Abstract
Context
Computerized drug alerts for psychotropic drugs are expected to reduce fall-related injuries in older adults. However, physicians over-ride most alerts because they believe the benefit of the drugs exceeds the risk.
Objective
To determine whether computerized prescribing decision support with patient-specific risk estimates would increase physician response to psychotropic drug alerts and reduce injury risk in older people.
Design
Cluster randomized controlled trial of 81 family physicians and 5628 of their patients aged 65 and older who were prescribed psychotropic medication.
Intervention
Intervention physicians received information about patient-specific risk of injury computed at the time of each visit using statistical models of non-modifiable risk factors and psychotropic drug doses. Risk thermometers presented changes in absolute and relative risk with each change in drug treatment. Control physicians received commercial drug alerts.
Main outcome measures
Injury risk at the end of follow-up based on psychotropic drug doses and non-modifiable risk factors. Electronic health records and provincial insurance administrative data were used to measure outcomes.
Results
Mean patient age was 75.2 years. Baseline risk of injury was 3.94 per 100 patients per year. Intermediate-acting benzodiazepines (56.2%) were the most common psychotropic drug. Intervention physicians reviewed therapy in 83.3% of visits and modified therapy in 24.6%. The intervention reduced the risk of injury by 1.7 injuries per 1000 patients (95% CI 0.2/1000 to 3.2/1000; p=0.02). The effect of the intervention was greater for patients with higher baseline risks of injury (p<0.03).
Conclusion
Patient-specific risk estimates provide an effective method of reducing the risk of injury for high-risk older people.
Trial registration number
clinicaltrials.gov Identifier: NCT00818285.
Keywords: Medical informatics, pharmacoepidemiology, patient safety
Introduction
Injuries are among the leading causes of morbidity and mortality in older adults.1 2 The majority of injuries are fall-related, and 5–10% are fatal.3–5 A further 9–27% lead to a permanent loss of capacity for independent living.2 6 Psychotropic drugs are a potentially preventable cause of injury.7–13 These drugs are commonly used in older adults, often for indications, such as insomnia and pain, where strong evidence of efficacy is lacking.14–16 Based on systematic reviews, the risk of injury is increased by 39%, 59%, and 50% with the use of benzodiazepines, antidepressants, and antipsychotics, respectively.7 Risks appear to be dose-dependent, particularly for antipsychotics and opioids, where the most rapid increase in use is seen for older adults.7 15 17–19
Effective management of psychotropic medication is challenging. In older adults, 21–33% of prescribed psychotropic medication is relatively contraindicated,20–22 and 29% in doses that exceed those recommended.22 Moreover, 20% of older adults use more than one psychotropic drug concurrently, and 69% have more than one physician prescribing treatment, increasing the risk of undetected cumulative toxicity.23
Computerized prescribing and decision support are expected to address preventable medication errors, as these digital technologies can guide dosing and provide alerts on drug treatment duplication, contraindications, and drug interaction errors, especially when integrated with information on all dispensed medication. However, the majority of drug alerts are over-ridden, particularly for psychotropic medication24–27 in both hospital-based and community-based studies. Even when drug alert systems are customized to present only clinically important interactions, physicians over-ride the majority of alerts, because they are deemed not clinically relevant and/or the benefit is believed to exceed the risk.24–26 28 29 Yet, the patient-specific risk is rarely known, even though it can be estimated by incorporating into drug alert systems predictive models of adverse events developed through pharmacoepidemiological studies.30 The advanced computing power available in today's electronic record systems and the focus on individualized medicine provides an unprecedented opportunity to integrate detailed patient data into complex predictive models for estimating patient risk.
In this study, we tested the hypothesis that the incorporation of patient-specific risk estimates into a computerized prescribing decision-support system in primary care would increase physician response to alerts for psychotropic medication and reduce the risk of psychotropic drug-related injury in older adults, particularly for patients with a higher baseline risk.
Methods
Context
The study was conducted in Quebec, Canada, a province with 8.5 million residents and 16 000 physicians. The provincial insurance agency (RAMQ) provides health insurance for all provincial residents, and pays all physicians and community pharmacies on a fee-for-service basis. Beneficiary, medical billing and pharmacy claims data can be used to create longitudinal health histories for each patient. These data have been validated and are often used for health services and epidemiological research.31–34 In 2003, MOXXI, an experimental community-based clinical information system, was the first to link to these databases and integrate this information into electronic health record systems to support clinical decision-making.35
Design and study population
A cluster randomized controlled trial was conducted to test the hypothesis that prescribing decision support that provided patient-specific risk assessment would reduce the risk of injury in older adults. The trial was conducted in a population of 81 family physicians and 5628 of their older patients from September 2008 to July 2010. This sample size was expected to detect a difference in risk of 5% assuming 80 physician clusters, 50 patients per physician, a cluster correlation of 0.01, and a Type I and II error of 5% and 20%, respectively. Injury risk reduction was assessed at the end of the follow-up period (July 2010).
Potentially eligible physicians were identified from the roster of active primary care physicians in the Quebec provincial regulatory authority and invited to participate in the MOXXI primary care research network to evaluate the use of computerized drug and chronic disease management systems in primary care.14–16 27 36 37 To be enrolled in the network, physicians needed to practice actively in an urban community setting in Montreal or Quebec City, and use the computerized drug management system successfully to write a minimum of 10 patient prescriptions per week.
Patients were eligible if they were age 65 years or older, had an active dispensed prescription for a psychotropic drug, or were prescribed a new psychotropic drug at a visit during the follow-up period. Psychotropic drugs included those with central nervous system side effects that increased the risk of injury: benzodiazepines, antidepressants, antipsychotics, anticonvulsants, antihistamines, and opiates.38
Randomization and blinding
To optimize balance in the intervention and control group, we stratified physicians by the number of patients in their practice who were prescribed psychotropic medication in the past year, with a minimum of two physicians per stratum. Within each stratum, an equivalent number of physicians were randomized by the biostatistician to intervention and control groups using a random number table. Physicians were not blinded to the intervention status, but were blinded to the specific study outcomes that were measured. Unless advised by their physicians, patients were blinded to the intervention status.
