Abstract
Emerging evidence suggests that chromatin adopts a nonrandom 3D topology and that the organization of genes into structural hubs and domains affects their transcriptional status. How chromatin conformation changes in diseases such as cancer is poorly understood. Moreover, how oncogenic transcription factors, which bind to thousands of sites across the genome, influence gene regulation by globally altering the topology of chromatin requires further investigation. To address these questions, we performed unbiased high-resolution mapping of intra- and interchromosome interactions upon overexpression of ERG, an oncogenic transcription factor frequently overexpressed in prostate cancer as a result of a gene fusion. By integrating data from genome-wide chromosome conformation capture (Hi-C), ERG binding, and gene expression, we demonstrate that oncogenic transcription factor overexpression is associated with global, reproducible, and functionally coherent changes in chromatin organization. The results presented here have broader implications, as genomic alterations in other cancer types frequently give rise to aberrant transcription factor expression, e.g., EWS-FLI1, c-Myc, n-Myc, and PML-RARα.
Mounting evidence suggests that many genes dynamically colocalize to shared nuclear compartments that favor gene activation or silencing (1–3). As demonstrated by chromosome conformation capture (3C) (4), ligand-bound androgen receptors (AR) and estrogen receptors mediate looped chromatin structures resulting in coordinated transcription of target genes (5, 6). In embryonic carcinoma cells, the PolyComb complex subunit EZH2 represses some of its target genes via the formation of similar looped chromatin structures (7). Trans-interactions that regulate gene expression have also been reported (8–10). These data suggest that oncogenic transcriptional regulators are capable of inducing changes in chromatin structures. These studies have mainly focused on local chromatin structures, and it is still unclear whether more global changes occur in the process of oncogene-mediated transformation. A broader implication of these observations is that global chromatin organization changes could impact functional and phenotypic aspects of cancer.
To globally investigate oncogene-mediated chromatin structure changes we focused on ERG, the ETS-family transcription factor most frequently rearranged and overexpressed in prostate cancer through the TMPRSS2-ERG and other gene fusions involving androgen-responsive promoters (11–13). ERG interacts with several cofactors (14) and other transcription factors including AR to regulate the expression of thousands of genes that favor dedifferentiation, cell invasion, and neoplastic transformation of prostate epithelium when overexpressed (15–20). We therefore hypothesized that changes in global gene expression induced by ERG overexpression could be associated with global changes in the 3D structure of chromosomes.
Results
ERG Overexpression Is Associated with Chromatin Topology.
To test our hypothesis, we used stable isogenic, normal benign prostate epithelial cell lines (RWPE1) (21) that differ with respect to ERG overexpression (17) (Fig. S1A). To test whether ERG overexpression is associated with global changes in chromatin structure, we performed unbiased chromatin interaction mapping using the Hi-C technique (22) from both RWPE1-ERG and RWPE1-GFP cells, with biological replicates (Fig. 1A and Fig. S1A). Successful fill-in and ligation were determined as previously reported (22) by testing for a known interaction between two distant genomic loci located on chromosome 6 (23) (Fig. S1B). The Hi-C libraries were paired-end sequenced using an Illumina GAIIx platform. Following alignment to the human genome (hg18) and filtering to remove unligated and self-ligated DNA, we identified intrachromosomal (or cis-) and interchromosomal (or trans-) interactions in both RWPE1 cell lines. Correlation matrices obtained from independent biological replicates were highly similar in both cell lines (Pearson’s correlation coefficient r = 0.99), indicating that the interaction patterns are highly reproducible. Biological replicate interactions were therefore combined for further analyses. In total, we identified 18.4 million intrachromosomal (or cis-) and 18.3 million interchromosomal (or trans-) interactions in RWPE1-ERG cells (Dataset S1). We also identified 16.9 million cis- and 18.6 million trans-interactions in RWPE1-GFP cells.
Fig. 1.
Specific cis-interactions that depend on ERG expression. (A) Schematic representation of the experimental flow of the Hi-C experiments. Red symbols refer to ERG protein and white symbols refer to other DNA-interacting proteins. (B) Circos plot depicting chromosomes 1–22 and X annotated for ERG binding on the basis of ChIP-seq data (normalized reads shown in orange) and 1,266 differentially expressed genes annotated on the basis of RNA-seq data, indicating over- (red) and underexpressed genes in RWPE1-ERG relative to RWPE1-GFP cells. Lines indicate significantly different cis- and trans-interactions in which line color represents interactions enriched in RWPE-ERG cells (orange) or RWPE-GFP cells (blue).
