Abstract
Insulin resistance and defective insulin secretion are the two major features of type 2 diabetes. The adapter protein APPL1 is an obligatory molecule in regulating peripheral insulin sensitivity, but its role in insulin secretion remains elusive. Here, we show that APPL1 expression in pancreatic β cells is markedly decreased in several mouse models of obesity and diabetes. APPL1 knockout mice exhibit glucose intolerance and impaired glucose-stimulated insulin secretion (GSIS), whereas transgenic expression of APPL1 prevents high-fat diet (HFD)-induced glucose intolerance partly by enhancing GSIS. In both pancreatic islets and rat β cells, APPL1 deficiency causes a marked reduction in expression of the exocytotic machinery SNARE proteins (syntaxin-1, synaptosomal-associated protein 25, and vesicle-associated membrane protein 2) and an obvious decrease in the number of exocytotic events. Such changes are accompanied by diminished insulin-stimulated Akt activation. Furthermore, the defective GSIS and reduced expression of SNARE proteins in APPL1-deficient β cells can be rescued by adenovirus-mediated expression of APPL1 or constitutively active Akt. These findings demonstrate that APPL1 couples insulin-stimulated Akt activation to GSIS by promoting the expression of the core exocytotic machinery involved in exocytosis and also suggest that reduced APPL1 expression in pancreatic islets may serve as a pathological link that couples insulin resistance to β-cell dysfunction in type 2 diabetes.
Type 2 diabetes mellitus (T2DM) is a heterogeneous metabolic disease resulting from a combined defect in both actions and secretion of insulin. β-cell dysfunction is the major contributor to the development of T2DM (1). Impaired first-phase insulin secretion is often observed in the very early stage of T2DM and also in impaired glucose tolerance (2). However, the molecular mechanism underlying the pathogenesis of β-cell dysfunction is vaguely understood.
Although the peripheral metabolic tissues are the main targets of insulin, mounting evidence from animal and human studies suggests that the β cell itself also possesses an insulin signaling system, which plays a critical role in regulating β-cell mass, survival, insulin biosynthesis, and secretion (3). β-cell–specific inactivation of several components involved in insulin signaling, including insulin receptor (IR), insulin receptor substrate (IRS)-2, class IA phosphatidylinositol 3-kinase (PI3K), and Akt, leads to impaired insulin secretion and/or decreased β-cell mass (4–7). By contrast, transgenic expression of active Akt or IRS-2 in β cells increases β-cell mass and enhances insulin secretion, thereby rendering the mice resistant to experimental diabetes (8, 9). In humans, the expression levels of several insulin signaling molecules (IR, IRS-2, and Akt2) are reduced in pancreatic islets isolated from patients with T2DM (10). On the other hand, early intensive insulin therapy can improve β-cell function and glycemic control in patients with T2DM, further supporting the beneficial effect of insulin on β-cell functions (11). However, the molecular pathways that couple insulin signaling to insulin secretion in the β cells remain enigmatic.
APPL1, an adapter protein containing an NH2-terminal Bin/Amphiphysin/Rvs domain (BAR), a central pleckstrin homology (PH) domain, and a COOH-terminal phosphotyrosine-binding domain (PTB), is a critical regulator of endocytotic pathways (12). As a binding partner of Akt2 (13), this adapter protein has emerged as an important mediator of insulin actions in the liver (14), skeletal muscles (15), adipocytes (16), and endothelium (17). In the liver, APPL1 potentiates the inhibitory effects of insulin on hepatic gluconeogenesis via Akt activation, and overexpression of APPL1 in liver alleviates hyperglycemia in db/db diabetic mice (14). In skeletal muscles and adipocytes, APPL1 mediates insulin-stimulated glucose uptake via controlling the translocation of cytosolic glucose transporter 4 to the plasma membrane (15, 16). In the blood vessels, APPL1 counteracts obesity-induced vascular insulin resistance by shifting the vasoconstrictor effect of insulin to nitric oxide-dependent vasodilatation (17). Furthermore, APPL1 interacts with the adiponectin receptors, thereby mediating the insulin-sensitizing effects of this adipokine (18, 19).
Although APPL1 is abundantly expressed in the pancreas (13), its roles in insulin secretion and β-cell functions have never been explored. During the metabolic phenotyping analysis of APPL1 knockout (KO) mice (17), we observed a marked impairment of glucose-stimulated insulin secretion (GSIS) in this rodent model. In this study, we further investigated the pathophysiological role of APPL1 in modulating insulin secretion in both lean and obese mice, and elucidated the molecular mechanisms underlying APPL1 functions in pancreatic β cells.
Results
APPL1 Expression in Pancreatic Islets Is Decreased in both Dietary and Genetic Obese Mice.
