Abstract
Coccolithophores are an important component of the Earth system, and, as calcifiers, their possible susceptibility to ocean acidification is of major concern. Laboratory studies at enhanced pCO2 levels have produced divergent results without overall consensus. However, it has been predicted from these studies that, although calcification may not be depressed in all species, acidification will produce “a transition in dominance from more to less heavily calcified coccolithophores” [Ridgwell A, et al., (2009) Biogeosciences 6:2611–2623]. A recent observational study [Beaufort L, et al., (2011) Nature 476:80–83] also suggested that coccolithophores are less calcified in more acidic conditions. We present the results of a large observational study of coccolithophore morphology in the Bay of Biscay. Samples were collected once a month for over a year, along a 1,000-km-long transect. Our data clearly show that there is a pronounced seasonality in the morphotypes of Emiliania huxleyi, the most abundant coccolithophore species. Whereas pH and CaCO3 saturation are lowest in winter, the E. huxleyi population shifts from <10% (summer) to >90% (winter) of the heavily calcified form. However, it is unlikely that the shifts in carbonate chemistry alone caused the morphotype shift. Our finding that the most heavily calcified morphotype dominates when conditions are most acidic is contrary to the earlier predictions and raises further questions about the fate of coccolithophores in a high-CO2 world.
Keywords: phytoplankton, North Atlantic, climate change
Coccolithophores contribute between ∼1% and 10% of marine primary production (1), dominate the pelagic calcium carbonate flux (2), and alter ocean albedo (3). Model predictions suggest that, if CO2 emissions continue unabated, global surface ocean pH will decrease by 0.3–0.5 units by 2100, leading to a halving of the carbonate ion concentration (4). Along with other calcifiers, coccolithophores such as Emiliania huxleyi are considered susceptible to this ocean acidification (OA). This hypothesis is contentious, however, with diverse calcification responses reported for culture experiments. Many experiments on E. huxleyi (the most common coccolithophore) have found depressed calcification at elevated CO2 concentration and the associated low pH and low CaCO3 saturation state (Ω) (5–11), whereas others have found elevated calcification (12, 13) or no trend (10). An in-depth discussion on the reasons behind the contrasting results of Riebesell et al. (5) and Iglesias-Rodriguez et al. (12) can be found in refs. 14 and 15. In a recent study, four different strains of E. huxleyi cultured under identical environmental conditions exhibited varying responses to elevated CO2 (16), as was also found between coccolithophore species (17).
