Abstract
Drought-induced proline accumulation is widely observed in plants but its regulation and adaptive value are not as well understood. Proline accumulation of the Arabidopsis accession Shakdara (Sha) was threefold less than that of Landsberg erecta (Ler) and quantitative trait loci mapping identified a reduced function allele of the proline synthesis enzyme Δ1-pyrroline-5-carboxylate synthetase1 (P5CS1) as a basis for the lower proline of Sha. Sha P5CS1 had additional TA repeats in intron 2 and a G-to-T transversion in intron 3 that were sufficient to promote alternative splicing and production of a nonfunctional transcript lacking exon 3 (exon 3-skip P5CS1). In Sha, and additional accessions with the same intron polymorphisms, the nonfunctional exon 3-skip P5CS1 splice variant constituted as much as half of the total P5CS1 transcript. In a larger panel of Arabidopsis accessions, low water potential-induced proline accumulation varied by 10-fold and variable production of exon 3-skip P5CS1 among accessions was an important, but not the sole, factor underlying variation in proline accumulation. Population genetic analyses suggest that P5CS1 may have evolved under positive selection, and more extensive correlation of exon 3-skip P5CS1 production than proline abundance with climate conditions of natural accessions also suggest a role of P5CS1 in local adaptation to the environment. These data identify a unique source of alternative splicing in plants, demonstrate a role of exon 3-skip P5CS1 in natural variation of proline metabolism, and suggest an association of P5CS1 and its alternative splicing with environmental adaptation.
Keywords: amino acid metabolism, drought adaptation, stress gene expression, osmoprotectant, compatible solute
Proline acts as an osmoprotectant and cryoprotectant in organisms as diverse as bacteria, plants, and insects (1, 2). Many plants accumulate proline in response to low water potential (ψw) and dehydration caused by drought or freezing. The mechanisms by which proline may promote drought resistance include osmoprotectant functions, as well as newly emerging functions of proline metabolism in NADP/NADPH balance and transfer or storage of energy and reducing potential (1–3). However, the overall importance of proline in drought adaptation is not as firmly established. In Arabidopsis thaliana, transcriptional up-regulation of Δ1-pyrroline-5-carboxylate synthetase1 (P5CS1) is essential for low ψw-induced proline accumulation, and proline accumulation of p5cs1 mutants is only 15–20% of the wild-type level (4–6). Additional regulation of proline metabolism is likely but not understood. The timing and duration of water limitation, as well as other environmental variables, all influence drought-adaptation strategies used by plants. Thus, we may expect substantial natural variation of these traits within a widely distributed species, such as Arabidopsis (7–10), with many of these differences related to the local conditions to which a particular accession has adapted (11–13). Several studies have identified quantative trait loci (QTL) controlling differences in metabolite content (14–18), but such studies have yet to be extended to drought-induced metabolic changes, such as proline accumulation.
Here we used natural variation and QTL mapping of low ψw-induced proline accumulation to reveal a unique mechanism whereby intron sequence variation in P5CS1 was sufficient to drive alternative splicing and production of a nonfunctional P5CS1 transcript variant, and thus affect the level of P5CS1 protein. This alternative splicing was an important factor underlying variation in proline accumulation across a panel of Arabidopsis accessions. Correlation with climate variation, as well as population genetic analysis of P5CS1 and its alternative splicing, further support P5CS1 as a gene under selection for environmental adaptation.
Results
Large Effect QTL Controls Differential Proline Accumulation Between Arabidopsis Accessions Landsberg erecta and Shakdara.
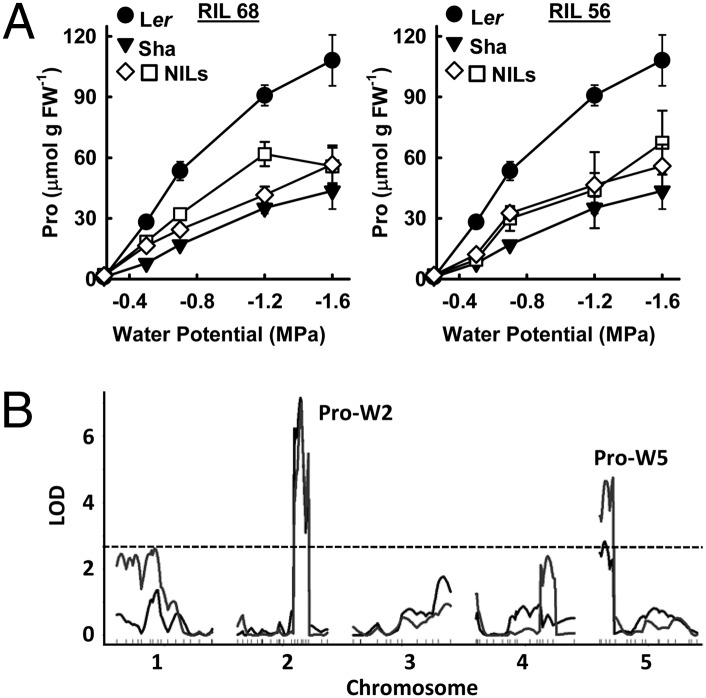
Landsberg erecta (Ler) and Shakdara (Sha; also known as Shahdara) differed by three- to fourfold in low ψw-induced proline accumulation (Fig. 1A and Fig. S1A). To find the causal genetic polymorphisms, we scored an existing Ler × Sha recombinant inbred line (RIL) population (19) for proline content at low ψw (−0.7 MPa and −1.2 MPa) and identified major QTL on chromosomes 2 and 5 (Fig. 1B, and Tables S1 and S2). We focused further efforts on the chromosome 2 QTL (Pro-W2) and narrowed its location to ∼4 cM. Interestingly, this interval contained P5CS1, which encodes the first enzyme of proline synthesis. Pro-W2 may correspond to a proline QTL found under unstressed conditions in Col × C24 (14).
