Abstract
Resting dendritic cells (DCs) induce tolerance of peripheral T cells that have escaped thymic negative selection and thus contribute significantly to protection against autoimmunity. We recently showed that CD4+Foxp3+ regulatory T cells (Tregs) are important for maintaining the steady-state phenotype of DCs and their tolerizing capacity in vivo. We now provide evidence that DC activation in the absence of Tregs is a direct consequence of missing DC–Treg interactions rather than being secondary to generalized autoimmunity in Treg-less mice. We show that DCs that lack MHC class II and thus cannot make cognate interactions with CD4+ T cells are completely unable to induce peripheral CD8+ T-cell tolerance. Consequently, mice in which interactions between DC and CD4+ T cells are not possible develop spontaneous and fatal cytotoxic T lymphocyte-mediated autoimmunity.
DCs are bone marrow (BM)-derived, short-lived cells that play a key role in regulating immune responses. They are located at low frequency in lymphoid and nonlymphoid organs throughout the body, where they act as sentinels for invading pathogens. On recognition of pathogen-associated cues, DCs undergo a series of functional changes termed maturation, which is characterized by the up-regulation of costimulatory molecules such as CD80, CD86, and CD70, by the production of cytokines, such as IL-12, and by the expression of homing receptors, such as CCR7, that direct DC migration into the T-cell areas of secondary lymphoid organs. Together these changes allow DCs to efficiently activate naive T cells.
Over the past decade, it has become clear that steady-state DCs play an important role in the maintenance of self-tolerance (1–3) and T-cell responsiveness (4). Because DCs can either prime or tolerize naive T cells, depending on their activation status, they are master regulators of adaptive immunity. How the maturation status of DCs translates into differential effects on naive T cells involves a variety of costimulatory and coinhibitory interactions between T cells and DCs. For example, we showed that tolerance induction depends on PD-1 and CTLA-4 (5), whereas blockade of CD70, a costimulatory molecule that is up-regulated on activated DCs, prevents priming of CD8+ T cells even in the context of an infection (6). Along the same line, constitutive transgenic expression of CD70 on all DCs prevents the induction of tolerance and results in priming of a functional T-cell response by steady-state DCs (7).
DC maturation can be triggered by numerous exogenous and endogenous stimuli that are usually associated with infection, inflammation, or damage (8). It is conceivable that minor perturbations of the steady state could induce DC maturation, even when a full-blown immune response would not be required or would be detrimental. Under such circumstances, regulatory mechanisms are required to counterbalance the activating signals and keep DCs in a resting state. We recently demonstrated that these suppressive signals can be provided by Foxp3+ CD25+ CD4+ regulatory T cells (Tregs) (9). Foxp3+ CD25+ CD4+ Tregs are important mediators of immune homeostasis and peripheral tolerance, and the absence of Tregs results in fatal autoimmune disease in mice and humans (10–14). Tregs may control self-reactive T-cell responses by direct interaction with conventional T cells or, alternatively, may act on DCs (15). Evidence for the latter comes from experiments in which depletion of Tregs results in Flt3-dependent increases in the number of DCs as well as in DC activation (9, 11, 16).
When we combined the DIETER model of peripheral tolerance induction by steady-state DCs and the DEREG model of conditional ablation of Tregs, we found that depletion of Tregs not only resulted in DC activation, but also impaired induction of tolerance by DCs (9). Whether DC activation and impaired tolerance induction after depletion of Tregs are a direct result of the loss of Treg control over the DC or a consequence of systemic autoimmunity after Treg depletion is unclear, however.
To address this question, we generated mixed BM chimeric mice in which half of the antigen-presenting cells (APCs) were negative for MHC class II and thus could not have cognate interaction with regulatory T cells. We found that the release of steady-state DCs from CD4+ T-cell control resulted in DC activation, completely abolished induction of peripheral CD8+ T-cell tolerance, and resulted in spontaneous fatal CTL autoimmunity.
Results
Induction of Peripheral Tolerance by DCs Is Impaired in the Absence of CD4+ T Cells.