Intervention and control group
Both the intervention and control groups used the MOXXI community-based clinical information system (CIS). A link to the provincial insurance agency (RAMQ) was used to pre-populate demographic information for the practice population based on a study physician's billings from the previous year. For patients who consented to participate in the research network, all medical services and prescription drugs provided for the past year were loaded into the MOXXI CIS, and thereafter all new records of medical services and prescriptions were refreshed on a daily basis. These data were used to update the profile of dispensed medications, dates and reasons for emergency department visits and hospitalizations, medical and surgical procedures, and health problems.39 40 A commercial drug alert system (http://www.vigilance.ca) automatically reviewed each new prescription for potential contraindications, including therapy duplication, dosing error, cumulative toxicity, and drug–disease, drug–drug, and drug–allergy interactions. Physicians could set the threshold for the alert system to one of three levels (1, severe alerts only; 2, moderate and severe alerts; 3, all alerts), which restricted alerts generated automatically during the prescribing process. By default, the system was set to level 2. However, all alerts generated for a patient were available for the physician to review in a drug alert summary in the patient's electronic chart. Physicians randomized to the control and intervention groups had access to these standard features of the MOXXI CIS.
Physicians randomized to the intervention group received a patient-specific risk of injury alert when a patient was prescribed a psychotropic medication that increased the risk of injury. The personalized alert used a published predictive model38 to estimate the risk of injury based on the patient's age, sex, injury history, presence of cognitive impairment, gait, and balance problems, and doses of selected psychotropic medication (selective serotonin / nor-epinephrine reuptake inhibitors antidepressants, antipsychotics, low-, intermediate- and high-potency opiates, intermediate- and long-acting benzodiazepines, anticonvulsants, and first-generation antihistamines41 42). We set a relatively low threshold for showing the alert—an increase in risk of 1 per 1000—to enable us to assess the likelihood of changes in drug treatment as a function of the magnitude of patient risk.
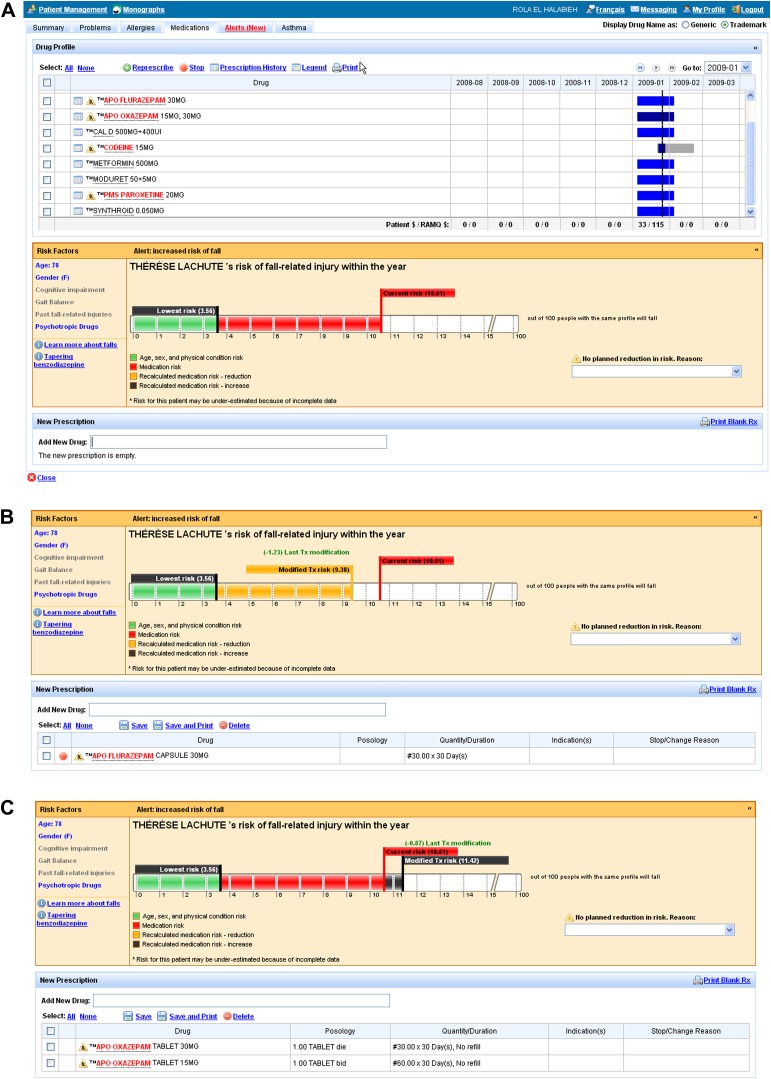
Graphics, in the form of risk thermometers, were created to show physicians the patient's risk of injury in the next 12 months related to psychotropic medication as well as non-modifiable characteristics (eg, age, sex) (figure 1A). Physicians would see the risk thermometer, annotated with numeric values generated by the risk calculation described above, when they opened the patient drug profile or when they prescribed a psychotropic drug. Drugs that contributed to the risk calculation were highlighted in the patient's drug profile. If the physician attempted to reduce the risk of injury by stopping or decreasing the dose of a psychotropic medication, the absolute and relative reduction in risk would be shown as an adjustment in the level of the thermometer and a change in the numeric values (figure 1B). If a new psychotropic drug was started or the dose was increased, the absolute and relative increase in the risk would be shown (figure 1C). If no change in medication was instituted (or the risk was increased by a medication change), physicians had to select a reason for the decision from a standardized pick-list (eg, prescribed by another physician). A reference section was available with publications on the risk of injury related to psychotropic drug use and methods of tapering benzodiazepines.