To visualize global patterns of cis-interactions, we binned the genome (chromosomes 1–22, X) into 1-Mb intervals and calculated the ratio between observed and expected number of interactions connecting each interval pair. We then generated correlation heat maps depicting the extent to which pairs of genomic intervals interact with the same intervals, on the basis of the assumption that if two loci are close in nuclear space, their patterns of interaction with other loci should be highly correlated. Confirming prior observations (22), the heat maps showed plaid patterns, indicating that each chromosome is composed of domains that are either enriched (red to yellow) or depleted (green) for cis-interactions (Fig. S2).
The patterns of enriched and depleted cis-interactions were predominantly similar between the two cell lines for each chromosome but some differences were visible (Fig. S3 A–C). Because gains and losses of a correlation can be due to gains and losses of other interactions shared by the two loci, we wanted to quantitatively identify differences in cis- and trans-interactions between the two cell lines. For this, we evaluated the statistical significance of the differences in interaction counts, using Fisher’s exact tests coupled with Benjamini–Hochberg corrections (SI Computational Analysis). Using an adjusted P-value threshold <0.05 (5% FDR), we identified 8,611 cis- and 86 trans-interactions with significant differences between the two cell lines (Fig. 1B). Among the 8,611 cis-interactions, 2,501 and 6,110 increased and decreased in proximity in RWPE1-ERG vs. RWPE1-GFP cells, respectively. Interestingly, we found a differential interaction between loci containing two genes highly relevant for prostate cancer, TMPRSS2 (the most common ERG fusion partner and itself an ERG-target gene) (20) and TFF3 (a gene that is ∼800 kb away from TMPRSS2 and also an ERG target gene) (17) in RWPE1-GFP cells. This interaction was abrogated in the presence of ERG (P = 0.01, Fisher’s exact test; Fig. S3D). To test this result, we performed targeted PCR from 3C libraries (3C-PCR) generated from asynchronous cells from the two cell lines. Consistent with the Hi-C data, the interaction between the promoters of TMPRSS2 and TFF3 was enriched in control cells (RWPE1-GFP) and depleted in the ERG overexpressing cells (Fig. S3D).
One of the most statistically significant cis-interaction changes involved two distant regions on chromosome 6 (regions between p22.3–12.3 and q22.31–25.32); these regions interact significantly more in cells overexpressing ERG compared with control cells (Fig. 2A and Fig. S3A). We also observed loss of interactions in RWPE1-ERG cells [e.g., chromosome 10 (regions between p15.3–p13 and q21.3–25.1, Fig. S3B) and chromosome 13 (regions between q12.11–13.1 and q13.1–21.1, Fig. S3C)]. Consistent with previous findings (22), we detected more trans-interactions between small, gene-rich chromosomes (e.g., chromosomes 16–22) than between larger chromosomes in both RWPE1-ERG and RWPE1-GFP cells (P = 1.4 × 10−9 and P = 4.1 × 10−6, respectively, Mann–Whitney tests; Fig. S4A). Moreover, specific regions of chromosomes tended to interact preferentially on the basis of high-resolution heat maps of normalized interactions between all cytogenetic bands (Fig. S4B). RWPE-1 cells have been reported to be primarily diploid and to harbor specific translocations involving chromosomes 9, 12, 18, and 21 in at least 90% of the cells (21). To determine whether additional, unreported translocations accounted for the enriched Hi-C interactions, we performed spectral karyotyping of both cell lines (24). The karyotypes observed in the two cell lines were identical, barring a der(13)t(13;15)(q12;q12) unique to ERG overexpressing cells (Fig. S5A), and also detectable using chromosome painting (Fig. S5B). Accordingly, our Hi-C data show a specific increase in interaction between chromosome 13 and chromosome 15 in RWPE1-ERG cells (Figs. S4B and S5C). The spectral karyotyping results thus indicate that changes in interchromosomal interactions detected by Hi-C sometimes represent translocations. However, further inspection of the Hi-C and spectral karyotype data shows that several ERG- or GFP-specific trans-interactions revealed by Hi-C are unlikely to be translocations. This result includes Hi-C–predicted interactions between chromosomes 4 and 21 and between chromosomes 4 and 13 in ERG cells, both of which involve regions that are ∼3 Mb long, and between chromosomes 5 and 9 in both cells, which involve much larger regions of each chromosome (Fig. 1B and Figs. S4B and S5A).