Fig. 1.
A chromosome 2 QTL causes reduced proline accumulation in Sha. (A) Proline accumulation across a range of ψw for Ler and Sha seedlings as well as NILs generated by twice backcrossing RILs 56 and 68 (Fig. S2) to Ler. Data are means ± SE, n = 6–10. (B) Plot of LOD scores for ψw-induced proline of the Ler × Sha RIL population. The gray line is for proline at −0.7 MPa and the darker line is for −1.2 MPa. The dashed line indicates the significance threshold (LOD = 2.5).
To determine the extent of proline variation controlled by Pro-W2, two RILs (56 and 68) (Fig. S2 A and B) were twice backcrossed to Ler and scored with markers flanking the Pro-W2 interval or with a marker within P5CS1 itself (see below) to recover near isogenic lines (NILs), which captured the Pro-W2 QTL region from Sha in a predominantly Ler genomic background. These NILs had reduced proline across a range of ψw (Fig. 1A), indicating that the Pro-W2 QTL was responsible for as much as 70% of the variation between Ler and Sha. Conversely NILs that were selected for the Ler genotype at P5CS1 after the second backcross had high proline (Fig. S2C).
Alternative Splicing Leading to Reduced P5CS1 Protein Level Is the Basis for the Pro-W2 QTL and Low Proline Accumulation of Sha.
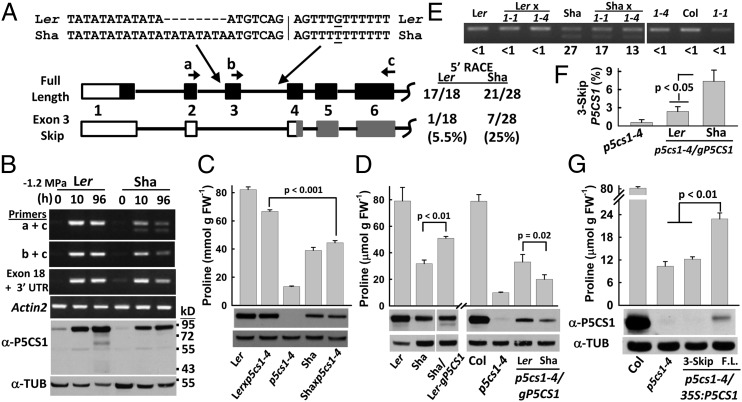
P5CS1 was the main candidate gene for Pro-W2 yet no substantial differences in gene expression of P5CS1, or other proline metabolism enzymes, were found between Ler and Sha (Fig. S1B). Sequencing of full-length P5CS1 cDNA from Ler and Sha found only a silent change of a GAT to GAC codon at Asp-644. However, 5′-RACE of Ler, Sha, and Col-0 identified deletion of exon 3 as an alternative splicing event occurring at substantial frequency in Sha P5CS1, but much less frequently in Ler (Fig. 2A) or Col-0. RT-PCR using primers flanking P5CS1 exon 3 (Fig. 2A, primers a and c) confirmed that Sha had more of the shorter fragment generated from the exon 3-skip transcript than Ler (Fig. 2B). Primers b and c that only amplified the full-length P5CS1 transcript (Fig. 2A), showed less full-length transcript in Sha (Fig. 2B), but downstream primers indicated similar amounts of total P5CS1 transcript in both accessions (exon 18 and 3′ UTR) (Fig. 2B), consistent with quantitative PCR results (Fig. S1B). Quantification of the relative intensity of the two bands produced by primers a and c showed that 30% of P5CS1 transcript in Sha was the exon 3-skip variant compared with 1% in Ler and Col-0.
Fig. 2.
Differences in P5CS1 intron sequence are the basis of the Pro-W2 QTL and are sufficient to promote production of nonfunctional exon 3-skip P5CS1 transcript. (A) Diagram of the 5′ portion of the two alternative P5CS1 transcripts detected by 5′-RACE analysis. Boxes indicate exons with dark portions indicating the coding region. Gray boxes indicate possible coding region of the exon 3-skip transcript starting from an alternative downstream ATG. The number of clones of each variant found is indicated along with sequence of the 3′ ends of P5CS1 introns 2 and 3; see Dataset S2 for complete intron 2 and 3 sequence. (B) Semiquantitative RT-PCR using primers indicated in A to detect P5CS1 splice variants before low ψw treatment (0 h) or after 10 and 96 h at −1.2 MPa. Actin2 was used as a control gene. P5CS1 protein in samples from the same treatments was detected by Western blot (50 μg protein loaded). Tubulin antibody was used as a loading control. (C) Proline content of Ler × p5cs1-4 or Sha x p5cs1-4 F1 seedlings after 96 h at −1.2 MPa. For both Ler and Sha, reciprocal crosses gave identical results and combined data are shown. Data are means ± SE (n = 6–9) with P value of the comparison of the two sets of F1 seedlings indicated. Western blot detection of P5CS1 and tubulin (Lower) are also shown. (D) Proline and P5CS1 level of plants transformed with P5CS1 genomic fragments (gP5CS1). Sha was transformed with the Ler genomic P5CS1 clone (Sha/Ler-gP5CS1) and p5cs1-4 transformed with either Ler or Sha gP5CS1(p5cs1-4/gP5CS1). Note that p5cs1-4 is in the Col-0 genetic background. Proline data are combined from three-to-five independent T3 homozygous lines and are means ± SE (n = 6–12); Western blot is for one representative transgenic line. (E) Exon 3-skip P5CS1 after 96 h at -1.2 MPa in F1 seedlings of Ler and Sha crossed to p5cs1-1 (1–1) or p5cs1-4 (1–4). RT-PCR was performed for 24 cycles when both P5CS1 transcripts were in the linear range of amplification. Quantitation of exon 3-skip percentage is indicated by numbers along bottom of the gel. Reciprocal crosses gave similar results and representative data are shown. (F) Percentage of exon 3-skip P5CS1 transcript produced by Ler or Sha gP5CS1 in p5cs1-4. For both gP5CS1 constructs, data shown are means ± SE of three to four independent T3 homozygous lines. (G) Proline content and P5CS1 level for p5cs1-4 transformed with either the exon 3-skip or full-length (F.L.) P5CS1 cDNA under control of the 35S promoter (p5cs1-4/35S:P5CS1). Proline data are means ± SE (n = 6–12) of three-to-four independent T3 homozygous lines and Western blot is from one representative transgenic line. Western blot of additional transgenic lines is shown in Fig. S3.