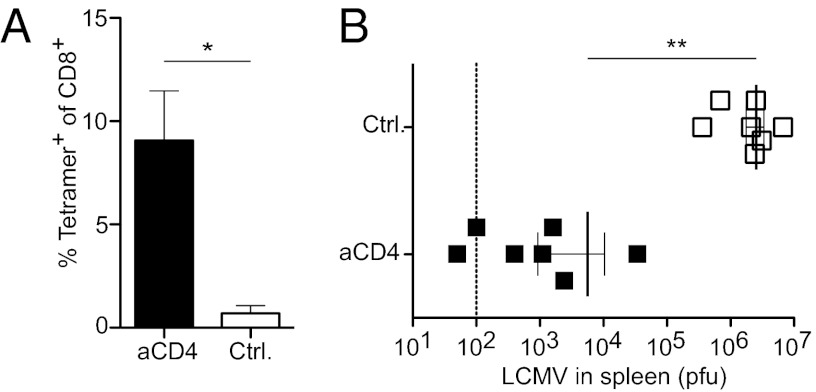
Injection of DIETER mice with tamoxifen leads to expression and presentation of transgene-encoded CTL epitopes by DCs, which results in the induction of robust peripheral T-cell tolerance (3), which is severely compromised in the absence of Tregs (9). Rudensky et al. (11) showed that autoimmunity as well as the expansion of DCs that occurs after depletion of Tregs depend on autoreactive CD4+ T cells. Thus, we tested whether the impaired tolerance induction by steady-state DCs after Treg depletion in DIETER mice was secondary to CD4+ T-cell–dependent autoimmunity. We compared tolerance induction by steady-state DCs in DIETER mice depleted of CD4+ T cells and undepleted DIETER mice. As expected, we found no substantial expansion of transgene-specific CD8+ T cells in DIETER mice 8 d after tamoxifen injection (Fig. 1A) (3). In contrast, we observed massive expansion of lymphocytic choriomeningitis virus (LCMV) GP33–41 CD8+ T cells in CD4+ T-cell–depleted mice, which lack both regulatory and conventional CD4+ T cells (Fig. 1A). To test whether the CD8+ T cells primed by steady-state DCs in the absence of CD4+ cells were functional effector CTLs, we challenged the mice with 200 pfu of the LCMV strain WE and determined virus titers in the spleen 5 d later. We found significantly reduced virus titers in CD4-depleted mice (Fig. 1B), indicating that antigen presentation by steady-state DCs in the absence of CD4+ T cells results in priming of functional effector CTLs. Interestingly, CTL priming by steady-state DCs after depletion of all CD4+ T cells was considerably stronger than observed after depletion of FoxP3+ cells using DEREG transgenic mice that express a diptheria toxin receptor in FoxP3+ cells (9). This raised the question of whether other suppressive CD4+ T cells besides FoxP3+ Tregs might be involved in maintaining the tolerogenic state of steady-state DCs.
Fig. 1.
Steady-state DCs induce priming of functional CTLs instead of tolerance after CD4 depletion. DIETER mice were injected i.v. with 0.5 mg of GK1.5 (closed symbols) or isotype control (open symbols) on day −1. On day 0, antigen presentation was induced by injection of 2 mg of tamoxifen i.p. (A) On day 8, expansion of GP33-41 specific CTLs was detected in the blood by staining with MHC class I tetramers. (B) On day 8, mice were challenged with 200 pfu of LCMV-WE i.v., and virus titers in the spleen were determined on day 13. n = 7 per group. Results are representative of four independent experiments.
Given that the CD4-depleting antibody is much more efficient in depleting FoxP3+ cells compared with depletion in the DEREG model, in which 10% of FoxP3+ cells remain after diphtheria toxin treatment (14), we made use of FoxP3.LuciDTR-5 transgenic mice, which allow depletion of >95% of FoxP3+ cells (17). We found comparable CTL priming after efficient depletion of FoxP3+ cells and CD4 depletion, indicating that FoxP3+ Tregs are the critical suppressive CD4+ T-cell population that maintain steady-state DC in a tolerogenic state (Fig. S1).
Comparing the expression of activation markers on DCs isolated from spleen and lymph nodes in untreated and CD4-depleted mice showed significant up-regulation of CD80, CD86, and CD40 cells in the latter (Fig. S2). This phenotype was similar to the activation observed in mice depleted of FoxP3+ cells only (9). However, in line with the observations of Kim et al. (11) in SMARTA × Rag−/− mice, which do not develop Tregs but lack self-reactive T cells, we found no increase in the number of DCs in CD4-depleted mice (Fig S2). Thus, DC activation and compromised tolerance induction stem directly from the lack of CD4+ T-cell–mediated suppression, whereas the increased numbers of DCs seen after Treg depletion, but not after CD4 depletion, appears to be a consequence of the activation of autoreactive CD4+ T cells in the absence of Tregs.
Because the transgene in DIETER mice encodes for both the CTL epitopes and the immunodominant I-Ab restricted LCMV CD4+ T-cell epitope GP61-81, it seems conceivable that CD4+ T-cell suppression of DCs in our model was specifically directed against this transgene-encoded determinant. However, induction of antigen presentation in DIETER mice by tamoxifen injection did not induce proliferation of LCMV GP61-81–specific SMARTA TCR transgenic CD4+ T cells (Fig. S3), indicating that the transgene-encoded fusion protein does not enter the MHC class II presentation pathway. Thus, the suppressive CD4+ T cells required for CD8+ T-cell tolerance induction are presumably specific for MHC class II-restricted self-peptides.
DCs Require Cognate Interactions with CD4+ T Cells to Induce CD8+ T-Cell Tolerance.
