Abstract
Intestinal epithelium has the capacity to self-renew and generate differentiated cells through the existence of two types of epithelial stem cells: active crypt base columnar cells (CBCs) and quiescent +4 cells. The behaviors of these cells are regulated both by intrinsic programs and by extrinsic signals sent by neighboring cells, which define the niche. It is clear that the β-catenin pathway acts as an essential intrinsic signal for the maintenance and proliferation of CBC, and it was recently proposed that Paneth cells provide a crucial niche by secreting Wingless/Int (Wnt) ligands. Here, we examined the effect of disrupting the intestinal stem cell niche by inducible deletion of the transcription factor Math1 (Atoh1), an essential driver of secretory cell differentiation. We found that complete loss of Paneth cells attributable to Math1 deficiency did not perturb the crypt architecture and allowed the maintenance and proliferation of CBCs. Indeed, Math1-deficient crypt cells tolerated in vivo Paneth cell loss and maintained active β-catenin signaling but could not grow ex vivo without exogenous Wnt, implying that, in vivo, underlying mucosal cells act as potential niche. Upon irradiation, Math1-deficient crypt cells regenerated and CBCs continued cycling. Finally, CBC stem cells deficient in adenomatous polyposis coli (Apc) and Math1 were able to promote intestinal tumorigenesis. We conclude that in vivo, Math1-deficient crypts counteract the absence of Paneth cell-derived Wnts and prevent CBC stem cell exhaustion.
The small intestinal epithelium is characterized by rapid and perpetual cell proliferation (1). This continuous regeneration is carried out by an active intestinal stem cell population, which gives rise to proliferating progenitors that differentiate into the five types of epithelial cells. These include two lineages: an absorptive one composed of enterocytes; and a secretory one composed of goblet cells, enteroendocrine cells, Paneth cells, and the recently characterized tuft cells (2). Differentiation of all of these cell types takes place during migration from the crypts to the villi, except Paneth cells, which complete their differentiation at the crypt base intercalated between a population of a particular type of stem cell: the crypt base columnar cells (CBCs). Indeed, available evidence suggests that two populations of stem cells reside in the crypt base: the actively cycling CBCs, and a less-abundant and slower-cycling population of quiescent stem cells (3, 4). CBCs have been relatively well-characterized. Microarray experiments have defined the CBC transcriptome and many of the genes expressed in CBCs, such as leucine-rich repeat containing G-protein-coupled-receptor 5 (Lgr5), Achaete scute-like 2 (Ascl2), SRY-box 9 (SOX9), and TNF receptor superfamily (Tnfrsf)19, are Wingless/Int (Wnt)/β-catenin-targets (5). In contrast, fewer markers, including polycomb gene Bmi-1, HOP homeobox gene (Hopx), and mouse telomerase reverse transcriptase (mTert), have been reported so far for the slower-cycling population of intestinal stem cells located above the crypt base (4, 6, 7). Remarkable progress has been made in identifying and characterizing intestinal stem cells but their particular niches remain poorly defined. The intestinal crypt is surrounded by subepithelial myofibroblasts, which are believed to secrete paracrine signals that regulate neighboring stem cells (8). In addition, Wnt factors have been clearly shown to be absolutely required within the intestinal stem cell niche. Ablation of Wnt signaling, either by overexpression of the Wnt inhibitor Dickkopf-1 (Dkk1) or by genetic deletion of T-cell factor 4 (Tcf4), results in a loss of intestinal crypts and underscores a specific role for Wnt signaling in the development and maintenance of intestinal stem cells (9–13). Intestinal stem cells reside in a Wnt-rich environment because of the constant secretion of Wnt ligands by the Paneth cells, which are interdigitated among the CBCs (14, 15). It has been recently proposed that Paneth cells provide an essential niche to support CBC maintenance and self-renewal (15). Furthermore, cells expressing a Paneth cell-like genetic program are found in mouse and human intestinal tumors, and this function might be conserved in tumors (16, 17). However, mice are able to tolerate the mosaic depletion of Paneth cells in several genetic contexts, supporting the idea that the intestine can overcome this defect. In particular, “escaper crypts” can repopulate the epithelium by stimulating crypt fission (18–20). In this study, we investigated the effects of depleting Math1 [atonal homolog 1 (Atoh1)], a basic helix–loop–helix (bHLH) transcription factor important for determining secretory cell fate, the absence of which leads to a complete loss of Paneth cells. Specifically, we examined the consequences of Math1 depletion, alone or in combination with adenomatous polyposis coli (Apc) gene deletion, on CBC self-renewal during homeostasis and during pathological proliferation or after intestinal injury.