The exon 3-skip P5CS1 transcript cannot produce protein starting from the same start codon because deletion of exon 3 changes the reading frame and introduces a stop codon after 53 amino acids. Instead, the exon 3-skip transcript is annotated (TAIR10) as producing a truncated P5CS1, starting from an alternative downstream start codon (Fig. 2A). However, Western blotting with P5CS1-specific polyclonal antisera (Fig. S3A) detected no evidence of this smaller form of P5CS1 and showed reduced level of P5CS1 in Sha compared with Ler (Fig. 2B). A single P5CS1 band was detected, which consistently ran 8–10 kDa above the expected molecular weight of full-length P5CS1 (∼87 kDa compared with an expected molecular weight of 77.7 kDa), possibly because of posttranslational modification.
To test whether difference in P5CS1 was a basis of the Pro-W2 QTL, we first crossed both Ler and Sha to the null mutant p5cs1-4 (3, 5). F1 seedlings of Ler × p5cs1-4 had proline content only slightly less than that of Ler and significantly higher than Sha or F1 seedlings of Sha × p5cs1-4 (Fig. 2C). Western blotting confirmed that both Sha and Sha × p5cs1-4 had reduced levels of P5CS1 compared with Ler or Ler × p5cs1-4 (Fig. 2C). These results indicated that the Sha allele of P5CS1 had reduced function.
We also transformed Sha with a Ler P5CS1 genomic clone including 1 kb of promoter (gP5CS1) and transformed p5cs1-4 with both Sha and Ler gP5CS1. Sha transformed with Ler gP5CS1 had increased proline (Fig. 2D), indicating that P5CS1 expression was limiting for proline accumulation in Sha. Higher proline and P5CS1 protein levels were observed in p5cs1-4 transformed with Ler gP5CS1 than with Sha gP5CS1 (Fig. 2D), again indicating reduced function of the Sha allele. In the Sha and p5cs1-4 complemented lines, the transgene produced much less P5CS1 protein than the endogenous gene (Fig. 2D), consistent with the partial complementation of proline accumulation. Such data were also consistent with experiments in our laboratory where Col-0 P5CS1 constructs were unable to fully complement p5cs1 mutants or where transgenic P5CS1promoter:reporter fusions were not stress-induced in the same manner as endogenous P5CS1. Despite this limitation, the combined data were consistent with variation in P5CS1 as the main factor underlying the low proline effect of the Pro-W2 QTL. We cannot, however, exclude the possibility that the Pro-W2 QTL interval also contains an additional variation that has smaller effects on proline accumulation.
P5CS1 Intron Sequence Variation Is Sufficient to Increase the Frequency of a Nonfunctional Exon 3-Skip Splice Variant.
These data indicated that Sha had a reduced function P5CS1 allele, likely related to alternative splicing. As the Pro-W2 QTL mapped to P5CS1 rather than a component of the splicing machinery, the basis for the increased exon 3-skip transcript should be intrinsic to P5CS1. Sequencing of the P5CS1 genomic region found an insertion of four TA-repeats near the 3′ end of intron 2 in Sha (Fig. 2A). Both Ler and Sha had a stretch of TA-repeats, with Sha having more of these repeats than Ler. Sha also had a G-to-T substitution relative to Ler in the 3′ end of intron 3, as well as a 32-bp intron 3 insertion (Dataset S2) (this larger insertion was used to score NILs after the second backcross; see above).
We quantified exon 3-skip P5CS1 percentage in F1 seedlings of Sha crossed to p5cs1-1 or p5cs1-4 and found that the F1 seedlings had a similar amount of exon 3-skip P5CS1 transcript as Sha itself (Fig. 2E). In Sha × p5cs1-4 F1 seedlings the percentage of exon 3-skip P5CS1 was half of the Sha level (13% vs. 27%) (Fig. 2E). This finding was consistent with the P5CS1 transcript in those F1 seedlings being an approximately equal mixture of the Sha allele and truncated P5CS1 transcript containing exon 3 produced by the endogenous Col-0 P5CS1 [the T-DNA insertion in p5cs1-4 is in exon 14 (5)]. A higher exon 3-skip percentage was seen in Sha × p5cs1-1, in which expression of Col-0 P5CS1 was reduced because of a promoter T-DNA insertion (5). These data were consistent with variation within P5CS1, rather than Sha alleles of other genes that would be heterozygous in the F1 seedlings, as the basis of increased exon 3-skip P5CS1production. Transgenic expression of Ler or Sha gP5CS1 in p5cs1-4 also demonstrated that the Sha P5CS1 allele produced more exon 3-skip P5CS1 transcript than the Ler allele (Fig. 2F). The difference between the observed (7%) and expected (13–15%) exon 3-skip P5CS1 in the p5cs1-4 transgenic plants was likely because the transgenic Sha P5CS1 was expressed at lower level than the endogenous Col-0 allele.