Figure 1.
(A) Screenshot of the user interface for the ‘risk of injury alert’ showing current risk and lowest possible risk. (B) Screenshot of the user interface for the risk of injury alert showing a reduction in risk due to modified treatment. (C) Screenshot of the user interface for the risk of injury alert showing risk increase with modified treatment.
Physicians in the intervention group received a 5-minute training program on the risk of injury alert that outlined how to interpret the risk thermometer information, the expected changes in risk with new or discontinued medication, the completion of reasons for not changing therapy if applicable, and the location of reference information. Physicians were advised that stopping or reducing the dose of highlighted medications in the patient's drug profile would reduce the patient's risk of injury.
Physician characteristics
Physician age, sex, and years of practice experience were documented at enrollment. Annual measures of practice size, number of practice settings, workdays, and daily practice volume were calculated for the year before randomization from each physician's RAMQ billing data using methods developed previously.40 43 Utilization of the drug management component of the MOXXI CIS was measured by calculating the number of electronic prescriptions written per 100 patient visits billed in the year before randomization. Skill in using the prescribing system was assessed by timed assessment of prescribing speed for four standardized prescriptions 3 months after the initial training for the MOXXI CIS.
Patient characteristics
Patient age, sex, and residential address were documented at enrollment from the RAMQ insurance records. Residential address was linked to neighborhood-level Statistics Canada census information to obtain estimates of patient household income, classified by low- (<$31 753), middle- ($31 754–$80 000) and high- (>$80 000) income neighborhoods. Relevant comorbidity was measured using diagnostic and procedure codes in RAMQ billing claims and the electronic health record problem list for each patient. Injury history was assessed by calculating the number of temporally distinct episodes of billing claim treatments for fractures and soft-tissue injuries in the past 24 months using algorithms that were previously validated.32 Cognitive impairment and gait and balance problems were defined in accordance with International Classification of Disease Ninth Revision (ICD9) codes included in the predictive model for injury.38 Cognitive impairment included dementia (291), alcohol-related cognitive impairment (292), and Alzheimer's and other cerebral degeneration (331). Gait and balance problems included degenerative diseases of the extrapyramidal system (332 333), seizure disorders (345), vertiginous syndromes (386), syncope (780), and orthostatic hypotension (458). The Charlson comorbidity index,44 45 number of emergency department visits, and number of hospital admissions in the past year were also measured using RAMQ billing data to provide descriptive information about population characteristics.
Outcomes
The primary study outcome was the risk of injury at the end of the follow-up period. To assess whether the effect of the intervention was greater for patients at higher risk, each patient's risk of injury was measured twice, once at the patient's first visit after randomization (baseline risk) and again on the last day of follow-up (July 15, 2010) for the intervention and control groups (outcome risk). Overall injury risk was measured using the predictive model used in the intervention, which reflected the probability of an injury in the next year based on age, sex, comorbidity, and daily dispensed doses of benzodiazepines, opiates, antipsychotics, antidepressants, anticonvulsants, and antihistamines (figure 2). Drug doses were based on active dispensed prescriptions on the first and last day of follow-up. To permit multiple drugs within the same therapeutic category to be combined, doses were standardized, using the same approach as the predictive model, by dividing the daily dose by the WHO-recommended daily dose for adults for each drug.46 For example, a patient prescribed 20 mg diazepam and 10 mg lorazepam daily would be using three standardized doses of benzodiazepine (20 mg diazepam/10 mg (WHO-recommended dose) + 10 mg lorazepam/10 mg (WHO-recommended dose) = three doses).
Figure 2.
Formula for estimating the risk of injury based on individual patient characteristics.37 NB Baseline risk was specified at 2.06%, representing the incidence of injury among 65-year-old men without any other risk factors. SSRI, selective serotonin reuptake inhibitors; SSNRI, selective serotonin-norepinephrine reuptake inhibitors.
In a secondary analysis, we assessed the physician's response to the injury risk alert, and changes in the use and dose of psychotropic medications in the intervention and control groups. Alert response was retrieved from the MOXXI audit trail for physicians in the intervention group. The audit trail recorded each action taken in relationship to psychotropic medication and its impact on overall and modifiable risk. Actions were classified into three mutually exclusive categories: (1) over-rode alert, (2) reviewed options but made no change, and (3) modified dose or drug to reduce risk. Reasons for over-riding alerts were tabulated.
To assess change in use and dose of medication, we estimated differences between the intervention and control groups in (1) the number of active psychotropic medications prescribed or dispensed, and (2) the standardized dose of psychotropic medication by therapeutic category between the baseline and follow-up period. Data were retrieved from records of all medications dispensed from the daily updates of the drug profile by the RAMQ.
Analysis
To test the hypothesis that the patients in the intervention group would have a greater reduction in the risk of injury than patients in the control group, we used multivariate linear regression within a generalized estimating equation framework. An exchangeable correlation structure was used to account for clustering of patients within physician. Each patient's calculated risk of injury on the last day of follow-up was the continuous outcome, and experimental status (intervention vs control) was the predictor. The patient's baseline risk score was used to test the hypothesis that the benefits of the intervention would be greater for high-risk patients. The interaction term between baseline risk score and experimental status was included in the model, and the Wald χ2 stxatistic was used to assess statistical significance. We used the same approach to estimate the change in the number of psychotropic medications and dose by therapeutic category between the baseline and follow-up period. For medication dose, we estimated one model per therapeutic category.
