Abstract
Life-history theory suggests that animals may skip reproductive events after initial maturation to maximize lifetime fitness. In iteroparous teleosts, verifying past spawning history is particularly difficult; the degree of skipped spawning at the population level therefore remains unknown. We unequivocally show frequent skipped spawning in Northeast Arctic cod (NEAC) in a massive field and laboratory effort from 2006 to 2008. This was verified by postovulatory follicles in temporarily arrested ovaries close to the putative spawning period. At the population level, “skippers” were estimated to be approximately equally abundant as spawning females in 2008, constituting ∼24% of the females 60–100 cm. These females never truly started vitellogenesis and principally remained on the feeding grounds when spawners migrated southward, avoiding any migration costs. The proximate cause of skipping seems to be insufficient energy to initiate oocyte development, indicating that skipped spawning may partly be a density-dependent response important in population regulation. Our data also indicate more skipping among smaller females and potential tradeoffs between current and future reproductive effort. We propose that skipped spawning is an integral life-history component for NEAC, likely varying annually, and it could therefore be an underlying factor causing some of the currently unexplained large NEAC recruitment variation. The same may hold for other teleosts.
Keywords: codfish, population dynamics, reproductive biology, total egg production, stock reproductive potential
Population demography changes according to reproduction and mortality. In high latitude marine ectotherms, including teleosts, survival of the youngest age classes varies substantially, resulting in large fluctuations in population size (1). Historic spawning stock biomass (SSB) is used to forecast future fish recruitment, although the amount of unexplained variation indicates that SSB may not accurately reflect offspring production (2, 3). One factor that could significantly impact egg production is skipped spawning—the failure of iteroparous spawners to use each spawning opportunity after sexual maturity sensu (4). For most teleosts this phenomenon and its impact on population demography has received scant attention, but theoretical models indicate that up to 30% of the mature individuals may skip spawning and that it is an adaptive strategy optimizing lifetime fitness (5). To date, skipped spawning has been documented in more than 30 species (4, 6), including freshwater (7), marine (8), and anadromous teleosts (9). It has been documented for fish with both indeterminate and determinate fecundity and sequential hermaphrodites (6) and in species with as diverse life histories as the European horse mackerel Trachurus trachurus (10) and the orange roughy Hoplostethus atlanticus (8). Given the difficulty in conclusively establishing past spawning, reports of skipped spawning in teleosts are commonly anecdotal (4).
The major process of energy transfer and oocyte growth in marine fish is vitellogenesis (11). At its onset energy is sequestered into previtellogenic oocytes, causing them to increase in size and enter the cortical alveoli oocyte stage. Vitellogenesis proceeds with the yolk granule oocyte stage, which lasts until oocytes are hydrated and the eggs spawned (11). After spawning, the gonad shrinks rapidly in size, visually appearing immature, and contains only previtellogenic oocytes, until the next maturation cycle. Fish mature at different sizes, and the presence of only previtellogenic oocytes in “large females” cannot be used as conclusive evidence for truly quantifying the numbers of skippers (e.g., ref. 12). Similarly, Yaragina (13) recently consulted macroscopic staging information on Northeast Arctic cod (Gadus morhua) (NEAC) and estimated the proportion of fish of uncertain maturity status (i.e., putative, but not certain, skippers) to constitute up to 21% of the sexually mature fraction in the years 1984–2006.
Unequivocal evidence of past spawning is provided by the presence of postovulatory follicles (POFs) in female gonads (4), but typically POFs are ephemeral and may last for only 1 d after spawning (14). In cod, POFs persist for more than 1 y (15), providing a unique marker of past spawning. POFs are primarily identified through time-consuming histological examination, and this hampers widespread use of the technique.
We aimed to elucidate the frequency and underlying mechanisms of female skipped spawning in the world’s largest cod population, presently NEAC, through the POF spawning marker, measurements of individual energy reserves, size, and age, and the application of state-of-the art assessment methodology. In the following we will define females with developing oocytes as “developing,” we will use “nondeveloping” to denote all females not developing oocytes. Nondeveloping females with POFs (10) were termed “skippers,” and those without were termed “immatures.”