The lower level of P5CS1 protein produced by Sha gP5CS1 compared with Ler gP5CS1 (Fig. 2D) and our inability to see a shorter P5CS1 isoform by Western blotting suggested that exon 3-skip P5CS1 transcript does not produce protein. To confirm this finding, we transformed p5cs1-4 with the full-length P5CS1 cDNA or the exon 3-skip cDNA. Transgenic lines expressing the full-length cDNA produced P5CS1 protein of the expected size and had increased proline content, but the exon 3-skip cDNA did not produce detectable P5CS1 nor increase proline content (Fig. 2G and Fig. S3B). The exon 3-skip cDNA was also unable to produce YFP-tagged fusion protein (Fig. S3C). These results demonstrated that P5CS1 of Sha was itself sufficient to increase production of a nonfunctional exon-3 skip transcript and thereby decrease P5CS1 protein and proline accumulation.
P5CS1 Alternative Splicing Contributes to Proline Variation Among Arabidopsis Accessions.
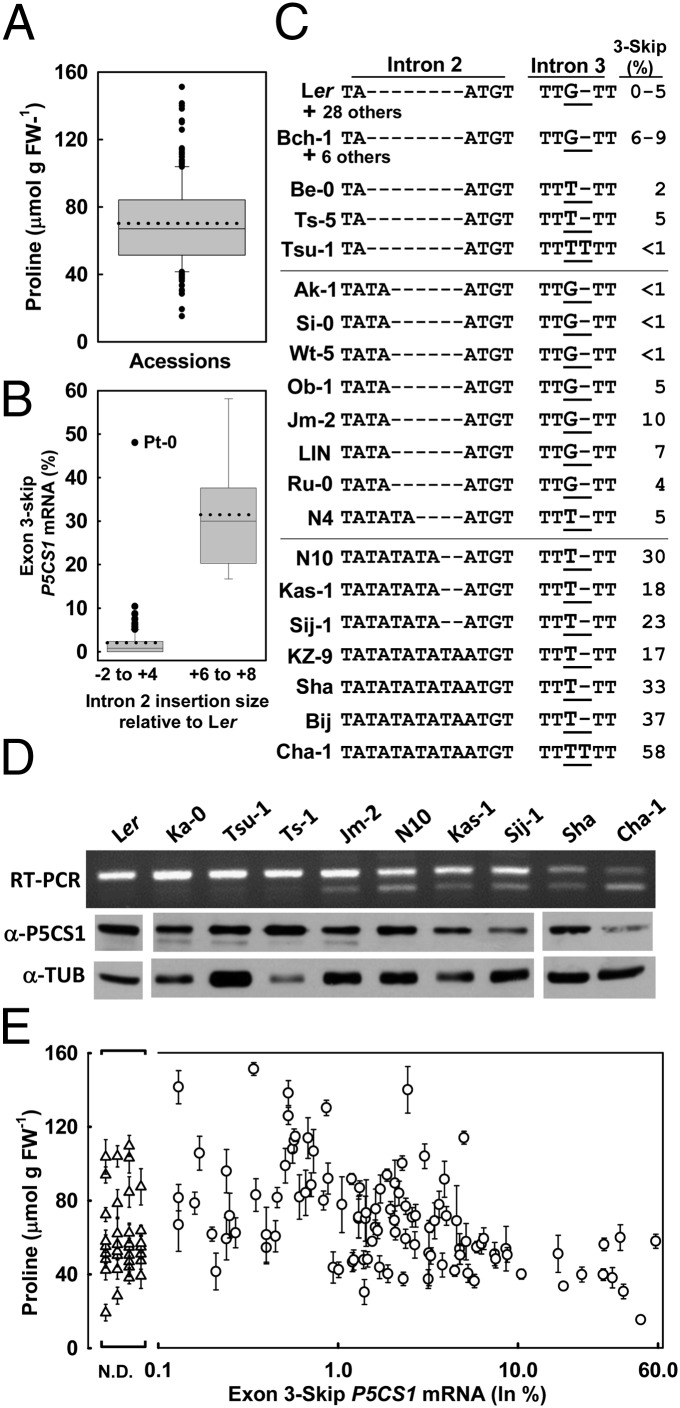
We found a negative relationship between proline and exon 3-skip P5CS1 across a large set of accessions. Proline content at low ψw (−1.2 MPa) varied nearly 10-fold between the highest and lowest accessions with a mean and median of ∼65 micromoles per gram fresh weight (μmol⋅g⋅F.W.−1) (Fig. 3A and Dataset S1). For 140 accessions, we isolated RNA from seedlings after 96 h at −1.2 MPa to quantify the percentage of exon 3-skip P5CS1 transcript and also used PCR to determine the size of their intron 2 insertion relative to Ler. Most (129 accessions) had either no or a small intron 2 insertion (−2 to +4) (Fig. 3B) and a low or nondetectable level of exon 3-skip P5CS1. A smaller number (nine accessions) had intron 2 insertions of similar size as Sha (+6 to +8) (Fig. 3B) and a high percentage of exon 3-skip P5CS1. In between these two groups were 14 accessions that had an intermediate level of exon 3-skip P5CS1 but where an intron 2 insertion could not be detected by gel-based scoring (outliers in the −2 to +4 category of Fig. 3B). The differences in both percent exon 3-skip P5CS1 and proline accumulation between the −2 to +4 and +6 to +8 groups of accessions were highly significant (ANOVA on log-percent exon 3-skip P5CS1+1; R2 = 0.46, P < 10−16; log proline abundance; R2 = 0.06, P = 0.004).
Fig. 3.