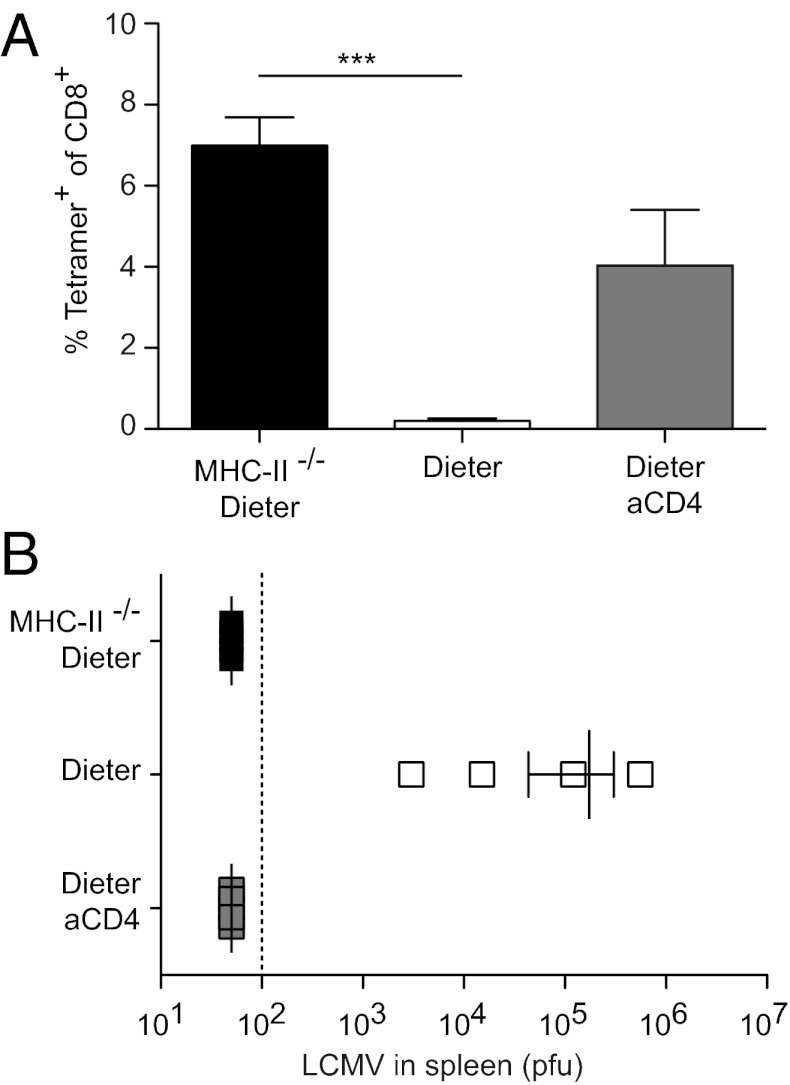
To establish whether the crucial role of CD4+ T cells in peripheral tolerance induction involves cognate interactions between DCs and suppressive CD4+ T cells, we created a model situation in which the DCs that express the antigen of interest lack MHC class II and thus are unable to form cognate interactions with CD4+ T cells. We bred DIETER mice to I-Ab α-chain–deficient animals, which are devoid of MHC class II expression (II−/−DIETER). We then generated mixed BM chimeric mice by reconstituting lethally irradiated C57BL/6 mice with equal numbers of II−/−DIETER and of C57BL/6(CD45.1) BM cells. Control mice were reconstituted with equal numbers of MHC class II-proficient DIETER and of C57BL/6(CD45.1) BM cells. To verify equal reconstitution in the DC compartment, we analyzed CD45.1/CD45.2 ratios in various cell populations isolated from blood, spleen, and lymph nodes of mice at 6 wk after BM transplantation. We found that although B cells were derived mainly from WT BM, T cells and DCs were equally derived from WT and MHC class II-deficient BM. Tamoxifen injection of mixed chimeric mice resulted in massive expansion of GP33-41-specific CD8+ T cells when the GP33-41–presenting DCs (DIETER DCs) lacked expression of MHC class II (Fig. 2A) and the size of the response was comparable to that seen in CD4-depleted DIETER mice (Fig. 1A). Again, the expanded CD8+ T cells were functional effector CTLs, providing protection against an LCMV challenge infection (Fig. 2B). Thus, DCs that cannot form cognate MHC class II/TCR interactions with CD4+ T cells fail to tolerize naïve CD8+ T cells, but instead prime functional effector CTLs.
Fig. 2.
Antigen presentation on MHC class II-deficient DCs results in functional CTL priming instead of tolerance induction. Mixed irradiation BM chimeric mice that had been reconstituted with equal numbers of MHC-II−/− DIETER + WT (CD45.1) (closed bars) or equal numbers of DIETER + WT (CD45.1) (open bars) BM cells were i.p. injected with 2 mg of tamoxifen on day 0 to induce antigen presentation on DCs. As controls, DIETER + WT mixed chimeric mice were depleted of CD4+ T cells on day −1. (A) On day 8, expansion of GP33-41–specific CTLs was detected in the blood by staining with MHC class I tetramers. (B) On day 8, mice were challenged with 200 pfu of LCMV-WE i.v., and virus titers in the spleen were measured on day 13. **P < 0.01. n = 4 per group. Results are representative of four independent experiments.
To exclude the possibility that impaired peripheral tolerance induction in II−/−DIETER mixed BM chimeras was due to a defect in the Treg compartment, we compared the frequency of FoxP3+ Tregs in different lymphoid compartments of these chimeras and found no differences (Fig. 3). Moreover, CD25+ CD4+ regulatory T cells isolated from chimeras lacking MHC class II expression on half of their APCs demonstrated normal suppressive function in vitro and in vivo. They were as effective as Treg cells isolated from WT mice in suppressing proliferation of T cells in vitro (Fig. S4). When cotransferred together with Treg-depleted CD4+ T cells into RAG1-deficient mice, they were fully capable of suppressing the autoimmunity that develops on transfer of Treg-depleted CD4+ T cells alone (Fig S5) (17). This indicates that expression of MHC class II on half of the APCs in mixed BM chimeric mice is sufficient to select and maintain normal numbers of FoxP3+ Tregs with intact suppressive capacity.
