Abstract
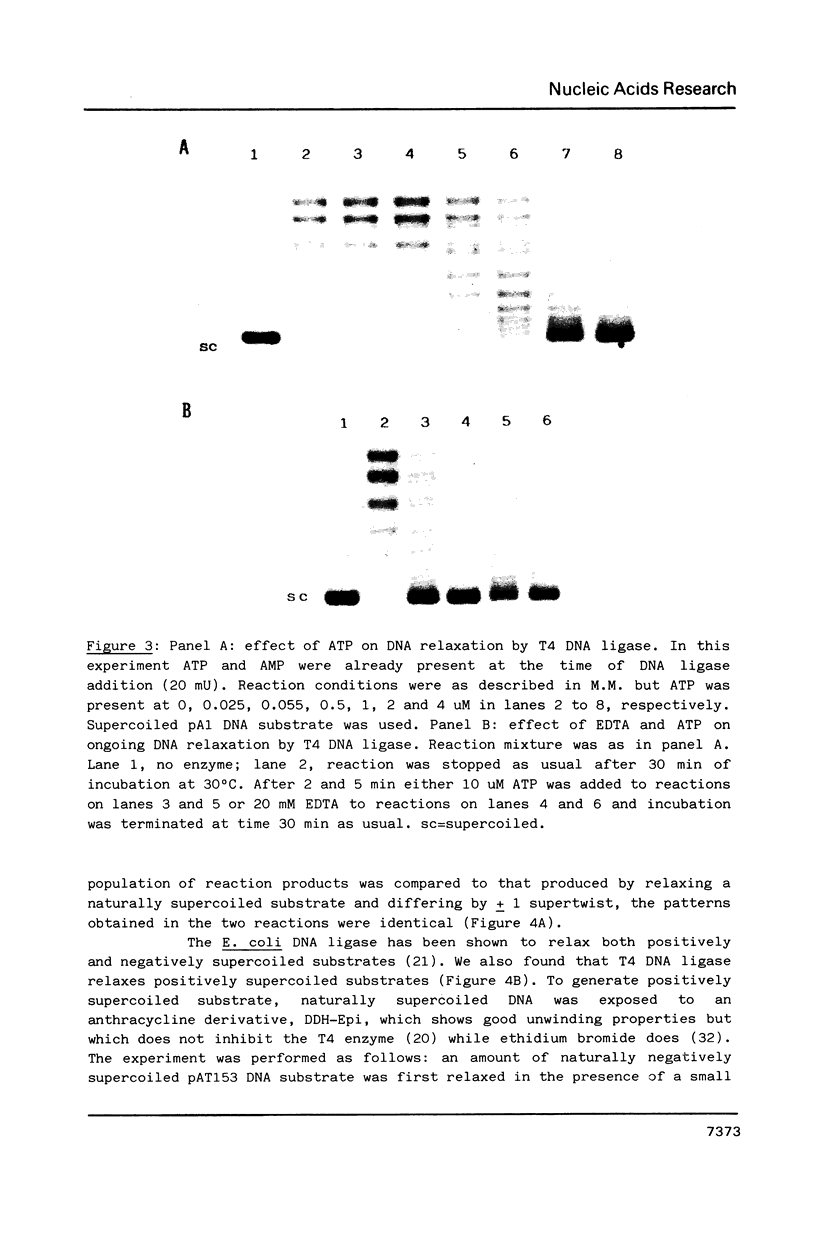
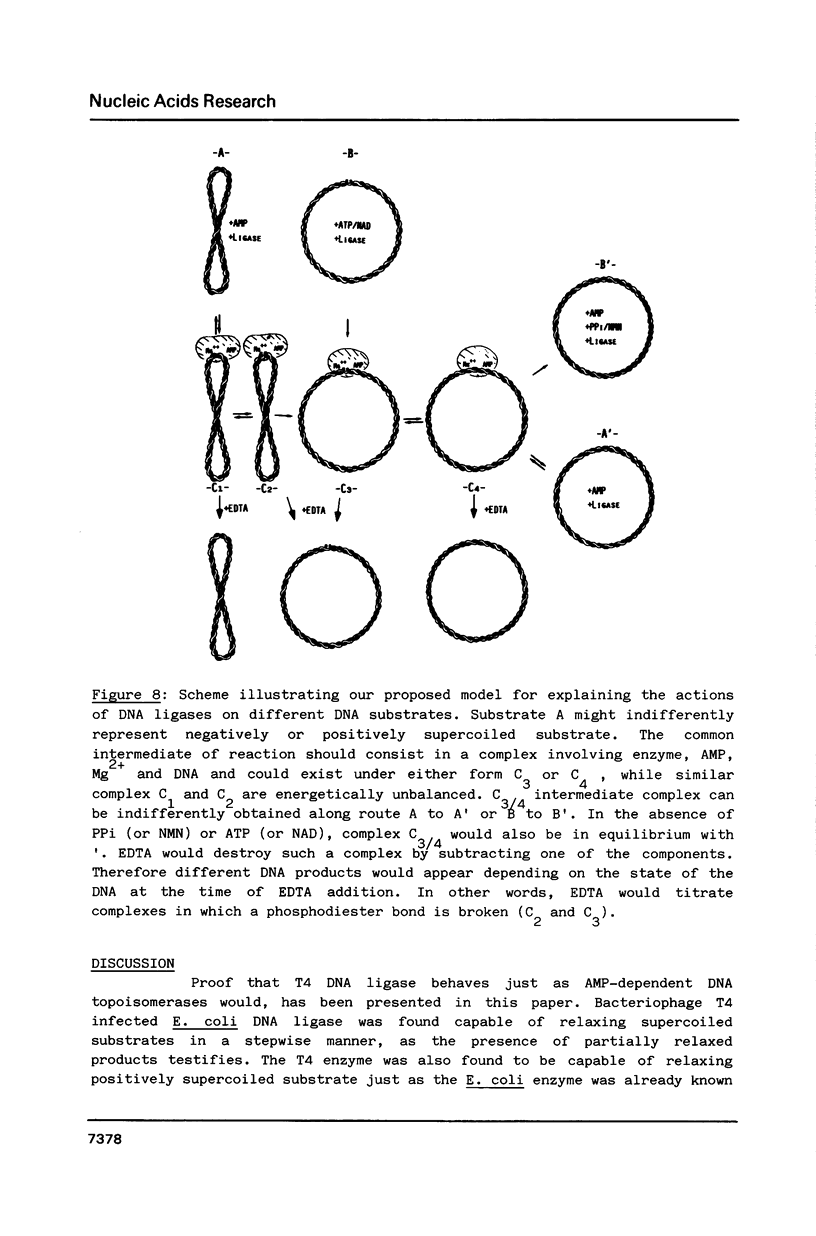
In the presence of AMP and Mg2+, a covalently closed duplex DNA containing negative superhelical turns was treated with DNA ligase isolated from bacteriophage T4-infected E. coli. This resulted in the gradual and not sudden loss of superhelical turns as for example in the case of type I DNA topoisomerase. All DNA products remain covalently closed. Since T4 enzyme-mediated DNA relaxation is inhibited by both pyrophosphate and by ATP this suggests that DNA relaxing and DNA joining activities probably coincide. EDTA addition in the presence of a large excess of enzyme, induces the formation of nicked DNA products while protein denaturing treatments are not very effective. Our observations might suggest an involvement of the relaxing activity of DNA ligase during the ligation process.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. G., Johnson A. L., Johnston L. H. An improved assay for DNA ligase reveals temperature-sensitive activity in cdc9 mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):458–462. doi: 10.1007/BF00425731. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Melechen N. E., Jovin T. M., Kornberg A. Polynucleotide cellulose as a substrate for a polynucleotide ligase induced by phage T4. Biochem Biophys Res Commun. 1967 Aug 23;28(4):578–586. doi: 10.1016/0006-291x(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. II. The structural gene for polynucleotide ligase in bacteriophage T4. Proc Natl Acad Sci U S A. 1967 Aug;58(2):665–672. doi: 10.1073/pnas.58.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre P., Mirambeau G., Jaxel C., Nadal M., Duguet M. High positive supercoiling in vitro catalyzed by an ATP and polyethylene glycol-stimulated topoisomerase from Sulfolobus acidocaldarius. EMBO J. 1985 Aug;4(8):2123–2128. doi: 10.1002/j.1460-2075.1985.tb03902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Becker A., Hurwitz J. The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc Natl Acad Sci U S A. 1967 Jul;58(1):240–247. doi: 10.1073/pnas.58.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc Natl Acad Sci U S A. 1967 Jan;57(1):148–155. doi: 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. Enzymatic joining of polynucleotides. VI. Activity of a synthetic adenylylated polydeoxynucleotide in the reaction. J Biol Chem. 1969 Jan 10;244(1):43–47. [PubMed] [Google Scholar]