Alternative splicing of P5CS1 is a major factor underlying variation in low ψw-induced proline accumulation between Arabidopsis accessions. (A) Proline contents of 180 Arabidopsis accessions after 96-h low ψw (−1.2 MPa) treatment. Box encompasses the 25–75 percentiles with the solid line indicating the median and dashed line the mean proline content of all accessions. Whiskers show the 10–90 percentiles and dots indicate outliers. Proline data for each accession is listed in Dataset S1. (B) Percentage of exon 3-skip P5CS1 mRNA as related to size of P5CS1 intron 2 insertion. Percentage of exon 3-skip P5CS1 was determined for seedlings exposed to −1.2 MPa for 96 h. The intron 2 insertion size was estimated by PCR and gel electrophoresis. Presentation of data are as described in A. n = 129 for the −2 to +4 insertion size and n = 9 for +6 to +8. Exon 3-skip percentage and intron 2 insertion size for each accession are listed in Dataset S1. (C) P5CS1 intron 2 and 3 sequences of selected accessions having varying intron 2 insertion size and exon 3-skip P5CS1 percentage. Complete intron 2 and 3 sequence alignments can be seen in Dataset S2. (D) Exon 3-skip P5CS1 and P5CS1 protein level of selected accessions. (Upper) RT-PCR using primers a and c (Fig. 2A). (Lower) Western blot detection of P5CS1 and tubulin (loading control). (E) Relationship of Pro accumulation at −1.2 MPa to percentage of exon 3-skip P5CS1 mRNA. Triangles indicate accessions where the exon 3-skip P5CS1 mRNA could not be detected (N.D., not detected).
Sequencing of P5CS1 introns 2 and 3 from a subsample of accessions showed that most low exon 3-skip accessions had the same intron 2 and 3 sequence as Ler (Fig. 3C and Dataset S2). Some accessions having an intermediate level of exon 3-skip P5CS1 (outliers in the −2 to +4 category of Fig. 3B) also had the same sequence as Ler. Other accessions had insertion of one or two TA repeats in intron 2 but lacked the intron 3 G-to-T transition. A few accessions (Be-0, Ts-5, Tsu-1, and N4) (Fig. 3C) had no or small intron 2 insertion coupled with the intron 3 G-to-T transversion found in Sha. These accessions also had a relatively low level of exon 3-skip P5CS1. The data indicated that neither a small intron 2 TA-insertion nor the intron 3 polymorphism by itself was sufficient to cause frequent exon 3-skip transcript formation. However, the high exon 3-skip P5CS1 accessions (with exception of Pt-0) all had both the intron 3 G-to-T transition and insertion of three or four TA repeats in intron 2 (Fig. 3C). Thus, it was the combination of these intron 2 and 3 changes that was associated with the highest levels of exon 3-skip P5CS1 formation. These data also suggested how an accession could transition from low to high levels of exon 3-skip P5CS1 transcript by adding TA repeats in intron 2 and acquiring the intron 3 G-to-T transversion. The 32-bp insertion in Sha intron 3 was not found in any other accession, indicating that it was not associated with alternative splicing. Western blotting of several accessions indicated that increased frequency of nonfunctional exon 3-skip P5CS1 transcript was associated with decreased levels of P5CS1 protein (Fig. 3D).
Consistent with increased exon 3-skip P5CS1 leading to reduced P5CS1 protein, there was a significant negative correlation between the frequency of exon 3-skip P5CS1 transcript and proline content in seedlings at low ψw (Fig. 3E), both when analyzing raw data (ANOVA, R2 = 0.09, P = 0.0004) and log-proline content vs. log exon 3-skip P5CS1 percentage + 1 (ANOVA, R2 = 0.11, P = 0.00006). In accessions where the proportion of exon 3-skip P5CS1 was 6–8% percent or higher, it was the dominating factor as all of these accessions had low or moderate levels of proline accumulation.
Although variation in percentage exon 3-skip P5CS1 had a substantial influence on proline accumulation, it was not the only factor. Accessions with low or nondetectable exon 3-skip P5CS1 covered the range from high to low proline accumulation (Fig. 3E). Some of the accessions with the highest and lowest levels of proline did not differ in P5CS1 (Fig. S4). In addition, Pt-0 was an interesting outlier accession in that it had a high level of exon 3-skip P5CS1 without the intron 2 insertion (Fig. 3B). Pt-0 also had a low level of total P5CS1 transcript and a nearly undetectable level of P5CS1 protein (Fig. S4). Pt-0 had the lowest proline content of any accession in our panel, comparable to p5cs1-4. Thus, Pt-0 is essentially a naturally occurring p5cs1-deficient mutant.
We also analyzed the association of P5CS1 sequence variation with exon 3-skip percentage and proline content using publicly available SNP datasets (13) as a way to control for population structure and minimize false-positive associations. Average genome-wide SNP similarities between accessions were used to infer population structure. A sliding window across P5CS1 and 10 kb on either side was used to characterize haplotype variation in 5-SNP intervals. Accessions sharing Ler and Sha 5-SNP haplotypes differed significantly in exon 3-skip P5CS1 and proline at seven haplotype windows while accounting for kinship (linear mixed model on log-percent exon 3-skip P5CS1 +1, α = 0.05). Sha haplotypes (n = 8) had 16% more exon 3-skip P5CS1 than Ler (n = 53) haplotypes at the most divergent 5-SNP interval (P < 10−5). The same 5-SNP interval was also where accessions with Ler (n = 67) vs. Sha (n = 8) haplotypes differed most in proline, with Ler haplotypes averaging 20 μmol⋅g F.W.−1 more proline than Sha haplotypes (linear mixed model on log proline abundance, P = 0.01). More generally, we found that within this interval, all unique 5-SNP haplotypes with more than one accession were significantly different in percent exon 3-skip P5CS1 (n = 100 accessions, P < 10−6) and proline abundance (n = 129 accessions, P = 0.004).
Exon 3-Skip P5CS1 Abundance Is Associated with Climate Variation.