Results
The target size (i.e., fish ≥60 cm) for sampling (Table S1) was directed toward females likely to be developing gonads (up-coming “spawners”) (16), but large numbers of nondeveloping females were also present (Fig. 1A). Relative gonad size [i.e., gonadosomatic index (GSI) = 100 × gonad weight × total weight−1] was closely linked to oocyte development; 98.8% (n = 410) of the females with GSI <1.1% were nondeveloping, whereas 97.9% (n = 335) of those females with GSI >1.5% were developing (Fig. 1B). Discriminant analyses yielded similar results. The threshold GSI value separating nondeveloping and developing females was estimated to be 1.51%, 1.29%, and 1.35% for sampling season 1 (2006–2007), 2 (2007–2008), and both sampling seasons pooled, respectively. We conclude that relative gonad size is an accurate indicator of present developmental status of individual NEAC females, but it could not be used to separate between immatures and skippers on the basis of their GSI only (Table S2).
Fig. 1.
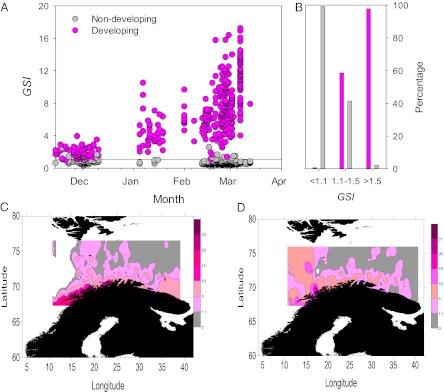
Gonad size in relation to developmental status and spatial distribution of NEAC. (A) Relationship between relative gonad size (i.e., GSI = 100 × gonad weight × total weight−1) and gonad development; pink, developing gonads; gray, nondeveloping gonads (n = 773). Line, GSI value of 1.1%. (B) Percentage of developing and nondeveloping fish for GSI <1.1%, GSI 1.1–1.5%, and GSI >1.5%—both sampling seasons [i.e., (i) November 2006 to April 2007 and (ii) November 2007 to April 2008, pooled]. (C and D) Geographic distribution of females ≥60 cm according to GSI values in February–April 2007 (C) and February–April 2008 (D). To construct C and D, individual GSI values, along with sample location (latitude and longitude), were subjected to kriging to create a contour map of the GSI values using the program SURFER (version 9; Golden Software). The map represents the distribution of weighted mean GSI over the area studied. Gray areas, GSI <1.1% (i.e., females not developing gonads).
Histological screening gave a homogenous picture of oocyte stages within groups and showed that oocyte maturation had not progressed beyond the previtellogenic stage in virtually all nondeveloping females, whereas developing females had predominantly reached advanced vitellogenic yolk granule stage (Table 1). The intermediate cortical alveoli stage was rare, effectively creating a bimodal distribution of oocyte stages (Table 1).
Table 1.
Percentage of NEAC in different ovarian developmental stages in different sampling months
| Potential skippers |
Developing |
|||||
| Month | PVO | E-CAO | n | L-CAO | YGO | n |
| Nov | 73.7 | 26.3 | 19 | 5.4 | 95.0 | 37 |
| Dec | 81.4 | 18.6 | 43 | 22.2 | 77.8 | 27 |
| Jan | 100 | 0 | 6 | 2.8 | 97.2 | 36 |
| Feb | 91.3 | 8.7 | 287 | 0 | 100 | 125 |
| Mar | 96.5 | 3.5 | 86 | 0 | 100 | 27 |
Classification criterion: most advanced oocyte stage, determined by histology (17) (n = 693). Potential skippers were divided into those showing previtellogenic oocytes (PVO) and those showing early cortical alveoli oocytes [E-CAO, LC20 <300 μm not detectable by image analyses (IA)] (18). LC20, leading diameter, from measurement of 20 largest oocytes (18). POFs were subsequently consulted to exclude immatures (see text). Developing fish were divided into late cortical alveoli (L-CAO, LC20 >300 μm detectable by IA) (18) or yolk granule oocyte (YGO) stages. Results are pooled across sampling seasons. All samples were collected within the Barents Sea except the January samples (caught at 70–71° N, 16–17° E).
