Type 2 diabetes is caused by relative insulin deficiency (1). Both impaired peripheral insulin signaling and defective insulin secretion contribute to this condition. Over the last century, especially the last 30 years, major research progress has been made in understanding molecular pathways in insulin signaling and molecular regulation of insulin secretion (2–4). Although the major targets of peripheral insulin actions are muscle, liver, and fat tissue, multiple components in the insulin signaling have been shown to regulate insulin secretion from pancreatic β-cells, and inactivation of insulin signaling by genetic deletions of key insulin signaling components is associated with impaired insulin secretion. However, the molecular cross-talk between insulin signaling and insulin secretion remains poorly understood. In PNAS, the work by Cheng et al. (5) describes that adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1 (APPL1), a binding partner of Akt2 and a key regulator of insulin signaling, is involved in the regulation of insulin secretion.
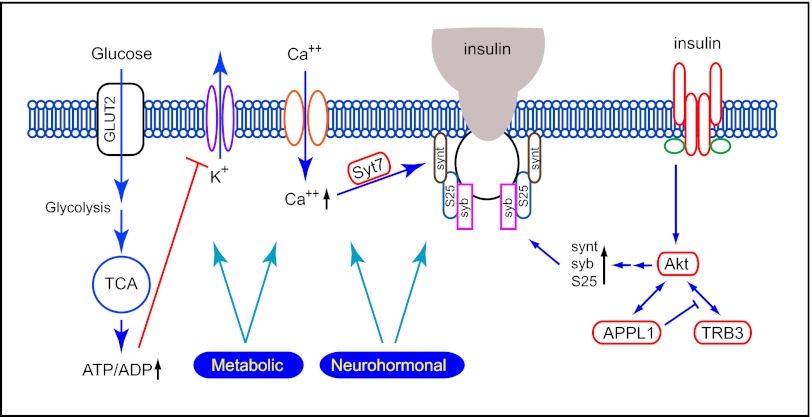
The cellular events and molecular players for insulin secretion are fairly well-understood (1). In physiological conditions, pancreatic β-cells secrete insulin in response to elevated blood glucose levels. Once inside β-cells, glucose undergoes glycolysis and the tricarboxylic acid cycle, resulting in increased ATP/ADP ratio. This process, in turn, shuts down the ATP-sensitive potassium channels on the plasma membrane and causes membrane depolarization, opening of L-type calcium channels, and calcium influx (Fig. 1). Like in neurotransmitter release, increased intracellular calcium levels serve as a triggering signal for insulin granule exocytosis. The triggering signal is relayed by calcium-sensing proteins to soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, which execute the fusion of insulin granule and plasma membrane to release insulin from β-cells. For insulin secretion, synaptotagmin-7 has been shown to be a calcium sensor (Fig. 1) (1). However, deletion of synaptotagmin-7 causes only 40–50% reduction of insulin secretion, indicating that additional calcium-sensing proteins are involved in regulating insulin secretion (1). The identities of the other calcium sensors remain to be determined. SNARE proteins, including syntaxin-1, synaptosomal-associated protein of 25 kDa (SNAP25), and synaptobrevin-2 [or vesicle-associated membrane protein-2 (VAMP2)], function in the final steps of membrane fusion, likely by providing the energy for lipid bilayer merger (6, 7). Although we are still in the process of understanding the interaction between the SNARE proteins and their regulatory proteins in the control of insulin granule exocytosis, there is strong experimental evidence that some of these proteins are also under the regulation of various signaling pathways, including insulin signaling (8).
Fig. 1.
Cross-talk between insulin secretion and insulin signaling. Multiple components of insulin secretion are under the regulation of metabolic and neurohormonal signaling, including insulin signaling. APPL1 is involved in the direct regulation of insulin secretion, possibly by up-regulating the expression of SNARE proteins. GLUT2, glucose transporter 2; S25, SNAP-25; syb, synaptobrevin; synt, syntaxin; Syt7, synaptotagmin-7; TCA, tricarboxylic acid cycle; TRB3, tribble-3.
Among the multiple signaling cascades activated by insulin, its metabolic actions are mediated predominantly by the PI3K/Akt pathway (9, 10). Akt is a central player in insulin signaling, and it regulates a number of cellular functions that are critical to glucose and lipid homeostasis. Impaired Akt activation is a hallmark of insulin resistance in animals and humans. The Akt activity is tightly regulated by its interacting partners, including Tribble-3 (11) and APPL1 (12) (Fig. 1). For example, Akt plays a critical role in insulin-stimulated glucose transporter 4 storage vesicle translocation and fusion with the plasma membrane in skeletal muscle and adipocytes, and this action is at least partially mediated by APPL1.
In this study, Cheng et al. (5) investigate the function of APPL1 in insulin secretion. Besides regulating Akt activation and determining substrate specificity (13), APPL1 interacts with adiponectin receptors and mediates the insulin-sensitizing effects of adiponectin in muscle and endothelial cells (14, 15). The first hint that APPL1 may participate in the regulation of insulin secretion comes from the glucose phenotyping analysis of APPL1 KO mice. Deletion of APPL1 results in higher fasting glucose and insulin levels, glucose intolerance, and more importantly, impaired glucose- and arginine-stimulated insulin secretion in vivo. The work by Cheng et al. (5) also shows that glucose-stimulated insulin secretion (GSIS) and high KCl-stimulated insulin secretion are also impaired in isolated islets. Because arginine and KCl directly depolarize the β-cells, bypassing the steps of glucose uptake and metabolism, and KATP channel closure, the observed insulin secretion impairment must be because of defects at or downstream of calcium channel activity.