- Harvey C. L., Gabriel T. F., Wilt E. M., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. IX. Synthesis and properties of the deoxyribonucleic acid adenylate in the phage T4 ligase reaction. J Biol Chem. 1971 Jul 25;246(14):4523–4530. [PubMed] [Google Scholar]
- Howell S. H., Stern H. The appearance of DNA breakage and repair activities in the synchronous meiotic cycle of Lilium. J Mol Biol. 1971 Feb 14;55(3):357–378. doi: 10.1016/0022-2836(71)90323-8. [DOI] [PubMed] [Google Scholar]
- Kessler B. Isolation, characterization and distribution of a DNA ligase from higher plants. Biochim Biophys Acta. 1971 Jul 29;240(4):496–505. doi: 10.1016/0005-2787(71)90706-4. [DOI] [PubMed] [Google Scholar]
- Lehman I. R. DNA ligase: structure, mechanism, and function. Science. 1974 Nov 29;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Edelman G. M. Polynucleotide ligase from myeloid and lymphoid tissues. Proc Natl Acad Sci U S A. 1968 Oct;61(2):680–687. doi: 10.1073/pnas.61.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic characterization of a mutant of Escherichia coli with an altered DNA ligase. Proc Natl Acad Sci U S A. 1971 May;68(5):1002–1005. doi: 10.1073/pnas.68.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R., Wang J. C. Enzymatic joining of polynucleotides. XI. Reversal of Escherichia coli deoxyribonucleic acid ligase reaction. J Biol Chem. 1972 Oct 10;247(19):6370–6372. [PubMed] [Google Scholar]
- Nilsson S. V., Magnusson G. Sealing of gaps in duplex DNA by T4 DNA ligase. Nucleic Acids Res. 1982 Mar 11;10(5):1425–1437. doi: 10.1093/nar/10.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Hall Z. W., Lehman I. R. Enzymatic joining of polynucleotides, V. A DNA-adenylate intermediate in the polynucleotide-joining reaction. Proc Natl Acad Sci U S A. 1968 Sep;61(1):237–244. doi: 10.1073/pnas.61.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini A. M., Ciarrocchi G. Inhibition of Micrococcus luteus DNA topoisomerase I by UV photoproducts. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1787–1791. doi: 10.1073/pnas.80.7.