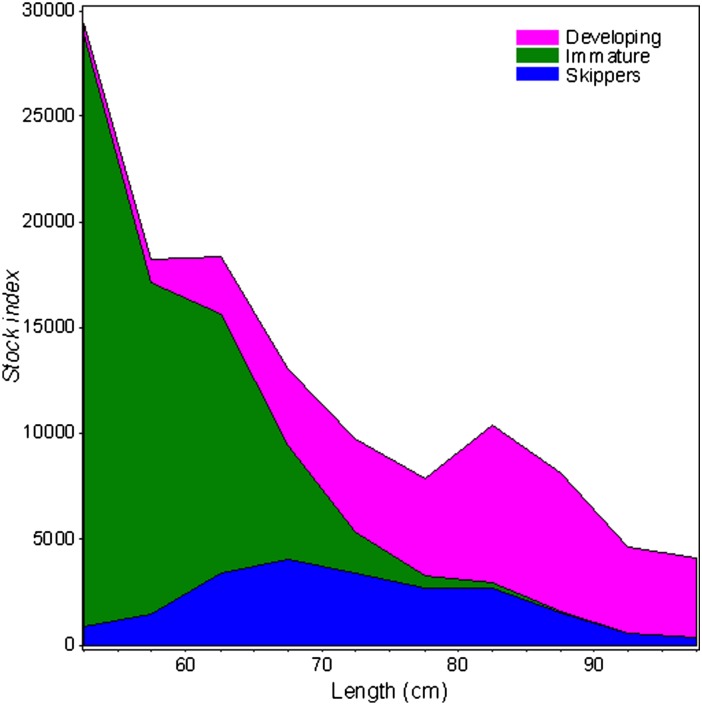
Nondeveloping fish dominated (84%) the February and March samples of females ≥60 cm in the central Barents Sea (Fig. 1 C and D). Only 5.2% of all females ≥60 cm observed on the NEAC spawning grounds in 2008 had nondeveloping ovaries. Our histological analyses revealed that a large portion of the nondeveloping females had spawned before, although this depended on female length (Wald χ2 = 60.61, df = 1, P < 0.0001; Tables S3 and S4), with many of the smaller fish in our length range being immatures (Figs. 2 and 3C). In 2008, 47% [95% confidence interval (CI) 40–56%] of the nondeveloping females were skippers. In terms of biomass the corresponding value was 58% (95% CI 50–65%). In the total population we estimate that 24% (90% CI 13–34%) of the females between 60 and 100 cm skipped spawning, 25% were developing oocytes, and 51% were immatures in 2008 (Fig. 2).
Fig. 2.
Calculated numbers of skipping (blue), immature (green), and developing (pink) females, according to total length for the NEAC population in 2008. Values <60 cm represent extrapolation of model results. Statistical details are listed in SI Methods.
Fig. 3.
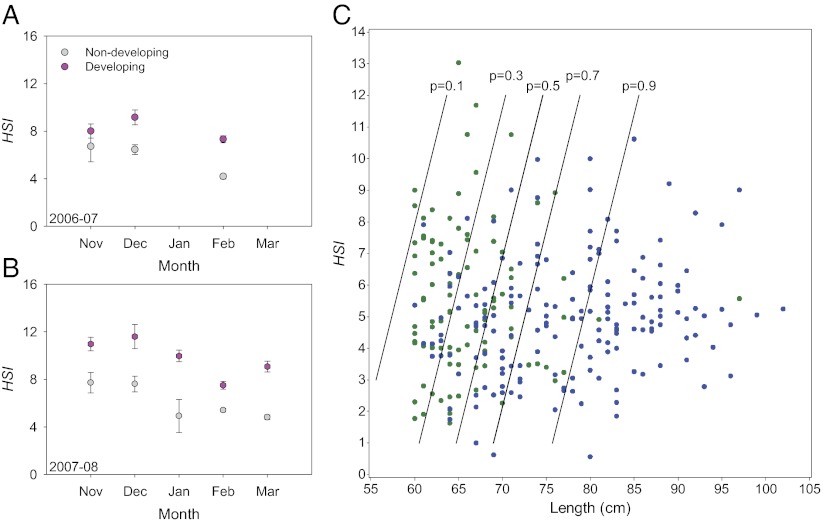
Explanatory variables of skipped spawning. Relative liver size [(hepatosomatic index, HSI) = 100 × liver weight × (total weight − gonad weight)−1] in different months for developing (pink) and nondeveloping (gray) females, 2006–2007 (A) and 2007–2008 (B). Error bars represent the SE. (C) HSI values of skippers (blue) and immatures (green) in February–March 2008, with calculated probability lines of being a skipper fitted for P values from 0.1 to 0.9 (n = 280).
