Abstract
CCAAT/enhancer-binding protein δ (C/EBPδ) recently emerged as an essential player in the inflammatory response to bacterial infections. C/EBPδ levels increase rapidly after a proinflammatory stimulus, and increasing C/EBPδ levels seem to be indispensable for amplification of the inflammatory response. Here we aimed to elucidate the role of C/EBPδ in host defense in community-acquired pneumococcal pneumonia. We show that C/EBPδ−/− mice are relatively resistant to pneumococcal pneumonia, as indicated by delayed and reduced mortality, diminished outgrowth of pneumococci in lungs, and reduced dissemination of the infection. Moreover, expression of platelet-activating factor receptor (PAFR), which is known to potentiate bacterial translocation of Gram-positive bacteria, was significantly reduced during infection in C/EBPδ−/− mice compared with WT controls. Importantly, cell stimulation experiments revealed that C/EBPδ potentiates PAFR expression induced by lipoteichoic acid and pneumococci. Thus, C/EBPδ exaggerates bacterial dissemination during Streptococcus pneumoniae-induced pulmonary infection, suggesting an important role for PAFR-dependent bacterial translocation.
Keywords: lung barrier, tanscytosis
CCAAT/enhancer-binding protein δ (C/EBPδ) is a member of the C/EBP family of transcription factors (1). Expression of C/EBPδ is typically low in most cell types but is rapidly induced by various extracellular stimuli, including IL-1, IL-6, LPS, and TNF-α (2, 3). Several in vitro studies have suggested that C/EBPδ is an important player in inflammation, especially during the acute-phase response (2, 4). Moreover, C/EBPδ expression levels seem to be associated with the magnitude of the inflammatory response toward LPS (5), and C/EBPδ regulates COX-2 expression in different cell types (6). Overall, these data suggest an important role for C/EBPδ during the acute-phase response in pulmonary infection.
In line with in vitro data, an important role of C/EBPδ in innate immunity has been established by showing that C/EBPδ plays a dual role during Escherichia coli-induced peritonitis (7). Indeed, C/EBPδ−/− mice are resistant to transient infection with a low, nonlethal dose of E. coli but are highly susceptible to persistent infection with a higher, lethal, dose. C/EBPδ contributes to protective immunity in persistent infection by amplifying the NF-κB–driven inflammatory response essential for bacterial elimination and survival. In contrast to the role of C/EBPδ in severe E. coli peritonitis, C/EBPδ−/− mice are protected against sterile inflammation. Indeed, C/EBPδ−/− mice demonstrated prolonged survival compared with WT mice in a double-hit LPS model for disseminated intravascular coagulation (8). Moreover, C/EBPδ deficiency limits LPS-induced systemic inflammation, as reflected by reduced TNF-α and IL-6 levels. Therefore, C/EBPδ appears to be an essential component of the inflammatory response, although its exact role in infectious disease remains elusive and needs further exploration.
Here we aimed to further characterize the involvement of C/EBPδ during infectious disease. To this end, we subjected C/EBPδ−/− mice to a murine model of Streptococcus pneumoniae-induced pneumonia, a clinically relevant infection model. S. pneumoniae is the leading causative pathogen in community-acquired pneumonia, associated with high morbidity and mortality (9). S. pneumoniae accounts for up to 36% of adult community-acquired pneumonia, and an estimated 570,000 cases of pneumococcal pneumonia occur annually, including 175,000 hospitalized cases and a fatality rate of 5–7% (10). Worldwide S. pneumoniae is responsible for an estimated 10 million deaths annually and causes more deaths than any other bacterial pathogen (11). Given the high incidence of S. pneumoniae, further efforts to understand the host response mechanisms involved in pneumococcal pneumonia are of essential importance (12).
Pneumococcal infections are initiated by asymptomatic colonization of the nasopharynx with subsequent dissemination into the lung, blood, and peripheral organs. To disseminate from the pulmonary compartment, S. pneumoniae binds to the platelet-activating factor receptor (PAFR) via cell wall phosphorylcholine, after which the bacteria are endocytosed (13). Subsequently, bacteria-containing vacuoles are directed away from lethal lysosomal trafficking toward transcytosis (14). In line with an essential role of PAFR in pneumococcal transcytosis, PAFR−/− mice were found to be relatively resistant to pneumococcal pneumonia, as demonstrated by delayed and reduced mortality, diminished bacterial outgrowth, and reduced dissemination into the bloodstream (15).
Here we show that C/EBPδ deficiency prolongs survival, reduces bacterial dissemination, and limits the inflammatory response after pneumococcal pneumonia. Moreover, we show that S. pneumoniae induces C/EBPδ expression levels in pulmonary epithelial cells, and that C/EBPδ subsequently induces PAFR expression. These findings suggest that C/EBPδ aggravates pneumococcal-induced pneumonia by facilitating transcytosis in a PAFR-dependent manner. Our data thus reveal a mechanism underlying the high mortality of pneumococcal infections and may potentially lead to the development of unique therapeutic modalities for the treatment of this severe infectious disease.
Results
Pneumococcal Infection Induces C/EBPδ Expression.