Laboratory studies are unrealistic in many respects and, because of their typically short timescales, preclude the possibility of evolutionary adaptation to the imposed change, a key uncertainty in OA research (17–19). It is therefore vital to complement laboratory experiments with observational studies of coccolithophores living in the natural habitats to which they are evolutionarily adapted.
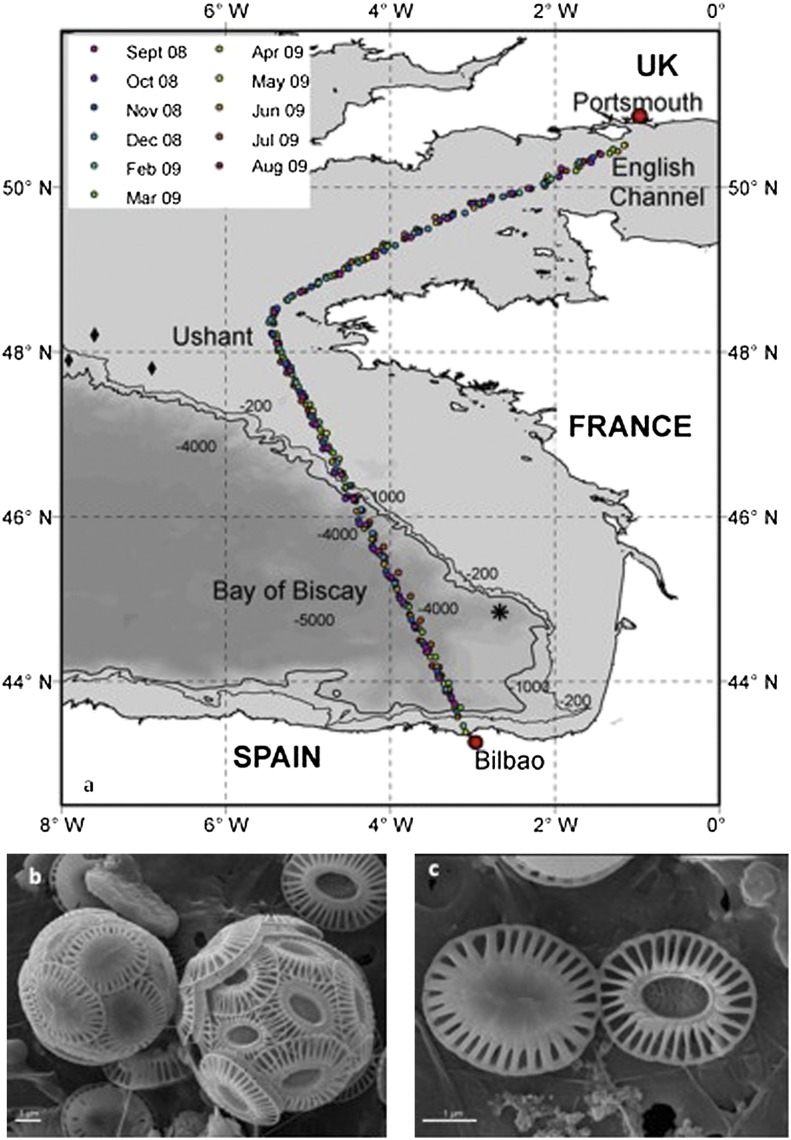
Here we report results from such a study. Coccolithophores, seawater carbonate chemistry, and other environmental variables (Methods) were sampled monthly between September 2008 and August 2009 along a 1,000-km route, including over deep oceanic waters in the Bay of Biscay (Fig. 1A). Our study was partly prompted by earlier results (20) from sediment traps at depths of 2,400 m and 3,000 m (asterisk in Fig. 1A). These results indicated that E. huxleyi type A overcalcified cells (Fig. 1 B and C) were more numerous (average of ∼60% of all E. huxleyi cells) than type A normal (type A) cells during summer but not during winter (∼30%). Such a pattern in morphotypes would be expected if calcification is inhibited by low Ω conditions during winter. We looked to confirm this seasonal pattern by sampling the surface waters directly.
Fig. 1.
Sampling locations and main morphotypes of E. huxleyi. (A) Sampling locations in the English Channel, adjacent continental shelf waters, deep ocean waters of the Bay of Biscay, and the Iberian shelf (color of symbol denotes month of sample collection as shown in the key). Asterisk shows location of the Beaufort and Heussner sediment trap (placed at 1,890-m water depth) (20). Filled black diamonds show locations of May 2008 shelf-edge sampling by Lei Chou and colleagues (Université libre de Bruxelles, Brussels; SI Appendix, Table S24). (B) The two main morphotypes of E. huxleyi observed during the crossings: on the left is a type A overcalcified cell, and on the right is a type A cell. (C) Coincidental juxtaposition of two individual coccoliths, one of each morphotype, in an SEM image. More calcium carbonate is invested in the type A overcalcified coccolith (left), as evidenced by infilling of the central area and thicker distal shield elements.
Results and Discussion
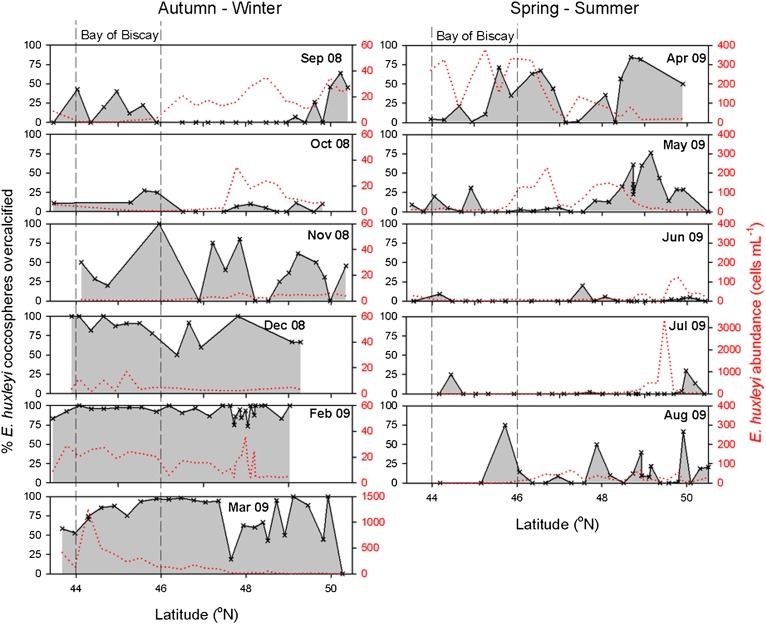
Our monthly sampling survey revealed a seasonal cycle in morphotypes opposite to the sediment-trap data. Surprisingly, the overcalcified morphotype was found to dominate the E. huxleyi population in winter. In the deep Bay of Biscay (44° N to 46° N), where we focus our analysis, the population switched from >50% type A in late summer 2008 to >90% type A overcalcified in winter 2008/2009, reverting to >90% type A in early summer 2009 (Fig. 2 and SI Appendix, Tables S1–S11). For example, whereas all 53 cells in the September 2008 SEM images were type A, as were 207 of 209 cells in May 2009, in February 2009 a total of 243 of the 254 cells examined were type A overcalcified. In the shallower and more tidally mixed conditions of the English Channel and near Ushant, France, a similar but more variable seasonal switch was seen to take place.
