Abstract
Background
We sought to measure population-level adherence to antihyperlipidemics, antihypertensives, and oral hypoglycemics, and to develop a model for early identification of subjects at high risk of long-term poor adherence.
Methods
Prescription-filling data for 2 million subjects derived from a payor's insurance claims were used to evaluate adherence to three chronic drugs over 1 year. We relied on patterns of prescription fills, including the length of gaps in medication possession, to measure adherence among subjects and to build models for predicting poor long-term adherence.
Results
All prescription fills for a specific drug were sequenced chronologically into drug eras. 61.3% to 66.5% of the prescription patterns contained medication gaps >30 days during the first year of drug use. These interrupted drug eras include long-term discontinuations, where the subject never again filled a prescription for any drug in that category in the dataset, which represent 23.7% to 29.1% of all drug eras. Among the prescription-filling patterns without large medication gaps, 0.8% to 1.3% exhibited long-term poor adherence. Our models identified these subjects as early as 60 days after the first prescription fill, with an area under the curve (AUC) of 0.81. Model performance improved as the predictions were made at later time-points, with AUC values increasing to 0.93 at the 120-day time-point.
Conclusions
Dispensed medication histories (widely available in real time) are useful for alerting providers about poorly adherent patients and those who will be non-adherent several months later. Efforts to use these data in point of care and decision support facilitating patient are warranted.
Keywords: Adherence, pharmacoepidemiology, pharmacovigilance, monitoring the health of populations, personal health records and self-care systems
Introduction
Although medications may be the single most important healthcare intervention for chronic disease in the developed world,1 2 their impact is modulated by patient adherence. Even for conditions where using a single medication just once a day is known to improve outcomes, only about 50% of patients adhere to the correct regimen.3–5 Studies of patients taking antihypertensives, antidepressives, hypoglycemics, antipsychotics, and antihyperlipidemics show that poor adherence is predictive of poor outcomes and high costs, including future hospitalizations and treatment discontinuation.6–18 Conversely, consistent adherence to statins, antihypertensives, antidiabetic agents, and antiviral therapies is associated with positive health outcomes.3 16 17 19
Causes of non-adherence are multifactorial, including tolerability, side effects, complexity of prescribing and filling procedures, level of understanding of the importance of taking the medication, cultural norms, out of pocket cost of the medication, and the possible lack of discernable effects of the medication.1 20–24 Healthcare providers tend to overestimate medication adherence.25–30
Surveillance of adherence can be achieved by monitoring the records of dispensed prescription medications.31 32 Prescription-filling data provide an indirect measure of a patient's adherence to prescription medication. Although filled prescription medications are not always taken, it is generally true that patients who do not fill a prescription are non-adherent. Early identification of patients at high risk of poor adherence would enable early interventions by clinicians that could improve health outcomes.30 33–35 Attempts to predict poor adherence using only patient characteristics (such as socio-economic status and comorbidities) have found those variables to be weak predictors.24 36 However, these studies did not consider the predictive potential of early prescription-filling patterns. We sought to measure the adherence of patients filling prescriptions for three classes of chronic drugs: antihyperlipidemics, antihypertensives, and oral hypoglycemics. Furthermore, we sought to develop models for early identification of patients who will likely be long-term poor adherers to these classes of drugs.
Methods
This study is a retrospective analysis of temporally sequenced dispensed medication data. We use a two-phase prediction model for early identification of patients at risk of poor adherence to chronic medications. The first phase is a straightforward approach to identifying prolonged gaps in prescription-filling patterns. The second phase uses a regression model to isolate more complex and subtle patterns predictive of future poor adherence.
Data
The subjects for this study were identified from more than 8.5 million beneficiaries of a large private insurance plan covered during the period of January 1999 to December 2006, who were taking at least one of three classes of drugs: antihyperlipidemics, antihypertensives, and oral hypoglycemics. The three drug classes were selected because for these chronic medications, improved health outcomes require consistent adherence over time. The individual drugs included for each category are given in online supplementary appendix table A1. The prescription-filling data included the date, National Drug Code (NDC), quantity dispensed (in days), and information about whether the prescription was filled through a mail order or retail mechanism in addition to other administrative information. The age and gender of the subjects were also available, as well as information about hospitalizations and dates of membership of the insurance plan.