Fig. 3.
Normal numbers of FoxP3+ CD4+ regulatory T cells in mice lacking MHC class II expression on half their APCs. Frequencies of FoxP3+ CD4+ T cells among all lymphocytes in WT + WT (Upper) and MHC class II−/−/WT (Lower) mixed BM chimeric mice were determined by flow cytometry at 7 wk after reconstitution. Data are mean (SEM). n = 5.
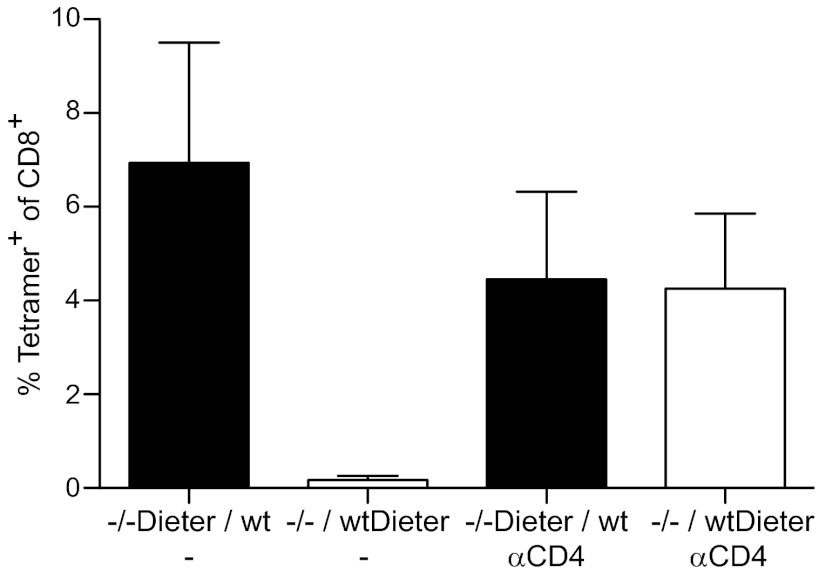
To further verify that the inability to induce peripheral tolerance in MHC-II−/−DIETER + WT chimeric mice is not due to a defect in suppressive CD4+ T cells or any other systemic defects in these chimeras, but rather is restricted to the MHC class II-deficient DCs in cis, we generated mixed BM chimeric mice in the reverse way, such that the DIETER DCs that present GP33-41 are MHC class II-proficient and thus can establish cognate interactions with CD4+ T cells and the WT DCs are MHC class II-deficient. Tamoxifen injection did not result in expansion of GP33-41–specific CTLs (Fig. 4), in sharp contrast to the mice in which the DIETER DCs are MHC class II-deficient. These data clearly demonstrate that in chimeric mice that lack MHC class II expression on half of their APCs, only DCs that lack MHC class II cannot induce peripheral CD8+ T-cell tolerance, whereas MHC class II-expressing DC can do so. This finding indicates that the inability of MHC class II-deficient DCs to induce peripheral CD8+ T-cell tolerance is caused not by a defect in the Treg compartment, but presumably by the inability of suppressive CD4+ T cells to interact with steady-state DCs that present antigens to CD8+ T cells.
Fig. 4.
Loss of tolerizing capacity by MHC class II-deficient, but not MHC class II-competent, DCs in mice lacking MHC class II expression on half of their APCs. Mixed irradiation BM chimeric mice that had been reconstituted with equal numbers of MHC-II−/−DIETER and WT (CD45.1) BM cells (closed bars) or equal numbers of MHC class II−/− and WT DIETER BM cells (open bars) were injected with 2 mg of tamoxifen i.p. on day 0 to induce antigen presentation on DCs. Some mice had been depleted of CD 4+ T cells on day −1. On day 8, expansion of LCMV GP33-41–specific CD8+ T cells was detected in the blood by staining with MHC class I tetramers. n = 5 per group. Results are representative of two independent experiments.
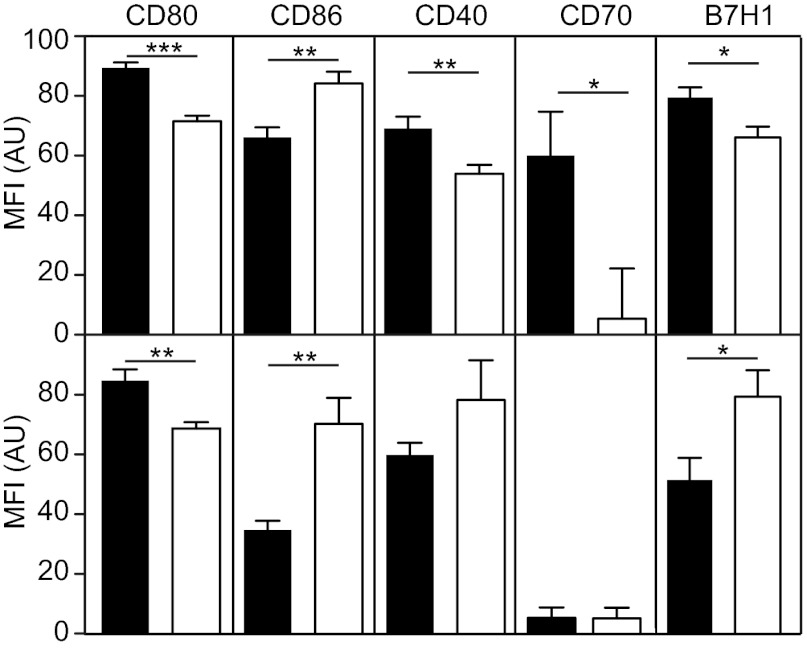
Flow cytometric analysis of MHC class II-deficient and MHC class II-proficient DCs from lymph nodes and spleen of MHC class II−/− + WT chimeric mice revealed greater expression of costimulatory molecules in MHC class II-deficient DCs than in WT DCs (Fig. 5). MHC class II-deficient DCs from the spleen expressed more CD80, CD40, CD70, and B7H1 cells than WT DCs. Activation of MHC class II-negative DCs was less obvious on lymph node DCs, where only CD80 was significantly up-regulated. Unexpectedly, CD86 expression was reduced on MHC class II-deficient DCs in both spleen and lymph nodes; however, we have no explanation for this finding, and no data on the implications.