Developing NEAC females had larger livers and consequently higher liver energy reserves (19) than nondeveloping females (F1,683 = 235.6, P < 0.0001; Fig. 3 A and B). Immatures also had larger livers than skippers (Wald χ2 = 5.62, df = 1, P < 0.05; Table S4) in February–March 2008.
Discussion
We unequivocally show that skipped spawning is a common occurrence in NEAC. Furthermore, our data also provide important insights into the likely evolutionary and state-dependent mechanisms underlying our observations.
Teleosts exhibit three different modes of skipping: (i) resting skippers do not start oocyte development, (ii) reabsorbing skippers start oocyte development but later go through mass atresia and resorption of all vitellogenic oocytes, and (iii) retaining skippers complete oocyte development but then retain their fully developed eggs (4). The latter mode is hardly ever observed in the wild (4), and mass atresia of yolk granule oocytes seems to be rare in NEAC (19). NEAC skippers thus predominantly exhibit a resting strategy (Table 1) very rarely advancing beyond the early cortical alveoli stage (Table 1) (17). Spawning omission through mass atresia has previously been noted in various cod stocks (4, 20). Minor degrees of atresia are normally seen in teleostean gonads, typically at the vitellogenic stage (11). Although some energy undoubtedly is lost in this process, the resorption of developing oocytes allows females to recover oocyte energy if nutritional conditions become unfavorable (21).
NEAC undertake a long-distance spawning migration (∼800 km each way) (22), starting in early December (23). Like other animal migrations (24–26), this migration incurs considerable energetic costs (27). An aborted spawning run would cost a female the energy associated with atresia as well as the energy spent on the spawning migration itself. An adaptive advantage would likely accrue by committing to oocyte maturation before this migration starts. Indeed, nondeveloping fish dominated the February and March catches in the central Barents Sea, after the bulk of migrating fish had left the area (23) (Fig. 1 C and D), but were rarely observed on the NEAC spawning grounds in 2008 (Fig. 1 C and D). Thus, any skippers will principally remain in the Barents Sea (Fig. 1 C and D) with arrested ovaries (17) (Table 1). That nonreproducing “mature” individuals do not undertake migrations associated with spawning has also been noted in other teleosts (7, 8). If spawning stock abundance is measured in spawning aggregations, and it is assumed that all fish spawn, this has the important implication that skipped spawning may result in an underestimation of the numbers of large individuals in the population.
Numerically, our estimates thus indicate that skippers were approximately equally abundant as spawners in the length range 60–100 cm in 2008, comparable with theoretically based estimates for NEAC (5) but somewhat higher than the maximum indicated by Yaragina (13). Hence, skipped spawning is clearly a common occurrence in NEAC, resulting in annual reproductive productivity that is typically considerably lower than would be expected if all mature individuals participated in spawning.
Animals commonly adjust their reproductive investment in a way likely to enhance their lifetime reproductive success. Reproductive investment may vary according to factors such as territory quality and age (28–30), and similarly, the “decision” to skip spawning is likely influenced by internal physiological state. We found that developing females had larger energy reserves than nondeveloping females (Fig. 3 A and B) and that immatures also had larger energy reserves than skippers in February–March 2008 (Fig. 3C). The latter result may possibly reflect differential energy allocation between body growth and storage in the past year, or incomplete recovery from last year’s spawning activity. Regardless, our results support the theory that skipping is associated with insufficient energy reserves to initiate vitellogenesis (31, 32). Low energy reserves during spawning increases postspawning mortality (33), and skipping when energy reserves get below a critical threshold is therefore likely an adaptive strategy (5). This latter result also suggests that skipped spawning may function as a density-dependent response and thereby act as an important mechanism in population regulation for this marine teleost. It is known that high population densities can lead to food limitation, reduced growth, and limited energy reserves (34, 35). As such it may be expected that more individuals fail to build up sufficient energy reserves to spawn and thereby skip spawning at large population sizes. Tentatively agreeing with this, our study showing frequent skipped spawning occurred at a time when the NEAC population was estimated to very be large (36). Intriguingly, skipped spawning may thus also be an alternative or additional underlying compensatory mechanism, in addition to density-dependent mortality, explaining the form of stock–recruitment relationships such as the Ricker curve (37).