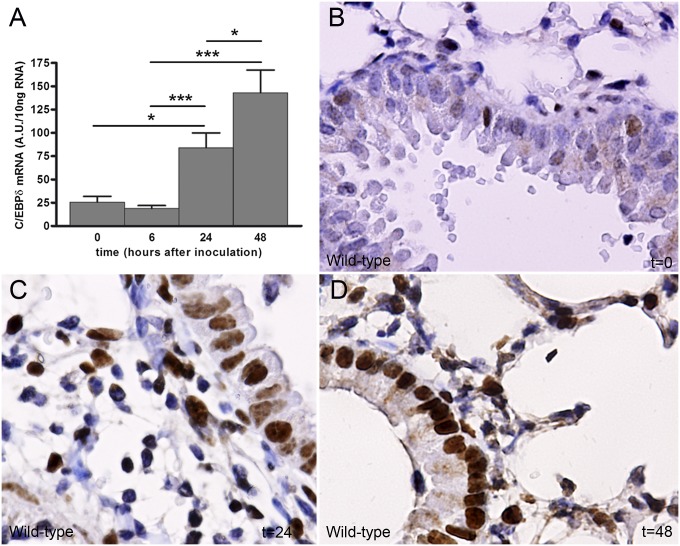
We first sought to determine c/ebpδ expression levels during experimental S. pneumoniae-induced pneumonia. To this end, WT mice were intranasally inoculated with 1 × 104 colony-forming units (CFU) and c/ebpδ mRNA levels were measured in lung tissue. c/ebpδ mRNA expression was low in uninfected lungs, whereas c/ebpδ levels were increased at 24 h and 48 h after S. pneumoniae inoculation (Fig. 1A). In line with this finding, C/EBPδ protein levels also increased over time, as demonstrated by immunohistochemistry analysis (Fig. 1 B–D). Interestingly, C/EBPδ was expressed mainly in bronchial epithelial and inflammatory cells in uninfected lungs, whereas C/EBPδ expression was significantly increased in both cell types after infection.
Fig. 1.
C/EBPδ expression increases during S. pneumoniae-induced pneumonia. C/EBPδ mRNA (A) and protein expression from WT lung tissue (B–D) were measured at different time points after intranasal inoculation with S. pneumoniae. Data are mean ± SEM (n = 4–8).
C/EBPδ Deficiency Improves Host Defense During S. pneumoniae-Induced Pulmonary Infection.
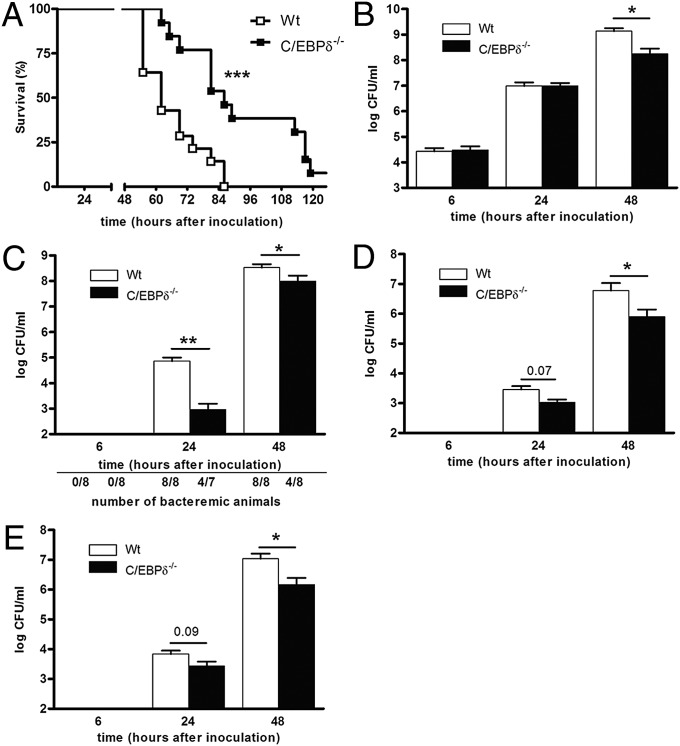
To define the biological role of C/EBPδ in pneumonia, we subjected WT and C/EBPδ−/− mice to experimental S. pneumoniae-induced pneumonia. WT mice began to die at 55 h after the intranasal inoculation of S. pneumoniae, and all mice succumbed within 86 h (Fig. 2A). C/EBPδ−/− mice began to die only after 64 h, and 1 of the 13 mice survived for longer than 120 h (the maximal observation time). Median survival was 62 h in the WT mice and 86 h in the C/EBPδ−/− mice. Notably, all WT mice had already succumbed due to infection at the median survival time of the C/EBPδ−/− mice. These findings suggest that C/EBPδ plays an essential detrimental role in S. pneumoniae pneumonia.
Fig. 2.
C/EBPδ deficiency protects against S. pneumoniae-induced pneumonia and limits bacterial dissemination. (A) Survival curves of WT and C/EBPδ−/− mice after intranasal inoculation with S. pneumoniae (n = 14 for WT and 13 for C/EBPδ−/− mice). (B–E) Bacterial outgrowth in lungs (B), blood (C), liver (D), and kidney (E) of WT and C/EBPδ−/− mice after intranasal inoculation with S. pneumoniae. Data are mean ± SEM (n = 7–8). *P < 0.05; **P < 0.01; ***P < 0.001.