Results and Discussion
Analysis of CBC Stem Cells and the Paneth Cell Lineage upon Removal of Math1.
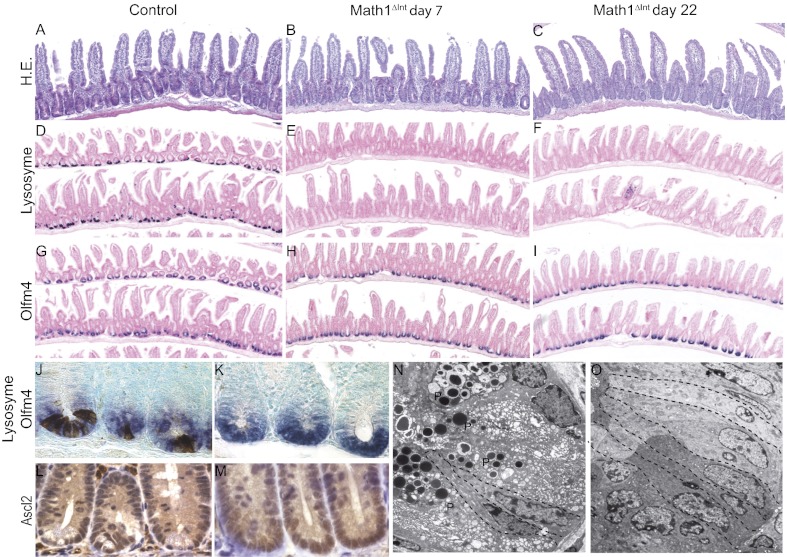
To investigate the effect of Paneth cell ablation on stem cell maintenance and proliferation, we used inducible deletion of intestinal Math1 in a mouse model that we established previously (21). In these Math1lox/loxVilCreERT2 mice, hereafter referred to as either Math1Δint or Math1 mutant mice, i.p. injections of tamoxifen (Tam) led to Cre recombinase activation in all intestinal epithelial cells, which efficiently deleted both Math1 alleles. This inducible genetic mouse model is unique, because it leads to the complete and persistent loss of Paneth cells, which can be verified by the absence of lysozyme expression and by ultrastructural analyses (Fig. 1 D–F and O). Although the lifespan of Paneth cells is relatively long and estimated to be between 20 and 50 d (22, 23), the complete ablation of these cells 7 d after Tam injection revealed that Math1 is essential for Paneth cell maintenance. Despite the absence of Paneth cells, crypt architecture in the intestines of Math1Δint mice was similar to that of control mice (Fig. 1 A–C). We first attempted to determine the consequences of Math1 deletion on intestinal stem cells by injecting Tam during the third postnatal week to produce Math1Δint mice at a stage when crypt numbers are increasing through crypt fission. We also injected Tam into 3-mo-old adults, in which the intestinal epithelium is undergoing constant self-renewal. We then determined the immediate molecular consequences of intestinal Math1 deficiency by using oligonucleotide microarrays to analyze gene expression in the small intestine 5 d after Math1 deletion at both stages (young and adult). We identified 101 genes that were significantly misexpressed in the intestines of young and adult Math1-deficient mice compared with those in the intestines of control mice (Table S1). Among all of the genes for which expression was affected by Math1 deletion, 66% of them (67/101) were down-regulated in the intestines of Math1Δint mice compared with the intestines of control mice. Among these genes, secretory cell lineage markers were abundant (32% of the down-regulated genes), and their expression levels were dramatically reduced (by up to 1,360-fold) in Math1Δint mice compared with those in wild-type mice. These results confirmed that Math1 is absolutely required to maintain the secretory cell lineage. Because no intestinal stem cell markers were among the list of genes affected by Math1 deletion, we used quantitative PCR to carefully examine the impact of Paneth cell loss on the expression of highly specific and robust markers of stem cells, which included CBC markers (Lgr5, Olfm4, and Ascl2) and the +4 marker Bmi-1 (Fig. S1). These markers were analyzed in mice 7 and 22 d after Tam injection to analyze the short- and long-term effects of Paneth cell ablation. We observed no significant changes in the expression of these stem cell markers except for Ascl2, which was unexpectedly induced in Math1-deficient mice at both time points. Indeed, Ascl2 plays an essential role in the maintenance of adult intestinal stem cells (5). In agreement with these findings, immunohistochemistry and in situ hybridization results indicated that the crypt bases of Math1Δint mice were occupied entirely by Olfm4- and Ascl2-positive cells (Fig. 1 G–M). Ultrastructural analyses of Math1Δint mice confirm the complete ablation of Paneth cells and the maintenance of typical CBCs with basal nuclei (Fig. 1 N and O). Recent data indicated that in a case of complete ablation of CBCs, Bmi-expressing stem cells were able to serve as an alternative stem cell pool and to replenish the CBCs (24). Quantification of apoptotic cells indicated that loss of Math1 does not affect the overall number of apoptotic crypt cells (Fig. S2A). Careful examination at the bottom of the crypt (from +1 to +10) did not indicate a specific loss of CBC in Math1Δint mice (Fig. S2 C–E). Then, under Math1 deletion, it appears unlikely that Bmi-1+ cells compensate and act as an alternative stem cell pool.