Results
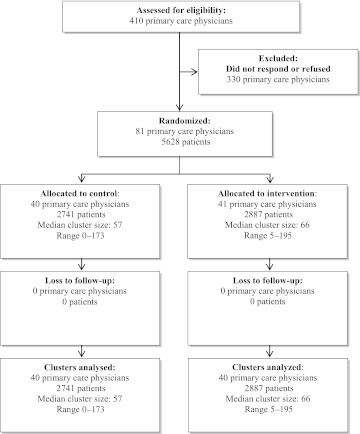
Among the 410 physicians eligible, 81 consented to participate, representing 5628 eligible patients. All consenting physicians and their patients were included in the analysis (figure 3).47 Physicians in the intervention and control groups were predominantly male, French speaking, and in practice for 25 or more years (table 1). Physicians in both groups worked, on average, in 2.2 practice settings, saw 16–17 patients per day, and used the MOXXI CIS with equivalent frequency and speed of writing electronic prescriptions.
Figure 3.
Consort diagram of physicians and patients eligible and enrolled in the trial.
Table 1.
Characteristics of the 81 physicians and the 5628 patients in the intervention and control groups
| Control, N=40 | Intervention, N=41 | |||
| Physician demographics | N | % | N | % |
| Sex | ||||
| Male | 20 | 50.0 | 22 | 55.0 |
| Female | 20 | 50.0 | 18 | 45.0 |
| Language | ||||
| English | 14 | 35.0 | 12 | 30.0 |
| French | 26 | 65.0 | 28 | 70.0 |
| Practice experience (years) | ||||
| <15 | 5 | 12.5 | 4 | 10.0 |
| 15–24 | 11 | 27.5 | 10 | 25.0 |
| ≥25 | 24 | 60.0 | 26 | 65.0 |
| Practice characteristics | Mean | SD | Mean | SD |
| Annual practice size | 1137.4 | 555.9 | 1330.6 | 711.6 |
| Number of practice settings | 2.2 | 1.3 | 2.2 | 1.0 |
| Number of clinic days worked | 190.6 | 43.6 | 186.6 | 39.1 |
| Number of patients/ clinic day | 16.7 | 7.6 | 17.4 | 6.5 |
| Use of MOXXI CIS | Mean | SD | Mean | SD |
| Electronic prescription/100 visits | 40.5 | 28.7 | 39.2 | 24.4 |
| Speed in prescription 4 standard scripts (min) | 3.08 | 0.88 | 3.04 | 1.09 |
| Minimum alert setting | ||||
| Level 1 (severe only) | 31 | 77.5 | 24 | 58.5 |
| Level 2 (moderate to severe) | 5 | 12.5 | 12 | 29.3 |
| Level 3 (all alerts) | 4 | 10.0 | 5 | 12.2 |
| Control, N=2741 | Intervention, N=2887 | |||
| Patient demographics | Mean | SD | Mean | SD |
| Age (years) | 75.63 | 7.47 | 74.81 | 6.84 |
| Patient sex | N | % | N | % |
| Female | 1876 | 68.4 | 1901 | 65.8 |
| Male | 865 | 31.6 | 986 | 34.2 |
| Socioeconomic status | ||||
| Low income (<$31 753) | 868 | 31.7 | 790 | 27.4 |
| Middle income ($31 754–$80 000) | 1682 | 61.3 | 1918 | 66.4 |
| High income (>$80 000) | 191 | 7.0 | 179 | 6.2 |
| Patient comorbidity | ||||
| Number injuries past 24 months | ||||
| No injury | 2618 | 95.5 | 2766 | 95.8 |
| 1 injury | 108 | 3.9 | 107 | 3.7 |
| 2 injuries | 10 | 0.4 | 13 | 0.5 |
| ≥3 injuries | 5 | 0.2 | 1 | 0.0 |
| Gait or balance problem | 505 | 18.4 | 476 | 16.5 |
| Cognitive impairment | 150 | 5.5 | 130 | 4.5 |
| Charlson comorbidity index | ||||
| 0 | 1379 | 50.3 | 1565 | 54.2 |
| 1–2 | 1039 | 37.9 | 1048 | 36.3 |
| 3–4 | 191 | 7.0 | 166 | 5.7 |
| ≥5 | 132 | 4.8 | 108 | 3.7 |
| Healthcare utilization | ||||
| ≥1 Emergency department visit | 1011 | 36.9 | 927 | 32.1 |
| ≥1 Hospitalization | 617 | 22.5 | 608 | 21.1 |
Patients in the intervention and control groups were similar: on average 75–76 years of age, predominantly female, and residing in middle-income neighborhoods (table 1). Approximately 5% of patients had treatment for at least one injury in the past year, and a similar proportion had cognitive impairment. The prevalence of gait and balance problems was slightly higher in the control group as were the proportion of patients with at least one emergency department visit in the past year.
At the first visit after randomization, the most prevalent psychoactive medication used was an intermediate-acting benzodiazepine, taken by approximately one-third of patients, followed by antidepressants, anticonvulsants, and antipsychotics (table 2). For intermediate-acting benzodiazepines, the mean daily dose prescribed was 10–20% over the recommended adult dose, primarily because 4.6% of patients in the control group and 3.8% in the intervention group were using more than one intermediate-acting benzodiazepine concurrently. This was also true for antidepressants, where 1–1.2% of patients were using more than one antidepressant. The overall risk of injury at baseline was comparable in the two groups—the risk of injury in the next year was 4.03 per 100 in the control group, and 3.85 in the intervention group, of which an equivalent amount (control, 0.51; intervention, 0.49) was related to the use of psychoactive medication. The distribution was skewed, with a median of 3.55, an IQR of 2.88–4.51, and a range 2.09 to 45.30.
Table 2.