To substantiate our findings on the role of C/EBPδ in host defense against pneumococcal pneumonia, we next examined bacterial outgrowth in lungs. Bacterial loads were increased in the lungs of WT mice from ∼3 × 104 at 6 h after inoculation to ∼1 × 109 after 48 h (Fig. 2B). Bacterial outgrowth in lungs of C/EBPδ−/− mice was similar to that in WT mice after 6 h and 24 h, whereas C/EBPδ−/− mice had a 50-fold lower bacterial load at 48 h postinoculation. Given that pneumonia is frequently complicated by systemic dissemination of the primary infection, we assessed bacterial outgrowth in blood as well. Bacterial dissemination was not observed at 6 h after inoculation, but significant dissemination was detected at 24 h and 48 h postinoculation in WT mice (Fig. 2C). Interestingly, bacterial outgrowth was lower in C/EBPδ−/− mice than in WT mice. Strikingly, S. pneumoniae disseminated into the bloodstream in all of the WT mice but in only 57% of the C/EBPδ−/− mice after 24 h and 50% of the C/EBPδ−/− mice after 48 h. The average bacterial load was significantly lower in these C/EBPδ−/− mice compared with the WT mice, however. In line with this finding, dissemination of S. pneumoniae to peripheral organs was observed at 24 h and 48 h after inoculation in both WT and C/EBPδ−/− mice, although to a lesser extent in the C/EBPδ−/− mice (Fig. 2 D and E). These results suggest that C/EBPδ aggravates S. pneumoniae-induced pulmonary infections and increases bacterial dissemination.
The widespread dissemination of bacteria suggests that the mice succumb to systemic disease leading to multiple organ failure; thus, we analyzed organ damage at 48 h postinoculation. Levels of the liver injury markers aspartate transaminase and alanine transaminase and the general tissue injury marker lactate dehydrogenase were strongly increased in the S. pneumoniae-infected WT mice, with significantly lower levels detected in the C/EBPδ−/− mice (Fig. S1).
C/EBPδ Deficiency Limits S. pneumoniae-Induced Inflammation.
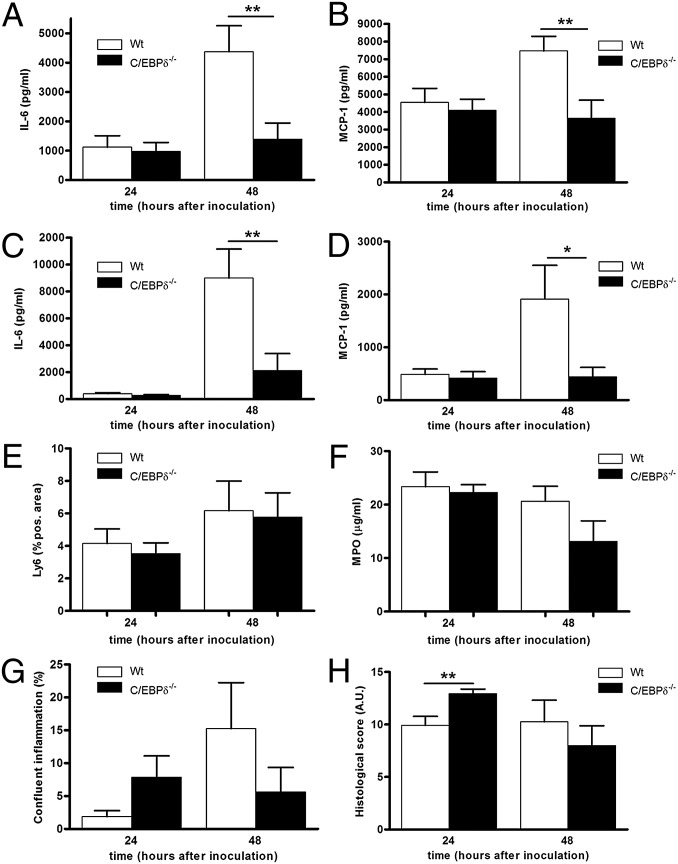
We next measured cytokine and chemokine levels in lung, blood, and peripheral organs. All cytokines/chemokines tested exhibited typically low expression at 6 h after inoculation, with increasing expression over time in both lung and plasma. IL-6 and MCP-1 levels increased to a similar extent in C/EBPδ−/− and WT mice by 24 h postinoculation in lung and plasma, whereas significantly reduced levels were observed in the C/EBPδ−/− mice at 48 h postinoculation (Fig. 3 A–D). Similar expression patterns were observed in peripheral organs (Fig. S2 A–D) and for TNF-α, KC, and MIP-2 (Fig. S2 E–J). These findings suggest that C/EBPδ−/− mice demonstrate a reduced inflammatory response during sustained S. pneumoniae infection.
Fig. 3.
C/EBPδ deficiency limits S. pneumoniae-induced inflammation. (A–D) IL-6 and MCP-1 levels in lung (A and B) and plasma (C and D) of WT and C/EBPδ−/− mice after inoculation with S. pneumoniae. (E and F) Neutrophil numbers (E) and neutrophil activity (F) in lung homogenates after intranasal inoculation with S. pneumoniae. (G and H) Histological analysis of WT and C/EBPδ−/− lung sections after intranasal inoculation with S. pneumoniae. (G) Confluent inflammation as indicated by the percentage of infiltrates of the total lung area. (H) Graphical representation of lung inflammation in WT and C/EBPδ−/− mice determined according to the scoring method described in Materials and Methods. Data are mean ± SEM (n = 7–8). *P < 0.05; **P < 0.01.
Given that neutrophil recruitment to the site of infection is an important hallmark of host defense during pneumonia (16), we next evaluated neutrophil influx in the lungs of WT and C/EBPδ−/− mice after inoculation with S. pneumoniae. The number of neutrophils increased over time to a similar extent in both WT and C/EBPδ−/− mice (Fig. 3E). In line with this finding, neutrophil content, as determined by myeloperoxidase level, was similar in both groups of mice (Fig. 3F). These data show that C/EBPδ does not influence neutrophil influx during pneumococcal pneumonia.