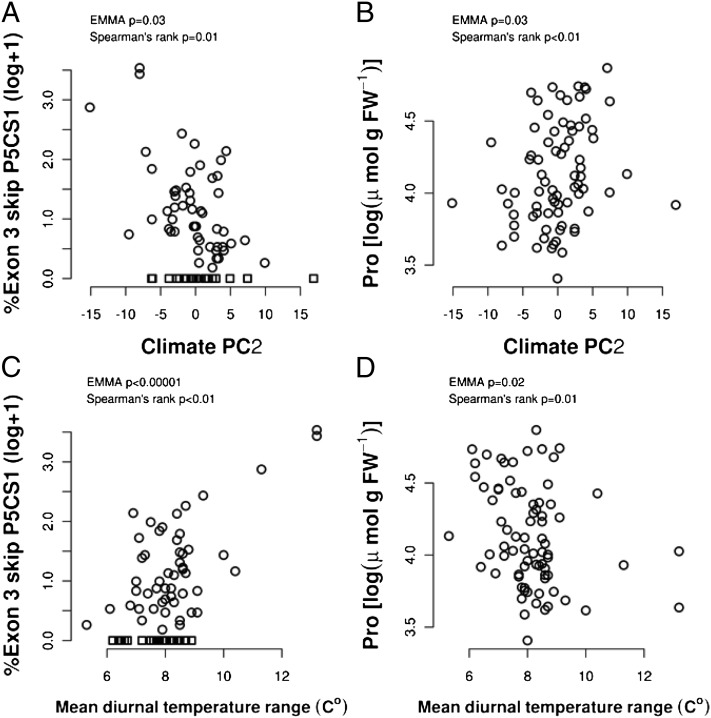
To understand the importance of proline and P5CS1 splicing in a broader context, we asked whether variation in exon 3-skip P5CS1 or proline was associated with environmental variation while statistically controlling for population structure (12, 20). Spatial variation in the exon 3-skip P5CS1 percentage was strongly nonrandom across the native Eurasian range of Arabidopsis; the percentage of exon 3-skip P5CS1 increased in eastern Eurasia, with longitude explaining 28% of (log-percent + 1) variation independently of a SNP-based kinship matrix (a proxy for population structure; EMMA, P < 0.0001) (Fig. S5). Spatial variation in exon 3-skip P5CS1 percentage was strongly associated with climate and the second principal component (PC2) of climate across Eurasia (explaining 21% of climatic variation) (Dataset S3 and SI Materials and Methods). The percent of exon 3-skip P5CS1 decreased along PC2 (Fig. 4A) as climates became wetter and had less temporal variation in temperature (explaining 16% of variation in exon 3-skip P5CS1; EMMA, P = 0.03). Conversely, proline accumulation increased with PC2 (explaining 7% of proline variation), indicating greater proline accumulation in locations with less variable temperature and more precipitation (Fig. 4B).
Fig. 4.
Association of exon 3-skip P5CS1 percentage and proline content with climatic factors. (A) Negative association of exon 3-skip P5CS1 percentage with the PC2 of climate (SI Materials and Methods and Dataset S3). Accessions where exon 3-skip P5CS1 could not be detected are shown as squares. Only accessions with SNP data and reliable collection locations (SI Materials and Methods) are included (n = 76). (B) Positive association of proline content at low ψw with climate PC2. (C) Increased frequency of exon 3-skip P5CS1 in accessions from more variable temperature environments. Mean diurnal temperature range was the climate variable most strongly associated with exon 3-skip P5CS1 percentage (Dataset S4). (D) Proline accumulation had an opposite, but weaker, correlation to mean diurnal temperature range and an overall weaker association with climate variables (Dataset S5).
When specific climate variables were tested, significant associations (α = 0.05) with exon 3-skip P5CS1 percentage, independent of kinship, were found for 39 of 101 variables tested (Dataset S4). Diurnal temperature range had the strongest loading on PC2 and one of the strongest associations with exon 3-skip P5CS1 percentage, explaining 32% of its variation (Fig. 4C). Proline content was also significantly correlated to some climatic variables, although associations were not as strong as for exon 3-skip P5CS1 percentage (11 of 101 climate variables had P < 0.05) (Dataset S5). For example, mean diurnal temperature range showed the opposite trend for proline compared with exon 3-skip P5CS1 percentage but with a weaker association (explaining only 6% of the proline variation) (Fig. 4D). Although log transformation reduced the skewness of exon 3-skip P5CS1 percentage, residuals were not normal and thus the associated P values are approximate. The opposite relationships of exon 3-skip P5CS1 percentage and proline accumulation with variables such as climate PC2 is consistent with the molecular data, showing that increasing exon 3-skip P5CS1 transcript leads to reduced P5CS1 protein and reduced proline accumulation.
P5CS1 Shows a Signature of Natural Selection for Local Adaptation to Climate.
P5CS1 also had a population genetic signature consistent with an involvement in local adaptation to the environment. Two lines of evidence suggest that neutral evolution at P5CS1 can be rejected and that adaptation to climate factors may drive patterns of genetic variation at this locus. First, we found a slight excess of segregating nonsynonymous substitutions in the protein-coding domain of P5CS1 (McDonald–Kreitman test marginally nonsignificant; G = 2.915, P = 0.087). Excess nonsynonymous substitutions suggest either that P5CS1 is a recurrent target of selection across the range of Arabidopsis or that selection on the intron 2 and 3 polymorphisms associated with high exon 3-skip P5CS1 has reduced the strength of purifying selection on tightly linked variants. Because tests based on models of sequence evolution can be sensitive to the effects of population structure, we also used the test of Toomajian et al. (21) that compares the haplotype structure at a given locus to a genome-wide distribution of haplotype lengths. This test provides evidence that selection has acted to increase the frequency of a P5CS1 haplotype in the recent past (pair-wise haplotype sharing score = 1.834, P = 0.059). Moreover, this SNP has a significantly elevated value of Wright’s fixation index for subdivided populations (FST = 0.265, P = 0.024), suggestive of local adaptation at the P5CS1 locus, and also shows significant associations with measures of aridity (ρ = 0.143, P = 0.0041) and light intensity (measured as photosynthetically available radiation; ρ = 0.137, P = 0.0027). These latter analyses are derived from previously reported genome-wide scans of European samples (12). These patterns may be because of the intron polymorphism we characterized or additional polymorphisms. In either case, the combined results suggest that P5CS1 is evolving nonneutrally.
