Abstract
Removal of the parametrial fat pads (partial lipectomy) from female SKH-1 mice fed a high-fat diet inhibited UVB-induced carcinogenesis, but this was not observed in mice fed a low-fat chow diet. Partial lipectomy in high-fat–fed mice decreased the number of keratoacanthomas and squamous cell carcinomas per mouse by 76 and 79%, respectively, compared with sham-operated control mice irradiated with UVB for 33 wk. Immunohistochemical analysis indicated that partial lipectomy increased caspase 3 (active form) positive cells by 48% in precancerous epidermis away from tumors, by 68% in keratoacanthomas, and by 224% in squamous cell carcinomas compared with sham-operated control mice. In addition, partial lipectomy decreased cell proliferation away from tumors and in tumors. RT-PCR analysis for adipokines revealed that mRNAs for TIMP1, MCP1, and SerpinE1 (proinflammatory/antiapoptotic cytokines) in the parametrial fat pads of sham-operated control mice were 54- to 83-fold higher than levels in compensatory fat that returned after surgery in partially lipectomized mice at the end of the tumor study. Feeding mice high-fat diets for 2 wk increased levels of TIMP1 and other adipokines in serum and epidermis, and these increases were inhibited by removal of the parametrial fat pads. Our results are a unique demonstration that surgical removal of a specific tissue fat results in inhibition of carcinogenesis in obese mice. This inhibition was associated with an increase in apoptosis and a decrease in proliferation in tumors and in precancerous areas away from tumors.
Keywords: obesity, skin cancer
Sunlight-induced nonmelanoma skin cancer is a major cancer in the United States (1–4), and rates of obesity are also dramatically increasing, leading to an alarming rise in morbidity and mortality caused by heart disease, diabetes, and cancer. How obesity contributes to skin cancer and the molecular changes induced by obesity that promote skin cancer development remain poorly understood. Therefore, approaches for preventing these cancers are of considerable importance. Early studies in our laboratory indicated that orally administered caffeine to UVB-pretreated “high-risk” SKH-1 mice decreased tissue fat as measured by the size of the parametrial fat pads and the thickness of the dermal fat layer (5), and this treatment also decreased tumor formation (6). Mechanistic studies suggested that the stimulatory effect of caffeine administration on locomotor activity contributed to the effect of caffeine on tissue fat (7). Our results also showed that voluntary running wheel exercise decreased the weight of the parametrial fat pads, which was associated with decreased UVB-induced formation of skin tumors as well as the size of the tumors (8). Additional observations indicated that a combination of caffeine administration and running wheel exercise was more effective than either treatment alone at decreasing tissue fat and enhancing UVB-induced apoptosis (9). In a recent study, we found that running wheel exercise together with oral administration of caffeine had a greater inhibitory effect on UVB-induced skin carcinogenesis than either treatment alone (10). Because these effects of caffeine and exercise were associated with decreased tissue fat, we studied the effect of removal of the parametrial fat pads (partial lipectomy) on UVB-induced apoptosis. The results indicated that partial lipectomy stimulated UVB-induced apoptotic sunburn cells, but this treatment had no effect in the absence of UVB (11). These results suggest that the parametrial fat pads can significantly influence UVB-induced skin carcinogenesis. In the present study, we investigated the effect of partial lipectomy on UVB-induced skin carcinogenesis in mice fed either a 40%-kcal high-fat diet rich in omega-6 fatty acids or a low-fat chow diet. Our previous studies showed that this high-fat diet rich in omega-6 fatty acids (described in SI Materials and Methods) enhanced UVB-induced skin tumor formation compared with mice fed a high-fat diet rich in omega-3 fatty acids (12). The results of the present study demonstrate that removal of the parametrial fat pads markedly inhibits UVB-induced carcinogenesis in high-fat–fed mice.
Results
Surgical Removal of the Parametrial Fat Pads Inhibits UVB-Induced Skin Tumor Formation in Mice Fed a High-Fat Diet, but Not in Mice Fed a Low-Fat Chow Diet.
As expected, mice fed a 40%-kcal high-fat diet during the course of UVB irradiation for 33 wk were heavier than the mice fed the low-fat chow diet (Fig. 1 A and B). Surgical removal of the parametrial fat pads did not influence body weight in mice fed either the 40%-kcal high-fat diet or the low-fat chow diet over the 33 wk of UVB treatment (Fig. 1 A and B). Surgical removal of the parametrial fat pads markedly inhibited UVB-induced skin tumorigenesis in mice fed the high-fat diet (Fig. 1A), but this outcome was not observed in mice fed the low-fat chow diet (Fig. 1B).
Fig. 1.
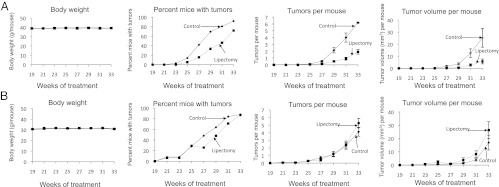
Surgical removal of the parametrial fat pads inhibits UVB-induced skin tumor formation In mice fed a high-fat diet, but not in mice fed a regular low-fat chow diet. (A) Eighty female SKH-1 mice were given a 40%-kcal high-fat diet rich in omega-6 fatty acids as described by Reddy et al. (56) for 2 wk, and (B) another 80 female SKH-1 mice were given a low-fat chow diet for 2 wk. Mice on each diet were then equally divided into two groups. One group of mice had their parametrial fat pads removed and the other group of mice was a sham-operated control. The high-fat and the chow diets were continued throughout the study. Two weeks after the surgery, all of the mice were treated with UVB (30 mJ/cm2) twice a week for 33 wk. Body weight and tumors were measured every 2 wk.