On histopathologic examination, the lungs of WT mice exhibited confluent inflammation accompanied by pleuritis, bronchitis, edema, interstitial inflammation, and vasculitis. At 24 h after inoculation, the C/EBPδ−/− mice showed significantly more signs of bronchitis and slightly more (but not significantly so) signs of confluent inflammation (Fig. 3G), edema, and endothelialitis (Fig. S3), which resulted in a significantly higher histological score compared with WT mice (Fig. 3H). At 48 h after inoculation, all histological parameters were similar in WT and C/EBPδ−/− mice, although confluent inflammation was slightly lower in the C/EBPδ−/− mice (Fig. 3G). Overall, cytokine/chemokine levels are lower in C/EBPδ−/− mice compared with WT mice, but this has no affect on neutrophil influx and histopathological inflammation parameters.
C/EBPδ Does Not Influence Systemic Infection, but Promotes Bacterial Dissemination in a PAFR-Dependent Mechanism.
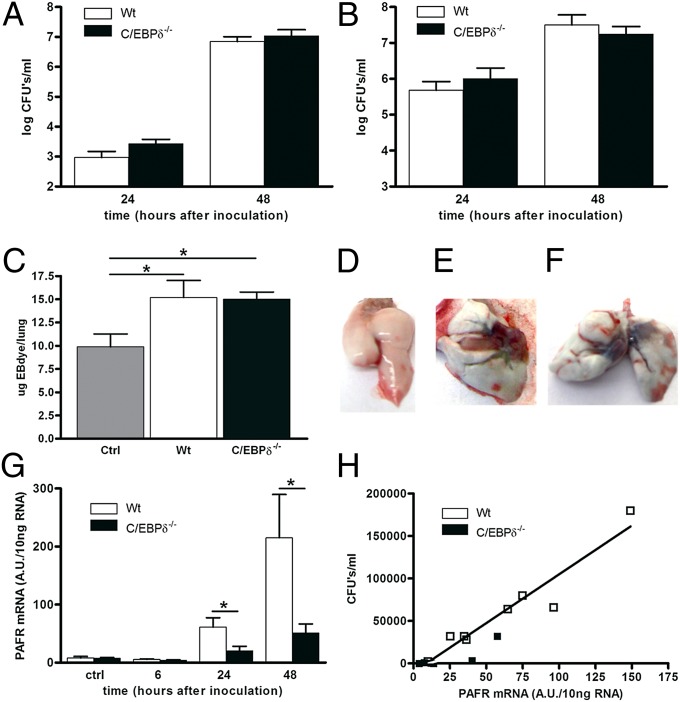
One of the most intriguing findings of our study so far is the lower bacterial loads in blood and distant organs in C/EBPδ−/− mice. A potential explanation for this finding may be that C/EBPδ deficiency improves bacterial clearance within the bloodstream. To prove or refute this hypothesis, we induced sepsis by injecting S. pneumoniae i.v. and measured bacterial outgrowth in blood and lungs. Bacterial outgrowth increased over time in the blood and lungs of both WT and C/EBPδ−/− mice to a similar extent, refuting our hypothesis that C/EBPδ would limit bacterial clearance in the systemic compartment (Fig. 4 A and B). Moreover, our data show that bacterial extravasation from blood into the lung is not affected by C/EBPδ. Thus, it is tempting to speculate that C/EBPδ specifically influences bacterial translocation from the lung into the blood.
Fig. 4.
C/EBPδ does not influence systemic infection but promotes bacterial dissemination in a PAFR-dependent mechanism. Bacterial outgrowth in blood (A) and lungs (B) of WT and C/EBPδ−/− mice after intravenous inoculation with S. pneumoniae. Data are mean ± SEM (n = 8). (C–F) Lung barrier function in WT and C/EBPδ−/− mice after intranasal inoculation with S. pneumoniae as determined by EB dye content in the lung and indicated as micrograms of EB dye in the lung (C). Data are mean ± SEM (n = 5–6). Representative pictures of uninfected WT (D), WT (E), and C/EBPδ−/− (F) mice inoculated intranasally with S. pneumoniae. (G) Pafr mRNA levels in lungs of WT and C/EBPδ−/− mice at different time points after intranasal inoculation with S. pneumoniae. Data are mean ± SEM (n = 7–8). (H) Correlation between pafr mRNA levels and bacterial outgrowth in blood of WT and C/EBPδ−/− mice at 24 h after intranasal inoculation with S. pneumoniae (R2 = 0.90; P < 0.0001). *P < 0.05.
Bacteria may cross the lung–blood barrier by directly inducing alveolar epithelial cell injury independent of leukocytes (17). To assess the potential role of C/EBPδ in blood–lung barrier integrity associated with S. pneumoniae infection, we evaluated S. pneumoniae-induced Evans blue (EB) dye leakage (18). S. pneumoniae induced increased the permeability of the blood–lung barrier, as evidenced by increased EB dye levels in the lungs of infected mice (Fig. 4 C–F). However, S. pneumoniae-induced leakage increased to similar extent in WT and C/EBPδ−/− mice, demonstrating showing that C/EBPδ does not play a role in lung barrier protection in S. pneumoniae infection. Moreover, these data suggest that the observed difference in bacterial dissemination is caused by an alternative mechanism.
Along with disrupting the epithelial barrier, S. pneumoniae may bind to the PAFR or the Ig receptor (pIg-R) on epithelial cells, leading to intracellular uptake of bacteria and subsequent transcytosis (15). To assess whether C/EBPδ could affect S. pneumoniae transcytosis, we determined pafr and pIg-R mRNA levels in the lung. pafr mRNA levels increased over time, with high expression seen at 24 h and 48 h after S. pneumoniae inoculation in WT mice (Fig. 4G). Pafr mRNA levels in C/EBPδ−/− mice also increased over time, although to lesser extent than in WT mice. pIg-R mRNA levels increased after S. pneumoniae inoculation, but with no differences between WT and C/EBPδ−/− mice (Fig. S4A). Strikingly, pafr mRNA levels positively correlated with bacterial dissemination of S. pneumoniae independent of the C/EBPδ genotype (Fig. 4H and Fig. S4B).