The incidence of skipped spawning also seemed more prevalent in smaller, younger fish (Fig. 2), perhaps reflecting higher migration cost for smaller individuals (38), lesser total energy reserves, and thereby decreased survival after spawning. However, evolutionary theory also predicts that older females are expected to invest more in the present reproductive opportunity, given their limited number of future reproductive opportunities (39, 40) compared with younger females, which may increase their lifetime reproductive success by investing in growth (5). Accordingly, spawning omission may be caused by age-dependent differential energy allocation rather than limited energy reserves per se, and some skippers may be expected to grow more than developing fish in the year of missed migration and spawning. This issue could be addressed in future studies by, for example, back-calculating individual otolith growth patterns.
Our results conclusively demonstrate that skipped spawning is occurring frequently in NEAC. The resting mode of skipped spawning exhibited by NEAC females may be an adaptive strategy linked to the long-range spawning migration undertaken by this population. The proximate cause of skipping seems to be a failure to build up sufficient energy reserves to initiate oocyte maturation in the autumn, and this also suggests that skipped spawning may act as a density-dependent response to limited energy reserves occurring more frequently at larger population sizes. The higher frequencies of skippers among the smaller females could indicate age- or size-dependent differential energy allocation during the feeding season and thereby a tradeoff between current and future reproductive success. From a fisheries management perspective, the link between energy reserves and skipped spawning suggests that skipping will not only heavily impact the total egg production of NEAC but may also vary substantially annually and could therefore be an underlying factor explaining some of the large recruitment variation currently unexplained (41). If SSB is measured purely as a function of the number of individuals above a certain age or size or is a function of a maturity ogive, this is likely to result in an overestimation of the total production when skipped spawning is prevalent. The same is likely true for other commercially and culturally important teleosts (6). This phenomenon therefore needs consideration in the construction of realistic stock–recruitment models.
Methods
Full detailed descriptions of the collection of samples and data analyses are provided in SI Methods.
We collected females ≥60 cm (total length) in two field seasons (2006–2007 and 2007–2008) from mid-November to late March (Fig. S1 and Table S1), during the vitellogenic prespawning period (19, 42). We measured total length of each fish and whole-body, gonad, and liver weight, to indicate energy reserves (e.g., ref. 3). The otoliths were collected and used to age each fish by breaking the otoliths along the transversal axis and reading the number of annual increments directly under a stereo microscope. Gonad sections were preserved for subsequent histology (n = 693) and image analysis (n = 773) in the laboratory (further information in SI Methods). The image analysis identified females developing oocytes (18), whereas oocyte stage and past spawning history was determined histologically. We defined females with developing oocytes as “developing” and used “nondeveloping” to denote all females not developing oocytes. Nondeveloping females with POFs (15) were termed “skippers,” and those without “immatures.” We used quadratic discriminant analyses to link relative gonad size to developmental status and an ANOVA model to examine energy reserves of fish in relation to their developmental status (i.e., developing or nondeveloping). The likelihood of a nondeveloping female being a skipper was modeled as a function of length and relative liver size. For the latter test we used the Wald χ2 statistic to examine significance. The sufficient spatial sample coverage then allowed us to estimate the number of skippers in the population in 2008, but not in 2007 (further information in SI Methods).
Supplementary Material
Acknowledgments
We thank V. Mangerud and B. Njøs Strand for laboratory assistance, the crews aboard various commercial fishing and Institute of Marine Research (Norway) research vessels for their effort during sample collection, and C. Jørgensen for comments and feedback during the development of the manuscript. The study was funded by the Research Council of Norway Projects 173341/S40 and 190228.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200223109/-/DCSupplemental.