Fig. 1.
Paneth cell ablation does not alter CBCs in Math1Δint mice. (A–C) Hematoxylin and eosin (H.E.) staining in control and Math1-deficient mice 7 and 22 d after Tam injections. (D–I) In situ hybridization of lysozyme and Olfm4 were carried out on serial sections from control and Math1-deficient mice 7 and 22 d after Tam injections. (J and K) Double-staining of lysozyme (brown) and Olfm4 (blue) in representative crypts of control and Math1-deficient mice. (L and M) Ascl2 immunostaining of representative crypts of control and Math1-deficient mice. (N and O) Transmission electron microscopy showing ablation of the Paneth cell lineage and the presence of normal CBCs with numerous supranuclear mitochondria and prominent supranuclear Golgi apparatus in Math1-deficient mice (O) compared with control mice (N). Dashed lines delimit the CBCs and P indicates the Paneth cells.
Together, these results confirm and extend previous analyses of the Math1-deficient intestine and show that Math1 is a critical secretory cell fate determinant (25, 26). However, Math1-deficient crypt cells appear to survive and maintain their CBC after Paneth cell ablation.
Analysis of Wnt Sources and β-Catenin Signaling in Math1-Deficient Mice.
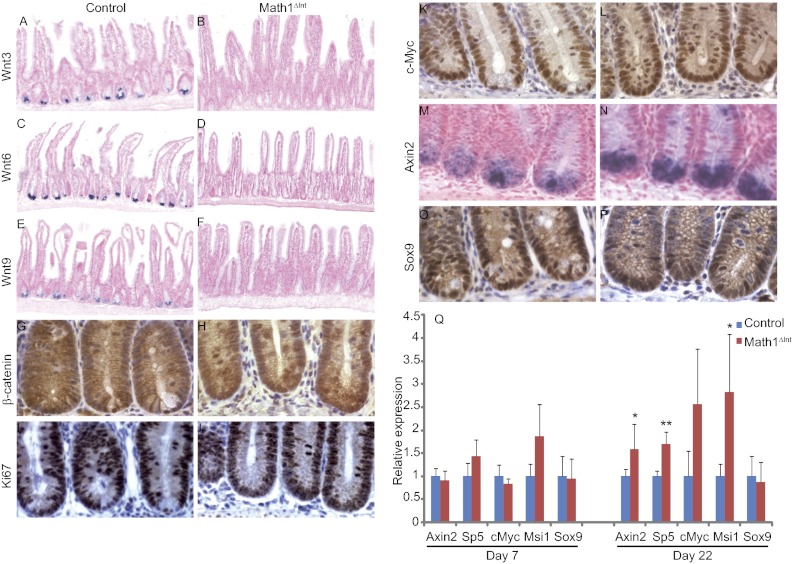
It has been proposed that Paneth cells supply essential niche signals by producing several Wnt factors, such as Wnt3, Wnt6, and Wnt9 (14, 15). Accordingly, by in situ hybridization, we found that these Wnt ligands were completely absent from the small intestine of Math1Δint mice (Fig. 2 A–F). We then analyzed the consequences of the absence of Wnt-producing Paneth cells on β-catenin signaling within the crypt compartment of Math1-deficient mice. In control mice, Paneth cells exhibit strong nuclear accumulation of β-catenin (Fig. 2G). After Tam injections, β-catenin staining of crypts in the Math1Δint mice was similar to that of control crypts, indicating that the CBCs occupying the entire crypt base were strongly positive for nuclear β-catenin, a marker for active Wnt signaling (Fig. 2H).