Psychoactive medication at the first visit after randomization
| Control, N=2741 | Intervention, N=2887 | |||
| Distribution by therapeutic class | N | % | N | % |
| Benzodiazepines | ||||
| Intermediate-acting | 1516 | 55 | 1648 | 57 |
| Long-acting | 109 | 4 | 111 | 4 |
| Antidepressants | 712 | 26 | 721 | 25 |
| Anticonvulsants | 451 | 16 | 404 | 14 |
| Antipsychotics | 258 | 9 | 278 | 10 |
| Opiates | ||||
| High potency | 22 | 1 | 5 | 0 |
| Intermediate potency | 96 | 4 | 104 | 4 |
| Low potency | 174 | 6 | 195 | 7 |
| Antihistamines | 7 | 0 | 4 | 0 |
| Standardized daily dose among users | Mean | SD | Mean | SD |
| Benzodiazepines | ||||
| Intermediate-acting | 1.20 | 1.71 | 1.10 | 1.52 |
| Long-acting | 0.81 | 0.45 | 0.74 | 0.37 |
| Antidepressants | 1.04 | 0.55 | 1.06 | 0.58 |
| Anticonvulsants | 0.51 | 0.41 | 0.54 | 0.42 |
| Antipsychotics | 0.59 | 2.02 | 0.79 | 3.38 |
| Opiates | ||||
| High potency | 0.29 | 0.85 | 0.16 | 0.15 |
| Intermediate potency | 0.43 | 0.38 | 0.50 | 0.63 |
| Low potency | 0.88 | 0.77 | 0.89 | 0.70 |
| Antihistamines | 0.13 | 0.04 | 0.79 | 3.38 |
| Overall use and risk | Mean | SD | Mean | SD |
| Number of psychoactive medications | 1.28 | 0.61 | 1.25 | 0.58 |
| Proportion prescribed by study doctor | 0.81 | 0.38 | 0.78 | 0.41 |
| Combined standardized dose | 1.18 | 1.59 | 1.15 | 1.69 |
| Overall risk of injury | 4.03 | 1.87 | 3.85 | 1.70 |
| Risk related to psychoactive medication | 0.51 | 0.80 | 0.49 | 1.14 |
Among the 2887 injury alerts generated at the first visit for patients in the intervention group, 13.7% were for new psychoactive medications that were being started by the physician during the visit, and the remaining alerts (86.3%) were for existing medications (table 3). Physicians responded to 2404 (83.3%) risk of injury alerts, by reviewing options but deciding not to change the treatment (n=1694, 58.7%), by reducing the dose, or by discontinuing psychoactive medications (n=710, 24.6%). Of interest, physicians were more likely to change the treatment plan for existing medication (25.7%) than for newly started therapy (17.7%). The most common reason for not changing treatment was the belief that the benefit would exceed the risk (n=1840, 84.5%), followed by patient resistance to change in therapy (n=179, 8.2%). While the majority of physicians changed their alert settings from the default setting to view only the most severe alerts (table 1), there were no statistically significant differences in the alert levels selected by the control and intervention groups.
Table 3.
Response to the TRIPP alert in the intervention group by type of prescription
| Renewing an existing psychoactive medication | Starting a new psychoactive medication | |||
| N | % | N | % | |
| Number of alerts | 2491 | 396 | ||
| Changed prescription | 640 | 25.7 | 70 | 17.7 |
| Reviewed but no change | 1399 | 56.2 | 295 | 74.5 |
| No review + over-rode alert | 452 | 18.1 | 31 | 7.8 |
| Reasons for over-riding alert | ||||
| Benefit greater than risk | 1545 | 62.02 | 295 | 74.49 |
| Patient demand/resistance | 161 | 6.46 | 18 | 4.55 |
| Need to consult prescribing doctor | 53 | 2.13 | 3 | 0.76 |
| Will review next time | 47 | 1.89 | 3 | 0.76 |
| Drug information incorrect | 45 | 1.81 | 7 | 1.77 |
On average, patients in the intervention and control groups were followed for 467 and 452 days, respectively. Although there was an overall trend for the number of drugs and doses in all psychotherapeutic classes (except intermediate-acting benzodiazepines) to be reduced in the intervention group, no single class decreased significantly in dose, when modeled alone (table 4). However, there were significant interactions between baseline risk and intervention status for antipsychotics (interaction term: p=0.02) and anticonvulsants (interaction term: p=0.03), with reductions in doses occurring for patients with greater baseline risk. The most prevalent medication affected in these therapeutic groups was risperidone for antipsychotics (37.5% of patients (n=201)) and pregabalin and gabapentin for anticonvulsants (71.1% of patients (n=608)). The most common indications for prescribing antipsychotics was psychosis/bipolar disorder (32.2% of patients) and dementia/delirium (20.4% of patients), and for anticonvulsants it was pain (72.6%).
Table 4.