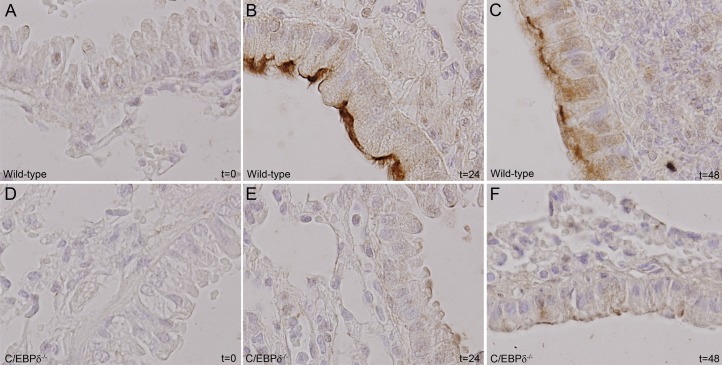
To confirm induction of PAFR expression during S. pneumoniae, we performed immunohistochemical analysis of pulmonary PAFR expression during the course of infection. PAFR expression was low in uninfected mice (Fig. 5 A and D) and increased significantly over time in epithelial cells of WT mice (Fig. 5 A–C) and only marginally in C/EBPδ−/− mice (Fig. 5 D–F). Overall, these data suggest that C/EBPδ-mediated PAFR expression is of pivotal importance for S. pneumoniae dissemination into the bloodstream.
Fig. 5.
C/EBPδ deficiency limits S. pneumoniae-induced PAFR expression. PAFR expression in WT (A–C) and C/EBPδ−/− (D–F) lung tissue was determined at different time points after intranasal inoculation with S. pneumoniae.
C/EBPδ Enhances PAFR Expression Directly and on Stimulation with Lipoteichoic Acid and/or S. pneumoniae.
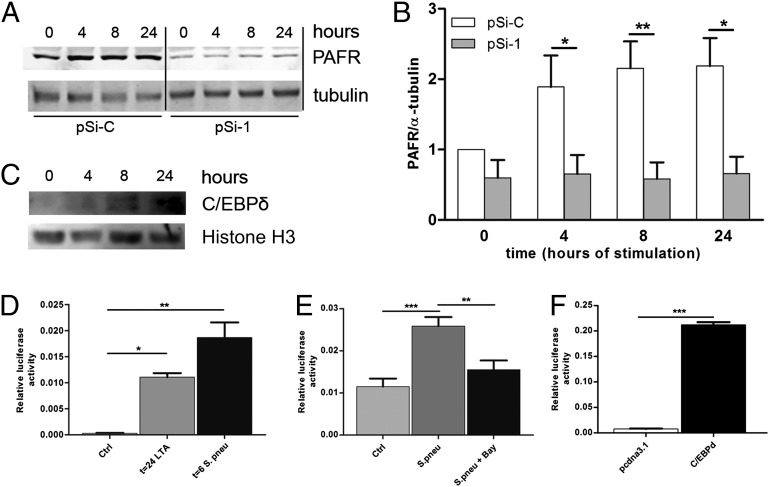
To confirm that C/EBPδ drives PAFR expression, we evaluated the effect of C/EBPδ on baseline PAFR levels in alveolar epithelial cells. To this end, A549 cells were stably transfected with C/EBPδ silencing vector or control vector. Silencing C/EBPδ expression reduced PAFR levels by approximately twofold (Fig. 6 A and B). Moreover, stimulation of these cells with lipoteichoic acid (LTA) induced C/EBPδ expression (Fig. 6C) and PAFR expression within 4–24 h. Interestingly, LTA did not induce PAFR expression in C/EBPδ-silenced A549 cells (Fig. 6 A and B).
Fig. 6.
C/EBPδ potentiates PAFR expression induced by the Gram-positive bacterial cell wall component LTA and by growth-arrested S. pneumoniae. (A) Representative Western blot of PAFR and α-tubulin levels in A549 lung epithelial cells stimulated with 10 ng/mL of LTA for the times indicated in the presence of control (pSi-C) or C/EBPδ (pSi-1) silencing plasmids. (B) Quantitative analysis of Western blots shown in A (n = 3). Shown are PAFR levels relative to unstimulated pSi-C–transfected cells after correction for α-tubulin levels, which serve as loading control. (C) Western blot analysis of C/EBPδ and Histone H3 in nuclear extracts of A549 lung epithelial cells under basal conditions and stimulated with 10 ng/mL of LTA for the times indicated. (D) PAFR luciferase activity of A549 cells stimulated with LTA (10 ng/mL) or S. pneumoniae (1 × 108). Shown is a representative figure of three independent experiments (n = 6). (E) PAFR luciferase activity of A549 cells stimulated with S. pneumoniae (1 × 108) in the absence or presence of the NfκB inhibitor. Shown is a representative figure of two independent experiments (n = 6). (F) PAFR luciferase activity of A549 cells transfected with either a C/EBPδ overexpression construct or an empty vector. Shown is a representative figure of two independent experiments (n = 6). *P < 0.05; **P < 0.01.