Fig. 2.
(A) Correlation matrices for chromosome 6 obtained from RWPE1-ERG cells (Left) and RWPE1-GFP cells (Center) and the difference between the data from two cell lines [(RWPE1_ERG) − (RWPE1_GFP)] (Right). A schematic of chromosome 6 is given (Above and Left of each matrix). (B) Pie chart of the 6,398 ERG ChIP-seq peaks corresponding to the distribution of ERG binding relative to the nearest gene in RPWE1-ERG cells. Binding was broken down into the following four categories: within promoters (±2 kb from transcription start sites), intronic, 3′ from the end of the last exon (3′ End), and distal (>2 kb away from known genes). (C) Graph showing the amount of ERG binding (kb) at each of the ranked (N) differential interacting chromatin loci of 1-Mb regions plus a 1-Mb region flanking either side (3 Mb total window size) showing the top interaction loci are strongly enriched with ERG binding sites.
ERG Binding Is Enriched at Loci Whose Interactions Differ Between the Cell Types.
To characterize ERG binding and ERG-mediated gene expression changes in these cells, we performed chromatin immunoprecipitation combined with high-throughput sequencing (ChIP-seq) and RNA sequencing (RNA-seq). ERG was bound to 6,398 sites in RPWE1-ERG cells, of which 23.4% were associated with promoters (±2 kb from transcription start sites), 30.8% of ERG binding sites were intronic, and 42.9% occurred >2 kb away from known genes (Fig. 2B). Unbiased DNA motif analysis (25) detected the ETS response element as the most represented element within the ERG peaks (Fig. S5D and Dataset S2). ERG was bound to its own promoter as well as to known ERG target genes in prostate, e.g., ADRB2, CDH1, DAB2IP, FKBP5, SLC45A3, LAMC2, KCNS3, and SERPINE1 (18, 20), and in nonprostate tissue, e.g., ICAM2 and CDH5 (26). Importantly, our results indicate that hotspots of differential chromatin interaction, i.e., loci that have the most differential interactions with other loci, are highly enriched in ERG binding (P < 10−11; Mann–Whitney test; Fig. 2C and Fig. S5E).
ERG-Associated Chromatin Topological Changes Are Associated with Coordinated Gene Expression.
We next investigated the association between gene expression and chromatin interactions. On the basis of paired-end RNA-seq data from both cell lines, 1,266 genes were differentially expressed (more than twofold change), indicating that ERG is highly active in RWPE1-ERG cells and induces global gene expression changes. Similar to Lieberman-Aiden et al. (22), we observed that the most highly expressed genes are in closer nuclear proximity than expected by chance, comparing randomly selected genes from both cell lines (P < 10−299 in GFP and ERG; Fig. S6A). This observation would support the view that active genes reside in transcription-prone nuclear areas, i.e., transcription factories or hubs. We also found that the 1,266 differentially expressed genes between ERG and GFP cells were in close proximity in ERG cells but also in GFP cells (P < 10−11, Fig. S6 B and C). However, we found that 777 (65%) of the 1,266 ERG-regulated genes are mapped to differentially interacting genomic regions between ERG and GFP cells (Fig. 3A). This set of 777 genes is hereafter denoted as the “interacting gene set.” The other 489 (35%) genes were not found to be significantly interacting or showed no difference in interaction between ERG and GFP cells (Fig. 3A). On the basis of Gene Ontology analyses (Fig. 3B and Dataset S3) the interacting gene set was significantly enriched with genes encoding for proteins that promote cell motility and invasion (adjusted P = 0.017, Fisher’s exact test), which are phenotypes induced by ERG overexpression (16, 18). Genes associated with urogenital development (e.g., HOXA, -B, and -C gene cluster members PYGO1 and NKX3.1) were also significantly enriched (adjusted P = 0.004). This result is consistent with ERG expression disrupting the normal lineage-specific prostate cell differentiation and promoting an EZH2-mediated dedifferentiation program (20). Cluster analysis using RNA-seq expression of the interacting gene set partitioned 50 human prostate cancer samples into subgroups that are associated with ERG rearrangement status (Fig. S7, P = 0.0195, Fisher’s exact test), suggesting that gene expression changes associated with ERG-associated cis- and trans-interactions are highly relevant in the context of aggressive prostate cancer.