For each subject, all instances of prescription fills for a specific drug were sequenced chronologically into drug eras.37 Each drug era represented the prescription-filling history of a particular subject for a specific drug during the time period included in the dataset.
Study period and cohort construction
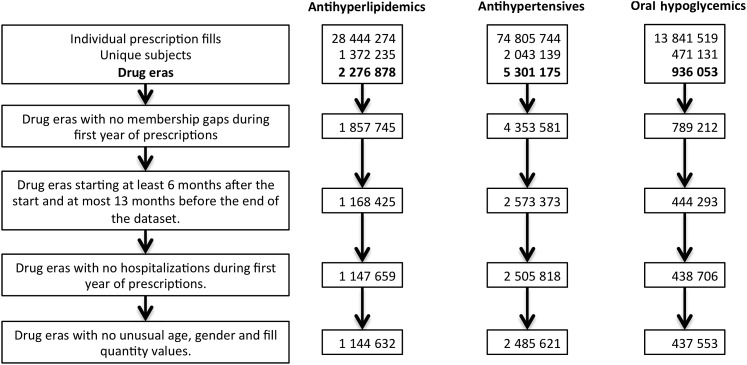
Drug eras coinciding with a gap in membership of the insurance program during the first year of prescriptions were excluded (figure 1). Left and right censoring was performed by excluding the drug eras beginning within the first 6 months of the study period, as well as those beginning in the last 13 months of the study period. Drug eras coinciding with hospitalizations during the first year of prescriptions were excluded. A final exclusion was performed to remove drug eras with unrealistic or unusual data including ages greater than 200 years, quantities greater than 200 supply days, and unlisted genders.
Figure 1.
Cohort construction. Analysis of prescription fills for three chronic drug categories: antihyperlipidemics, antihypertensives, and oral hypoglycemic. For each subject, prescription fills for the same drug were sequenced temporally into drug eras. Drug eras were excluded from analysis if there were: (1) gaps in membership in the insurance plan during the first year of prescriptions; (2) drug eras that began before a 6-month wash-out period, or in the last 13 months of the dataset (left and right censoring); (3) hospitalizations during the first year of prescriptions; and (4) unusual age, gender, and fill quantities likely due to human errors during data entry.
Adherence analysis
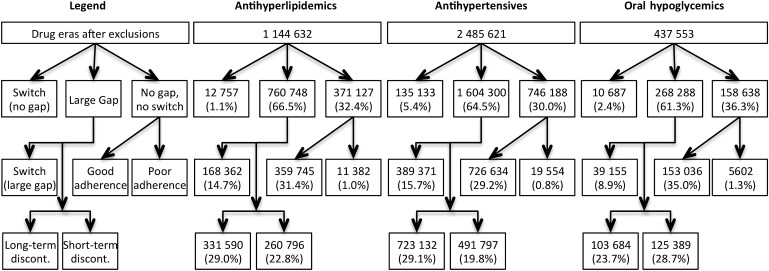
The drugs eras were first evaluated based on medication gaps during the first year of prescription fills (figure 2). Those containing at least one medication gap greater than 30 days were flagged as poor adherers. This did not include drug eras where the subject switched to another drug in the same category within 30 days of running out of their last prescription. A switch to a new drug must have been initiated no sooner than 90 days before the last prescription fill.
Figure 2.
Classification of drug eras by adherence during the first year of prescription fills. The drug eras were initially separated into three categories: (1) subject switched to another drug in the same category; (2) flagged by the large-gap detector because they accumulated a medication gap greater than 30 days; and (3) neither switch to another drug nor accumulation of a large medication gap. The drug eras that accumulated a large medication gap were further categorized into those with a switch to another drug in the same category within 90 days after the flagging date, long-term discontinuers who never filled a prescription for that same drug again in the dataset, and short-term discontinuers who did fill a prescription for the same drug sometime after the flagging date. The drug eras that exhibited consistent prescription filling for the entire year without any large gaps (‘No gap, no switch’) were further categorized into those that had a medication possession ratio greater than 0.80 (‘Good adherence’) and those who fell below the threshold (‘Poor adherence’).