References
- 1.Rothschild BJ, Chen CS, Lough RG. Managing fish stocks under climate uncertainty. ICES J Mar Sci. 2005;62:1531–1541. [Google Scholar]
- 2.Marshall CT, Kjesbu OS, Yaragina NA, Solemdal P, Ulltang O. Is spawner biomass a sensitive measure of the reproductive and recruitment potential of Northeast Arctic cod? Can J Fish Aquat Sci. 1998;55:1766–1783. [Google Scholar]
- 3.Marshall CT, Yaragina NA, Lambert Y, Kjesbu OS. Total lipid energy as a proxy for total egg production by fish stocks. Nature. 1999;402:288–290. [Google Scholar]
- 4.Rideout RM, Rose GA, Burton MPM. Skipped spawning in female iteroparous fishes. Fish Fish. 2005;6:50–72. [Google Scholar]
- 5.Jørgensen C, Ernande B, Fiksen O, Dieckmann U. The logic of skipped spawning in fish. Can J Fish Aquat Sci. 2006;63:200–211. [Google Scholar]
- 6.Rideout RM, Tomkiewicz J. Skipped spawning in fishes: More common than you might think. Mar Coast Fish. 2011;3:176–189. [Google Scholar]
- 7.Milton DA, Chenery SR. Movement patterns of barramundi Lates calcarifer, inferred from Sr-87/Sr-86 and Sr/Ca ratios in otoliths, indicate non-participation in spawning. Mar Ecol Prog Ser. 2005;301:279–291. [Google Scholar]
- 8.Bell JD, et al. Spatial variation in reproduction, and occurrence of nonreproductive adults, in orange roughy, Hoplostethus atlanticus Collett (Trachichthyidae), from south-eastern Australia. J Fish Biol. 1992;40:107–122. [Google Scholar]
- 9.Jonsson N, Hansen LP, Jonsson B. Variation in age, size and repeat spawning of adult Atlantic salmon in relation to river discharge. J Anim Ecol. 1991;60:937–947. [Google Scholar]
- 10.Ndjaula HON, Hansen T, Kruger-Johnsen M, Kjesbu OS. Oocyte development in captive Atlantic horse mackerel Trachurus trachurus. ICES J Mar Sci. 2009;66:623–630. [Google Scholar]
- 11.Tyler CR, Sumpter JP. Oocyte growth and development in teleosts. Rev Fish Biol Fish. 1996;6:287–318. [Google Scholar]
- 12.Rideout RM, Rose GA. Suppression of reproduction in Atlantic cod Gadus morhua. Mar Ecol Prog Ser. 2006;320:267–277. [Google Scholar]
- 13.Yaragina NA. Biological parameters of immature, ripening, and non-reproductive, mature northeast Arctic cod in 1984–2006. ICES J Mar Sci. 2010;67:2033–2041. [Google Scholar]
- 14.Mylonas CC, et al. Preparation and administration of gonadotropin-releasing hormone agonist (GnRHa) implants for the artificial control of reproductive maturation in captive-reared Atlantic bluefin tuna (Thunnus thynnus thynnus) Rev Fish Sci. 2007;15:183–210. [Google Scholar]
- 15.Witthames PR, Thorsen A, Kjesbu OS. The fate of vitellogenic follicles in experimentally monitored Atlantic cod Gadus morhua (L.): Application to stock assessment. Fish Res. 2010;104:27–37. [Google Scholar]
- 16.Nash RDM, Pilling GM, Kell LT, Schon PJ, Kjesbu OS. Investment in maturity-at-age and -length in northeast Atlantic cod stocks. Fish Res. 2010;104:89–99. [Google Scholar]
- 17.Skjæraasen JE, et al. Mechanisms regulating oocyte recruitment and skipped spawning in Northeast Arctic cod (Gadus morhua) Can J Fish Aquat Sci. 2009;66:1582–1596. [Google Scholar]
- 18.Thorsen A, Kjesbu OS. A rapid method for estimation of oocyte size and potential fecundity in Atlantic cod using a computer-aided particle analysis system. J Sea Res. 2001;46:295–308. [Google Scholar]
- 19.Skjæraasen JE, et al. Liver energy, atresia and oocyte stage influence fecundity regulation in Northeast Arctic cod. Mar Ecol Prog Ser. 2010;404:173–183. [Google Scholar]
- 20.Rideout RM, Morgan MJ, Lilly GR. Variation in the frequency of skipped spawning in Atlantic cod (Gadus morhua) off Newfoundland and Labrador. ICES J Mar Sci. 2006;63:1101–1110. [Google Scholar]
- 21.Kjesbu OS, Klungsoyr J, Kryvi H, Witthames PR, Walker MG. Fecundity, atresia, and egg size of captive Atlantic cod (Gadus morhua) in relation to proximate body composition. Can J Fish Aquat Sci. 1991;48:2333–2343. [Google Scholar]