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin B. A., Chase J. W. DNA ligase from Drosophila melanogaster embryos. Substrate specificity and mechanism of action. J Biol Chem. 1987 Oct 15;262(29):14105–14111. [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaramella V., Van de Sande J. H., Khorana H. G. Studies on polynucleotides, C. A novel joining reaction catalyzed by the T4-polynucleotide ligase. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1468–1475. doi: 10.1073/pnas.67.3.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Ciarrocchi G., Falaschi A. Purification and properties of a polynucleotide ligase from human cell cultures. Eur J Biochem. 1971 Sep 13;22(1):75–78. doi: 10.1111/j.1432-1033.1971.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Spadari S., Pedrali-Noy G., Focher F., Montecucco A., Bordoni T., Geroni C., Giuliani F. C., Ventrella G., Arcamone F., Ciarrocchi G. DNA polymerases and DNA topoisomerases as targets for the development of anticancer drugs. Anticancer Res. 1986 Sep-Oct;6(5):935–940. [PubMed] [Google Scholar]
- Spadari S., Sutherland B. M., Pedrali-Noy G., Focher F., Chiesa M. T., Ciarrocchi G. Alteration of DNA tertiary structure by physical and chemical carcinogens: involvement in DNA repair processes. Toxicol Pathol. 1987;15(1):82–87. doi: 10.1177/019262338701500111. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. DNA ligases of eukaryotes. FEBS Lett. 1976 Aug 1;67(1):1–8. doi: 10.1016/0014-5793(76)80858-7. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Sumikawa T., Tsukada K. Purification of DNA ligase II from calf thymus and preparation of rabbit antibody against calf thymus DNA ligase II. J Biol Chem. 1986 May 25;261(15):6888–6892. [PubMed] [Google Scholar]
- Tsukada K., Ichimura M. Polynucleotide ligase from rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1156–1161. doi: 10.1016/0006-291x(71)90026-x. [DOI] [PubMed] [Google Scholar]
- Tsukada K., Nishi A. Polynucleotide ligase from cultured plant cells. J Biochem. 1971 Sep;70(3):541–542. doi: 10.1093/oxfordjournals.jbchem.a129669. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Macromolecular crowding allows blunt-end ligation by DNA ligases from rat liver or Escherichia coli. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5852–5856. doi: 10.1073/pnas.80.19.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]