Fig. 2.
E. huxleyi abundance from September 2008 to August 2009. Variation in E. huxleyi abundance (dotted red line, right-hand axes; note different scale on some plots) and morphotype dominance (solid black line and gray shading, left-hand axes; same scale on all plots) with latitude. Each subplot shows a different month through from September 2008 to August 2009. Vertical dashed lines mark the region of deep water in the Bay of Biscay (44° N to 46° N) where analysis is focused. Cell densities increased through the winter but remained low (<40 cells⋅mL−1) throughout. At least four cells were viewed for each data point shown.
The combination of simple methods, large volumes of data, and comparison with other data allowed the existence of this seasonal switch to be established with a high degree of certainty. The evidence for the phenomenon is based on direct sampling of surface waters and uses a straightforward technique (visual examination of SEM images; 3,500 cells were examined in the Bay of Biscay and 8,900 cells were examined in total). Our findings agree with other data: (i) the same pattern recurred along our transect in 2009–2010 (SI Appendix, Tables S12–S21); (ii) summer dominance of type A morphotypes was also seen during crossings in 2006 (SI Appendix, Table S22) and 2007 (SI Appendix, Table S23) (winters were not sampled in these years); (iii) the same trend in morphotypes was seen in the more abundant detached coccoliths (SI Appendix, section 1 and Fig. S1); (iv) E. huxleyi were almost exclusively type A in May 2008 surface samples from the Biscay shelf edge (filled black diamonds in Fig. 1A and SI Appendix, Table S24); and (v) a similar morphotype seasonality was seen in less-frequently collected data from farther west in the Atlantic (21). Horizontal advection of a patch of water cannot explain a seasonal shift occurring all along the transect (deep water and shelf) and in multiple years. Although many fewer cells were imaged in winter, statistical analysis confirms that the differences are highly significant (P ≪ 0.001; SI Appendix, section 2). The results of this observational program show clearly that there is a seasonal alternation in the dominant E. huxleyi morphotype.
Although the results of our direct sampling conflict with the sediment-trap observations of Beaufort and Heussner (20), deep sediment traps are known to be subject to various biases. These include the possibility of lateral advection of previously deposited sediment material from the nearby shelf or slope (20, 22), which is of particular relevance given the location of their trap. The seasonal oscillation in morphotypes probably takes place in all years but cannot always be seen in deep sediment-trap sampling.