To further substantiate the importance of C/EBPδ to PAFR expression, we transfected cells with a PAFR promoter-driven luciferase construct. Stimulation with LTA or growth arrested S. pneumoniae-induced PAFR promoter activity (Fig. 6D). In line with this finding, S. pneumoniae stimulation of airway epithelial Calu3 cells also induced both C/EBPδ and PAFR expression (Fig S5).
NF-κB and C/EBPδ have been shown to act synergistically on different promoters (19); thus, we examined whether S. pneumoniae-induced PAFR luciferase activity would be affected by a specific NF-κB inhibitor. S. pneumoniae-induced PAFR expression was partly inhibited by BAY 11-7082, suggesting that C/EBPδ and NF-κB indeed synergistically drive PAFR expression during infection (Fig. 6E). Interestingly however, C/EBPδ overexpression to higher levels than seen in our in vitro LTA or S. pneumoniae stimulations dramatically induced PAFR expression by itself (Fig. 6F), demonstrating that C/EBPδ acts as direct transcriptional activator of the pafr gene. C/EBPδ thus regulates PAFR expression during S. pneumoniae infection.
Discussion
This study shows that C/EBPδ deficiency protects mice from S. pneumoniae-induced mortality. Our data demonstrate that C/EBPδ is highly induced during S. pneumoniae infection, and that it aggravates bacterial dissemination over the lung–blood barrier, leading to multiple organ failure and subsequent shorter overall survival. Of particular interest, we found that pulmonary PAFR levels are induced by S. pneumoniae in a C/EBPδ-dependent manner, and that PAFR levels are directly correlated with the amount of bacteria disseminated into the bloodstream. Moreover, we also found that C/EBPδ overexpression directly induces PAFR promoter activity in vitro. The potential importance of C/EBPδ-driven PAFR expression in pneumococcal pneumonia is in line with previous data showing that PAFR−/− mice are less likely to develop invasive disease and have more effective host defenses during pneumococcal pulmonary infection (15). Remarkably, PAFR−/− mice exhibit a similar phenotype as C/EBPδ−/− mice, with reduced bacterial dissemination, diminished bacterial loads in the lung, and prolonged survival. Although these data support the idea that PAFR-mediated transcytosis is a prime mechanism through which pneumococci disseminate, bacteria do disseminate in the absence of PAFR, suggesting that S. pneumoniae-induced direct alveolar epithelial injury also contributes (at least in part) to S. pneumoniae-induced lethality.
C/EBPδ has been previously shown to be involved in the inflammatory response during persistent bacterial infection and has been suggested to act as an amplifier of the inflammatory response (7). Indeed, C/EBPδ−/− mice were able to resist a transient peritoneal infection with low-dose E. coli, but were highly susceptible to persistent peritoneal infection with high-dose E. coli. At a first glance, our data showing that C/EBPδ plays an essential aggravating role in pneumococcal pneumonia seem to contradict these observations. However, several important differences between our pneumonia model and the peritonitis model may explain this apparent discrepancy. Most notably, Gram-positive S. pneumoniae and Gram-negative E. coli express different pathogen-associated molecular patterns, of which phosphorylcholine (PC) might be the most important difference in this perspective. Indeed, PAFR-dependent transcytosis is facilitated by PC (13), and thus PC-negative E. coli will not disseminate in a PAFR-dependent manner (20). However, the fact that host defenses against PC-positive Pseudomonas aeruginosa are aggravated in PAFR−/− mice (21) suggests that an alternative mechanism may be responsible, at least in part. Another difference between our model and the foregoing model is in the site of primary infection, and it would be interesting to evaluate the role of PAFR in E. coli peritonitis. Independent of the actual mechanism, the role of C/EBPδ in innate immunity is not as straightforward as once expected, and C/EBPδ may play a detrimental role in antibacterial defense as well.
NF-κB and C/EBPδ have been shown to act synergistically on different promoters (19). Moreover, PAFR is directly induced by NF-κB on TNF-α stimulation in MonoMac-1 cells (22), whereas C/EBPδ also amplifies NF-κB–driven LPS-induced IL-6 levels (7). Here we have shown that C/EBPδ regulates PAFR levels, and that S. pneumoniae-induced PAFR promoter activity is dependent in part on NF-κB. Thus, it seems that C/EBPδ and NF-κB act synergistically to regulate PAFR expression during infection. Interestingly however, C/EBPδ overexpression to higher levels than seen in our in vitro LTA or S. pneumoniae stimulations dramatically induced PAFR expression by itself, indicating that C/EBPδ may act as a direct transcriptional activator of the pafr gene under nonphysiological circumstances. Further experiments should aim to elucidate the exact interplay between C/EBPδ and NF-κB in PAFR regulation during infectious disease.
Our data shed light on previous experiments showing that PAFR−/− mice are protected against LTA-induced lung inflammation (23). Those investigators proposed that LTA may be a ligand for PAFR or, alternatively, that LTA-TLR2–induced inflammation instigates the generation of endogenous mediators that serve as ligands for PAFR. However, our data suggest that LTA-dependent TLR2 activation induces PAFR expression in a C/EBPδ−dependent manner, and the lack of LTA-induced PAFR expression in PAFR−/− mice may actually explain the observed phenotype.
Considering that C/EBPδ has been shown to directly regulate proinflammatory cytokine expression (4, 7), we expected to find lower cytokine levels in the C/EBPδ−/− mice compared with the WT mice. Indeed, at 48 h after inoculation, IL-6, TNF-α, and MCP-1 levels were significantly lower in the absence of C/EBPδ in both lungs and peripheral organs; however, at earlier time points, no difference in inflammatory response was observed between the different genotypes. Thus, we speculate that the observed differences in cytokine levels at 48 h are a mere reflection of the bacterial load and not related to the genotype of the mice. This supposition is in line with previous studies reporting that the extent of inflammation in this particular model closely follows the bacterial burden in the lungs (16, 24), and that pulmonary bacterial loads are strongly correlated with proinflammatory cytokine concentrations (25).