Fig. 5.
MHC II-deficient DCs are activated compared with WT DCs. Expression of CD80, CD86, CD40, CD70, and B7-H1 on CD45.1+ (WT, open bars) and CD45.1− (MHC class II−/−, filled bars) DCs isolated from spleen (Upper) or pooled peripheral lymph nodes (Lower) of MHC class II−/− + WT (CD45.1) mixed BM chimeric mice was quantified by flow cytometry. *P < 0.05; **P < 0.01; ***P < 0.001. Median fluorescence intensities of 10 mice are shown. Results are representative of three independent experiments.
DCs That Cannot Interact with CD4+ T Cells Induce CTL-Mediated Autoimmunity.
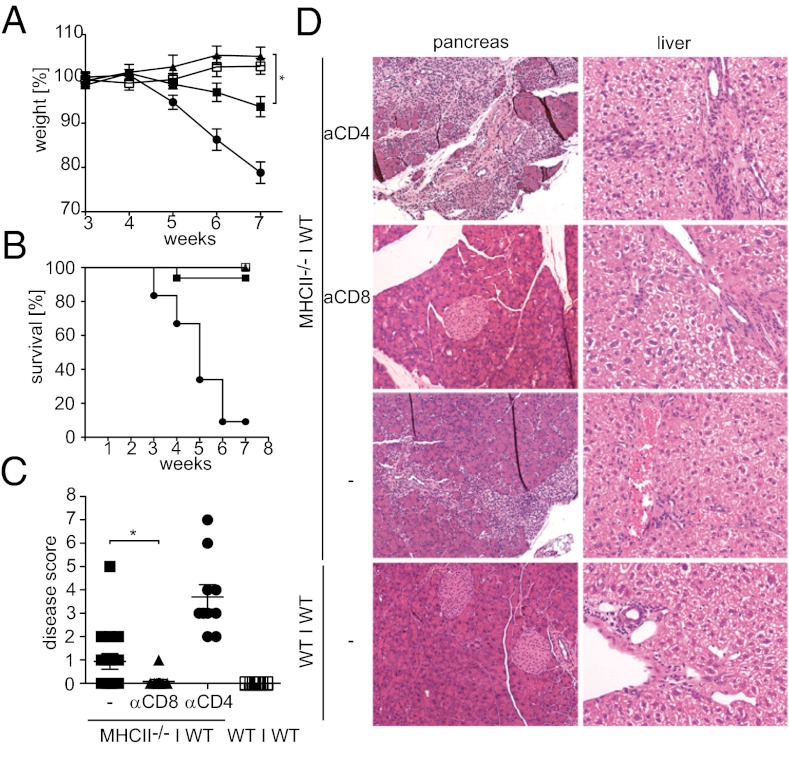
We noticed that mixed BM chimeric mice that received BM from MHC class II-deficient animals exhibited progressive deterioration in their general condition beginning at 4–6 wk after BM transplantation. Mice that had received 50% MHC class II-deficient BM exhibited moderate weight loss, with some animals showing additional disease symptoms, including hunched posture, scaly skin, and lack of flight; in contrast, none of the chimeras that had received only WT BM showed any disease symptoms (Fig. 6 A–C). To identify the cell type causing the disease in MHC class II−/− + WT mixed chimeras, we used monoclonal antibodies to deplete these mice of CD4+ or CD8+ T cells starting at 3 wk after transplantation. Depletion of CD8+ T cells completely prevented disease, suggesting that autoreactive CD8+ T cells are crucial (Fig. 6 A–C). In stark contrast, depletion of CD4+ T cells severely exacerbated disease (Fig. 6 A–C); all CD4+ T-cell–depleted mice exhibited multiple disease symptoms by 6 wk after transplantation and had to be killed by 10 wk after reconstitution. Histological examination of the animals revealed mononuclear infiltrates in the pancreas and liver of MHC class II−/− + WT mixed chimeras (Fig. 6D). No infiltrates were found in CD8-depleted chimeras, whereas CD4-depleted chimeras exhibited large infiltrates in the liver and almost complete destruction of the exocrine pancreas (Fig. 6D). Occasionally some focal infiltrates were found in the colon of CD4+-depleted chimeras, but no infiltrates were found in lung, skin, stomach, small intestine, or kidney. The severe liver and pancreas pathology in MHC class II + WT chimeras after CD4 depletion was also manifested by elevated serum transaminase levels and decreased serum levels of pancreatic amylase and lipase (Fig. S6). Consistent with CD8+ T-cell–mediated autoimmunity, we found a high percentage of activated (CD44high, CD62Llow) CD8+ T cells in the lymph nodes draining the inflamed pancreas, as well as in a peripheral lymph node unrelated to an evidently affected organ (Fig. S7).
