coding gene (patient 2), but experienced significant toxicity after the first cycle of 5-fluorouracil, demonstrated a good tolerance to UFT. As patient 2 was the only one who received FOLFOX, some of her toxicities might have been related to oxaliplatin as well.
Severe toxicity is expected in DPD-deficient patients that are treated with any fluoropyrimidines. Here, we demonstrate a complete absence of severe toxicity in all patients and cycles analysed. Despite the small number of patients involved in this pilot study, the data herein represent a proof of principle of the safety of UFT in patients with partial DPD deficiency. Given the importance of fluoropyrimidines in the treatment of colorectal neoplasia, this finding has a large practical application and proposes a new treatment alternative for this group of patients.
However, our study does have limitations. Owing to the restricted number of patients involved in this study and because all patients were females, we exert caution when generalizing these data to other patients. Another point is that we have not performed the pharmacodynamic analyses of UFT at our series, and this issue should be studied in future works. Since the use of fluoropyrimidines, even at low doses, can be very toxic in patients with severe DPD deficiency, we want to emphasize that this strategy should only be considered for patients with partial DPD deficiency.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare there are no financial or personal relationships with other people or organizations that could inappropriately influence (bias) the results of this article.
Contributor Information
Daniel I.G. Cubero, Department of Oncology and Haematology, ABC Foundation School of Medicine, Av. Príncipe de Gales, n. 821, anexo 3, Santo André/SP, 09060-650, Brazil
Felipe Melo Cruz, Department of Oncology and Haematology, ABC Foundation School of Medicine in Santo André/SP, Brazil.
Patrícia Santi, Department of Oncology and Haematology, ABC Foundation School of Medicine in Santo André/SP, Brazil.
Ismael Dale C.G. Silva, Department of Gynaecology, São Paulo School of Medicine [Escola Paulista de Medicina] – Federal University of São Paulo [UNIFESP] in São Paulo/SP, Brazil
Auro del Giglio, Department of Oncology and Haematology, ABC Foundation School of Medicine in Santo André/SP, Brazil and Medical Coordinator Oncology Section at Albert Einstein Jewish Hospital in São Paulo/SP, Brazil.
References
- Ashley A.C., Sargent D.J., Alberts S.R., Grothey A., Campbell M.E., Morton R.F., et al. (2007) Updated efficacy and toxicity analysis of irinotecan and oxaliplatin (IROX) : intergroup trial N9741 in first-line treatment of metastatic colorectal cancer. Cancer 110: 670–677 [DOI] [PubMed] [Google Scholar]
- Borner M.M., Schoffski P., de Wit R., Caponigro F., Comella G., Sulkes A., et al. (2002) Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 38: 349–358 [DOI] [PubMed] [Google Scholar]
- Carmichael J., Popiela T., Radstone D., Falk S., Borner M., Oza A., et al. (2002) Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20: 3617–3627 [DOI] [PubMed] [Google Scholar]
- Cascinu S., Graziano F., Ferraù F., Catalano V., Massacesi C., Santini D., et al. (2002) Raltitrexed plus oxaliplatin (TOMOX) as first-line chemotherapy for metastatic colorectal cancer. A phase II study of the Italian Group for the Study of Gastrointestinal Tract Carcinomas (GISCAD). Ann Oncol 13: 716–720 [DOI] [PubMed] [Google Scholar]
- Douillard J.Y., Hoff P.M., Skillings J.R., Eisenberg P., Davidson N., Harper P., et al. (2002) Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 17: 3605–3616 [DOI] [PubMed] [Google Scholar]
- Etienne M.C., Lagrange J.L., Dassonville O., Fleming R., Thyss A., Renée N., et al. (1994) Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 11: 2248–2253 [DOI] [PubMed] [Google Scholar]
- Ezzeldin H.H., Lee A.M., Mattison L.K., Diasio R.B. (2005) Methylation of the DPYD promoter: an alternative mechanism for dihydropyrimidine dehydrogenase deficiency in cancer patients. Clin Cancer Res 11: 8699–8705 [DOI] [PubMed] [Google Scholar]
- Ferlay J., Autier P., Boniol M., Heanue M., Colombet M., Boyle P., et al. (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18: 581–592 [DOI] [PubMed] [Google Scholar]
- Fraile R.J., Baker L.H., Buroker T.R., Horwitz J., Vaitkevicius V.K. (1980) Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res 40: 2223–2228 [PubMed] [Google Scholar]
- Gamelin E., Boisdron-Celle M., Guérin-Meyer V., Delva R., Lortholary A., Genevieve F., et al. (1999) Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: A potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage; J Clin Oncol 4: 1105–1110 [DOI] [PubMed] [Google Scholar]