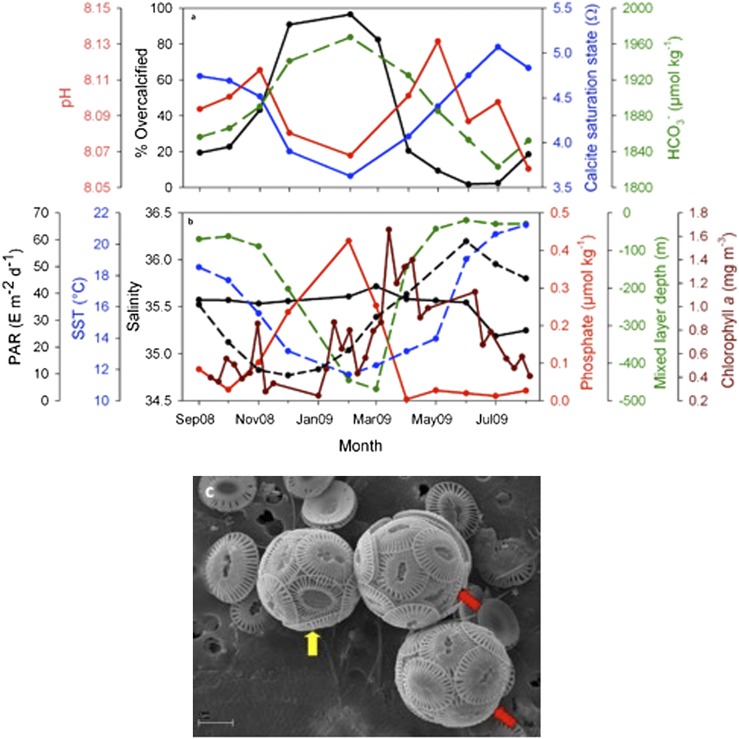
The cause of the phenomenon is unclear. Several factors have been found to stimulate calcification in laboratory experiments but are rejected as possible explanations of this phenomenon because they are inconsistent with our in situ data. E. huxleyi cellular CaCO3 content increases in cultures at low phosphate levels (23–25), but in situ phosphate levels are highest in winter (Fig. 3B). Bulk (community) calcification rates are correlated with light intensity in the Atlantic (26), but wintertime is the period with both the lowest surface-incident irradiance and the deepest mixing (Fig. 3B) and, hence, the lowest average light intensities in the mixed layer. However, it has to be noted that calcification rates do not necessarily covary with the amount of CaCO3 in each coccolith. It has been suggested that E. huxleyi morphometrics vary consistently with salinity, allowing them to be used as a paleo-proxy for salinity (27); however, there is only a subtle seasonal cycle in salinity (Fig. 3B). All physiological rates are potentially facilitated by higher temperatures, but in some culture experiments cellular calcification by E. huxleyi was in fact higher at lower temperatures (at 10° C, 12° C, and 13 °C) (28, 29). In situ temperatures (Fig. 3B) are lowest (∼12 °C) in February, and so this explanation remains a possibility. The trend could also be attributable to differences in the growth rate of different morphotypes at low temperatures (16). Furthermore, the morphotype switch could potentially be explained by seasonal grazing patterns or be a response to another seasonally varying environmental variable that we did not measure.
Fig. 3.
Carbonate chemistry, environmental variables, and coccolith type. (A) Seasonal changes in the proportion of E. huxleyi cells that are type A overcalcified (black line) as well as the saturation state of surface seawater with respect to calcite (Ω; blue line), pH (red line), and bicarbonate ion (HCO3−) concentration (μmol⋅kg−1; green dashed line). There are no carbonate chemistry data for March 2009. (B) Seasonal changes in various environmental variables: average mixed-layer light intensity in terms of photosynthetically available radiation (Einstein’s m−2⋅d−1; black dashed line), sea-surface temperature (°C; blue dashed line), sea-surface salinity (black solid line), surface phosphate concentration (μmol⋅kg−1; red solid line), mixed-layer depth (green dashed line), and surface chlorophyll a data (8-d composite; mg⋅m−3; brown solid line). For both A and B, there are no ship-derived data for Jan 2009 when the ship was in refit, and all points represent averages over the Bay of Biscay part of the route (44° N to 46° N). (C) Different coccolith types on a single coccosphere (yellow arrow) and coccoliths with intermediate degrees of calcification (red arrows) (from March 2009).
A further possibility is that overcalcified cells are specialized life stages for survival through harsh winter conditions. Diatoms produce resting spores as environmental conditions become unfavorable (30), and dinoflagellates produce cysts. Similarly, perhaps overcalcified E. huxleyi cells are especially hardy. The phenomenon is unlikely to be connected to any difference in sinking rates between the two morphotypes (CaCO3 is 2.7 times denser than water) because individual cells are so small that they have very slow sinking speeds (≤0.5 m⋅d−1) (31) and are instead transported downward primarily within marine snow and fecal pellets (32).
Underlining our lack of understanding of the cause of this phenomenon, it is unclear whether it consists of a shift in genotypes or in phenotypes. The two morphotypes may be (i) genetically distinct subspecies, (ii) different life stages of the same genetic species (cf. butterflies and caterpillars), or (iii) alternate phenotypes of the same genetic species, and at the same life stage, but with environmentally determined ontogeny. It may be relevant that some of the observed coccospheres exhibit intermediate calcification (red arrows in Fig. 3C) or, less frequently, a mix of coccolith types (yellow arrow in Fig. 3C). Our understanding could be improved by genetically characterizing the two E. huxleyi morphotypes as they occur in nature and by investigating whether it is possible to induce type A to turn into type A overcalcified in culture.