The drug eras that were flagged by the large-gap detector were further subdivided into three categories: (1) those that switched to another drug in the same category within 90 days of the flagging date; (2) long-term discontinuers who never filled another prescription for that particular drug in this dataset; and (3) short-term discontinuers who filled at least one more prescription for the same drug after the flagging date.
Drug eras that were neither flagged by the large-gap detector nor switched to another drug in the same category (‘No gap, no switch’ category in figure 2) exhibited consistent filling of prescriptions for at least 1 year. For this group of drug eras, we developed models to identify those exhibiting poor adherence by consistently filling prescriptions late.
Model development and evaluation
The outcome variable was adherence as measured by the medication possession ratio (MPR), a standard measure of possession of filled prescription medication over time.13 The study period considered was the first year of the drug era, measured from the first observed prescription fill. An MPR of 0.80 is the accepted standard indicator of good adherence.11 The goal of our modeling was to identify the subjects who did not meet this criterion of good adherence at the 1-year time point (‘Poor adherence’ category in figure 2). Only drug eras that did not contain medication gaps greater than 30 days during the first year were considered (‘No gap, no switch’ category in figure 2).
The drug eras were used to build and test a logistic regression model for predicting the outcome variable using two independent measures: age and the latest calculated MPR value. Gender and drug names were also initially considered as variables but were found not to significantly contribute to the models, and were excluded. Three models were developed to make predictions at three time points early in the drug era (days 60, 90, and 120). Each of the models was trained with one third of the drug eras to identify those that would not meet the criterion for good adherence 1 year after the first filled prescription. To demonstrate the value of the fill data patterns, comparison models for each drug category were also developed for performance comparison using only the gender and age of the subject. The models were tested on the remaining two thirds of the dataset and evaluated using the area under the receiver operator characteristic curve (AUC). Additional metrics for performance included the specificity, accuracy, positive predictive value, and negative predictive value, with the sensitivity of the models set to 90.0%.
Results
Over 2 million subjects were identified (table 1). After the exclusion criteria were applied, the antihypertensives class had the most subjects (1 294 521) followed by antihyperlipidemics (790 883) and oral hypoglycemics (278 029). The antihypertensives class contained more than four times as many drugs (74) as the two other classes (17 and 16 for antihyperlipidemics and oral hypoglycemic, respectively) (online supplementary appendix table A1). In all three classes, between 50% and 60% of subjects filled prescriptions for more than one drug in the same class, resulting in 1.4 to 1.9 times as many drug eras as unique subjects in each class. The final cohort had slightly more females than males in all three classes, and the average age on the date of the first prescription was between 62 and 67 years (table 1).
Table 1.
Cohort characteristics on date of first prescription fill
| Antihyperlipidemics | Antihypertensives | Oral Hypoglycemics | |
| Number of unique subjects | 790 883 | 1 294 521 | 278 029 |
| Percent female | 50.2% | 53.5% | 51.1% |
| Age (in years) at first prescription fill (mean±SD) | 62.5±12.9 | 66.3±15.2 | 62.8±13.8 |
| Number of drug eras per subject (mean±SD) | 1.4±0.8 | 1.9±1.3 | 1.6±0.8 |
A two-phase adherence monitor was applied. First, a large-gap detector, identifying periods of more than 30 days between expected fills, was applied. For antihypertensives, antihyperlipidemics, and oral hypoglycemics, respectively, 66.5%, 64.5%, and 61.3% of drug eras contained medication gaps greater than 30 days. These include long-term discontinuations, where the subject never again filled a prescription for that drug in the dataset, which represent 29.0%, 29.1%, and 23.7% of all the drug eras and 59.5%, 68.6%, and 51.8% of the drug eras containing large medication gaps. Among the long-term discontinuations, 50.4%, 50.3%, and 36.6% had only one prescription fill. On average, long-term discontinuation drug eras had 1.7, 1.6, and 2.2 prescription fills, and 71.3, 59.6, and 59.5 dispensed supply days. The drug eras defined as short-term discontinuations—containing large gaps followed by prescription fills (short-term discontinuations)—had average gap lengths of 105.6, 120.0, and 107.6 days for antihyperlipidemics, antihypertensives, and oral hypoglycemic, respectively.