Fig. 3.
(A) Pie chart of the 1,266 ERG-differentially expressed genes as a function of significantly interacting on the basis of the Hi-C data. These genes are divided into two groups: the interacting genes (housed in loci that are significantly interacting differently in the ERG compared with the control cell lines) and the noninteracting genes (housed in loci that are not significantly interacting differently in the ERG compared with the control cell lines). (B) Gene Ontology (GO) analysis results showing a subset of the significant categories (adjusted P value <0.05), using the interacting gene set. There were no significant categories based on the noninteracting gene set.
Many of the genomic loci containing differentially expressed genes are spatially interconnected, as shown using a network representation (Fig. S8). In Fig. S8, nodes correspond to 1-Mb genomic regions containing the differentially expressed gene(s) and the lines connecting the regions (edges) indicate interactions either enriched in RWPE1-ERG (orange edges) or depleted (blue edges) compared with RWPE1-GFP. Node color and shape indicate expression status of the indicated gene(s) in RWPE1-ERG relative to RWPE1-GFP cells and ERG binding, respectively. As shown in Fig S8A, the network of differentially interacting genes associated with ERG overexpression consists of several unconnected smaller subnetworks. One subnetwork (Fig. 4 A and B) linked several genes located throughout chromosome 6 and includes FYN, a known prostate cancer oncogene with a role in cell invasion (27, 28), and MOXD1, a gene up-regulated in primary compared with metastatic prostate cancer (29). These genes are bound by ERG proximally or distally (Fig. S8B). This network analysis revealed a variety of patterns of interaction and expression changes that are consistent with ERG-associated formation or disappearance of transcription or repression factories. For some genes, increase in proximity correlated with increased expression, consistent with the formation of an active transcription factory. For example, the MOXD1 gene specifically interacts with FYN, SERPINB9, and ARMC2 in RWPE1-ERG cells, and all of these genes are overexpressed in these same cells. In other cases, a decrease in interaction in RWPE1-ERG cells was associated with increased expression, consistent with potential escape from a repression factory. For example, MOXD1 and HEY2, SYNE1 are farther apart in RWPE1-ERG cells than in RWPE1-GFP cells and these genes are up-regulated in RWPE1-ERG cells, similar to what we observed for TMPRSS2 and TFF3. We then sought to validate a subset of these interactions using fluorescent in situ hybridization (FISH) and 3C-PCR. We also validated expression changes of genes located within these regions, using quantitative RT-PCR. Using the MOXD1 genomic locus as the anchor point, 3C-PCR data revealed an increased number of interactions involving distant loci enriched in RWPE1-ERG cells (20.6 Mb, MOXD1-FYN interactions; and 129.69 Mb, MOXD1-SERPINB9 interactions; Fig. 4 B and C) or in the RWPE1-GFP cells (6.65 Mb, MOXD1-HEY2 interactions). FISH analysis also confirmed the enriched interaction between loci containing MOXD1 and SERPINB9 or FYN and HEY2 in the RWPE1-ERG cells compared with RWPE1-GFP cells. We then sought to determine whether certain chromosomal interactions are dynamically orchestrated during the cell cycle. FISH analyses on synchronized cell lines showed significantly enriched interactions between regions associated with MOXD1 and SERPINB9 in cells enriched during the G1 phase (P < 0.0001, two-tailed Fisher’s exact test) compared with the G2/M phase (P = 0.5004, Fig. S9). These results confirm the cell-cycle dependency of some of the interactions initially identified by Hi-C from unsynchronized cells. We then wanted to know whether ERG-associated interactions could be observed in another isogenic cell type overexpressing ERG. For this analysis we generated two independent 3C libraries from DU145 prostate cancer cells, with (DU145-ERG) or without (DU145-GFP) stable ERG overexpression (Fig. 5A). As a control, we generated a DU145-ERG cell line stably expressing an shRNA that targets ERG mRNA (DU145-ERG shRNA-ERG). As observed in the RWPE1 cells, we found that ERG expression is associated with increased interaction between the chromosome 6 loci containing MOXD1 and SERPINB9 and with the overexpression of both genes (Fig. 5B). Knocking down the ERG protein in the DU145-ERG shRNA-ERG cells led to decreased interaction between these loci and abrogated the overexpression of both genes, providing further evidence of the dynamic nature of ERG-associated chromosome interactions. Although FYN was overexpressed in conjunction with ERG overexpression (Fig. 5C), we did not find any difference in interaction between MOXD1 and FYN in DU145 cells as we found in RWPE1-ERG cells. This result indicates that a subset of ERG-associated interactions is also dependent on cellular context and genetic background and does not always form upon ERG overexpression.