Fig. 2.
β-Catenin signaling and cell proliferation of progenitors and CBC in Math1-deficient mice. (A–F) In situ hybridization of Wnt3 (A and B), Wnt6 (C and D), and Wnt9 (E and F) in control and Math1-deficient mice 7 d after Tam injection. (G–P) β-Catenin (G and H), Ki67 (I and J), c-Myc (K and L), and Sox9 (O and P) immunostaining and in situ hybridization of Axin2 (M and N) in control and Math1-deficient mice 7 d after Tam injection. (Q) Real-time quantitative RT-PCR analyses of β-catenin/Tcf target genes in control and Math1Δint mice 7 and 22 d after Tam injections. Significant differences between the expression levels of control and Math1-deficient mice are marked by asterisks: *P < 0.05; **P < 0.01.
In addition, the number of Ki67+ proliferating cells per crypt was similar in Math1Δint and in control mice and the percentage of Ki67+ at the bottom of the crypt was unchanged (Fig. S2 B and F). Indeed, the normal cell proliferation detected in Math1Δint mice by Ki67 staining in crypt compartments, which also expressed the Wnt target, cMyc, likely reflects normal β-catenin signaling (Fig. 2 I–L). In addition, expression of Axin2, transcription factor 5 (SP5), Musashi-1 (Msi1), and Sox9, which are four other β-catenin/Tcf target genes expressed in the base of the crypt, are also indicative of active β-catenin signaling in Math1-deficient mice (Fig. 2 M–Q). Moreover, these data are in agreement with the maintenance of Wnt-related stem cell marker expression in Math1Δint mice (Fig. 1 and Fig. S1).
These results indicate that in vivo Math1-deficient crypt cells are able to maintain active β-catenin signaling in the absence of the Wnt ligands (Wnt3, Wnt6, Wnt9) normally provided by Paneth cells. Therefore, this suggest that other signals, such as other Wnt ligands or other growth factors able to sustain β-catenin stability, may act as potential niche to maintain and activate the CBCs.
Wnt Source Is Necessary for Survival of Math1 Mutant Crypts in the Absence of a Subepithelial Cellular Niche.
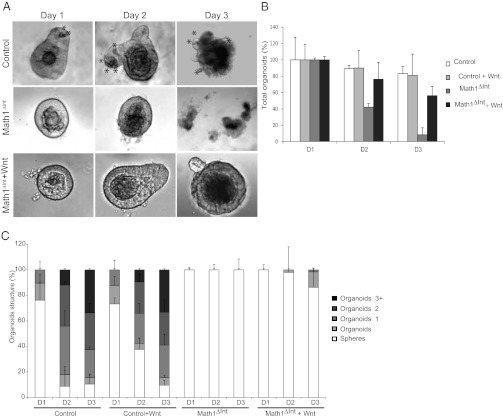
To determine whether Math1-deficient crypt cells are able to self-renew without the influence of the mesenchyme, we isolated crypts from Math1Δint mice and control mice 7 d after Tam injection and cultured the crypt organoids in vitro (27). In contrast to crypts from wild-type mice, which give rise to organoids, all of the crypts from the Math1Δint mice died within a few days of preparation (Fig. 3A). Following isolation, they first appeared as hollow spheres, disaggregated within 2–3 d, and died within a week (Fig. 3 B and C). The presence of Matrigel and specific growth factors ex vivo were insufficient to allow Math1-deficient crypt cells to self-renew and expand in culture. However, addition of Wnt3a in the medium led to a persistent rescue of Math1Δint crypts, allowing some of them to form budding crypt-like structures in 3 d, indicating that they grow at a slower rate (Fig. 3 A–C). To confirm this discrepancy between the ability of Math1-deficient crypts to grow in vivo but not ex vivo, we used another previously described mouse model for intestinal Math1 deletion in which mosaic deletion in the distal small intestine is driven by a synthetic fatty acid binding protein liver (Fabpl)-Cre transgene (Math1lox/lox; Fabpl4X AT132) (25). In these mice, patches of crypts retain Math1 (called Math1WT crypts) adjacent to patches of crypts that lack Math1 (Math1Fabp mutant crypts). As observed in our Math1Δint mice, Math1Fabp mutant crypts, identified by lack of Paneth cell granules, were unable to grow ex vivo, except in rare cases where nearby Math1WT crypts formed nascent organoids (Fig. S3 A–H). However, mutant organoids grew at a significantly slower rate and diminished capacity compared with wild type organoids as seen at 4 and 7 d (Fig. S3 A–D). As observed in Math1Δint crypts, addition of Wnt3a to the culture media protects against the death of Math1Fabp mutant crypts and allowed the growth of rescued organoids(Fig. S3 F–H).