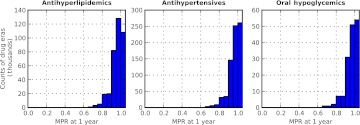
After applying the large-gap detector, we applied an algorithm to detect non-adherence defined as an MPR of less than 0.80 at 1 year. The MPR, a standard measure of adherence, is defined as the days supply of medication divided by the days between refills. Among drug eras not containing large gaps between fills (‘No gap, no switch’ category in figure 2), those which had MPRs less than 0.80 at the 1-year outcome date (‘Poor adherence’ category in figure 2) met our definition of long-term poor adherence. For antihyperlipidemics, antihypertensives, and oral hypoglycemic, respectively, these drug eras represented 1.0%, 0.8%, and 1.3% of all the drug eras, and 3.1%, 2.6%, and 3.5% of the drug eras in the ‘No gap, no switch’ category. The distributions of the 1-year MPR values for the drug eras in this group are similar for the three drug categories (figure 3). These drug eras were used to build logistic regression models for predicting long-term poor adherence. Models were built for making predictions at three early time-points in the drug era: 60, 90, and 120 days after the first prescription fill.
Figure 3.
Distribution of medication possession ratios (MPRs) measured 1 year after the first prescription fill. Data include drug eras remaining after the removal of those containing medication gaps greater than 30 days during that time period and those that switched to another drug in the same category (‘No gap, no switch’ category in figure 2).
The logistic regression models are in the form:
where x1 is the latest available MPR value and x2 is the age of the subject at the beginning of the drug era. The intercepts (β0) and coefficients (β1 and β2) for the nine models are given in table 2.
Table 2.
Coefficients for the logistical regression models where the risk of poor adherence is calculated using the latest available MPR value and the age of the subject at the beginning of the drug era
| Day 60 model | Day 90 model | Day 120 model | |
| Antihyperlipidemics | |||
| β0 (intercept) | 7.195 | 11.791 | 16.462 |
| β1 (MPR) | −8.850 | −14.481 | −20.350 |
| β2 (age) | −0.039 | −0.034 | −0.031 |
| Antihypertensives | |||
| β0 (intercept) | 5.911 | 10.430 | 14.397 |
| β1 (MPR) | −8.140 | −13.420 | −18.260 |
| β2 (age) | −0.031 | −0.029 | −0.027 |
| Oral hypoglycemics | |||
| β0 (intercept) | 6.406 | 10.759 | 14.524 |
| β1 (MPR) | −8.436 | −13.595 | −18.260 |
| β2 (age) | −0.031 | −0.029 | −0.026 |
MPR, medication possession ratio.
The performance of the prediction models developed for each drug category and each time point to detect drug eras with poor adherence is shown in figure 4. The models performed similarly for the three drug classes, and performance improved across the three time points of prediction when starting at a later point. For antihyperlipidemics, antihypertensives, and oral hypoglycemics, respectively, for predictions made at 120 days after the first fill, compared with 60 days, the areas under the curve (AUC) improved from 0.83, 0.81, and 0.83 to 0.93, 0.92, and 0.93 (figure 4).
Figure 4.
Model performance for prediction of drug eras with medication possession ratios of less than 0.80 (poor adherence) at 1 year from the vantage point of 60, 90, and 120 days after beginning the first fill. The data represent drug eras remaining after the large medication gap filter was applied (‘No gap, no switch’ category). The areas under the receiver-operator characteristic curve (AUC) show the improving performance of the models with increasing number of days since the first prescription fill.
With sensitivity held constant at 90%, table 3 shows the performance statistics for each model, which like the AUC, improved when making predictions at later time points. Specificity, accuracy, positive predictive value, and negative predictive value all increased from below 50% to above 80% between days 60 and 120. The negative predictive value remained constant at 99% for all three drug categories and the three time points.