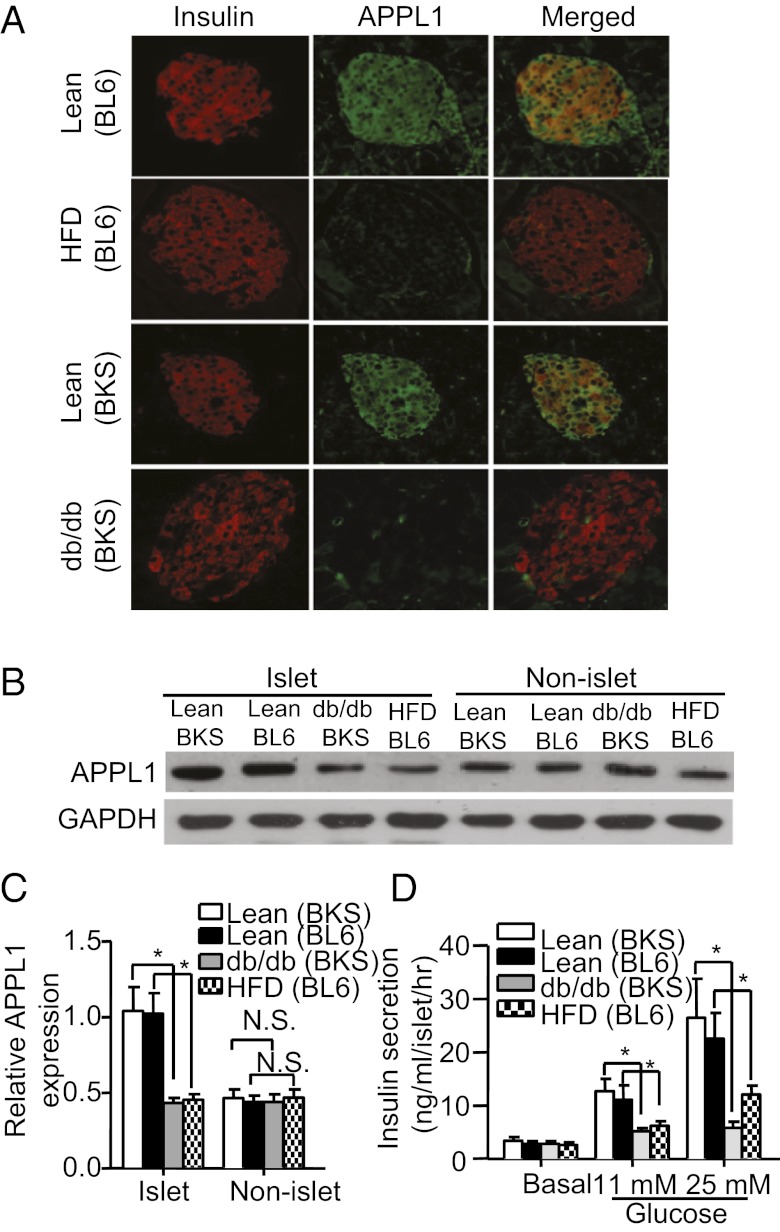
A previous study has detected a high level of pancreatic APPL1 mRNA expression in mice (13). We further examined the expression and distribution pattern of APPL1 in pancreatic tissues. Both immunofluorescence staining and cell fractionation analysis detected a much higher level of APPL1 protein in islet fraction compared with nonislet fraction (exocrine cells) in the pancreas of C57 lean mice (Fig. 1 A and B). In pancreatic islets, a large proportion of APPL1 colocalized with insulin, suggesting that this adapter protein is abundantly expressed in β cells. Notably, APPL1 expression was significantly decreased in islet cells of both high-fat diet (HFD)-induced obese mice and genetically inherited db/db obese/diabetic mice compared with those of lean controls (Fig. 1 A–C), whereas there was no obvious difference in APPL1 expression in nonislet cells or other metabolic tissues, including liver and skeletal muscles (Fig. S1). In line with previous reports (20, 21), we found that pancreatic islets isolated from both diet-induced obese mice and db/db diabetic mice displayed a significant reduction in GSIS compared with age-matched lean controls (Fig. 1D).
Fig. 1.
Decreased APPL1 expression in pancreatic islets and impaired GSIS in both dietary and genetic obese mice. (A) Immunofluorescence staining of APPL1 (green) and insulin (red) in pancreas sections of 12-wk-old male C57BL/6J (BL6) lean mice on standard chow, or high-fat diet (HFD), or C57BKS db/+ lean mice or C57BKS (BKS) db/db obese/diabetic mice. (B) Western blot analysis of APPL1 in nonislet (exocrine cells) and islet fractions isolated from the pancreas of C57BL/6J lean, or HFD-fed obese mice, or C57BKS db/+ lean or db/db mice. (C) Densitometric analysis for the relative abundance of APPL1 as in B. (D) Static GSIS in islets isolated from different mouse models as specified. *P < 0.05 (n = 5). NS, not significant.
Genetic Ablation of APPL1 Causes Glucose Intolerance and Impairment of Insulin Secretion in Mice.