Fig. 3.
Growth of Math1 mutant intestinal organoids required exogenous Wnt. (A) Crypt organoids from adult control mice form efficiently during the first 3 d of culture. Note the presence of Paneth cells (indicated by asterisks) in the crypts from control mice. Progressive degeneration of Math1-deficient crypts occurred between days 2 and 3 in culture. Addition of Wnt3a supported the growth of Math1 mutant crypts into organoids. (B and C) Quantification of the total number of organoids (B) and the organoid structural complexity (C) of crypts from each mouse genotype during the first 3 d of culture in presence or absence of exogenous Wnt3a.
Altogether, ex vivo Math1-deficient crypt cells are not able to self-renew or survive without a Wnt signal. The maintenance of active β-catenin signaling in CBCs observed in vivo in Math1-deficient mice suggests that potential extraepithelial signals coming from mesenchyme exist.
Intestinal Regeneration in the Absence of Math1.
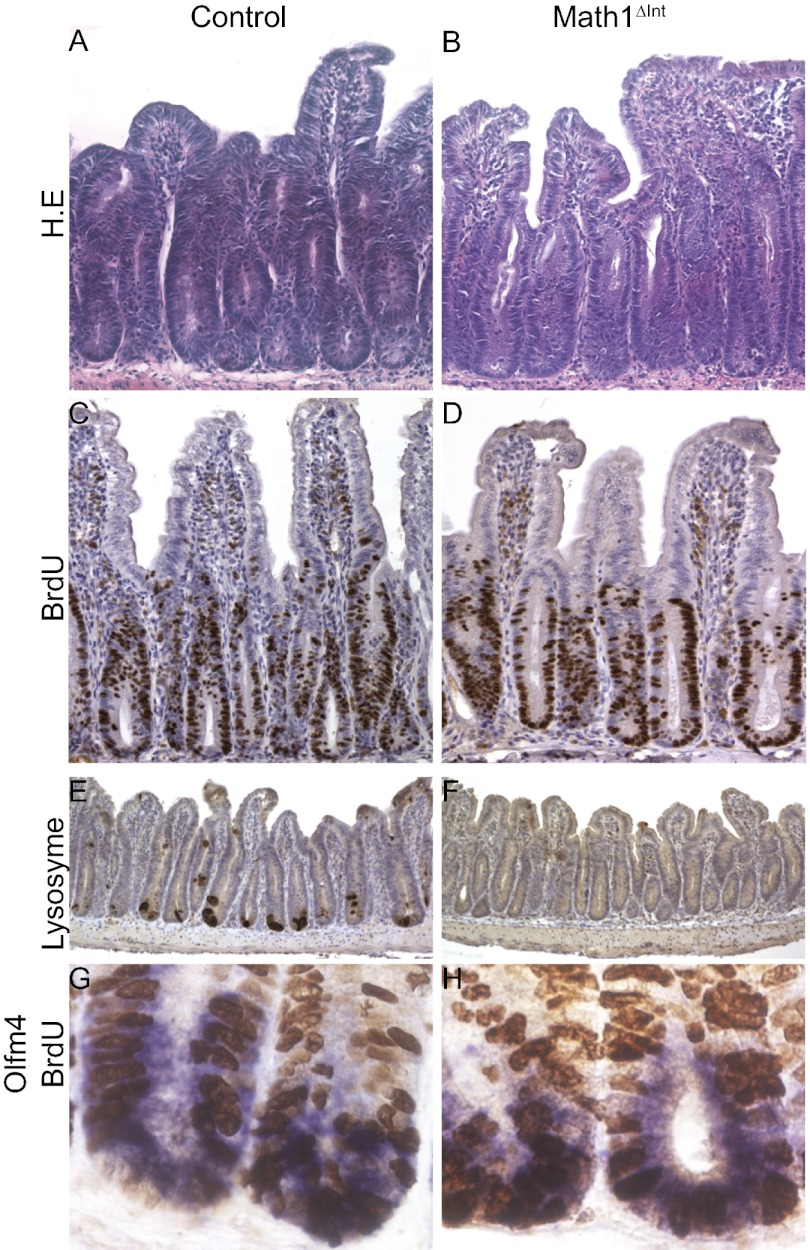
Next, we tested whether Math1-deficient stem cells were able to regenerate following γ-irradiation. Math1-deficient mice and control mice were injected with Tam, irradiated (10 Gy) 7 d later, and then killed 6 or 72 h after irradiation (Fig. S3). Following irradiation, crypts in the intestinal epithelium contain large numbers of apoptotic cells. This is followed by compensatory proliferation of intestinal stem cells and progenitor cells, which results in crypt regeneration. Six hours after irradiation, the apoptotic responses of all mice were similar, regardless of genotype (Fig. S4 A–E). Math1-deficient crypts underwent successful regeneration that resulted in large hyperproliferative crypts accompanied by prominent expansion of BrdU-positive cells that was similar to that observed in the control crypts (Fig. 4 A–D and Fig. S4F). Interestingly, Olfm4 expression decreased significantly after 6 h and then increased at 72 h in both genotypes (Fig. S4G). Double-staining for Olfm4 and BrdU showed that CBC stem cells were actively cycling in the Math1Δint mice, as well as in the control mice (Fig. 4 G and H). The absence of lysozyme staining showed that the regenerative crypts from Math1Δint mice had not escaped the Tam-induced recombination (Fig. 4 E and F). Together, these data suggest that Paneth cells are not required for CBC self-renewal in response to injury.
Fig. 4.
Math1 is dispensable for intestinal regeneration. (A–D) H.E. (A and B) and BrdU (C and D) staining of regenerative crypts from control and Math1-deficient mice 72 h following 10 Gy irradiation. (E and F) Lysozyme staining showing the efficient recombination of Math1-deficient crypts after Tam injection. (G and H) Costaining of Olfm4 (blue) and BrdU (brown) showing actively cycling CBCs in control and in Math1-deficient mice 72 h after irradiation.