Fig. 6.
Release of APCs from CD4+ T-cell control results in autoimmunity caused by CD8+ T cells. MHC-II−/− + WT mixed BM chimeric mice (closed symbols) and WT + WT control chimeric mice (open symbols) were either left untreated (squares) or depleted of CD8+ T cells (triangles) or CD4+ cells (circles) starting at 3 wk after reconstitution. (A) Body weight was measured weekly over 10 wk after BM reconstitution. (B) Survival of mixed BM chimeric mice after BM reconstitution. (C) Disease score (posture, weight loss, activity, fur texture, and skin integrity) of mixed BM chimeric mice was determined at 7 wk after BM reconstitution. (D) Histological analysis of pancreas and liver of untreated or CD4-depleted MHC-II−/− + WT and WT + WT mixed BM chimeric mice at 7 wk after reconstitution.
To establish whether the CD8+ T-cell autoimmunity in MHC II−/− + WT mixed chimeras is induced by MHC class II-deficient DCs, we bred MHC II−/− mice to CD11cDTR mice (18), which allow selective depletion of DC by diphtheria toxin, and generated MHC II−/−CD11cDTR + WT mixed BM chimeras. Depletion of the MHC class II-deficient DCs prevented the development of autoimmune disease (Fig. S8), demonstrating that CTL autoimmunity is indeed driven by DCs that cannot form cognate interactions with suppressive CD4+ T cells.
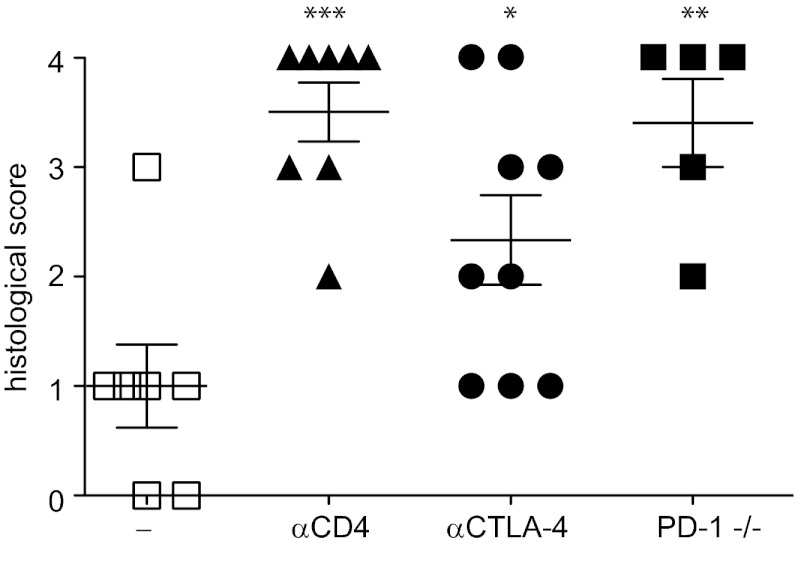
We previously reported that the inhibitory receptors PD-1 and CTLA-4 are required for tolerization of CD8+ T cells by steady-state DCs (5). Blockage of CTLA-4 in MHC II−/− + WT mixed chimeras by a monoclonal antibody produced a significant aggravation of autoimmune disease (Fig. 7). Moreover, reconstitution of irradiated mice with a mixture of MHC II−/− and PD-1−/− BM, so that half of the T cells were deficient for PD-1, resulted in significantly more severe autoimmune disease as assessed by mononuclear infiltration of the exocrine pancreas (Fig. 7). Thus, the critical roles of PD-1 and CTLA-4 for peripheral tolerance induction by steady-state DC are reflected by their requirement for suppression of autoimmune disease in MHC II−/− + WT mixed chimeras.
Fig. 7.
PD-1 and CTLA-4 are involved in suppression of CD8+ T-cell–mediated autoimmunity. MHC-II−/− + WT mixed BM chimeric mice and MHC-II−/− + PD-1−/− chimeric mice were either left untreated (squares) or depleted of CD4+ T cells (triangles) or treated with a monoclonal antibody blocking CTLA-4 (circles) starting at 3 wk after reconstitution. Comparisons with the untreated MHC-II−/− + WT group: P < 0.05; *P < 0.01; ***P < 0.001. At 6 wk after reconstitution, mononuclear infiltration to the pancreas was scored on H&E-stained sections.
Taken together, our data demonstrate that cognate interactions between steady-state DCs and CD4+ T cells are crucial to maintain peripheral CD8+ T-cell tolerance and prevent CTL-mediated autoimmunity.