GSIS exhibits a biphasic pattern consisting of a rapid first phase and a sustained second phase. The first phase requires rapid and marked elevation of calcium levels, whereas the second phase requires amplifying signals from glucose metabolism in addition to oscillatory Ca2+ concentrations (1). The work by Cheng et al. (5) examines the role of APPL1 in each phase by perifusion experiments of insulin secretion. Interestingly, deletion of APPL1 specifically affects the first phase, with no detectable changes in the second phase. It has been proposed that the first phase of insulin secretion is supported by docked granules. Consistent with this model, the work by Cheng et al. (5) finds that the number of docked granules is reduced in APPL1 KO β-cells.
Besides the loss-of-function approach by studying the APPL1 KO mice, the work by Cheng et al. (5) uses the same set of tests in transgenic mice with APPL1 overexpression. Consistent with the KO findings, the APPL1 transgenic mice seem to be resistant to high-fat diet-induced glucose intolerance and impaired insulin secretion. The transgenic mice under high-fat diet also exhibit milder hyperglycemia and hyperinsulinemia than their control group under the same diet. Pancreatic islets from the APPL1 transgenic mice also show improved GSIS and high KCl-stimulated insulin secretion and an increased number of docked granules. These results, along with the findings from APPL1 KO mouse analysis, establish that APPL1 indeed plays a key role in insulin secretion regulation.
Because the defect for the impaired insulin secretion is identified to be downstream of membrane depolarization, the work by Cheng et al. (5) examines calcium influx by measuring intracellular calcium concentrations under glucose stimulation, but it finds that the β-cells from APPL1 KO mice behave similarly to their control group. This finding thus identifies the defect to be downstream of the calcium influx, which may be at the calcium-sensing step and/or the SNARE-mediated fusion step. The work by Cheng et al. (5) then examines the expression levels of the key proteins involved in these steps and finds that SNARE proteins syntaxin 1, SNAP25,
The work by Cheng et al. shows the importance of a key regulator of insulin signaling in insulin secretion.
and synaptobrevin/VAMP2 are down-regulated, whereas Munc18 and synaptotagmin-7, two key regulators of insulin secretion, are not affected. Reintroducing APPL1 in APPL1 KO β-cells rescues the expression levels of syntaxin, SNAP25, and synaptobrevin and leads to increased glucose- and high KCl-stimulated insulin secretion. These findings support the notion that APPL1 is a positive regulator of insulin secretion, possibly through its actions in modulating SNARE protein levels.
Several groups have shown that APPL1 regulates insulin signaling by potentiating Akt activities in adipocytes (16), hepatocytes (12), and muscle cells (14), and therefore, the work by Cheng et al. (5) examines whether such an action also applies to β-cells. Indeed, insulin-stimulated Akt phosphorylation is impaired in APPL1 KO β-cells, whereas a parallel insulin-dependent ERK phosphorylation is not affected. The work by Cheng et al. (5) then reasons that, if the effect of APPL1 is because of reduced Akt phosphorylation, then increasing Akt action should restore the defect in APPL1 KO mice. Therefore, the work by Cheng et al. (5) expresses a constitutively active Akt in APPL1 KO islets and examines whether SNARE protein levels and GSIS could be restored. As predicted, Akt activation could bypass the requirement of APPL1 and largely rescues the secretion defects as observed in APPL1 KO islets.
As expected from significant studies, although the work by Cheng et al. (5) shows the importance of a key regulator of insulin signaling in insulin secretion, it also raises a number of important unanswered questions (5). First, how does APPL1 regulate SNARE protein transcription and translation? Does this require direct interaction of APPL1 with the affected target proteins? If so, does this interaction affect SNARE core complex formation? An alternative model may be that the regulation is mediated by indirect APPL1 action through Akt and possibly FoxO1. Second, APPL1 seems to affect only the first phase of insulin secretion. The biphasic insulin secretion is attributed to distinct pools of insulin granules based on their location, age, and/or biochemical state. If the molecular composition of distinct pools is identical (i.e., all of the insulin granules contain the same set of molecular machineries), then one would expect that both phases of insulin secretion should be affected. The work by Cheng et al. (5) seems to support the alternative model (i.e., each pool of granules is molecularly distinct), and only the pool for the first phase is regulated by APPL1. The notion of insulin granule pools of distinct molecular composition is supported by others (17); however, this model remains to be further validated.
Acknowledgments
Research in the laboratory of W.H. is supported by intramural funding from the Agency for Science Technology and Research (A*STAR) Biomedical Research Council.
Footnotes
The author declares no conflict of interest.
See companion article on page 8919.
References
- 1.Gustavsson N, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci USA. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustavsson N, Han W. Calcium-sensing beyond neurotransmitters: Functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci Rep. 2009;29:245–259. doi: 10.1042/BSR20090031. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 4.Gerber SH, Südhof TC. Molecular determinants of regulated exocytosis. Diabetes. 2002;51(Suppl 1):S3–S11. doi: 10.2337/diabetes.51.2007.s3. [DOI] [PubMed] [Google Scholar]
- 5.Cheng KKY, et al. APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc Natl Acad Sci USA. 2012;109:8919–8924. doi: 10.1073/pnas.1202435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 8.Gustavsson N, Wu B, Han W. Calcium sensing in exocytosis. Adv Exp Med Biol. 2012;740:731–757. doi: 10.1007/978-94-007-2888-2_32. [DOI] [PubMed] [Google Scholar]
- 9.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 10.Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 11.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 12.Cheng KK, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–427. doi: 10.1016/j.cmet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Schenck A, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Mao X, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 15.Cheng KK, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 16.Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- 17.Jewell JL, Oh E, Thurmond DC. Exocytosis mechanisms underlying insulin release and glucose uptake: Conserved roles for Munc18c and syntaxin 4. Am J Physiol Regul Integr Comp Physiol. 2010;298:R517–R531. doi: 10.1152/ajpregu.00597.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]