Change in intermediate outcomes: psychotropic drug dose and number of drugs
| Intermediate outcomes | Dose at the end of follow-up[y] | Dose change[y] (follow-up− baseline) | Cluster adjusted difference[z, x] | Interaction[x] baseline risk × intervention | |||
| Dose by therapeutic class[*] | Control | Intervention | Control | Intervention | Difference | 95% CI | p Value |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| Benzodiazepines | |||||||
| Intermediate-acting | 0.68±0.6 | 0.66±0.6 | −0.79±1.7 | −0.74±1.5 | −0.008 | −0.05 to 0.03 | 0.91 |
| Long-acting | 0.88±0.5 | 0.70±0.3 | −0.32±0.6 | −0.43±0.5 | −0.006 | −0.01− to −0.00 | 0.19 |
| Antidepressants | 1.04±0.5 | 1.07±0.6 | −0.41±0.8 | −0.46±0.8 | −0.011 | −0.03 to 0.01 | 0.48 |
| Anticonvulsants | 0.51±0.4 | 0.59±0.4 | −0.21±0.5 | −0.19±0.4 | 0.006 | −0.00 to 0.01 | 0.03 |
| Antipsychotics | 0.44±0.4 | 0.54±0.7 | −0.33±1.9 | −0.49±3.2 | 0.005 | −0.00 to 0.01 | 0.02 |
| Opiates | |||||||
| Intermediate potency | 0.38±0.4 | 0.56±0.6 | −0.24±0.5 | −0.26±0.7 | 0.001 | −0.00 to 0.01 | 0.65 |
| Low potency | 0.79±1.2 | 0.49±0.5 | −0.63±1.0 | −0.70±0.8 | −0.004 | −0.01 to 0.00 | 0.29 |
| By number of psychotropic drugs | Number drugs at the end of follow-up | Drug change (follow-up−baseline) | Cluster adjusted difference | Interaction baseline risk × intervention | |||
| Control | Intervention | Control | Intervention | Difference | 95% CI | p Value | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||||
| 0.67±0.8 | 0.62±0.7 | −0.60±0.8 | −0.63±0.8 | −0.02 | −0.09 to 0.05 | 0.96 | |
*There was an insufficient number of patients on high-potency opiates (n=27) or antihistamines (n=11) to model separately.
†Standardized drug dosage = daily prescribed dosage (mg)/WHO-recommended adult dosage (mg).
‡Each therapy class was modeled separately for drug dose in a model that included baseline dose and intervention group status. To test the hypothesis that the intervention was modified by baseline risk, baseline risk was added to the model as well as a two-way interaction term between intervention group status and baseline risk.
§Change in the number of psychotropic drugs was assessed in a model that included baseline number of psychotropic drugs and intervention group status. To test the hypothesis that the intervention was modified by baseline risk, baseline risk was added to the model as well as a two-way interaction term between intervention group status and baseline risk.
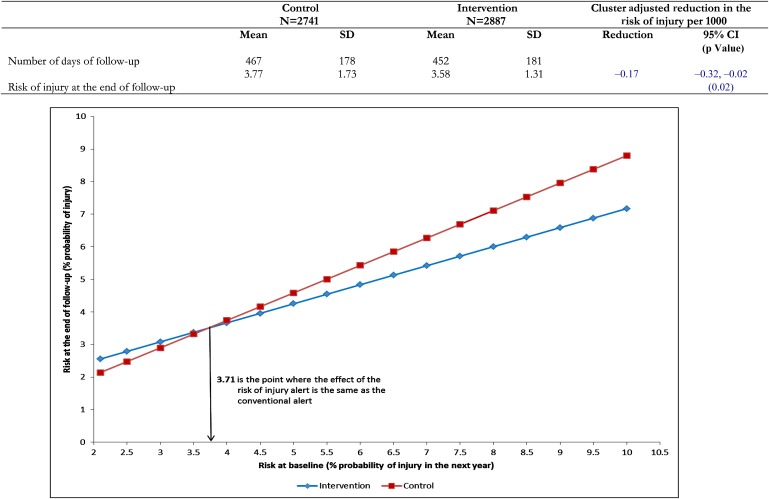
Congruent with these changes in therapy, the risk of injury in the intervention group was reduced by 1.7 injuries per 1000 patients (95% CI 0.2/1000 to 3.2/1000; p=0.02) compared with the control group (figure 4). The effect of the intervention was significantly greater for patients at higher risk of injury at the start of the trial (interaction between the baseline risk of injury and the intervention: p=0.03) (figure 4). For patients in the upper decile of risk of 5.76, the intervention produced a reduction in risk of 5.3 per 1000 (95% CI 3.8 to 6.8).
Figure 4.
Risk of injury at the end of follow-up in the intervention and control groups and modification of the effect of the intervention by the magnitude of the baseline risk. The model used to estimate the change in the effect of the intervention by baseline risk of injury was: follow-up risk (y)=intercept (0.3696) + baseline risk (0.8427) + intervention (0.9585) + baseline risk × intervention (−0.2584) (p=0).
Discussion
This study tested the benefits of using a new approach for drug safety alerts that provided patient-specific risk estimates of the likelihood of harm. The provision of patient-specific risk information resulted in a therapy review in over 80% of patients and significant reductions in the risk of injury related to psychoactive medication, particularly for those who were at greatest risk of fall-related injuries.
This study produced a greater response to alerts than previous studies,24–27 even those that used highly selected subsets of clinically relevant alerts.48 49 Not only did physicians review consequences of revising treatment for the vast majority of patients, they also modified drug therapy in one-quarter of instances.
Of interest, the most common reason for not changing therapy was that physicians perceived the benefit of treatment to be greater than the risk. This reason was particularly common when the patient was starting new medication, possibly because a physician who starts a patient on medication will generally have decided that the benefit exceeds the risk before prescribing, even if the precise risk and benefit are not known. In contrast, prescription renewals for prevalent users were more likely to be modified, possibly because the effects of treatment (or lack thereof) can be more precisely evaluated, and thus more precise risk information can be factored into renewal decisions. It may also be because the evidence supporting the benefits of treatment is less compelling to start with, particularly in some therapeutic categories. The most significant reductions in therapy in this study were for antipsychotics and anticonvulsants, therapeutic categories that have been reported to have both high levels of off-label use and weak to non-existent evidence of benefit.50 Indeed, 22% of anticonvulsant prescriptions were for gabapentin, and all of these prescriptions were for off-label use for pain.