- 22.Godø OR, Michalsen K. Migratory behaviour of north-east Arctic cod, studied by use of data storage tags. Fish Res. 2000;48:127–140. [Google Scholar]
- 23.Bergstad OA, Jørgensen T, Dragesund O. Life-history and ecology of the gadoid resources of the Barents sea. Fish Res. 1987;5:119–161. [Google Scholar]
- 24.Weber TP, Ens BJ, Houston AI. Optimal avian migration: A dynamic model of fuel stores and site use. Evol Ecol. 1998;12:377–401. [Google Scholar]
- 25.Hedenstrom A. Scaling migration speed in animals that run, swim and fly. J Zool (Lond) 2003;259:155–160. [Google Scholar]
- 26.Hedenström A. Adaptations to migration in birds: Behavioural strategies, morphology and scaling effects. Philos Trans R Soc Lond B Biol Sci. 2008;363:287–299. doi: 10.1098/rstb.2007.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen C, Dunlop ES, Opdal AF, Fiksen O. The evolution of spawning migrations: State dependence and fishing-induced changes. Ecology. 2008;89:3436–3448. doi: 10.1890/07-1469.1. [DOI] [PubMed] [Google Scholar]
- 28.Högstedt G. Evolution of clutch size in birds: Adaptive variation in relation to territory quality. Science. 1980;210:1148–1150. doi: 10.1126/science.210.4474.1148. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson IR, Bancroft DR. Fluctuating trade-offs favour precocial maturity in male Soay sheep. Proc Biol Sci. 1995;262:267–275. doi: 10.1098/rspb.1995.0205. [DOI] [PubMed] [Google Scholar]
- 30.Cam E, Monnat JY. Apparent inferiority of first-time breeders in the kittiwake: The role of heterogeneity among age classes. J Anim Ecol. 2000;69:380–394. [Google Scholar]
- 31.Rijnsdorp AD. The mechanism of energy allocation over reproduction and somatic growth in female North Sea plaice, Pleuronectes platessa L. J Sea Res. 1990;25:279–290. [Google Scholar]
- 32.Burton MP, Penney RM, Biddiscombe S. Time course of gametogenesis in Northwest Atlantic cod (Gadus morhua) Can J Fish Aquat Sci. 1997;54:122–131. [Google Scholar]
- 33.Dutil JD, Lambert Y. Natural mortality from poor condition in Atlantic cod (Gadus morhua) Can J Fish Aquat Sci. 2000;57:826–836. [Google Scholar]
- 34.Utz RM, Hartman KJ. Temporal and spatial variation in the energy intake of a brook trout (Salvelinus fontinalis) population in an Appalachian watershed. Can J Fish Aquat Sci. 2006;63:2675–2686. [Google Scholar]
- 35.Amundsen PA, Knudsen R, Klemetsen A. Intraspecific competition and density dependence of food consumption and growth in Arctic charr. J Anim Ecol. 2007;76:149–158. doi: 10.1111/j.1365-2656.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- 36.Sundby S, Nakken O. Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecadal climate oscillations and climate change. ICES J Mar Sci. 2008;65:953–962. [Google Scholar]
- 37.Ricker WE. Stock and recruitment. J Fish Res Board Can. 1954;11:559–623. [Google Scholar]
- 38.Alexander RM. Principles of Animal Locomotion. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 39.Creighton JC, Heflin ND, Belk MC. Cost of reproduction, resource quality, and terminal investment in a burying beetle. Am Nat. 2009;174:673–684. doi: 10.1086/605963. [DOI] [PubMed] [Google Scholar]
- 40.Kindsvater HK, Bonsall MB, Alonzo SH. Survival costs of reproduction predict age-dependent variation in maternal investment. J Evol Biol. 2011;24:2230–2240. doi: 10.1111/j.1420-9101.2011.02351.x. [DOI] [PubMed] [Google Scholar]
- 41.Ulltang Ø. Stock assessment and biological knowledge: Can prediction uncertainty be reduced? ICES J Mar Sci. 1996;53:659–675. [Google Scholar]
- 42.Kjesbu OS, et al. Thermal dynamics of ovarian maturation in Atlantic cod (Gadus morhua) Can J Fish Aquat Sci. 2010;67:605–625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.