Our data are well suited for elucidating OA impacts. Along a route of over 1,000 km, both carbonate chemistry and coccolithophores were simultaneously determined on the same long crossings every month for over a year. The seasonal pattern of surface-water carbonate chemistry changes that we measured in the Bay of Biscay (Fig. 3A and SI Appendix, Table S25) resembles that found elsewhere (33, 34): dissolved inorganic carbon (DIC) concentrations were higher in winter than in summer, and values for pH and Ω were lower (Fig. 3A). However, Ω never reached undersaturation, and the range of pH values encountered were much smaller than the changes expected over the next century under a high-CO2 emission scenario.
A comparison between our carbonate chemistry and coccolithophore data only contributes to the continuing controversy surrounding this topic. Past experimental laboratory studies (5–13, 16, 17) have come to divergent conclusions about the effect of OA on coccolithophore calcification, and paleo records of different events have been interpreted variously as evidence of either modest (35) or, conversely, serious (36) impacts of OA on coccolithophores. Whereas a recent observational and paleo study (37) found a positive correlation between carbonate ion concentration and degree of coccolithophore calcification, our observational data exhibit a distinct anticorrelation (Fig. 3A). As pH and Ω declined with the onset of winter in the Bay of Biscay, the overcalcified morphotype increased in both percentage and absolute abundance. We do not suggest that the changing carbonate chemistry was necessarily responsible for this shift in morphotypes seen in our study. However, if it was not, then the alternative is that carbonate chemistry is not the sole and overriding control over coccolithophore calcification. Although Beaufort et al. (37) based their conclusion on three different present-day datasets, one of their three datasets shows an anticorrelation, as does ours. Altogether, these two datasets seriously call into question their conclusion that OA will lead to a replacement of heavily-calcified coccolithophores by lightly-calcified ones.
Methods
Study Area.
Sampling was undertaken from P&O Ferries’ Pride of Bilbao between Portsmouth, UK, and Bilbao, Spain. The route (Fig. 1) was consistent throughout the sampling period (September 2008 to July 2009).
Sampling and Analysis.
Samples were collected every 50 km from the ship’s underway supply (∼5 m depth).
Coccolithophores.
Up to 2 L of seawater was prefiltered onto a 200-μm mesh to remove zooplankton. This water was passed through 1.2-μm, 25-mm Millipore Isopore membrane filters, which were then rinsed with alkaline (pH ∼9) freshwater solution, allowed to air-dry, and stored in sealed Petri dishes in the dark. The alkaline freshwater solution used for the rinsing of coccolithophore samples was prepared by adding 30 μL of concentrated ammonia solution to 1 L of Milli-Q water. The pH of the final solution was measured with a pH meter to verify it was ∼9.
Coccolithophore enumeration.
A radially cut portion of each filter was coated with gold, and 225 photographs (equivalent to 1 mm2) per filter were taken at 5,000× magnification with a LEO 1450 VP SEM (Carl Zeiss). Extra photographs were then taken when cells were scarce. E. huxleyi coccospheres were categorized into morphotypes (38). The two morphotypes of E. huxleyi that we most frequently observed, and which we counted, are type A and type A overcalcified (Fig. 1 A and B) as defined in ref. 38. Some types B and C morphotypes were observed, but these usually accounted for ≤10% of the total. For each sample, either 225 photographs [fields of view (FOV)] or 300 E. huxleyi cells (if encountered in fewer than 225 FOV) were enumerated and categorized into morphotypes (38). The percentage overcalcified was only reported and included in Fig. 2 for stations where at least four cells were viewed. Additional FOV were counted when abundance was very low but did not always raise the total number of cells counted to four or more. The minimum cell density below which we were unable to determine the percentage overcalcified varied between ∼1 and 10 cells⋅mL−1, depending on counting effort. The abundance of each morphotype was calculated as
where C is the total number of cells or coccoliths counted, A is the area investigated (mm2), F is the total filter area (mm2), and V is the volume filtered (mL).
Carbonate chemistry.
Samples for the determination of DIC and total alkalinity (TA) were drawn in 250-mL SCHOTT SUPRAX borosilicate glass bottles following the standard method to minimize gas exchange. A headspace of 1% was allowed for water expansion, and samples were poisoned with 50 μL of a saturated solution of mercuric chloride.