A ubiquitous complaint about drug alerts is that there are too many ‘nuisance alerts’, ie, alerts for problems of little clinical significance.28 49 51 This study is the first to identify a data-driven approach to identifying clinically meaningful alerts and quantifying the risk associated with an alert. Typically, ‘expert opinion’ is used to classify alerts as mild, moderate, and severe.48 This method of classification has already been shown to lack reproducible results so that different commercial systems will generate different subsets of alerts within these categories.52 53 Empirically estimating the probability of adverse effects in different subpopulations provides a more meaningful clinical metric that could be used to establish alert thresholds. In this study, physicians were more likely to alter treatment when the overall risk of injury was high, and, conversely, physicians were less likely to respond when the risk was low. To study this effect, the threshold to generate an alert was set at a low level of risk: an increased risk of 1 per 1000 (0.1%). We identified the threshold at which physicians were more likely to respond to an alert at an overall risk of 32 per 1000 (3 per 100). In the future, empirical estimates of the risk of adverse outcomes could be used to establish an alert threshold. Many national agencies are establishing methods of conducting robust pharmacosurveillance. Part of the mandate of post-market surveillance could be to assess the risk associated with both new drugs and drug interactions, thereby providing the data needed to establish and calibrate a new generation of smarter drug alert systems.
An important limitation of drug alerts, including this new generation of drug alert systems, is that information is presented only on the risk of treatment and not on the potential benefits. In the future, efforts should be made to generate empirical information on both the risks and benefits of treatment, ideally deriving this information from both clinical trials and post-market surveillance systems. In addition to providing clinicians and patients with relative risk and benefit information, ideally individualized to a patient's risk profile, alternate treatment options that have lower risk with equivalent benefit for the same treatment indication could be provided. Future research should be directed to methods of generating empirical estimates of comparative risk and benefit that can be incorporated into advanced systems of drug decision support, cognitive decision-making studies that will enable the best methods of presenting this information to physicians and patients to be identified, methods of establishing the threshold for generating drug alerts, and the potential benefits of displaying alternate therapy options by treatment indication.
In summary, this study shows that individual risk estimates displayed graphically and numerically are a more effective method of eliciting response to drug alerts and reducing the risk of medication-related injury in higher risk seniors. Future research should estimate the reduction in injury rate related to this new generation of alerts and determine whether similar effects can be achieved in other therapy classes for other important outcomes.
Footnotes
Funding: RT is supported by the Canadian Institutes of Health Research and the Canadian Patient Safety Institute. TE is supported by The CIHR Frederick Banting and Charles Best Canada Graduate Scholarship and CIHR Emerging Team Grant. DB is supported by a Canada Research Chair in Public Health Informatics.
Competing interests: None.
Ethics approval: Ethics approval was given by McGill University Institutional Review Board.
Contributors: All authors contributed significantly to the conception and design or analysis and interpretation of the data. All authors drafted the article or revised it for important intellectual content, and have given final approval of the version to be published.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970-2002. JAMA 2005;294:1255–9 [DOI] [PubMed] [Google Scholar]
- 2.Scuffham P, Chaplin S, Legood R. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Health 2003;57:740–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Public health and aging: nonfatal injuries among older adults treated in hospital emergency departments–United States, 2001. MMWR Morb Mortal Wkly Rep 2003;52:1019–22 [PubMed] [Google Scholar]
- 4.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med 2002;18:141–58 [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Doucette J, Claus E, et al. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc 1995;43:1214–21 [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 1997;337:1279–84 [DOI] [PubMed] [Google Scholar]
- 7.Bloch F, Thibaud M, Dugue B, et al. Psychotropic drugs and falls in the elderly people: updated literature review and meta-analysis. J Aging Health 2011;23:329–46 [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Anderson G, Mittmann N, et al. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998;351:1303–7 [DOI] [PubMed] [Google Scholar]
- 9.Nurminen J, Puustinen J, Piirtola M, et al. Psychotropic drugs and the risk of fractures in old age: a prospective population-based study. BMC Public Health 2010;10:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moden B, Merlo J, Ohlsson H, et al. Psychotropic drugs and falling accidents among the elderly: a nested case control study in the whole population of Scania, Sweden. J Epidemiol Community Health 2010;64:440–6 [DOI] [PubMed] [Google Scholar]
- 11.Kragh A, Elmstahl S, Atroshi I. Older adults' medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc 2011;59:863–8 [DOI] [PubMed] [Google Scholar]
- 12.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775–86 [DOI] [PubMed] [Google Scholar]
- 13.Vitry AI, Hoile AP, Gilbert AL, et al. The risk of falls and fractures associated with persistent use of psychotropic medications in elderly people. Arch Gerontol Geriatr 2010;50:e1–4 [DOI] [PubMed] [Google Scholar]
- 14.Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ 2000;162:225–33 [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan MD, Edlund MJ, Fan MY, et al. Trends in use of opioids for non-cancer pain conditions 2000-2005 in commercial and Medicaid insurance plans: the TROUP study. Pain 2008;138:440–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med 2006;260:76–87 [DOI] [PubMed] [Google Scholar]
- 17.Stroke Unit TC Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2002;(1):CD000197. [DOI] [PubMed] [Google Scholar]