Discussion
Mechanisms of peripheral T-cell tolerance are essential to control autoreactive T cells that have escaped thymic negative selection. Dominant mechanisms, involving cells that actively suppress the activation of self-reactive lymphocytes, as well as recessive or T-cell intrinsic mechanisms, which act by inactivation or deletion of self-reactive lymphocytes in the periphery, have been described previously (15). FoxP3+ CD4+ Tregs are of unique importance among the dominant mechanisms (15), whereas steady-state DCs play a central role in the recessive arm of peripheral tolerance induction (19). There is growing evidence that these two arms not only act synergistically, but also are highly interdependent. Steady-state DCs in both the intestinal environment and secondary lymphoid organs can induce FoxP3+ Tregs (iTregs), and DCs drive homeostatic proliferation of FoxP3+ Tregs that were generated in the thymus (nTregs) (16, 20, 21). On the other hand, Tregs can suppress DC activation (reviewed in ref. 15).
We show here that the tolerogenic function of steady-state DCs is absolutely dependent on the cognate interaction between CD4+ Tregs and DCs via MHC class II. Steady-state DCs that cannot present antigens to Tregs because of a lack of MHC class II expression induce differentiation of naïve T cells into effector CTLs instead of tolerance. A comparison of surface phenotypes of MHC class II-deficient and MHC class II-proficient DCs isolated from the same animal revealed increased expression of CD80, CD40 and CD70 on MHC class II-deficient DCs, suggesting that cognate suppression of DCs is necessary to prevent their spontaneous maturation in response to weak proinflammatory or pathogen-associated stimuli that are present even in the absence of an overt infection (22–24). Along with inhibiting DC maturation, cognate DC–Treg interactions might be important to induce tolerogenic mechanisms in DCs, such as the up-regulation of indeolamine 2,3-dioxygenase (25) and intracellular cAMP (26). Other suppressive mechanisms of Tregs that act on T cells directly, such as production of TGF-β or activation of latent TGF-β (27–29), generation of pericellular adenosine (30), or up-regulation of intracellular cAMP (31), may require simultaneous interaction of Tregs and naïve T cells with the same steady-state DCs for tolerance induction. Identification of the Treg effector mechanisms involved in peripheral tolerance induction by steady-state DCs is needed to further elucidate the interdependence of Tregs and DCs. However, the absolute dependence on the cognate interaction between Tregs and DCs for tolerance induction suggests that Tregs and steady-state DCs actually represent two sides of the same coin rather than complementary mechanisms of peripheral tolerance.
In the DIETER model for peripheral tolerance induction, the transgenic T-cell epitopes presented on DCs are all MHC class I-restricted (3). Thus, the MHC class II-dependent interaction of Tregs with steady-state DCs that is instrumental for CD8+ T-cell tolerance induction presumably involves MHC class II-restricted self-peptides. This is consistent with the notion that nTregs recognize constitutively expressed peripheral self-antigens (32).
BM chimeric mice in which a part of the APC population does not express MHC class II develop spontaneous autoimmune disease. Histological analysis of the diseased mice revealed massive mononuclear infiltrates in the exocrine pancreas, some infiltrates in the liver, and occasional infiltrates in the colon. The preferential infiltration of the exocrine pancreas is in line with previous studies in mice lacking MHC class II expression on all cells (33) or only on hematopoietic cells (34), both of which describe pancreatitis as the most prominent disease manifestation. Thus, the pancreas appears to be particularly susceptible to CD8+ T-cell–mediated autoimmunity when peripheral APCs lack MHC class II expression. Whether tolerance against antigens of the exocrine pancreas is especially dependent on peripheral mechanisms or whether the pancreas is particularly sensitive to attack by autoreactive CD8+ T cells remains to be determined. Some previous studies reported widespread destructive colitis in MHC class II-deficient mice (35, 36), which we did not observe. In the light of a recent study showing that germ-free mice developed more pancreatitis but less intestinal inflammation on depletion of Tregs (37), these different disease manifestations might be explained by environmental conditions.
CTL-mediated autoimmune disease in BM chimeras lacking MHC class II on hematopoietic cells has been shown to result from impaired control of autoreactive CD8+ T cells by FoxP3+ CD4+ Tregs (34). Although these chimeras have normal numbers of peripheral FoxP3+ CD4+ Tregs, they cannot be activated because of the lack of MHC class II in the periphery and thus lack suppressive function. We circumvented this problem by generating mixed BM chimeras in which only half of the APCs lacked MHC class II. We found that peripheral tolerance induction in DIETER mice was perfectly normal in these chimeric mice if transgenic MHC class I-restricted antigens were expressed on the MHC class II-expressing DCs, excluding the possibility that systemic defects, such as impaired Treg function, are responsible for impaired tolerance. However, massive CTL priming resulted when the transgenic MHC class I-restricted antigens were expressed on MHC class II-deficient DCs. These opposite outcomes of the interaction between naïve CD8+ T cells and steady-state DCs—tolerance if the DCs are MHC class II-proficient but priming if the DCs lack MHC class II—are reflected by the autoimmune disease in the mixed BM chimeras. Self-antigens that are recognized by the pathogenic T cells in these mice are presented simultaneously on both MHC class II-deficient and WT DCs; thus, the pathogenic T cells will have both activating and tolerizing encounters with DCs, resulting in a situation of partially suppressed autoimmunity. The animals have mononuclear infiltrations in the pancreas and liver and mild weight loss, but usually survive. In this situation, interference with the tolerizing capacity of the WT DCs by depletion of CD4+ T cells or blockade of PD-1 or CTLA-4 signaling results in severe aggravation and fatal outcome of the autoimmune disease.