- Hisamuddin I.M., Wehbi M.A., Yang V.W. (2007) Pharmacogenetics and diseases of the colon. Curr Opin Gastroenterol 23: 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249 [DOI] [PubMed] [Google Scholar]
- Johnson M.R., Yan J., Shao L., Albin N., Diasio R.B. (1997) Semi-automated radioassay for determination of dihydropyrimidine dehydrogenase (DPD) activity. Screening cancer patients for DPD deficiency, a condition associated with 5-fluorouracil toxicity. J Chromatogr B Biomed Sci Appl 696: 183–191 [DOI] [PubMed] [Google Scholar]
- Lembersky B.C., Wieand H.S., Petrelli N.J., O’Connell M.J., Colangelo L.H., Smith R.E., et al. (2006) Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol 24: 2059–2064 [DOI] [PubMed] [Google Scholar]
- Lima A.P., del Giglio A. (2005) Randomized crossover trial of intravenous 5-FU versus oral UFT both modulated by leucovorin: a one-centre experience. Eur J Cancer Care 14: 151–154 [DOI] [PubMed] [Google Scholar]
- Lu Z., Zhang R., Diasio R.B. (1993) Dihydropyrimidine dehydrogenase activity in human peripheral blood mononuclear cells and liver: population characteristics, newly identified deficient patients, and clinical implication in 5-fluorouracil chemotherapy. Cancer Res 53: 5433–5438 [PubMed] [Google Scholar]
- Mattison L.K., Ezzeldin H., Carpenter M., Modak A., Johnson M.R., Diasio R.B. (2004) Rapid identification of dihydropyrimidine dehydrogenase deficiency by using a novel 2-13C-uracil breath test. Clin Cancer Res 10: 2652–2658 [DOI] [PubMed] [Google Scholar]
- Mattison L.K., Ezzeldin H., Carpenter M., Modak A., Johnson M.R., Diasio R.B. (2006) Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with Caucasians. Clin Cancer Res 12: 5491–5495 [DOI] [PubMed] [Google Scholar]
- Meta-Analysis Group In Cancer (1998a) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16: 301–308 [DOI] [PubMed] [Google Scholar]
- Meta-Analysis Group In Cancer (1998b) Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. J Clin Oncol 16: 3537–3541 [DOI] [PubMed] [Google Scholar]
- Morel A., Boisdron-Celle M., Fey L., Soulie P., Craipeau M.C., Traore S.L., et al. (2006) Clinical relevance of different dihydropyrimidine dehydrogenase gene single nucleotide polymorphisms on 5-fluorouracil tolerance. Mol Cancer Ther 5: 2895–2904 [DOI] [PubMed] [Google Scholar]
- Niederhuber J.E., Cole C.E., Grochow L. (2004) Colon cancer. In Abeloff M.D., Armitage J.O., Niederhuber J.E., Kastan M.B., McKenna W.G. (eds), Clinical Oncology, 3rd edition Philadelphia, PA: Elsevier Churchill Livingstone, 2004; pp. 1928–1929 [Google Scholar]
- Paré L., Paez D., Salazar J., Del Rio E., Tizzano E., Marcuello E., et al. (2010) Absence of large intragenic rearrangements in the DPYD gene in a large cohort of colorectal cancer patients treated with 5-FU-based chemotherapy. Br J Clin Pharmacol 70: 268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raida M., Schwabe W., Häusler P., Van Kuilenburg A.B., Van Gennip A.H., Behnke D., et al. (2001) Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase (DPD) gene within the 5′-splice donor site of intron 14 in patients with severe 5-fluorouracil (5-FU)-related toxicity compared with controls. Clin Cancer Res 7: 2832–2839 [PubMed] [Google Scholar]
- Scheithauer W., Rosen H., Kornek G.V., Sebesta C., Depisch D. (1993) Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306: 752–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag D. (2004) The price tag on progress - chemotherapy for colorectal cancer. N Eng J Med 351: 317–319 [DOI] [PubMed] [Google Scholar]
- Seo B.G., Kwon H.C., Oh S.Y., Lee S., Kim S.G., Kim S.H., et al. (2009) Comprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI. Oncol Rep 22: 127–136 [PubMed] [Google Scholar]
- Simmonds P.C. (2000) Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000; 321: 531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuilenburg A.B., Abreu R., Van Gennip A. (2003) Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem 40: 41–45 [DOI] [PubMed] [Google Scholar]
- van Kuilenburg A.B., Haasjes J., Richel D.J., Zoetekouw L., Van Lenthe H., De Abreu R.A., et al. (2000) Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res 6: 4705–4712 [PubMed] [Google Scholar]
- Venook A. (2005) Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist 10: 250–261 [DOI] [PubMed] [Google Scholar]
- Volk J., Reinke F., van Kuilenburg A.B., van Gennip A.H., Schlichting C., Ganser A., et al. (2001) Safe administration of irinotecan, oxaliplatin and raltitrexed in a DPD-deficient patient with metastatic colon cancer. Ann Oncol 12: 569–571 [DOI] [PubMed] [Google Scholar]
- Wilson K.S., Fitzgerald C.A., Barnett J.B., Gill S., Khoo K.E. (2007) Adjuvant therapy with raltitrexed in colorectal cancer patients intolerant of 5-fluorouracil: British Columbia Cancer Agency experience. Cancer Invest 25: 711–714 [DOI] [PubMed] [Google Scholar]