Fig. 4.
ChIP-seq, RNA-seq, and Hi-C data integration. (A) Network of genes (visualized using Cytoscape) (35) that are represented in independent cis-interactions between 1-Mb chromosome loci on chromosome 6 and that are significantly differentially expressed between RWPE-GFP (Left) and RWPE-ERG (Right) cells. Node color represents over- (red) and underexpressed (green) genes in RWPE-ERG cells relative to RWPE-GFP cells. Pink indicates that the genes in the node are both over- and underexpressed in RWPE-ERG cells. Node shapes are defined by ERG binding peak calls from ChIP-seq data [triangle, bound to promoter; diamond, bound to gene body; square, bound to distal region (between 2 kb and 50 kb); circle, bound to far distal region (> 50 kb)]. Edge (lines connecting the nodes) color represents interaction (from Hi-C) enriched in RWPE-ERG cells (orange) or RWPE-GFP cells (blue). (B and C) Validation of a subset of cis-interactions on chromosome 6 that involved ERG-regulated genes (MOXD1, HEY2, FYN, and SERPINB9). (B) Schematic map of chromosome 6 showing a subset of interactions and deregulated genes from A is shown with loci and anchor regions indicated. (C) (Left) The mean frequencies of interactions for the indicated loci pair based on PCR products obtained from two independent 3C libraries (above) and mRNA levels of the indicated genes (below) generated from either RWPE1-ERG (orange) or RPWE1-GFP (blue). SDs are shown as error bars. (Upper Right) Representative FISH images of G1-enriched nuclei based on analyses using probes made from BACs housing, to the left, MOXD1 (loci 1, red) and SERPINB9 (loci 6, green) or, to the right, FYN (loci 6, red) and HEY2 (loci 5, green), show close or distant proximity of the two loci. (Lower Right) Graphical representation of the percentage of nuclei having overlapping signals from RWPE1-ERG (orange bars) or RWPE1-GFP (blue bars) cells based on 251 and 304 (MOXD1-SERPINB9) nuclei or 150 and 150 nuclei (HEY2-FYN) evaluated, respectively (P value based on a two-tailed Fisher’s exact test is shown above).
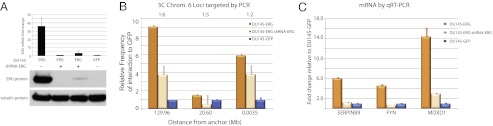
Fig. 5.
Validation of specific cis-interactions in DU145 prostate cancer cell lines overexpressing ERG alone or in the presence of shRNA targeting ERG mRNA. (A) ERG mRNA (Upper) and protein (Lower) levels measured by quantitative RT-PCR (17) and Western blot analysis in DU145 cells stably overexpressing ERG alone or in the presence of stable expression of shRNA targeting the ERG mRNA (two independent cell line clones are shown) or GFP as a control. shRNA expression led to a drastic reduction in transcript and protein levels. (B) Validation of a subset of cis-interactions on chromosome 6 that involved ERG-regulated genes (MOXD1, FYN, and SERPINB9) in the DU145 cell lines based on the primers described in the Fig. 3 legend (MOXD1 region is the anchor, and FYN regions are located 20.6 Mb and 129.96 Mb from the anchor region, respectively). (C) Validation of the overexpression of ERG-regulated genes (MOXD1, FYN, and SERPINB9) in the DU145 cell lines.