DIC and TA.
The analysis of DIC and TA was undertaken by using the VINDTA 3C system (Marianda). The DIC samples were analyzed coulometrically (coulometer 5011; UIC), and the TA samples were analyzed by using a closed-cell titration (39). The cell (100 mL) for the TA determination was equipped with a pH half-cell electrode (glass-bodied 8101SC; Orion) and an Ag/AgCl reference electrode (6.0729.100; Metrohm). The calculation of TA was based on a nonlinear curve-fitting (least-squares) approach (39). The analysis of the samples was temperature-regulated at 25 °C (±0.1 °C) with a water bath (F12; Julabo). The precision of the method was determined daily from repeated measurements on the same batch of seawater and was estimated with the whole dataset to be ±3.7 μmol⋅kg−1 for DIC and ±2.6 μmol⋅kg−1 for TA. Certified reference materials (from A. G. Dickson, Scripps Institution of Oceanography, La Jolla, CA) were analyzed as standards at the beginning and end of each day of analysis.
Carbonate System.
The carbonate system parameters (Ω calcite, pHT, and carbonate ion concentration) were calculated from the DIC, TA, temperature, salinity, and nutrient data. The CO2SYS program (version 1.05) (40) was used for the calculation with the pH total scale and the equilibrium constants from Mehrbach et al. (41) refitted by Dickson and Millero (42).
Ancillary data.
Nutrient samples were analyzed with standard methods on an autoanalyzer (43). Continuous in situ temperature, conductivity, and chlorophyll a fluorescence measurements were obtained from a MINI-pack system (Chelsea Technologies Group) installed on the ship. The salinity data were calibrated by using samples collected approximately every 50 km on each crossing and analyzed on a salinometer (8400 B Autosal; Guildline) back in the laboratory. The precision and accuracy of the salinity measurements were better than ±0.1. Nutrient samples were collected every half hour (∼25 km) on each crossing and analyzed in the laboratory by using standard methods on an autoanalyzer for silicate, total nitrate (nitrate plus nitrite), and phosphate (43). The precision and accuracy of the nutrient concentrations were ±0.1 μmol⋅L−1 for nitrate and silicate and ±0.02 μmol⋅L−1 for phosphate.
Mixed-layer depth.
The weekly data from four Argo floats located in the Bay of Biscay (44° N to 46.4° N) were used for the determination of the mixed-layer depth (http://www.coriolis.eu.org/Data-Services-Products/View-Download/Access-to-Argo-floats-by-WMO-number; Argo floats 1900621, 1900624, 6900323, and 6900324). The monthly averaged mixed-layer depth was consistent among the four floats, and the winter mixed-layer depth ranged between 400 and 525 m. However, for reasons of clarity, only data from Argo float 1900624 is presented in Fig. 3. The mixed-layer depth was estimated according to the temperature criterion of 0.5 °C difference from the sea-surface temperature (44).
Satellite Data.
The monthly SeaWiFS photosynthetically available radiation data, averaged for the Bay of Biscay region (44° N to 46.4° N), were obtained from the Giovanni Ocean Color Radiometry Online Visualization and Analysis system (Global Monthly Product; http://gdata1.sci.gsfc.nasa.gov/daac-bin/G3/gui.cgi?instance_id=ocean_month; SeaWiFS.R2009). The SeaWiFS 8-d composite chlorophyll a data averaged for the Bay of Biscay region (44° N to 46.4° N) were obtained from OceanColor (http://oceancolor.gsfc.nasa.gov/cgi/l3).
Supplementary Material
Acknowledgments
We acknowledge P&O Ferries for permission to travel on and sample from their ship. We thank the officers and crew of P&O Ferries’ Pride of Bilbao for their assistance during crossings and the members of the National Oceanography Centre Southampton FerryBox team for assistance with sampling, analyses, equipment maintenance, and other aspects. We are grateful for help from F. Sardenne and E. Jenner for coccolithophore counting in SEM images. We thank L. Chou for provision of samples from the Biscay shelf edge and A. Poulton, S. Widdicombe, B. Kelly-Gerreyn, R. Howlett, and two anonymous reviewers for critical discussions and comments. This work is a contribution to the European Project on Ocean Acidification (EPOCA), which received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 211384. This work was also made possible by Grant EVK2-CT-2000-00088 from the European Union to the CarboOcean Integrated Project and through funding from the Department for Environment, Food and Rural Affairs (Defra) for the Defra pH project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117508109/-/DCSupplemental.