Table 3.
Performance statistics (with sensitivity set to 90%) of models for detecting drug eras at high risk of future poor adherence among the drug eras remaining after the large medication gap filter was applied
| Day 60 model | Day 90 model | Day 120 model | |
| Antihyperlipidemics | |||
| Sensitivity | 89.9% | 90.0% | 90.0% |
| Specificity | 44.8% | 75.8% | 82.0% |
| Accuracy | 46.2% | 76.2% | 82.2% |
| Positive predictive value | 44.8% | 75.8% | 82.0% |
| Negative predictive value | 99.3% | 99.6% | 99.6% |
| Antihypertensives | |||
| Sensitivity | 90.0% | 90.0% | 90.0% |
| Specificity | 36.9% | 68.0% | 80.7% |
| Accuracy | 38.3% | 68.6% | 80.9% |
| Positive predictive value | 36.9% | 68.0% | 80.7% |
| Negative predictive value | 99.3% | 99.6% | 99.7% |
| Oral hypoglycemics | |||
| Sensitivity | 90.0% | 90.0% | 90.0% |
| Specificity | 46.8% | 72.6% | 81.4% |
| Accuracy | 48.3% | 73.2% | 81.7% |
| Positive predictive value | 46.8% | 72.6% | 81.4% |
| Negative predictive value | 99.2% | 99.5% | 99.5% |
All metrics improved across the three prediction dates.
Discussion
A high rate of non-adherence is evident in claims data. Similar data are available in real time from pharmacy benefit managers (PBMs) and pharmacies, suggesting a ready approach to real time adherence surveillance. Beyond simply detecting overt non-adherence, we have also shown that poor adherence is predictable very early based on patterns of medication fills.
Among the patients who continuously fill their prescriptions over the course of at least 1 year, the small percentage of long-term poor adherers can be identified as early as 60 days after the first prescription fill. Poor adherers in this class are harder to catch than those with significant medication gaps, and identifying them early in the treatment could greatly reduce the risks associated with long-term poor adherence.
Our analysis and modeling were conducted on claims data that included both mail order and retail mechanisms for acquiring medications. The main difference between the two mechanisms is that mail order prescriptions were generally for 90-day supplies, while retail prescriptions were for fewer supply days, most commonly 30 days. The mechanism for mail order still required that the beneficiary actively request each new medication course. We expect that in contrast, mail order mechanisms requiring minimal or no action on the part of the subject would make prescription-filling data less predictive of future adherence to chronic medications, potentially disguising non-adherence.
The MPR metric used in this model, although widely accepted, is still only an indirect metric of adherence to taking medication, as patients may fill the prescription but not take the medication. It is certainly reasonable to assume that in most cases, if the subject did not fill the prescriptions, then the subject was not taking sufficient medication. However, it is possible that the subjects were receiving supplies of the medication through methods not captured in the data available for this study, such as out of pocket payments, particularly when the copay would be higher than the advertised rate. An additional concern is pill splitting, which would be observed as poor adherence in the dataset, but might well be a physician-approved regimen. Samples provided by the physician at visit intervals may also have disrupted the perceived fill cycle. Further, although this could be corrected by including e-prescribing data in the model, without it, the method misses patients who are prescribed a medication and never fill.
The definition of poor adherence as an MPR value of less than 80%, although widely used in studies of adherence measure, is arbitrary and may not be appropriate in all cases.38 In the context of this study, the threshold of 80% adherence labeled a very small portion of the overall population as poor adherers. Although a single threshold was used in this study to define poor adherence, similar models can be built and evaluated with different threshold values.
The models presented here incorporate information widely available through pharmacies, pharmacy benefit managers, and insurers. Bringing these data and their interpretation to the point of care presents an opportunity for a clinician to quantify a patient's adherence to a specific medication regime and to intervene where appropriate. In practice, an automated system that uses both the large-gap detector and the regression model could identify the vast majority of patients at risk of poor outcomes due to poor adherence. By running the algorithm on a prescription-filling dataset on a daily basis, subjects would be identified as soon as they met the models' criteria for alert, rather than waiting until a future interaction with a clinician. Once a patient is identified as being at risk, a clinician could evaluate whether the subject is indeed non-adherent and initiate an interaction with the subject to attempt to improve adherence.