Initiation of Tumorigenesis Does Not Depend on Paneth Cells Following Math1 Deletion.
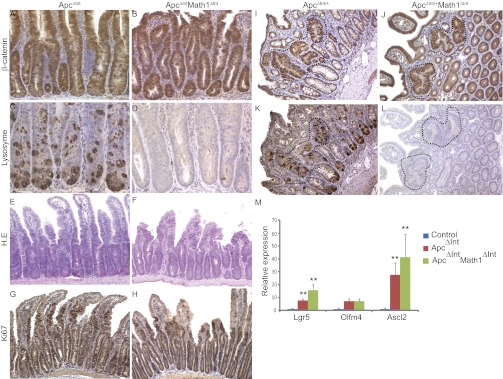
We and others have shown that β-catenin signaling is required for Paneth cell differentiation (16, 17, 28). Furthermore, cells expressing Paneth cell-like genetic programs are found in mouse and human tumors. The CBC has been described to act as cell of origin for intestinal tumorigenesis (29). We then wondered if Paneth cells, by producing several signaling molecules and growth factors, could influence the rapid initiation of tumorigenesis induced by Apc deletion within the CBC. For this, we examined whether the intestinal stem cells in Math1-deficient mice could undergo oncogenic transformation following Apc gene loss. We compared the hyperplasia phenotype induced by Apc deletion in the presence or absence of Math1 deletion. Thus, Math1lox/loxVilCreERT2 mice were crossed with mice carrying the Apc floxed allele to yield Apclox/loxMath1lox/loxVilCreERT2 mice (called ApcMath1 mutant mice or ApcΔintMath1Δint mice) (16). Math1 and Apc gene deletions were induced in these mice by Tam injection, and they were compared with Tam-injected Apclox/loxVilCreERT2 mice (called Apc mutant mice or ApcΔint). Five days after Tam injection, ApcMath1 mutant mice developed signs of ill health identical to those observed in Apc mutant mice (16). We confirmed deletion of the genes by staining for both lysozyme and β-catenin (Fig. 5 A–D) and then analyzed whether any differences in the respective hyperplasia phenotypes could be detected by comparing progenitor cell proliferation and CBC amplification in the mutant mice. ApcMath1 and Apc mutant mice both exhibited similarly higher levels of cell proliferation compared with that of control mice, as assessed by histological studies and Ki67 staining (Fig. 5 E–H and Fig. S5A). Next, we used quantitative RT-PCR to analyze the expression of the CBC stem cell markers Lgr5, Olfm4, and Ascl2. Apc mutant mice and ApcMath1 mutant mice both had amplified stem cell pools (Fig. 5M). Tam injection in heterozygous ApcΔint/+ mice led to spontaneous development of several adenomas after 70 d whereas the Tam-induced deletion of both Math1 and ApcΔint/+ synergized to promote intestinal tumorigenesis at 22 d (Fig. 5 I and K and Fig. S5 B and C). These results confirmed and extended our previous analyses, which indicated that the absence of Math1 rapidly promotes the development of intestinal tumors induced in mice (21, 30). Using serial sections and staining for β-catenin and lysozyme, we showed that in contrast to the microadenomas that developed in ApcΔint/+ mice, microadenomas that developed in ApcΔint/+Math1Δint mice were devoid of Paneth cells as seen in the surrounding nontumor epithelium (Fig. 5 J and L). Altogether, our results suggest that stem cells lacking adjacent Paneth cells are competent to initiate intestinal tumorigenesis.