References
- 1.Poulton AJ, Adey TR, Balch WM, Holligan PM. Relating coccolithophore calcification rates to phytoplankton community dynamics: Regional differences and implications for carbon export. Deep Sea Res Part II Top Stud Oceanogr. 2007;54:538–557. [Google Scholar]
- 2.Broecker W, Clark E. Ratio of coccolith CaCO3 to foraminifera CaCO3 in late Holocene deep sea sediments. Paleoceanography. 2009;24:PA3205. [Google Scholar]
- 3.Tyrrell T, Holligan PM, Mobley CD. Optical impacts of oceanic coccolithophore blooms. J Geophys Res-Oceans. 1999;104:3223–3241. [Google Scholar]
- 4.Orr JC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 5.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 6.Zondervan I, Rost B, Riebesell U. Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light-limiting conditions and different daylengths. J Exp Mar Biol Ecol. 2002;272:55–70. [Google Scholar]
- 7.Delille B, et al. Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Global Biogeochem Cycles. 2005;19:GB2023. [Google Scholar]
- 8.Engel A, et al. Testing the direct effect of CO2 concentration on a bloom of the coccolithophorids Emiliania huxleyi in mesocosm experiments. Limnol Oceanogr. 2005;50:493–507. [Google Scholar]
- 9.Sciandra A, et al. Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation. Mar Ecol Prog Ser. 2003;261:111–122. [Google Scholar]
- 10.Feng Y, et al. Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae) Eur J Phycol. 2008;43:87–98. [Google Scholar]
- 11.Hoppe CJM, Langer G, Rost B. Emiliania huxleyi shows identical responses to elevated pCO2 in TA and DIC manipulations. J Exp Mar Biol Ecol. 2011;406:54–62. [Google Scholar]
- 12.Iglesias-Rodriguez MD, et al. Phytoplankton calcification in a high-CO2 world. Science. 2008;320:336–340. doi: 10.1126/science.1154122. [DOI] [PubMed] [Google Scholar]
- 13.Shi D, Xu Y, Morel FMM. Effects of the pH/pCO2 control method on medium chemistry and phytoplankton growth. Biogeosciences. 2009;6:1199–1207. [Google Scholar]
- 14.Riebesell U, et al. Comment on “Phytoplankton calcification in a high-CO2 world”. Science. 2008;322 doi: 10.1126/science.1161096. 1466b, author reply 1466c. [DOI] [PubMed] [Google Scholar]
- 15.Iglesias-Rodriguez MD, et al. Response to comment on “Phytoplankton calcification in a high-CO2 world”. Science. 2008;322:1466c. doi: 10.1126/science.1154122. [DOI] [PubMed] [Google Scholar]
- 16.Langer G, Nehrke G, Probert I, Ly J, Ziveri P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences. 2009;6:2637–2646. [Google Scholar]
- 17.Langer G, et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem Geophys Geosyst. 2006;7:Q09006. [Google Scholar]
- 18.Gattuso JP, Buddemeier RW. Ocean biogeochemistry. Calcification and CO2. Nature. 2000;407:311–313. doi: 10.1038/35030280. [DOI] [PubMed] [Google Scholar]
- 19.Ridgwell A, et al. From laboratory manipulations to Earth system models: Scaling calcification impacts of ocean acidification. Biogeosciences. 2009;6:2611–2623. [Google Scholar]
- 20.Beaufort L, Heussner S. Seasonal dynamics of calcareous nannoplankton on a West European continental margin: The Bay of Biscay. Mar Micropaleontol. 2001;43:27–55. [Google Scholar]
- 21.Dandonneau Y, Montel Y, Blanchot J, Girardeau J, Neveux J. Temporal variability in phytoplankton pigments, picoplankton and coccolithophores along a transect through the North Atlantic and tropical southwestern Pacific. Deep Sea Res Part I Oceanogr Res Pap. 2006;53:689–712. [Google Scholar]
- 22.Hwang J, Eglinton TI, Krishfield RA, Manganini SJ, Honjo S. Lateral organic carbon supply to the deep Canada Basin. Geophys Res Lett. 2008;35:L11607. [Google Scholar]
- 23.Paasche E, Brubak S. Enhanced calcification in the coccolithophorid Emiliania huxleyi (Haptophyceae) under phosphorus limitation. Phycologia. 1994;33:324–330. [Google Scholar]