In conclusion, our data demonstrate that cognate interactions between steady-state DCs and regulatory CD4+ T cells are crucial to maintain peripheral CD8+ T-cell tolerance and prevent CD8+ T-cell–mediated autoimmunity. Our results suggest that in the face of DC-activating signals that are omnipresent even in the absence of overt infection, constant suppression of DCs by regulatory T cells is required to maintain the DCs’ tolerogenic steady state and avert the threat of autoimmunity.
Materials and Methods
Treatment of Mice.
Cre recombinase activity resulting in antigen presentation by steady-state DCs was induced in vivo by injecting DIETER BM recipients i.p. with 2 mg of tamoxifen, as described previously (3). In some experiments, CD4+ T cells were depleted 1 d before tamoxifen administration by i.v. injection of 0.5 mg of GK1.5. Priming of endogenous, GP33–41/Db- specific CD8+ T cells was measured 8 d after tamoxifen injection by tetramer staining. The effector function of GP33–41/Db-specific CD8+ T cells was assessed based on their capacity to protect against an i.v. challenge with 200 plaque-forming units (pfu) of LCMV-WE. CD8+ or CD4+ T cells were depleted by weekly i.v. injection of 0.5 mg of YTS169.4 or GK1.5, respectively. CTLA-4 was blocked by weekly i.v. injection of 0.5 mg of 4F10.
Systemic Analysis of Autoimmune Disease.
The body weight of BM chimeric mice was monitored weekly after reconstitution. Mice were killed when weight loss exceeded 25%. At 7 wk after reconstitution, the degree of autoimmune disease was assessed using a scoring system that sums changes in five clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index value, 10) (38).
Histology.
Mice were killed by cervical dislocation, and tissues for histology were fixed in 4% buffered formalin for at least 24 h. Tissue samples were embedded in paraffin, sectioned at a thickness of 3 μm, and stained with H&E. The severity of inflammation was determined by scoring the degree of the mononuclear infiltration into tissues (0, none; 1, mild; 2, moderate; 3, moderate and diffuse or severe but focal; 4, severe and diffuse).
Mice, reagents, generation of BM chimeras, DC isolation and phenotyping, and flow cytometric analysis are described in more detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Günter Hämmerling and Natalio Garbi (Deutsches Krebsforschungszentrum) for providing the FoxP3.LuciDTR-5 mice; Claudia Braun (Forschungszentrum Immunologie Mainz) for performing the histology analyses; the team of the central animal facility of the Johannes Gutenberg University of Mainz for providing expert animal care; and Maries van den Broek (Zurich University Hospital) for helpful discussions and a critical reading of the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grant GK1043 (to H.C.P. and H.S.) and the Mainzer Forschungsförderung (MAIFOR) Program of the Johannes Gutenberg University Medical Center Mainz (H.C.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110620109/-/DCSupplemental.
References
- 1.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady-state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonifaz L, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 4.Garbi N, Hämmerling GJ, Probst HC, van den Broek M. Tonic T cell signaling and T cell tolerance as opposite effects of self-recognition on dendritic cells. Curr Opin Immunol. 2010;22:601–608. doi: 10.1016/j.coi.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 6.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 7.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schildknecht A, et al. FoxP3+ regulatory T cells essentially contribute to peripheral CD8+ T-cell tolerance induced by steady-state dendritic cells. Proc Natl Acad Sci USA. 2010;107:199–203. doi: 10.1073/pnas.0910620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 13.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 14.Lahl K, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Darrasse-Jèze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suffner J, et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 18.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 20.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki S, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang B, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 24.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 25.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 26.Fassbender M, et al. Cyclic adenosine monophosphate and IL-10 coordinately contribute to nTreg cell-mediated suppression of dendritic cell activation. Cell Immunol. 2010;265:91–96. doi: 10.1016/j.cellimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Fahlén L, et al. T cells that cannot respond to TGF-β escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Travis MA, et al. Loss of integrin α(v)β8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bopp T, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Vallance BA, Hewlett BR, Snider DP, Collins SM. T cell-mediated exocrine pancreatic damage in major histocompatibility complex class II-deficient mice. Gastroenterology. 1998;115:978–987. doi: 10.1016/s0016-5085(98)70270-7. [DOI] [PubMed] [Google Scholar]
- 34.Poitrasson-Rivière M, et al. Regulatory CD4+ T cells are crucial for preventing CD8+ T cell-mediated autoimmunity. J Immunol. 2008;180:7294–7304. doi: 10.4049/jimmunol.180.11.7294. [DOI] [PubMed] [Google Scholar]
- 35.Mombaerts P, et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 36.Marguerat S, MacDonald HR, Kraehenbuhl JP, van Meerwijk JP. Protection from radiation-induced colitis requires MHC class II antigen expression by cells of hemopoietic origin. J Immunol. 1999;163:4033–4040. [PubMed] [Google Scholar]
- 37.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med. 2010;207:2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teshima T, et al. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood. 2003;102:429–435. doi: 10.1182/blood-2003-01-0266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.