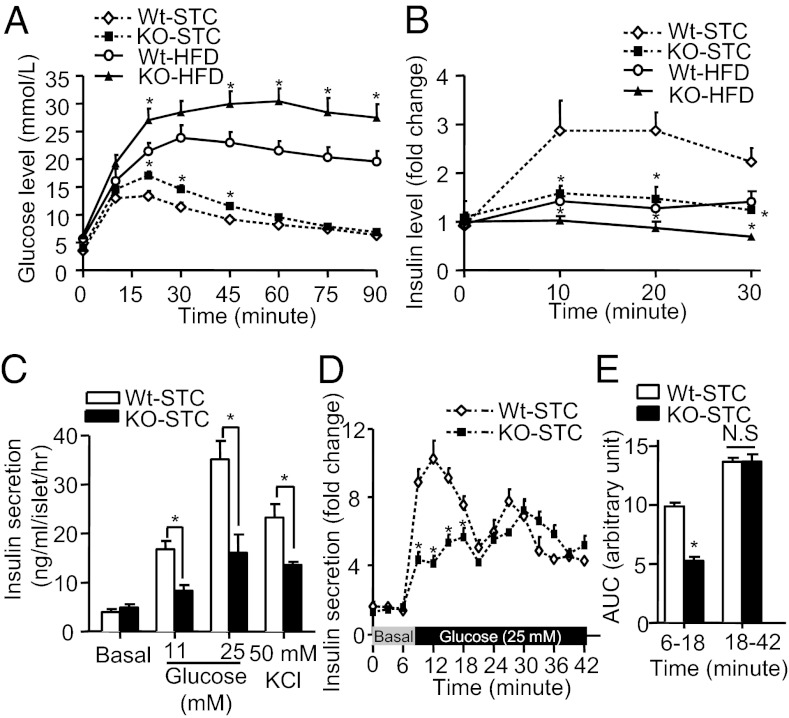
We next investigated the physiological role of APPL1 in insulin secretion and glucose metabolism using APPL1 KO mice as recently described (17). Body weight gain (Fig. S2A) and food intake (Table S1) were comparable between male APPL1 KO mice and wild-type (WT) littermates when they were fed with a standard chow (STC) or HFD for a period of 20 wk. APPL1 KO mice on either STC or HFD had significantly higher fasting glucose and insulin levels compared with WT controls (Table S1). Glucose tolerance test (GTT) revealed a mild but significant impairment in glucose disposal in STC fed-APPL1 KO mice relative to WT controls, whereas a much more severe glucose intolerance was observed in APPL1 KO mice fed with HFD (Fig. 2A). In APPL1 KO mice on both STC and HFD, insulin secretion during i.p. glucose challenge was markedly attenuated compared with their WT littermates (Fig. 2B). Furthermore, insulin secretion induced by l-arginine, which directly induces membrane depolarization of β cells, was compromised in APPL1 KO mice (Fig. S2B), suggesting that the lack of APPL1 causes a generalized defect in insulin secretion. APPL1 KO mice also developed more severe insulin resistance, as determined by insulin tolerance test (ITT) (Fig. S2 C and D). Taken together, these findings suggest that glucose intolerance in APPL1 KO mice is attributed in part to impaired insulin secretion.
Fig. 2.
Effects of APPL1 deficiency on glucose tolerance and insulin secretion in mice. (A) GTT in 20-wk-old male APPL1 KO and WT littermates fed with HFD or STC. (B) In vivo GSIS in APPL1 KO mice and WT littermates fed with STC or HFD (fold change over basal insulin level). (C) Glucose- and KCl-stimulated static insulin secretion in islets isolated from 20-wk-old male APPL1 KO mice and WT controls on STC. Insulin concentration was measured at 30 min after stimulation. (D) Dynamic insulin secretion of pancreatic islets isolated from APPL1 KO and WT mice in response to glucose (25 mM) using a perifusion system. (E) Area under curve (AUC) for the first phase (6–18 min) and second phase (18–42 min) of insulin secretion during the perifusion experiment as in D. *P < 0.05 (n = 6–8). NS, not significant.
Although the role of APPL1 in peripheral insulin actions is now well documented (14–16), whether it regulates insulin secretion has not been explored. Therefore, we further investigated the impact of APPL1 deficiency on insulin secretion in isolated mouse pancreatic islets. The islets from STC fed-APPL1 KO mice exhibited a comparable level of basal insulin secretion, but significantly lower GSIS compared with WT controls (Fig. 2C). In addition, insulin secretion stimulated by potassium chloride (KCl), which directly depolarizes the cell membrane and induces calcium influx, was blunted in the islets of APPL1 KO mice (Fig. 2C). Impairment of insulin secretion in response to both glucose and KCl stimulation was also observed in islets of APPL1 KO mice on HFD (Fig. S3A).
To further examine the dynamics of insulin secretion, perifusion experiment was performed in islets of APPL1 KO and WT mice. This analysis demonstrated that APPL1 deficiency led to a marked reduction in the first phase (6–18 min) of GSIS, but had little effect on the second phase (18–42 min) of GSIS (Fig. 2 D and E).
Consistent with the above findings in isolated mouse islets, rat INS-1E β cells infected with adenovirus encoding APPL1 RNAi exhibited an ∼75% reduction in APPL1 expression (Fig. S3B), and this change was accompanied by a significantly decreased insulin secretion induced by glucose and KCl (Fig. S3C). Taken together, these in vivo and in vitro findings support an obligatory role of APPL1 in insulin secretion.
Transgenic Expression of APPL1 Prevents Obesity-Induced Glucose Intolerance and Defective Insulin Secretion.