- 24.Shiraiwa Y. Physiological regulation of carbon fixation in the photosynthesis and calcification of coccolithophorids. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:775–783. doi: 10.1016/s1096-4959(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 25.Muller MN, Antia AN, LaRoche J. Influence of cell cycle phase on calcification in the coccolithophore Emiliania huxleyi. Limnol Oceanogr. 2008;53:506–512. [Google Scholar]
- 26.Poulton AJ, et al. Phytoplankton carbon fixation, chlorophyll-biomass and diagnostic pigments in the Atlantic Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2006;53:1593–1610. [Google Scholar]
- 27.Bollmann J, Herrle JO. Morphological variation of Emiliania huxleyi and sea surface salinity. Earth Planet Sci Lett. 2007;255:273–288. [Google Scholar]
- 28.Sorrosa JM, Satoh M, Shiraiwa Y. Low temperature stimulates cell enlargement and intracellular calcification of coccolithophorids. Mar Biotechnol (NY) 2005;7:128–133. doi: 10.1007/s10126-004-0478-1. [DOI] [PubMed] [Google Scholar]
- 29.De Bodt C, Van Oostende N, Harlay J, Sabbe K, Chou L. Individual and interacting effects of pCO2 and temperature on Emiliania huxleyi calcification: Study of the calcite production, the coccolith morphology and the coccosphere size. Biogeosciences. 2010;7:1401–1412. [Google Scholar]
- 30.McQuoid MR, Hobson LA. Diatom resting stages. J Phycol. 1996;32:889–902. [Google Scholar]
- 31.Lecourt M, Muggli DL, Harrison PJ. Comparison of growth and sinking rates of non-coccolith- and coccolith-forming strains of Emiliania huxleyi (Prymnesiophyceae) grown under different irradiances and nitrogen sources. J Phycol. 1996;32:17–21. [Google Scholar]
- 32.Honjo S, Manganini SJ, Krishfield RA, Francois R. Particulate organic carbon fluxes to the ocean interior and factors controlling the biological pump: A synthesis of global sediment trap programs since 1983. Prog Oceanogr. 2008;76:217–285. [Google Scholar]
- 33.Merico A, Tyrrell T, Cokacar T. Is there any relationship between phytoplankton seasonal dynamics and the carbonate system? J Mar Syst. 2006;59:120–142. [Google Scholar]
- 34.Findlay HS, Tyrrell T, Bellerby RGJ, Merico A, Skjelvan I. Carbon and nutrient mixed layer dynamics in the Norwegian Sea. Biogeosciences. 2008;5:1395–1410. [Google Scholar]
- 35.Gibbs SJ, Bown PR, Sessa JA, Bralower TJ, Wilson PA. Nannoplankton extinction and origination across the Paleocene-Eocene Thermal Maximum. Science. 2006;314:1770–1773. doi: 10.1126/science.1133902. [DOI] [PubMed] [Google Scholar]
- 36.Erba E, Bottini C, Weissert HJ, Keller CE. Calcareous nannoplankton response to surface-water acidification around Oceanic Anoxic Event 1a. Science. 2010;329:428–432. doi: 10.1126/science.1188886. [DOI] [PubMed] [Google Scholar]
- 37.Beaufort L, et al. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature. 2011;476:80–83. doi: 10.1038/nature10295. [DOI] [PubMed] [Google Scholar]
- 38.Young JR, et al. A guide to extant coccolithophore taxonomy. J Nannoplankton Res. 2003;(Special Issue 1):1–132. [Google Scholar]
- 39.Dickson AG, Sabine CL, Christian JR. Guide to Best Practices for Ocean CO2 Measurements. 2007. North Pacific Marine Science Organization (PICES) Special Publ 3. [Google Scholar]
- 40.Lewis E, Wallace DWR. Program Developed for CO2 System Calculations. ORNL/CDIAC-105. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; 1998. [Google Scholar]
- 41.Mehrbach C, Culberson CH, Hawley JH, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18:897–907. [Google Scholar]
- 42.Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987;34:1733–1743. [Google Scholar]
- 43.Grasshoff K. Determination of nutrients. In: Grasshoff K, Ehrhardt M, Kremling K, editors. Methods of Seawater Analysis. 2nd Ed. Basel: Verlag Chemie; 1983. pp. 125–188. [Google Scholar]
- 44.Monterey G, Levitus S. NOAA NESDIS Atlas 14: Seasonal Variability of Mixed Layer Depth for the World Ocean. Washington DC: US Government Printing Office; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.