Dispensed medication data for chronic medications could be useful in alerting providers about patients currently poorly adherent as well as those who will be non-adherent several months afterwards. Even moderate non-adherence early in the treatment course can be an indicator of future poor adherence. However, there are practical considerations around integrating an automated adherence screening tool into clinical practice including integrating a point of care application into clinician workflow and devising approaches to notifying physicians without inducing ‘alert fatigue.’ False alerts could be reduced if e-prescribing were incorporated into the algorithm, eliminating detection of intended medication discontinuations.
Studies of interventions have shown mixed results in improving adherence, and new approaches are being developed and studied,22 29 30 33 39 40 but none have used a predictive model. Pharmacies use prescription-filling data to target poor adherers with programs aimed at reducing gaps in medication possession, including mail order programs with refill reminders through email, phone or text, proactive interactive phone calls, counseling by pharmacists, and education on adherence for pharmacists.35 36 Furthermore, studies of adherence show that good adherers are systematically more health-seeking than non-adherers,41 which suggests that identifying patients at high risk of poor adherence may also help identify patients in need of intervention to improve not only adherence to chronic medications, but also other aspects of health.
Supplementary Material
Acknowledgments
We thank Shannon Manzi for helpful discussions and advice.
Footnotes
Funding: This work was supported by the National Institute of Child Health and Human Development training grant 5T32HD040128, Strategic Health IT Advanced Research Projects Award 90TR000101 from the Office of the National Coordinator of Health Information Technology, and National Library of Medicine grant 5R01LM007677.
Competing interests: None.
Ethics approval: Ethics approval was provided by the Children's Hospital Boston Committee on Clinical Investigation.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97 [DOI] [PubMed] [Google Scholar]
- 2.Avorn J. Medication use in older patients: better policy could encourage better practice. JAMA 2010;304:1606–7 [DOI] [PubMed] [Google Scholar]
- 3.Krousel-Wood M, Thomas S, Muntner P, et al. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol 2004;19:357–62 [DOI] [PubMed] [Google Scholar]
- 4.Patel BV, Leslie RS, Thiebaud P, et al. Adherence with single-pill amlodipine/atorvastatin vs a two-pill regimen. Vasc Health Risk Manag 2008;4:673–81 [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman RH, Pelletier EM, Smith PJ, et al. Can adherence to antihypertensive therapy be used to promote adherence to statin therapy? Patient Prefer Adherence 2009;3:265–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodgers PT, Ruffin DM. Medication nonadherence—Part I: the health and humanistic consequences. Manag Care Interface 1998;11:58–60 [PubMed] [Google Scholar]
- 7.Senst BL, Achusim LE, Genest RP, et al. Practical approach to determining costs and frequency of adverse drug events in a health care network. Am J Health Syst Pharm 2001;58:1126–32 [DOI] [PubMed] [Google Scholar]
- 8.McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother 2002;36:1331–6 [DOI] [PubMed] [Google Scholar]
- 9.Karve S, Cleves MA, Helm M, et al. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care 2008;46:1125–33 [DOI] [PubMed] [Google Scholar]
- 10.Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin 2009;25:2303–10 [DOI] [PubMed] [Google Scholar]
- 12.Karve S, Cleves MA, Helm M, et al. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health 2009;12:989–95 [DOI] [PubMed] [Google Scholar]
- 13.Vink NM, Klungel OH, Stolk RP, et al. Comparison of various measures for assessing medication refill adherence using prescription data. Pharmacoepidemiol Drug Saf 2009;18:159–65 [DOI] [PubMed] [Google Scholar]
- 14.Hansen RA, Dusetzina SB, Dominik RC, et al. Prescription refill records as a screening tool to identify antidepressant non-adherence. Pharmacoepidemiol Drug Saf 2010;19:33–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–41 [DOI] [PubMed] [Google Scholar]