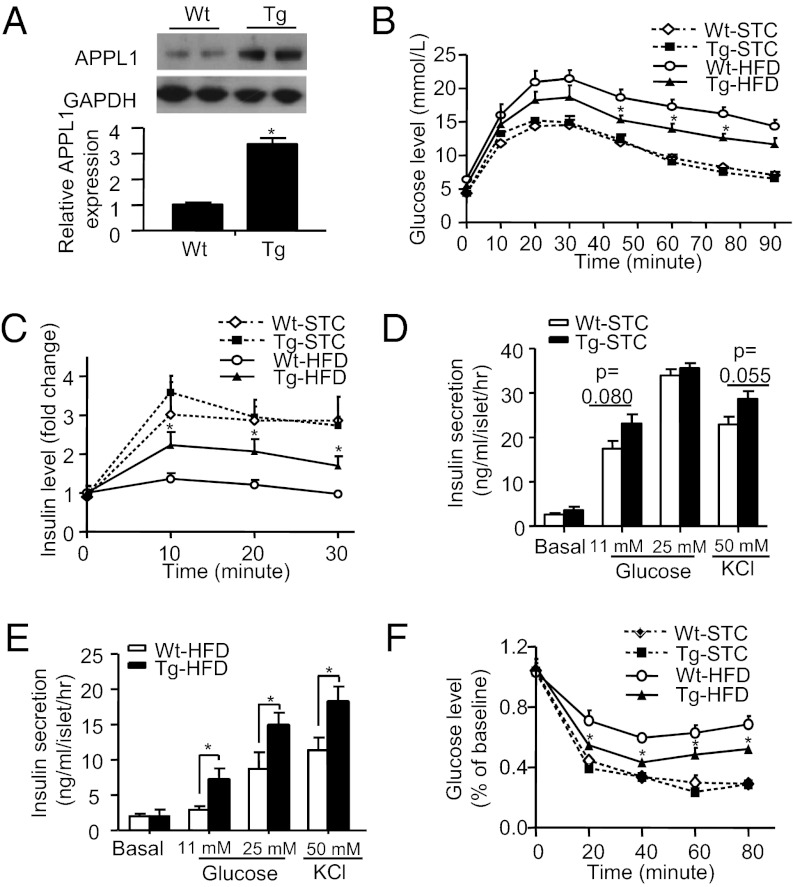
To test whether overexpression of APPL1 alleviates HFD-induced decrement of glucose tolerance and insulin secretion, we next performed GTT and GSIS in APPL1 transgenic mice (17) and WT controls on both STC and HFD. The expression level of APPL1 in pancreatic islets of the transgenic mice was increased by ∼3.5-fold compared with WT controls (Fig. 3A). Transgenic expression of APPL1 had no obvious impact on food intake and body weight gain, but caused a significant alleviation in HFD-induced fasting hyperglycemia and hyperinsulinemia (Table S1). In response to glucose challenge, glucose excursion curves were similar between APPL1 transgenic mice and WT controls fed with STC, whereas HFD-induced glucose intolerance was significantly reduced by transgenic expression of APPL1 (Fig. 3B). Furthermore, HFD-induced impairment in GSIS was partially reversed by the transgenic expression of APPL1 (Fig. 3C). The islets isolated from STC-fed APPL1 transgenic mice displayed a trend toward enhanced insulin secretion in response to glucose (11 mM) or KCl (50 mM) stimulation (Fig. 3D), and such changes became much more evident in the islets of HFD-fed APPL1 transgenic mice compared with the islets isolated from WT controls (Fig. 3E). Insulin sensitivity as assessed by ITT was similar between APPL1 transgenic mice and WT controls, whereas HFD-induced insulin resistance was significantly alleviated by transgenic expression of APPL1 (Fig. 3F).
Fig. 3.
Transgenic expression of APPL1 improves glucose tolerance and insulin sensitivity and enhances insulin secretion in HFD-fed obese mice. (A) Western blot analysis for APPL1 expression in isolated pancreatic islets of 12-wk-old male APPL1 transgenic (Tg) mice and WT littermates. (B) GTT in 16-wk-old APPL1 Tg mice and WT littermates fed with either STC or HFD. (C) In vivo GSIS in 16-wk-old male mice, and plasma insulin level was expressed as fold change over the baseline. (D and E) Static insulin secretion in response to glucose and KCl stimulation in islets from 20-wk-old APPL1 Tg and WT littermates on STC or HFD, respectively. (F) ITT in 20-wk-old APPL1 Tg mice and WT littermates fed with either STC or HFD. *P < 0.05 (n = 6–7).
APPL1 KO Mice Exhibit Decreased Expression of SNARE Proteins and Impaired Exocytosis.
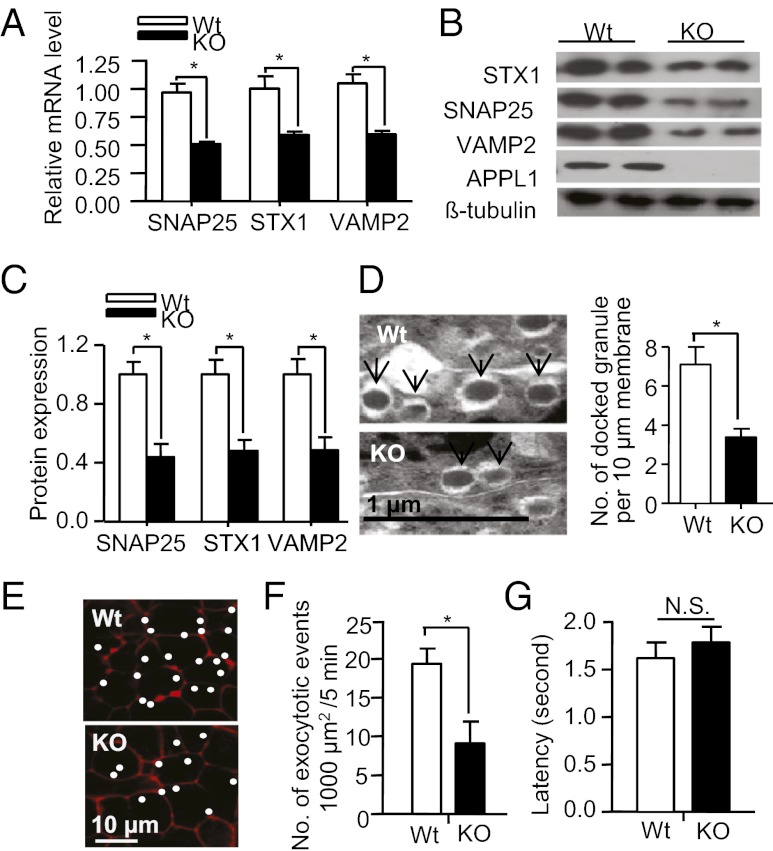
To further delineate the mechanism that causes defective insulin secretion in APPL1 KO mice, we analyzed β-cell mass, insulin content, ATP production, and calcium influx in response to glucose challenge. The pancreatic β-cell mass was significantly increased in both STC- and HFD-fed APPL1 KO mice compared with the WT controls (Fig. S4A). The total insulin content in the isolated pancreatic islets was not different between APPL1 KO mice and WT littermates (Fig. S4B). Glucose-induced ATP production and calcium influx in pancreatic islets were comparable between the two groups (Fig. S4 C and D), suggesting that defective insulin secretion in APPL1 KO mice occurs at a step downstream of calcium influx. The expression levels of key genes involved in the regulation of insulin synthesis (insulin and pancreatic and duodenal homeobox 1, PDX-1) and glucose metabolism (glucose kinase, GCK and glucose transporter 2, GLUT2) were also similar between the islets of APPL1 KO mice and WT controls (Fig. S4E). On the other hand, APPL1 KO mice exhibited a significant down-regulation in mRNA expression of several soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, including syntaxin-1 (STX1), synaptosomal-associated protein 25 (SNAP25), and vesicle-associated membrane protein 2 (VAMP2) (Fig. 4A), which are the core components of the exocytotic machinery of eukaryotic cells (22). The mRNA expression level of Sec1/Munc18 (Munc18), Rab3a, and synaptotagmin VII, which are also involved in the regulation of insulin secretion through SNARE proteins (23, 24), was comparable between the two groups of mice (Fig. S4E).