Discussion
This study provides the characterization of the correlation between the overexpression of an oncogenic transcription factor (ERG) and changes to the 3D chromosome structure. It has been proposed that ETS-rearranged prostate cancer, similar to other translocation-associated tumors, represents a distinct molecular subclass of prostate cancer (12). Our data show that overexpression of ERG is associated with broad chromatin topology changes and, interestingly, that ERG binding is significantly associated with hotspots of differential chromatin interactions. The shift in chromatin territories that may be associated with differential chromatin interactions appears to correlate with changes in the expression of a subset of genes relevant to aggressive prostate cancer. However, this correlation does not directly imply causality as the associations observed could be resultant of clonal selection of the cell lines assayed. Further experiments will be required to characterize more precisely the subset of ERG-associated interactions that are directly caused by ERG binding. Such characterization could be achieved, for example, using an inducible system in which the timing of ERG expression can be tightly controlled and alterations in proximity and DNA binding are quantified concurrently.
Of note, combining our Hi-C data with spectral karyotyping and chromosome painting led to the detection of a translocation between chromosomes 13 and 15 that is specific to ERG-overexpressed RWPE-1 cells. Moreover, we found that ERG binding is enriched at several loci in chromosomes 13 and 15 whose interaction status changed as a function of ERG overexpression compared with other genomic regions, e.g., two regions in q14.12 and q22.1 on chromosome 13 (P = 0.024, Mann–Whitney test; Fig. S5C). It has been shown that ERG induces DNA damage (30) and that there is a positive correlation between the location of breakpoints in ETS-rearranged tumor cells and transcriptional or chromatin compartments of active genes (31). This observation is intriguing given that others have shown that active genes (1, 22) and translocated genes (2) are in close spatial proximity. Data from a recent study combining Hi-C and high-throughput genome-wide translocation sequencing in G1-arrested, mouse pro-B cells suggest that spatial proximity may indeed guide translocations (32). Our data support the notion that conformational proximity associated with overexpression of an oncogenic transcription factor may play an important role in translocation partner choice. Taken together, these data—although currently correlative—lead us to speculate that ERG overexpression may favor the formation of secondary genomic alterations. Supporting this hypothesis, the TMPRSS2-ERG gene fusion is an early event in prostate cancer and is almost always associated with other genomic alterations. Nonetheless, it is important to remember that the observed correlation between ERG overexpression and a novel translocation does not imply causality. Moreover, although this translocation was observed in independent clonal populations of RWPE1-ERG cells, we cannot entirely exclude the possibility that this translocation did not arise through an incidental clonal selection of a nonregulated process. Further experiments, e.g., using an inducible ERG expression system as described above, are required to determine whether ERG binding is directly involved in mediating translocations such as the one observed here. We also note that, whereas the resolution for detecting rearrangements using our approach (multicolor FISH and G-banding) is ∼0.5–1.5 Mb (24, 33), we cannot entirely rule out translocations accounting for the trans-interactions involving smaller chromosomal regions that were enriched in the ERG overexpressing cells. Moving forward, high-resolution assessment of structural changes, e.g., using whole-genome sequencing, should accompany large-scale chromosome capture data interpretation to better resolve differences between proximity and genomic rearrangements.
Finally, our findings also extend beyond the context of the prostate as many driving genetic lesions in other cancer types involve aberrant expression of transcription factors due to genomic alterations, e.g., EWS-FLI1, c-Myc, n-Myc, and PML-RARα. We hypothesize that overexpression of other oncogenic transcription factors also alters 3D chromatin structure in different cellular contexts.
Methods
Human Cell Lines.
RWPE1 and DU145 cells were obtained from ATCC and maintained according to the manufacturer’s protocol. Isogenic cell lines generated to overexpress either truncated ERG (most commonly encoded isoform based on TMPRSS2-ERG fusion) have been previously described (17).
Hi-C Library Generation.
Fifty million RWPE1-ERG or RWPE1-GFP cells were fixed and processed to generate Hi-C and 3C libraries as previously reported (22). Briefly, cells were cross-linked and the chromatin was digested with HindIII, ligated after fill-in with biotin-conjugated dCTP, and purified using streptavidivin-conjugated magnetic beads. The Hi-C libraries were then paired-end sequenced using an Illumina GAIIx platform, resulting in replicate-combined 158.5 million and 159.2 million paired-end DNA sequence reads from RWPE1-ERG and RWPE1-GFP, respectively. Sequences for all primers used to validate Hi-C and RNAseq data are given in Dataset S4.