It is tempting to speculate that the suggested role of C/EBPδ in the induction of PAFR expression during S. pneumoniae infection may be part of a more general mechanism. In fact, the absence or antagonism of PAFR causes significant protection against bacterial translocation through intestinal epithelial cells (26) and influenza A-associated lethality (27), PAFR contributes to cancer progression and metastasis (28), and activation of PAFR plays a major role in the pathogenesis of experimental Dengue infection (29). If C/EBPδ also induced PAFR levels in these disorders, it might be a potential target for therapeutic modulation. Further study is needed to examine this provocative claim.
At a first glance, C/EBPδ-driven PAFR mediated bacterial dissemination may not seem to explain the observed reduced bacterial loads at the site of infection. However, it is well known that on dissemination, bacteria extravasate from the bloodstream into distant organs. Given that extravasation of S. pneumoniae to the lung on i.v. injection is not affected by C/EBPδ (Fig. 3B), it is tempting to speculate that lower systemic bacterial loads will lead to reduced secondary infection of the lung, thereby causing reduced “local” bacterial loads.
Taken together, our data show that C/EBPδ plays a detrimental role in pneumococcal pneumonia-induced mortality. We suggest that C/EBPδ aggravates pneumococcal pneumonia by facilitating transcytosis in a PAFR-dependent manner. Overall, our findings make a substantial contribution to the understanding of the complex role of C/EBPδ in innate immunity.
Materials and Methods
Mice.
C/EBPδ−/− mice generated as described previously (30) and C57BL/6 mice (purchased from Charles River) were maintained at the animal facility of the Academic Medical Center of Amsterdam with free access to food and water. All animal experiments were approved by the facility’s Institutional Animal Care and Use Committee.
Infection Models.
Pneumonia was induced by intranasal inoculation with S. pneumoniae (serotype 3, American Type Culture Collection 6303; 5 × 105 CFU in 50 μL of saline) as described previously (31). Sepsis was induced by inoculation into the tail vein of 5 × 105 CFU of S. pneumoniae (in 200 μL of saline) of the same strain. Control animals received saline only. At predefined time points after inoculation, organs were collected and homogenates prepared as described previously (31).
Inflammatory Assays.
TNF-α, IL-6, MCP-1, and IL-10 levels were determined using a cytometric bead array multiplex assay (BD Biosciences). KC and MIP-2 levels were measured by ELISA (R&D Systems). Myeloperoxidase was measured by ELISA (Hycult Biotechnology).
Histology.
After embedding, lungs were stained with H&E to score inflammation, or tissue sections were stained for C/EBPδ and PAFR using immunohistochemical procedures, as described in SI Materials and Methods.
LightCycler Assay.
LightCycler assays were performed on a LightCycler 480 Real-Time PCR System (Roche Diagnostics) using the primers listed in SI Materials and Methods.
Lung Permeability Assays.
Lung permeability was assessed as described previously (18). For details, see SI Materials and Methods.
Cell Culture, Transfection, and Stimulation.
A549 (human epithelial lung cells; American Type Culture Collection CCL-185) and Calu3 (human bronchial epithelial cells; American Type Culture Collection HTB-55) cells were cultured in RPMI-1640 or DMEM/Ham’s-F12 medium, respectively, supplemented with nonessential amino acids and 10% (vol/vol) FCS. Cells were transfected with C/EBPδ silencing constructs (pSilencer Si-1 and -C) (6) using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions. Stable cell lines were obtained by selection with 500 μg/mL of geneticin. Cells were stimulated with either 10 ng/mL of LTA or growth-arrested S. pneumoniae (1 × 108 CFU/mL), after which cells were quickly lysed and stored for further analysis.
Western Blot Analysis.
Cells were lysed in Laemmli buffer for total protein lysates or in ice-cold cell extract buffer [10 mM Hepes-KOH (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.2 mM phenylmethysulfonyl fluoride] to obtain nuclear lysates as described previously (32). Then samples were fractionated by SDS/PAGE under reducing conditions and blotted with goat polyclonal α-C/EBPδ, α-PAFR, or α-tubulin as described in SI Materials and Methods.
Luciferase Assay.
A549 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions with a PAFR reporter construct bearing the promoter region of the PAFR gene driving the luciferase gene (33) and a CMV Renilla construct at a 1,000:1 ratio. Cells were cotransfected with a C/EBPδ overexpression construct or with a pcDNA3.1 empty vector. Cells were stimulated with 1 × 108 CFU of UV-irradiated S. pneumoniae, after which the cells were lysed in 50 μL of lysis buffer from the dual luciferase reporter kit (E1910; Promega) and stored at −80 °C for 30 min. Luciferase and Renilla expression was measured according to the manufacturer’s recommendations using a BioTek Synergy HT.
Statistical Analysis.
Differences between groups were analyzed using the Mann–Whitney U test. For the survival experiment, a Kaplan–Meier analysis was performed using the log-rank test. Cell stimulations with C/EBPδ silencing constructs were analyzed by two-way ANOVA with Bonferroni correction. Analyses were performed using GraphPad Prism version 5.0. A P value < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank Marieke ten Brink, Regina de Beer, and Chris van der Loos for technical assistance. This work was supported by the Dutch Kidney Foundation Grant C06.2198 (to J.D.). The C/EBPδ−/− mice were a gift from Dr. Esta Sterneck, National Cancer Institute. The C/EBPδ-silencing RNA plasmids were a gift from Dr. Ju-Ming Wang, College of Bioscience and Biotechnology, Taiwan. The PAFR luciferase reporter construct was a kind gift from Dr. Jana Stankova, University of Sherbrooke.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202641109/-/DCSupplemental.