Fig. 5.
Paneth cells are not required to initiate the intestinal tumorigenesis driven by Apc loss. (A–H) β-catenin (A and B), lysozyme (C and D), H.E. (E and F), and Ki67 (G and H) staining of small intestine from ApcΔInt and ApcΔIntMathΔInt mice 5 d after Tam injections. (I–L) β-Catenin (I and J) and lysozyme(K and L) staining of adenomas from ApcΔInt/+ and ApcΔInt/+MathΔInt mice, respectively, 70 and 22 d after Tam injections. (M) Real-time quantitative RT-PCR analysis of stem cell markers in the small intestine of ApcΔInt and ApcΔIntMathΔInt mice 5 d after Tam injections.
In conclusion, our results indicate that in the absence of Math1, the maintenance and activation of CBC can be driven by a mechanism that is independent of Paneth cells. Of note, it has been shown in three other genetic mouse models for Paneth cell loss, that Paneth cells are essential in vivo for the maintenance of crypts and stem cells (15). Our data strongly contrast with these results; in this scenario, we hypothesized that another source of signals (Wnt or other growth factors) are able to maintain stem cell proliferation after Math1 depletion. Several Wnt/β-catenin target genes appeared to be more highly expressed in the context of Math1 deletion, which might reflect an alternative mechanism of β-catenin signaling activation. Our data suggest that mesenchymal cells act as potential source of niche factors, but it remains to be determined which signal, such as Wnt or other growth factors, leads to the activity of β-catenin in CBCs. Of note, classic Paneth cells are not normally found in the colon, indicating that other cell types must be the source of stem cell niche factors in this tissue. In conclusion, the intestinal stem cell niche appears more complex than recently proposed. A revisitation of other niche signals, potentially from mesenchymal cells, able to efficiently maintain intestinal stem cells seems to be necessary to consider.
Materials and Methods
Mouse Colonies.
Math1lox/loxVilCreERT2, Math1lox/lox; Fabpl4X AT132, and Apclox/loxVilCreERT2 mice have been described previously (16, 25). All mice had a C57BL/6 background. All experiments involving mice were carried out in accordance with French or U.S. governmental regulations. Mice were housed in conventional conditions. Cre-recombinase was activated by injecting Tam (1 mg; ICN) on 4 consecutive days, and the mice were killed 5, 7, or 22 d after the first Tam injection. For proliferation studies, mice were injected with BrdU (2.5 mg; Sigma-Aldrich) 2 h before they were killed. Mice were exposed to 137Cs source of γ-irradiation with a dose rate of 1.47 Gy/min.