Fig. 4.
APPL1 deficiency decreases the expression of SNARE proteins and reduces the number of docked insulin granules and exocytotic events in pancreatic islets. (A) Real-time PCR analysis for mRNA expression levels of the three SNARE proteins (SNAP25, STX1, and VAMP2) in pancreatic islets isolated from 20-wk-old male APPL1 KO mice or WT controls on STC. (B) Western blot analysis for the SNARE proteins in the isolated islets. (C) Densitometric analysis for the relative abundance of SNARE proteins as in B. (D) Representative electron microscopic images of docked insulin granules (Left, denoted with arrows), and quantification of the number of docked granules (Right) in the isolated islets. (E) Distribution of exocytotic events in isolated islets of APPL1 KO mice and WT controls as measured by two-photon excitation imaging. White dots represent sites where exocytotic events were observed during 5 min of glucose (20 mM)-stimulated insulin secretion. The underlying red image is sulforhodamine B fluorescence. (F) Average number of glucose-stimulated exocytotic events in a cell surface area of 1,000 μm2. (G) Latency (in seconds) for the onset of staining with sulforhodamine B relative to that of staining with 10 kDa dextran. *P < 0.05 (n = 5–6). NS, not significant.
Consistent with the aforementioned changes in mRNA expression, Western blot analysis demonstrated a marked reduction in protein levels of the three SNARE proteins in the islets of APPL1 KO mice, compared with their WT controls (Fig. 4 B and C). Notably, decreased expression of these SNARE proteins was accompanied by a reduced number of docked insulin granules at the plasma membrane in the islets of APPL1 KO mice (Fig. 4D). By contrast, the islets from APPL1 transgenic mice displayed an increase in docked insulin granules (Fig. S5). We next examined the role of APPL1 in insulin granule exocytosis and fusion pore dynamics with two-photon excitation imaging (25). In response to glucose stimulation (20 mM), the exocytotic events during the first 5 min were significantly reduced in the islets of APPL1 KO mice compared with WT controls (Fig. 4 E and F), further supporting an indispensable role of APPL1 in the first-phase insulin secretion. On the other hand, expansion of the fusion pore as detected by measuring latency for the onset of staining with fluorescent markers of different sizes was comparable between the islets of APPL1 KO mice and WT littermates (Fig. 4G), implying that APPL1 deficiency has no effect on the fusion dynamics.
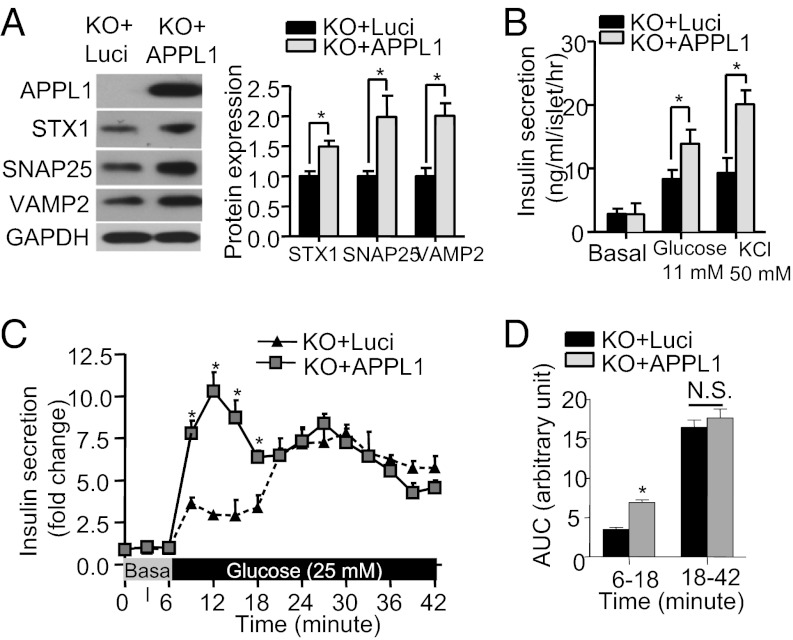
In INS-1E β cells, adenovirus-mediated knockdown of APPL1 expression resulted in a significant reduction in expression of SNARE proteins (Fig. S6A), whereas adenovirus-mediated expression of APPL1 augmented the expression of SNAP25, STX1, and VAMP2 in primary β cells isolated from APPL1 KO mice (Fig. 5A). The latter change was associated with increased glucose- and KCl-induced insulin secretion (Fig. 5 B–D). Taken together, these findings suggest that defective insulin secretion in APPL1 KO mice is attributed at least in part to decreased expression of the SNARE proteins in β cells.