- 16.Perreault S, Dragomir A, Blais L, et al. Impact of adherence to statins on chronic heart failure in primary prevention. Br J Clin Pharmacol 2008;66:706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006;333:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu PH, Yang CY, Yao ZL, et al. Relationship of blood pressure control and hospitalization risk to medication adherence among patients with hypertension in Taiwan. Am J Hypertens 2010;23:155–60 [DOI] [PubMed] [Google Scholar]
- 19.Yiannakopoulou EC, Papadopulos JS, Cokkinos DV, et al. Adherence to antihypertensive treatment: a critical factor for blood pressure control. Eur J Cardiovasc Prev Rehabil 2005;12:243–9 [DOI] [PubMed] [Google Scholar]
- 20.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA 2011;305:1669–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med 2011;171:814–22 [DOI] [PubMed] [Google Scholar]
- 22.Ross FM. Patient compliance—whose responsibility? Soc Sci Med 1991;32:89–94 [DOI] [PubMed] [Google Scholar]
- 23.Murray MD, Harrison J. Prescription abandonment: another path to medication nonadherence. Ann Intern Med 2010;153:680–1 [DOI] [PubMed] [Google Scholar]
- 24.Chan DC, Shrank WH, Cutler D, et al. Patient, physician, and payment predictors of statin adherence. Med Care 2010;48:196–202 [DOI] [PubMed] [Google Scholar]
- 25.Burnier M. Long-term compliance with antihypertensive therapy: another facet of chronotherapeutics in hypertension. Blood Press Monit 2000;5 Suppl 1:S31–4 [PubMed] [Google Scholar]
- 26.Miller LG, Liu H, Hays RD, et al. How well do clinicians estimate patients' adherence to combination antiretroviral therapy? J Gen Intern Med 2002;17:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murri R, Ammassari A, Trotta MP, et al. Patient-reported and physician-estimated adherence to HAART: social and clinic center-related factors are associated with discordance. J Gen Intern Med 2004;19:1104–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA 2002;288:2880–3 [DOI] [PubMed] [Google Scholar]
- 29.Cramer JA. Optimizing long-term patient compliance. Neurology 1995;45(2 Suppl 1):S25–8 [PubMed] [Google Scholar]
- 30.Cutrona SL, Choudhry NK, Stedman M, et al. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med 2010;25:1090–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkrishnan R. The importance of medication adherence in improving chronic-disease related outcomes: what we know and what we need to further know. Med Care 2005;43:517–20 [DOI] [PubMed] [Google Scholar]
- 32.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med 2007;120:26–32 [DOI] [PubMed] [Google Scholar]
- 33.Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry 2002;159:1653–64 [DOI] [PubMed] [Google Scholar]
- 34.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med 2007;167:847–52 [DOI] [PubMed] [Google Scholar]
- 35.Piette JD, Heisler M, Krein S, et al. The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med 2005;165:1749–55 [DOI] [PubMed] [Google Scholar]
- 36.Steiner JF. Can we identify clinical predictors of medication adherence. and should we? Med Care 2010;48:193–5 [DOI] [PubMed] [Google Scholar]
- 37.Ryan P. Establishing a Drug Era Persistence Window for Active Surveillance. Foundation for the National Institutes of Health, 2010. http://omop.fnih.org/sites/default/files/OMOP-Establishing%20a%20Drug%20Era%20Persistence%20Window%20for%20Active%20Surveillance-Jan%202010.pdf [Google Scholar]
- 38.Crystal S, Akincigil A, Bilder S, et al. Studying prescription drug use and outcomes with medicaid claims data: strengths, limitations, and strategies. Med Care 2007;45(10 Suppl 2):S58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patton K, Meyers J, Lewis BE. Enhancement of compliance among patients with hypertension. Am J Manag Care 1997;3:1693–8 [PubMed] [Google Scholar]
- 40.Tamblyn R, Reidel K, Huang A, et al. Increasing the detection and response to adherence problems with cardiovascular medication in primary care through computerized drug management systems: a randomized controlled trial. Med Decis Making 2010;30:176–88 [DOI] [PubMed] [Google Scholar]
- 41.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation 2009;119:2051–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.