Discussion
Alternative splicing has been shown to be a factor in plant response to the environment (22–25). In such reports, it has been variation in the expression or activity of splicing-related proteins that leads to alternative splicing of target genes. In contrast, sequence variation in P5CS1 introns 2 and 3 was sufficient to change the P5CS1 splicing pattern. Efficient splicing depends on both intron sequence, with properly spaced U-rich and UA-rich regions promoting intron recognition, and on optimal spacing between the 3′ end of the intron and the branch point (23, 26, 27). For P5CS1, extra intron 2 TA repeats may lead to less efficient recognition of intron 2, while the intron 3 G-to-T transition enhances recognition of that intron. The combined effect is to shift the balance toward splicing of exons 2 and 4 rather than exons 2 and 3. This hypothesized mechanism is illustrated in Fig. S6.
We are not aware of reports of similar intron polymorphisms causing alternative splicing in plants. In humans, TG and T insertions in the 3′ end of intron 8 of cystic fibrosis transmembrane regulator (CFTR) lead to skipping of exon 9 and production of a nonfunctional transcript associated with cystic fibrosis (28–30). Specific splicing factors promote exon skipping and splicing factor variation affects penetrance of disease-associated CFTR alleles (31, 32). This finding is qualitatively similar to our data where as much as 10% variation in exon 3-skip P5CS1 percentage can be seen for accessions with the same intron 2 and 3 sequence. This finding suggests that, in addition to the specific polymorphisms identified here, recognition and splicing of P5CS1 intron 2 may be influenced by natural variation in splicing machinery or RNA processing. However, because plant-splicing mechanisms are not well understood, how similar P5CS1 is to the well-studied CFTR example is not certain.
The value of proline in drought adaptation is a question of both interest and uncertainty in plant stress biology. The climate associations and population genetic analysis presented here suggest an association of proline metabolism, and P5CS1 specifically, with climate adaptation. Although these analyses are correlational and cannot by themselves establish cause and effect, they do raise questions about the role of proline metabolism. Particularly interesting is the overall stronger association of exon 3-skip P5CS1 percentage than proline level itself with climate variables, and the observation that higher proline was associated with wetter environments and more stable temperatures. One possible explanation for the latter observation is that accessions adapted to dryer or more variable climates have acquired additional stress-adaptive metabolic mechanisms to supplement proline accumulation. An example of this type of adaptation was observed in the Plumbaginaceae family, where species adapted to chronically dry environments convert proline to the more potent osmoprotectant proline-betaine (33). It has often been assumed that more proline accumulation is better in drought resistance; however, the stronger relationship of exon 3-skip P5CS1 percentage than proline content with climate variation is consistent with the amount of proline synthesis, rather than just the amount of proline accumulation, as a factor in environmental adaptation.
The overall importance of proline metabolism is also indicated by the P5CS1-deficient accession Pt-0. Pt-0 shows that P5CS1 can be reduced or lost without serious consequence for normal growth and development; however, the vast majority of accessions surveyed had little exon 3-skip P5CS1 and maintained the ability to induce high levels of P5CS1. Further study of this type of natural variation, such as transgenic introgression of naturally occurring P5CS1 alleles into different genetic backgrounds, promises to shed light on drought-adaptive mechanisms, as well as the novel alternative splicing mechanism exemplified by P5CS1.
Materials and Methods
Plant Material and QTL Mapping.
Arabidopsis accessions and the Ler × Sha RIL population (19) were obtained from the Arabidopsis Biological Resource Center. Details of low ψw treatment and QTL mapping can be found in SI Materials and Methods.
P5CS1 Alternative Splicing and Protein Detection.
RT-PCR and Western blot assays were performed using standard methods with primers and antisera described in SI Materials and Methods and Table S3.
Transgenic Plants.
Constructs and transgenic lines were generated as described in SI Materials and Methods. Data presented is from three to five homozygous T3 lines derived from independent transformation events, unless otherwise noted.
Climate Associations, Population Genetics, and P5CS1 Haplotypes.
We tested 101 climate variables and the principal components of those variables for association with either exon 3-skip P5CS1 percentage or proline accumulation (SI Materials and Methods). Associations were tested while accounting for population structure among accessions (EMMA) (20) or nonparametrically, ignoring population structure (Spearman’s rank correlation), and only included accessions for which 250 K SNP data are available (12) to estimate population structure and reliable collection location information are available (34).
Population genetic analyses were completed on the aligned coding sequences of P5CS1 from 80 accessions from the 1001 Genomes Project (35) using Arabidopsis lyrata P5CS1 coding sequence as an outgroup (SI Materials and Methods). Pair-wise haplotype sharing, Fst, and aridity/PAR patterns come from previously reported SNP datasets (12).
Identification of P5CS1haplotypes and accessions representing different haplotypes are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Mei-Jane Fang, Ang-Hsi Lin, Na Lin, and Ling-Shan Yu for assistance; and Dr. Wendy Hwang-Verslues for critical reading. This work was supported by an Academia Sinica Career Development Award (to P.E.V.); National Science Council of Taiwan Grant NSC-97-2311-B-001-005 (to P.E.V.) and a postdoctoral stipend (to R.K.); and US National Science Foundation Grants DEB 0618347 and IOS-0922457 (to T.E.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203433109/-/DCSupplemental.