Fig. 5.
Adenovirus-mediated expression of APPL1 enhances SNARE protein expression and insulin secretion in the islets of APPL1 KO mice. (A) Islets isolated from 20-wk-old male APPL1 KO mice on STC were infected with recombinant adenovirus expressing APPL1 or luciferase (Luci) for 36 h, followed by Western blot analysis to detect the expression levels of the three SNARE proteins. Bar chart on the Right is the densitometric analysis for the relative abundance of the three SNARE proteins in the infected islets. (B) Static insulin secretion in the infected islets measured after stimulation with glucose or KCl for 30 min. (C) First phase (6–18 min) and second phase (18–42 min) of GSIS of the infected islets using the perifusion system. (D) AUC for the first and second phase of insulin secretion during the perifusion experiment as in C. *P < 0.05 (n = 8). NS, not significant.
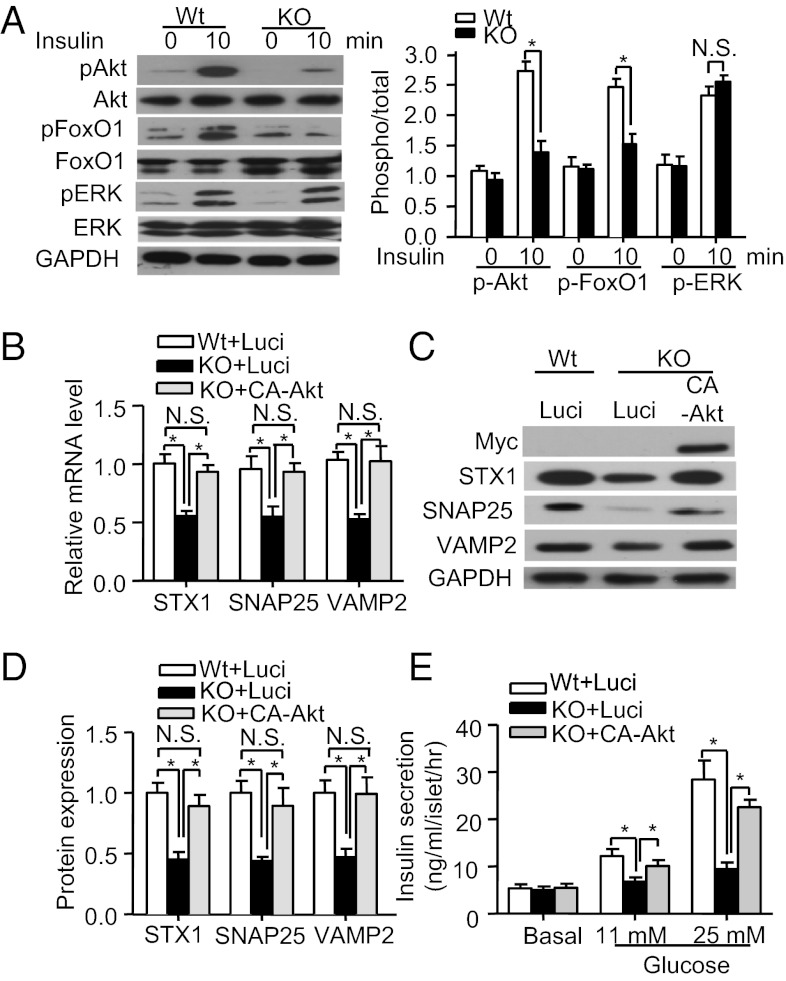
Defective Insulin Secretion in APPL1-null β Cells Is Rescued by Akt Activation.
The Akt/FoxO1 signaling cascade plays a pivotal role in the regulation of SNARE protein expression and insulin secretion (4, 26). Because APPL1 has been shown to potentiate Akt signaling in adipocytes (16), hepatocytes (14), and muscle cells (15), we next investigated whether APPL1 deficiency has any impact on this signaling cascade in pancreatic islets. Insulin-stimulated phosphorylation of Akt at Ser-473 and FoxO1 at Ser-256 in the islets of APPL1 KO mice was significantly decreased compared with those of WT controls, whereas there was no obvious difference in insulin-induced phosphorylation of ERK1/2 (Thr-202/Tyr-204) between the two groups of mice (Fig. 6A). Likewise, RNAi-mediated reduction in APPL1 expression in INS-1E cells significantly enhanced the interaction between Akt and its endogenous inhibitor tribble homolog 3 (TRB3) (Fig. S6B) and blunted insulin-stimulated phosphorylation of Akt and FoxO1 (Fig. S6C). On the other hand, adenovirus-mediated expression of APPL1 reversed the impairment in insulin-stimulated Akt/FoxO1 phosphorylation in APPL1-null β cells (Fig. S7A). Similarly, transgenic expression of APPL1 augmented the phosphorylation of Akt and FoxO1 induced by insulin in the islets of APPL1 transgenic mice on HFD (Fig. S7B).
Fig. 6.