ChIP-seq.
ChIP-seq data from 50 million RWPE1-ERG cells were generated as previously described (17). Eight separate chromatin samples were immunoprecipitated with rabbit anti-ERG (Epitomics; 2849-1) that we have shown to be highly specific for ERG protein expression in prostate cancer (34).
Details regarding all computational approaches and other methods are found in SI Methods and SI Computational Analysis.
Supplementary Material
Acknowledgments
The authors thank Naoki Kitabayashi, Kyung Park, Aparna Nigam, and Wasay Hussain for their technical support. This work was supported by funding from the Department of Defense New Investigator Award PC081337 (to D.S.R.), by National Science Foundation CAREER Grant 1054964 (to O.E.), by National Institutes of Health National Cancer Institute Grant CA125612 (to M.A.R.), and by the Starr Cancer Consortium (F.D. and M.A.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Hi-C and ChIPseq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE37752).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112570109/-/DCSupplemental.
References
- 1.Osborne CS, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CS, et al. Myc dynamically and preferentially relocates to a transcription factory occupied by Igh. PLoS Biol. 2007;5:1763–1772. doi: 10.1371/journal.pbio.0050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sexton T, Schober H, Fraser P, Gasser SM. Gene regulation through nuclear organization. Nat Struct Mol Biol. 2007;14:1049–1055. doi: 10.1038/nsmb1324. [DOI] [PubMed] [Google Scholar]
- 4.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 5.Fullwood MJ, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiwari VK, et al. PcG proteins, DNA methylation, and gene repression by chromatin looping. PLoS Biol. 2008;6:2911–2927. doi: 10.1371/journal.pbio.0060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolou E, Thanos D. Virus infection induces NF-kappaB-dependent interchromosomal associations mediating monoallelic IFN-beta gene expression. Cell. 2008;134:85–96. doi: 10.1016/j.cell.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 9.Schoenfelder S, et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet. 2010;42:53–61. doi: 10.1038/ng.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z, et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38:1341–1347. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]
- 11.Pflueger D, et al. N-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancer. Neoplasia. 2009;11:804–811. doi: 10.1593/neo.09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Pei H, Watson DK. Regulation of Ets function by protein - protein interactions. Oncogene. 2000;19:6514–6523. doi: 10.1038/sj.onc.1204035. [DOI] [PubMed] [Google Scholar]
- 15.Hollenhorst PC, et al. Oncogenic ETS proteins mimic activated RAS/MAPK signaling in prostate cells. Genes Dev. 2011;25:2147–2157. doi: 10.1101/gad.17546311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klezovitch O, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickman DS, et al. ERG cooperates with androgen receptor in regulating trefoil factor 3 in prostate cancer disease progression. Neoplasia. 2010;12:1031–1040. doi: 10.1593/neo.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlins SA, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlins SA, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh M, Franco OE, Hayward SW, van der Meer R, Abdulkadir SA. A role for polyploidy in the tumorigenicity of Pim-1-expressing human prostate and mammary epithelial cells. PLoS ONE. 2008;3(7):1–11. doi: 10.1371/journal.pone.0002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman-Aiden E, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder P, Gomez JA, Chadwick BP, Boss JM. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J Exp Med. 2008;205:785–798. doi: 10.1084/jem.20071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schröck E, et al. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273:494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 25.Elemento O, Slonim N, Tavazoie S. A universal framework for regulatory element discovery across all genomes and data types. Mol Cell. 2007;28:337–350. doi: 10.1016/j.molcel.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birdsey GM, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, et al. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci USA. 2011;108:6579–6584. doi: 10.1073/pnas.1103904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Dutta U, Shaw LM. SHP2 mediates the localized activation of Fyn downstream of the α6β4 integrin to promote carcinoma invasion. Mol Cell Biol. 2010;30:5306–5317. doi: 10.1128/MCB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandran UR, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:1–21. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner JC, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger MF, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell. 2012;148:908–921. doi: 10.1016/j.cell.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan YS, Siu VM, Jung JH, Xu J. Sensitivity of multiple color spectral karyotyping in detecting small interchromosomal rearrangements. Genet Test. 2000;4:9–14. doi: 10.1089/109065700316417. [DOI] [PubMed] [Google Scholar]
- 34.Park K, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cline MS, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2:2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.