References
- 1.Szabados L, Savouré A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Verslues PE, Sharma S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book. 2010;8:e0140. doi: 10.1199/tab.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S, Villamor JG, Verslues PE. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011;157:292–304. doi: 10.1104/pp.111.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S, Verslues PE. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ. 2010;33:1838–1851. doi: 10.1111/j.1365-3040.2010.02188.x. [DOI] [PubMed] [Google Scholar]
- 5.Székely G, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshiba Y, et al. Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. 1995;7:751–760. doi: 10.1046/j.1365-313x.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- 7.Verslues PE, Juenger TE. Drought, metabolites, and Arabidopsis natural variation: A promising combination for understanding adaptation to water-limited environments. Curr Opin Plant Biol. 2011;14:240–245. doi: 10.1016/j.pbi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Bouchabke O, et al. Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. Plos One. 2008;3:e1705. doi: 10.1371/journal.pone.0001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Des Marais DL, Juenger TE. Pleiotropy, plasticity, and the evolution of plant abiotic stress tolerance. Ann N Y Acad Sci. 2010;1206:56–79. doi: 10.1111/j.1749-6632.2010.05703.x. [DOI] [PubMed] [Google Scholar]
- 10.McKay JK, et al. Genetics of drought adaptation in Arabidopsis thaliana II. QTL analysis of a new mapping population, KAS-1 x TSU-1. Evolution. 2008;62:3014–3026. doi: 10.1111/j.1558-5646.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 11.Fournier-Level A, et al. A map of local adaptation in Arabidopsis thaliana. Science. 2011;334:86–89. doi: 10.1126/science.1209271. [DOI] [PubMed] [Google Scholar]
- 12.Hancock AM, et al. Adaptation to climate across the Arabidopsis thaliana genome. Science. 2011;334:83–86. doi: 10.1126/science.1209244. [DOI] [PubMed] [Google Scholar]
- 13.Horton MW, et al. Genome-wide patterns of genetic variation in worldwide Arabidopsis thaliana accessions from the RegMap panel. Nat Genet. 2012;44:212–216. doi: 10.1038/ng.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisec J, et al. Identification of metabolic and biomass QTL in Arabidopsis thaliana in a parallel analysis of RIL and IL populations. Plant J. 2008;53:960–972. doi: 10.1111/j.1365-313X.2007.03383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisec J, et al. Identification of heterotic metabolite QTL in Arabidopsis thaliana RIL and IL populations. Plant J. 2009;59:777–788. doi: 10.1111/j.1365-313X.2009.03910.x. [DOI] [PubMed] [Google Scholar]
- 16.Rowe HC, Hansen BG, Halkier BA, Kliebenstein DJ. Biochemical networks and epistasis shape the Arabidopsis thaliana metabolome. Plant Cell. 2008;20:1199–1216. doi: 10.1105/tpc.108.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentzell AM, et al. Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet. 2007;3:1687–1701. doi: 10.1371/journal.pgen.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu J, et al. System-wide molecular evidence for phenotypic buffering in Arabidopsis. Nat Genet. 2009;41:166–167. doi: 10.1038/ng.308. [DOI] [PubMed] [Google Scholar]
- 19.Clerkx EJM, et al. Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol. 2004;135:432–443. doi: 10.1104/pp.103.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang HM, et al. Efficient control of population structure in model organism association mapping. Genetics. 2008;178:1709–1723. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toomajian C, et al. A nonparametric test reveals selection for rapid flowering in the Arabidopsis genome. PLoS Biol. 2006;4:e137. doi: 10.1371/journal.pbio.0040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsukura S, et al. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol Genet Genomics. 2010;283:185–196. doi: 10.1007/s00438-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 23.Reddy ASN, Day IS, Göhring J, Barta A. Localization and dynamics of nuclear speckles in plants. Plant Physiol. 2012;158:67–77. doi: 10.1104/pp.111.186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez SE, et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature. 2010;468:112–116. doi: 10.1038/nature09470. [DOI] [PubMed] [Google Scholar]
- 25.Sugliani M, Brambilla V, Clerkx EJM, Koornneef M, Soppe WJJ. The conserved splicing factor SUA controls alternative splicing of the developmental regulator ABI3 in Arabidopsis. Plant Cell. 2010;22:1936–1946. doi: 10.1105/tpc.110.074674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cellini A, Felder E, Rossi JJ. Yeast pre-messenger RNA splicing efficiency depends on critical spacing requirements between the branch point and 3′ splice site. EMBO J. 1986;5:1023–1030. doi: 10.1002/j.1460-2075.1986.tb04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy ASN. Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annu Rev Plant Biol. 2007;58:267–294. doi: 10.1146/annurev.arplant.58.032806.103754. [DOI] [PubMed] [Google Scholar]
- 28.Hefferon TW, Groman JD, Yurk CE, Cutting GR. A variable dinucleotide repeat in the CFTR gene contributes to phenotype diversity by forming RNA secondary structures that alter splicing. Proc Natl Acad Sci USA. 2004;101:3504–3509. doi: 10.1073/pnas.0400182101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niksic M, Romano M, Buratti E, Pagani F, Baralle FE. Functional analysis of cis-acting elements regulating the alternative splicing of human CFTR exon 9. Hum Mol Genet. 1999;8:2339–2349. doi: 10.1093/hmg/8.13.2339. [DOI] [PubMed] [Google Scholar]
- 30.Pagani F, et al. Splicing factors induce cystic fibrosis transmembrane regulator exon 9 skipping through a nonevolutionary conserved intronic element. J Biol Chem. 2000;275:21041–21047. doi: 10.1074/jbc.M910165199. [DOI] [PubMed] [Google Scholar]
- 31.Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: A functional link with disease penetrance. Am J Hum Genet. 2004;74:1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buratti E, et al. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanson AD, et al. Osmoprotective compounds in the Plumbaginaceae: A natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci USA. 1994;91:306–310. doi: 10.1073/pnas.91.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastasio AE, et al. Source verification of mis-identified Arabidopsis thaliana accessions. Plant J. 2011;67:554–566. doi: 10.1111/j.1365-313X.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet. 2011;43:956–963. doi: 10.1038/ng.911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.