Microarray Analysis.
Microarray experiments were performed using Affymetrix Mouse Genome 430 2.0 GeneChips. Datasets were derived from three biological samples of each genotype and subjected to paired comparisons of multiple samples using Student’s t test with a false-discovery rate of 5%.
Immunohistochemistry and in Situ Hybridization.
Immunohistochemistry and in situ hybridization were performed as described previously (21). cRNA probes were generated from plasmids containing Axin2 (gift from F. Costantini, Department of Genetics and Development, Columbia University Medical Center, New York, NY), Olfm4, Wnt3, Wnt6, and Wnt9 (gifts from H. Clevers, Hubrecht Institute for Developmental Biology and Stem Cell Research and University Medical Centre Utrecht, Utrecht, Netherlands) cDNA sequences (14, 17, 31). Primary antibodies used for immunohistochemistry were specific for: lysozyme (Dako, 1/500), Ki67 (Novocastra; 1/300), β-catenin (Transduction Laboratories; 1/50), BrdU (Abcam; 1/500), Ascl2 (generously provided by H. Clevers; 1/5), cMyc (Santa Cruz; 1/50), and Sox9 (Millipore; 1/500). Total quantifications have been performed on three independent intestines from each group of mice; at least 50 crypts per sample were analyzed. The individual crypt position frequencies of apoptotic and mitotic cells were counted on at least 30 crypts per animal (n = 3).
RNA Extraction and Quantitative PCR.
Total RNA was extracted as described elsewhere (21). Reverse transcription was carried out using standard protocols (Roche Diagnostics), and quantitative PCR was performed on a LightCycler apparatus using LightCycler 480 SYBR Green I Master (Roche Diagnostics) reagents, and the values were expressed relative to 18S rRNA, as previously described. PCR primer sequences are available upon request. At least four animals of each genotype were analyzed in each group.
Crypt Isolation and Culture.
Crypt isolation and crypt culture were performed as previously described (27). Isolated crypts were plated on Matrigel with culture medium (Advanced DMEM/F12; Invitrogen) containing the growth factors: EGF (Peprotech); R-spondin 1 (R&D Systems); and noggin (Peprotech); and supplemented with N2 and B27 (Invitrogen). In some experiments, media were supplemented with 100 ng/mL human Wnt3a (Millipore). Three independent experiments were performed on control and Math1 mutant mice 7 d after Tam injection (control: n = 433 crypts; control + Wnt3: n = 640 crypts; Math1ΔInt: n = 621 crypts; Math1ΔInt + Wnt3: n = 995 crypts).
Electron Microscopy.
The tissues were fixed, treated, and stained as described previously (21).
Supplementary Material
Acknowledgments
We thank Dr. F. Costantini and Dr. H. Clevers for providing plasmids and antibodies. We thank M. Escobar and P. Jay for help with the organoid cultures, Etienne E. Dewailly and B. Radenen-Bussiere for technical assistance, all of the staff of the animal care facilities, and C. Lesaffre and M. Favier of the morphology and histology facility. This work was supported by grants from INCa, the Ligue Nationale Contre le Cancer, Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and NIH Grant CA142826.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201652109/-/DCSupplemental.
References
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Gerbe F, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 4.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Flier LG, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery RK, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda N, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 10.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 11.Kuhnert F, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorieff A, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreu P, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 17.Andreu P, et al. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol. 2008;324:288–296. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 19.Mori-Akiyama Y, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Bastide P, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peignon G, et al. Complex interplay between β-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166–176. doi: 10.1136/gut.2009.204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat. 1981;160:51–63. doi: 10.1002/aja.1001600105. [DOI] [PubMed] [Google Scholar]
- 23.Ireland H, Houghton C, Howard L, Winton DJ. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev Dyn. 2005;233:1332–1336. doi: 10.1002/dvdy.20446. [DOI] [PubMed] [Google Scholar]
- 24.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shroyer NF, et al. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 28.van Es JH, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 29.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 30.Kazanjian A, Noah T, Brown D, Burkart J, Shroyer NF. Atonal homolog 1 is required for growth and differentiation effects of notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010;139:918–928, 928, e1–e6. doi: 10.1053/j.gastro.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.