APPL1 induces SNARE protein expression and insulin secretion via Akt activation. (A) Islets from 20-wk-old male APPL1 KO mice and WT controls on STC were treated with insulin (50 nM) for various time points as indicated, followed by Western blot analysis using antitotal or phospho-FoxO1 (Ser-256), antitotal or phospho-Akt (Ser-473), antitotal or phospho-ERK1/2 (Thr-202/Tyr-204), or anti-GAPDH antibody as a loading control. Bar chart on the Right represents the relative fold change of phosphorylation as quantified by densitometry. (B) Real-time PCR analysis for mRNA expression levels of the three SNARE proteins in the islets infected with adenovirus encoding the constitutively active form of Akt (CA-Akt) or luciferase (Luci) for 48 h. (C) Western blot analysis for protein expression levels of the three SNARE proteins of the infected islets. Note that CA-Akt is tagged with a Myc epitope at the NH2 terminus and can be detected by an anti-Myc antibody. (D) Densitometric analysis for the relative abundance of the three SNARE proteins in the infected islets as in C. (E) Static GSIS in the infected islets (n = 4). *P < 0.05 (n = 6). NS, not significant.
To test whether the activation of Akt could reverse defective insulin secretion in APPL1-null β cells, islets isolated from APPL1 KO mice were infected with adenovirus encoding luciferase or a constitutively active form of Akt (CA-Akt). This analysis demonstrated that decreased expression of the three SNARE proteins in islets of APPL1 KO mice was largely reversed by adenovirus-mediated expression of CA-Akt but not by the luciferase control (Fig. 6 B–D), and this change was accompanied by the restoration of GSIS in the APPL1-null β cells (Fig. 6E).
Discussion
Defective insulin secretion is a major feature of β-cell dysfunction and a primary contributor to hyperglycemia in T2DM. However, because insulin secretion is a highly dynamic process regulated by complex mechanisms, the pathological pathways leading to β-cell dysfunction remain poorly characterized. In the present study, we provide both in vitro and in vivo evidence showing that APPL1, a multidomain adapter protein involved in the peripheral actions of insulin (14–16), is a physiological regulator of insulin secretion in pancreatic β cells. GSIS is blunted in APPL1 KO mice, but is augmented by transgenic expression of APPL1. Despite impaired GSIS, it is noteworthy that basal plasma insulin levels in APPL1 KO mice are much higher than those in WT littermates. Increased basal insulin secretion in APPL1 KO mice is perhaps due to the compensatory function of β cells, and may also explain why glucose intolerance is not obvious in young APPL1 KO mice (27).
Loss of first-phase insulin secretion occurs in the very early stage of type 2 diabetes (2). In isolated islets from APPL1 KO mice, we found that the impaired GSIS is mainly attributed to defective first-phase insulin secretion. This conclusion is also supported by our observation that the number of docked insulin granules, a major pool for first-phase insulin secretion (2), is decreased in APPL1-null islets but increased in APPL1 transgenic islets. Furthermore, our data from both gain-of-function and loss-of-function studies suggest that APPL1 may promote insulin exocytosis by increasing the expression of SNARE protein complex, a core component involved in calcium-induced docking and exocytosis of secretory granules (22, 28).
The three main components of SNARE proteins (STX1, SNAP25, and VAMP2) promote exocytosis of insulin granules by forming a heterotrimeric complex, thereby facilitating membrane fusion, priming and docking of secretory vesicles (22, 28). The importance of SNARE-mediated exocytosis in the first-phase insulin secretion has been documented in both in vitro and animal studies (29), and decreased expression of the three SNARE proteins has been suggested as a possible cause of defective insulin secretion in rodents and patients with obesity and T2DM (30, 31). Notably, β cells from STX1 KO mice display impaired first-phase insulin secretion and defective docking and fusion of insulin granules (29), a pattern similar to APPL1-null islets observed in our study. In both the pancreatic islets of APPL1 KO mice and rat INS-1E β cells with decreased APPL1 expression, we found that the expression levels of all three SNARE proteins are markedly decreased, whereas this change is reversed by adenovirus-mediated replenishment of APPL1. These findings suggest that decreased APPL1 expression in islet β cells may contribute to decreased expression of the SNARE proteins in obesity and diabetes, which in turn causes defective insulin secretion.
APPL1 has been reported to modulate both substrate specificity and activity of Akt (14–17, 32), a protein kinase that plays a central role in mediating the peripheral actions of insulin. In addition, the PI3K/Akt signaling pathway has been shown to control insulin secretion at the step of exocytosis, partly via enhancing the expression of SNARE proteins (4, 7). The promoter regions of the SNARE complex genes contain the binding sites for FoxO1, which suppresses the transcription of these genes (4). Akt activation in β cells induces the transcriptional activation of the SNARE complex genes by phosphorylating and inactivating FoxO1, but has no obvious effect on expression of several other exocytotic proteins (Rab3a, Munc18, and synaptotagmin) (4, 7). Pancreatic β-cell–specific ablation of PI3K leads to glucose intolerance and defective insulin secretion in mice, and PI3K-null β cells exhibit defective exocytosis of insulin granules due to the reduced expression of SNARE proteins and loss of cell–cell synchronization (4). These defects can be rescued by forced activation of Akt or inactivation of the Akt downstream target FoxO1 in PI3K-null cells (4). Likewise, transgenic mice with diminished Akt activity in β cells (7) display glucose intolerance due to defective insulin secretion at the level of exocytosis, whereas glucose-stimulated calcium influx is not altered. By contrast, transgenic mice with β-cell–specific activation of Akt can protect against streptozotocin-induced diabetes by preserving β-cell mass and GSIS (8). In line with these findings, several pieces of evidence from the present study demonstrated that APPL1-mediated expression of the three SNARE proteins is attributed to the ability of this adapter protein in potentiating insulin-evoked Akt activation. First, in the islets of APPL1 KO mice and rat INS-1E β cells with RNAi-mediated knockdown of APPL1, decreased expression of the three SNARE proteins is accompanied by a marked attenuation in insulin-elicited phosphorylation of Akt and its downstream target FoxO1. Second, adenovirus-mediated expression of constitutively active Akt is sufficient to rescue the reduction in expression of the three SNARE proteins in APPL1-null islet cells. Taken together, these data support the role of APPL1 as a key regulator that couples insulin signaling to insulin secretion in β cells.