- 18.Tamblyn R, Abrahamowicz M, du BR, et al. A 5-year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc 2005;53:233–41 [DOI] [PubMed] [Google Scholar]
- 19.Simoni-Wastila L. Increases in opioid medication use: balancing the good with the bad. Pain 2008;138:245–6 [DOI] [PubMed] [Google Scholar]
- 20.Mort JR, Aparasu RR. Prescribing potentially inappropriate medications to the ambulatory elderly. Arch Intern Med 2000;160:2825–31 [DOI] [PubMed] [Google Scholar]
- 21.Curtis LH, Ostbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004;164:1621–5 [DOI] [PubMed] [Google Scholar]
- 22.Pugh MJ, Fincke BG, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc 2005;53:1282–9 [DOI] [PubMed] [Google Scholar]
- 23.Tamblyn RM, McLeod PJ, Abrahamowicz M, et al. Do too many Cooks Spoil the Broth? Multiple physician Involvement in medical management and inappropriate prescribing in the elderly. Can Med Assoc J 1996;154:1177–84 [PMC free article] [PubMed] [Google Scholar]
- 24.Weingart SN, Toth M, Sands DZ, et al. Physicians' decisions to override computerized drug alerts in primary care. Arch Intern Med 2003;163:2625–31 [DOI] [PubMed] [Google Scholar]
- 25.van der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006;13:138–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor LK, Kawasumi Y, Bartlett G, et al. Inappropriate prescribing practices: the challenge and opportunity for patient safety. Healthc Q 2005;(8 Spec No):81–5 [DOI] [PubMed] [Google Scholar]
- 27.Tamblyn R, Huang A, Taylor L, et al. A randomized trial of the effectiveness of on-demand versus computer-triggered drug decision support in primary care. J Am Med Inform Assoc 2008;15:430–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grizzle AJ, Mahmood MH, Ko Y, et al. Reasons provided by prescribers when overriding drug-drug interaction alerts. Am J Manag Care 2007;13:573–8 [PubMed] [Google Scholar]
- 29.Kuperman GJ, Reichley RM, Bailey TC. Using commercial knowledge bases for clinical decision support: opportunities, hurdles, and recommendations. J Am Med Inform Assoc 2006;13:369–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Julien M, Hanley JA. Profile-specific survival estimates: making reports of clinical trials more patient-relevant. Clin Trials 2008;5:107–15 [DOI] [PubMed] [Google Scholar]
- 31.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes in medical services claims data. Can J Clin Pharmacol 2001;8:39. [DOI] [PubMed] [Google Scholar]
- 32.Tamblyn RM, Reid T, Mayo N, et al. Using medical services claims to assess injuries in the elderly: the sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol 2000;53:183–94 [DOI] [PubMed] [Google Scholar]
- 33.Levy AR, Tamblyn RFD, McLeod P, et al. Coding accuracy of hospital discharge data for elderly survivors of myocardial infarction. Can J Cardiol 1999;15:1277–82 [PubMed] [Google Scholar]
- 34.Tamblyn RM, Lavoie G, Petrella L, et al. The use of prescription claims databases in Pharmacoepidemiological research: the accuracy and Comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 1995;48:999–1009 [DOI] [PubMed] [Google Scholar]
- 35.Tamblyn R, Huang A, Perreault R, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ 2003;169:549–56 [PMC free article] [PubMed] [Google Scholar]
- 36.Tamblyn R, Huang A, Kawasumi Y, et al. The development and evaluation of an integrated electronic prescribing and drug management system for primary care. J Am Med Inform Assoc 2006;13:148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamblyn R, Reidel K, Huang A, et al. Increasing the detection and response to adherence problems with cardiovascular medication in primary care through computerized drug management systems: a randomized controlled trial. Med Decis Making 2010;30:176–88 [DOI] [PubMed] [Google Scholar]
- 38.Buckeridge D, Huang A, Hanley J, et al. Risk of injury associated with opioid use in older adults. J Am Geriatr Soc 2010;58:1664–70 [DOI] [PubMed] [Google Scholar]
- 39.Poissant L, Taylor L, Huang A, et al. Assessing the accuracy of an inter-institutional automated patient-specific health problem list. BMC Med Inform Decis Mak 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamblyn R, Huang A, Kawasumi Y, et al. The development and evaluation of an integrated electronic prescribing and drug Managment system for primary care. J Am Med Inform Assoc 2006;13:148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenblatt DJ, Harmatz JS, Shader RI. Clinical Pharmacokinetics of Anxiolytics and Hypnotics in the elderly: therapeutic Considerations (Part 1). Clin Pharmacokinet 1991;21:165–77 [DOI] [PubMed] [Google Scholar]
- 42.Greenblatt DJ. Pharmacology of benzodiazepine Hypnotics. J Clin Psychiatry 1992;53(Suppl 6):7–13 [PubMed] [Google Scholar]
- 43.Tamblyn R, Abrahamowicz M, Brailovsky C, et al. The association between licensing examination scores and resource use and quality of care in primary care practice. JAMA 1998;280:989–96 [DOI] [PubMed] [Google Scholar]
- 44.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83 [DOI] [PubMed] [Google Scholar]
- 45.Haas JS, Cleary PD, Guadagnoli E, et al. The impact of socioeconomic status on the intensity of ambulatory treatment and health outcomes after hospital discharge for adults with asthma. J Gen Intern Med 1994;9:121–6 [DOI] [PubMed] [Google Scholar]
- 46.WHO Collaborating Centre for Drug Statistics Methodology Anatomical Therapeutic Chemical Classification Index with Defined Daily Doses. Oslo: World Health Organization, 2000 [Google Scholar]
- 47.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. Br Med J 2004;328:702–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paterno MD, Maviglia SM, Gorman PN, et al. Tiering drug-drug interaction alerts by severity increases compliance rates. J Am Med Inform Assoc 2009;16:40–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah NR, Seger AC, Seger DL, et al. Improving acceptance of computerized prescribing alerts in ambulatory care. J Am Med Inform Assoc 2006;13:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayo NE, Wood-Dauphinee S, Cote R, et al. There's no place like home: an evaluation of early supported discharge for stroke.[comment]. Stroke 2000;31:1016–23 [DOI] [PubMed] [Google Scholar]
- 51.Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med 2009;169:305–11 [DOI] [PubMed] [Google Scholar]
- 52.Grant EN, Lyttle CS, Weiss KB. The relation of socioeconomic factors and racial/ethnic differences in US asthma mortality. Am J Public Health 2000;90:1923–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweidan M, Reeve JF, Brien JA, et al. Quality of drug interaction alerts in prescribing and dispensing software. Med J Aust 2009;190:251–4 [DOI] [PubMed] [Google Scholar]