References
- 1.Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 2.Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. [PubMed] [Google Scholar]
- 3.Juan TS, Wilson DR, Wilde MD, Darlington GJ. Participation of the transcription factor C/EBP δ in the acute-phase regulation of the human gene for complement component C3. Proc Natl Acad Sci USA. 1993;90:2584–2588. doi: 10.1073/pnas.90.7.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu HM, et al. The C/EBP bZIP domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem. 2000;275:16373–16381. doi: 10.1074/jbc.M910269199. [DOI] [PubMed] [Google Scholar]
- 5.Beck GC, et al. Heterogeneity in lipopolysaccharide responsiveness of endothelial cells identified by gene expression profiling: Role of transcription factors. Clin Exp Immunol. 2006;143:523–533. doi: 10.1111/j.1365-2249.2006.03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JM, Ko CY, Chen LC, Wang WL, Chang WC. Functional role of NF-IL6β and its sumoylation and acetylation modifications in promoter activation of cyclooxygenase 2 gene. Nucleic Acids Res. 2006;34:217–231. doi: 10.1093/nar/gkj422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litvak V, et al. Function of C/EBPδ in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slofstra SH, et al. Gene expression profiling identifies C/EBPδ as a candidate regulator of endotoxin-induced disseminated intravascular coagulation. Am J Respir Crit Care Med. 2007;176:602–609. doi: 10.1164/rccm.200609-1250OC. [DOI] [PubMed] [Google Scholar]
- 9.Mandell LA, et al. Infectious Diseases Society of America American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard GR, et al. Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett JG, et al. Infectious Diseases Society of America Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis. 2000;31:347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman C. Clinical relevance of antimicrobial resistance in the management of pneumococcal community-acquired pneumonia. J Lab Clin Med. 2004;143:269–283. doi: 10.1016/j.lab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 14.Radin JN, et al. β-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rijneveld AW, et al. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis. 2004;189:711–716. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- 16.Knapp S, Schultz MJ, van der Poll T. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock. 2005;24(Suppl 1):12–18. doi: 10.1097/01.shk.0000191385.41689.f3. [DOI] [PubMed] [Google Scholar]
- 17.Lim JH, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27:349–360. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Fu P, et al. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33:612–624. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia C, Cheshire JK, Patel H, Woo P. Cross-talk between transcription factors NF-κB and C/EBP in the transcriptional regulation of genes. Int J Biochem Cell Biol. 1997;29:1525–1539. doi: 10.1016/s1357-2725(97)00083-6. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie SH, Ainscough S, Dickens A, Lewin J. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J Med Microbiol. 1996;44:35–40. doi: 10.1099/00222615-44-1-35. [DOI] [PubMed] [Google Scholar]
- 21.van Zoelen MAD, et al. Platelet-activating factor receptor contributes to host defense against Pseudomonas aeruginosa pneumonia but is not essential for the accompanying inflammatory and procoagulant response. J Immunol. 2008;180:3357–3365. doi: 10.4049/jimmunol.180.5.3357. [DOI] [PubMed] [Google Scholar]
- 22.Dagenais P, Thivierge M, Stankova J, Rola-Pleszczynski M. Modulation of platelet-activating factor receptor (PAFR) gene expression via NFκB in MonoMac-1 cells. Inflamm Res. 1997;46(Suppl 2):S161–S162. doi: 10.1007/s000110050158. [DOI] [PubMed] [Google Scholar]
- 23.Knapp S, et al. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 24.Bergeron Y, et al. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giebelen IA, Leendertse M, Florquin S, van der Poll T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur Respir J. 2009;33:375–381. doi: 10.1183/09031936.00103408. [DOI] [PubMed] [Google Scholar]
- 26.Keely S, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell. 2010;21:538–546. doi: 10.1091/mbc.E09-07-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia CC, et al. Platelet-activating factor receptor plays a role in lung injury and death caused by influenza A in mice. PLoS Pathog. 2010;6:e1001171. doi: 10.1371/journal.ppat.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melnikova V, Bar-Eli M. Inflammation and melanoma growth and metastasis: The role of platelet-activating factor (PAF) and its receptor. Cancer Metastasis Rev. 2007;26:359–371. doi: 10.1007/s10555-007-9092-9. [DOI] [PubMed] [Google Scholar]
- 29.Souza DG, et al. Essential role of platelet-activating factor receptor in the pathogenesis of Dengue virus infection. Proc Natl Acad Sci USA. 2009;106:14138–14143. doi: 10.1073/pnas.0906467106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterneck E, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein δ. Proc Natl Acad Sci USA. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rijneveld AW, et al. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J Immunol. 2002;168:3507–3511. doi: 10.4049/jimmunol.168.7.3507. [DOI] [PubMed] [Google Scholar]
- 32.Queiroz KC, et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene. 2010;29:6314–6322. doi: 10.1038/onc.2010.375. [DOI] [PubMed] [Google Scholar]
- 33.Lukashova V, Chen ZG, Duhé RJ, Rola-Pleszczynski M, Stanková J. Janus kinase 2 activation by the platelet-activating factor receptor (PAFR): Roles of Tyk2 and PAFR C terminus. J Immunol. 2003;171:3794–3800. doi: 10.4049/jimmunol.171.7.3794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.