As an interacting partner of Akt (13), APPL1 has been shown to enhance Akt activity by competing with the pseudokinase TRB3 for Akt binding sites in hepatocytes and endothelial cells (14, 17). Likewise, the present study found that the impaired insulin-evoked Akt activation by knockdown of APPL1 expression is accompanied by enhanced interaction between Akt and TRB3 in β cells. TRB3 acts as an endogenous Akt inhibitor by trapping Akt within the cytosol and preventing its translocation to the plasma membrane (14). By contrast, APPL1 releases Akt trapped by TRB3 and promotes Akt translocation to the plasma membrane and endosomes for further activation (14). Notably, TRB3 expression is markedly elevated in islets from patients with T2DM as well as insulin receptor-deficient mice (26). Overexpression of TRB3 in both mouse β cells and human islet cells blocks docking and exocytosis of insulin granules by down-regulating the expression of the SNARE proteins (26). Therefore, APPL1 and TRB3 display opposite changes in the islets of obesity and diabetes and exert opposing effects in modulating the expression of SNARE proteins and insulin exocytosis, suggesting that these two Akt-binding proteins may act as a “yin-and-yang” pair to fine-tune insulin secretion, by tightly controlling the expression of key exocytotic genes in β cells.
In conclusion, the present study has identified APPL1 as an indispensable component that controls insulin secretion in pancreatic β cells, by modulating the expression of SNARE proteins via an Akt-dependent pathway. Our data, together with the previous findings showing that APPL1 mediates the metabolic effects of insulin in its peripheral targets, support the role of APPL1 as a master coordinator controlling both secretion and actions of insulin. These findings further highlight the importance of insulin signaling in modulating β-cell function and suggest that reduced APPL1 expression in pancreatic islets in obesity may serve as a mediator coupling insulin resistance to β-cell dysfunction, thereby accelerating the progression of T2DM.
Materials and Methods
Animals.
Male APPL1 KO mice and male APPL1 transgenic mice in C57BL/6J genetic background have been described in our previous publication (17). C57BKS db/+ mice were originally from Jackson Laboratories. All mice had free access to food and water and were kept in cages in a 12-h light/dark cycle. All animal experimental protocols were approved by the animal ethics committee of The University of Hong Kong.
Islet Isolation and Insulin Secretion Assay.
Pancreatic islets were isolated, cultured, and analyzed by following standard protocol or as described in SI Materials and Methods.
Additional methods are described in SI Materials and Methods. Primers used for real-time PCR analysis are listed in Table S2.
Supplementary Material
Acknowledgments
This work was supported by General Research Fund Grants HKU 781309M and 783010M (to A.X.), Theme-Based Research Scheme Grant T12-705/11, and Collaborative Research Fund Grant HKU4/CRF/10 from the Research Grants Council of Hong Kong, the National Basic Research Program of China Grant 2011CB504004, and its matching fund from The University of Hong Kong, and the National Science Foundation of China Grant 30811120429.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8795.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202435109/-/DCSupplemental.
References
- 1.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibiger IB, Leibiger B, Berggren PO. Insulin signaling in the pancreatic beta-cell. Annu Rev Nutr. 2008;28:233–251. doi: 10.1146/annurev.nutr.28.061807.155530. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko K, et al. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010;12:619–632. doi: 10.1016/j.cmet.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 6.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 7.Bernal-Mizrachi E, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuttle RL, et al. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 9.Hennige AM, et al. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest. 2003;112:1521–1532. doi: 10.1172/JCI18581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunton JE, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Weng J, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 12.Zoncu R, et al. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsuuchi Y, et al. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. doi: 10.1038/sj.onc.1203080. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KK, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417–427. doi: 10.1016/j.cmet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Cleasby ME, et al. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J Endocrinol. 2011;210:81–92. doi: 10.1530/JOE-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes. 2011;60:3044–3054. doi: 10.2337/db11-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao X, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 19.Cheng KK, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 20.Peyot ML, et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: Secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59:2178–2187. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou YP, Berggren PO, Grill V. A fatty acid-induced decrease in pyruvate dehydrogenase activity is an important determinant of beta-cell dysfunction in the obese diabetic db/db mouse. Diabetes. 1996;45:580–586. doi: 10.2337/diab.45.5.580. [DOI] [PubMed] [Google Scholar]
- 22.Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 23.Coppola T, et al. Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell. 2002;13:1906–1915. doi: 10.1091/mbc.02-02-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustavsson N, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci USA. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- 26.Liew CW, et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest. 2010;120:2876–2888. doi: 10.1172/JCI36849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan Y, You H, Coffey FJ, Wiest DL, Testa JR. Appl1 is dispensable for Akt signaling in vivo and mouse T-cell development. Genesis. 2010;48:531–539. doi: 10.1002/dvg.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi N, et al. SNARE conformational changes that prepare vesicles for exocytosis. Cell Metab. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Ohara-Imaizumi M, et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- 31.Nagamatsu S, et al. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: Restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999;48:2367–2373. doi: 10.2337/diabetes.48.12.2367. [DOI] [PubMed] [Google Scholar]
- 32.Schenck A, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.