Surgical removal of the parametrial fat pads in SKH-1 mice fed a high-fat diet increased the time needed for the development of skin tumors compared with sham-operated control animals. Lipectomized mice started developing tumors during the 25th week after the start of UVB exposure, whereas sham-operated control animals started developing tumors during the 23rd week after the start of UVB exposure (Fig. 1A). Thereafter, the tumor incidence in the lipectomized group was always lower than that in the sham-operated control mice. Kaplan–Meier estimates of the median tumor-free time for the lipectomized group was 31 wk of UVB exposure with a 95% confidence interval from week 29 to week 33. The median tumor-free time for the sham-operated control group was 29 wk of UVB exposure with a 95% confidence interval from week 27 to week 29. The log-rank test of the homogeneity of the tumor-free distribution function showed that the difference between the two treatment groups was statistically significant (P = 0.005) (Fig. 1A).
In both the lipectomy and sham-operated groups fed the high-fat diet, the number of tumors per mouse increased with time (Fig. 1A). The rate of increase (quadratic trend) in tumor numbers per mouse for the sham-operated group was statistically greater than that for the lipectomy group (P < 0.0001) (Fig. 1A). On average, at week 33, the tumor number per mouse for the sham-operated group was 3.3 times more than that for the lipectomy group (P < 0.0001). These results indicate that removal of the parametrial fat pads decreased the number of tumors per mouse.
The tumor volume per mouse increased over time in both groups fed the high-fat diet (Fig. 1A), as did the variation of the tumor volume per mouse. Although the quadratic time trend was not statistically different between the two groups, the linear trend for the sham-operated group was statistically larger than that for the lipectomy group (P < 0.0001). On average, at week 33, the tumor volume per mouse for the sham-operated control group was 4.5 times bigger than that for the lipectomy group (P < 0.0001) (Fig. 1A). These results indicate that partial lipectomy significantly decreased tumor size.
Histopathological examinations of all of the skin lesions from animals fed the high-fat diet revealed that lipectomy decreased the percentage of mice with squamous cell papillomas, keratoacanthomas, and squamous cell carcinomas by 38, 28, and 65%, respectively, compared with the percentage of the various tumors in sham-operated control mice (Table 1). Lipectomy decreased the number of squamous cell papillomas, keratoacanthomas, and squamous cell carcinomas per mouse by 37, 76, and 79%, respectively, compared with the various tumors in sham-operated control mice (Table 1). Lipectomy decreased the size of squamous cell papillomas, keratoacanthomas, and squamous cell carcinomas per mouse by 35, 90, and 88%, respectively, compared with the sham-operated control mice (Table S1). In an additional study, removal of the parametrial fat pads from SKH-1 mice fed a 60%-kcal very-high-fat diet inhibited UVB-induced formation of tumors per mouse by 70%, and the size of tumors per mouse was decreased by 82%, compared with sham-operated control mice.
Table 1.
Effect of lipectomy on the incidence and multiplicity of histologically characterized skin tumors in SKH-1 mice treated with UVB
| Squamous cell papillomas |
Keratoacanthomas |
Squamous cell carcinomas |
Total tumors |
||||||
| Treatment | No. of mice | Percent of mice with tumors | Tumors per mouse | Percent of mice with tumors | Tumors per mouse | Percent of mice with tumors | Tumors per mouse | Percent of mice with tumors | Tumors per mouse |
| Sham operated control | 36 | 8.33 | 0.08 ± 0.05 | 100.00 | 11.47 ± 1.73 | 22.22 | 0.39 ± 0.13 | 100.00 | 11.94 ± 1.80 |
| Lipectomy | 39 | 5.13 (38) | 0.05 ± 0.04 (37) | 71.79 (28) | 2.79 ± 0.46* (76) | 7.69* (65) | 0.08 ± 0.04* (79) | 71.79** (28) | 2.92 ± 0.49* (76) |
Eighty female SKH-1 mice were given a high-fat diet rich in polyunsaturated omega-6 fatty acids for 2 wk. Mice were then equally divided into two groups on the basis of body weight. One group of mice had their parametrial fat pads removed and another group of mice was a sham-operated control. Two weeks after the surgery, all of the mice were treated with UVB (30 mJ/cm2) once a day, twice weekly for 33 wk. The mice were killed at 24 h after the last dose of UVB, and all tumors were characterized by histopathology. Each value is the mean ± SE, and the numbers in parentheses represent percent decrease. *P < 0.01, **P < 0.05.
Surgical Removal of the Parametrial Fat Pads Stimulates Apoptosis and Inhibits Proliferation in Tumors and in Areas of the Epidermis away from Tumors in UVB-Treated Mice.
Immunohistochemical analysis with all tumor samples from high-fat–fed mice (Fig. 1A) indicated that lipectomy increased the percentage of caspase 3 (active form) positive cells in precancerous epidermis away from tumors by 48%, in keratoacanthomas by 68%, and in squamous cell carcinomas by 224%, compared with the sham-operated control mice (all P values were less than 0.01) (Fig. 2A). Partial lipectomy also inhibited cell proliferation. Removal of the parametrial fat pads decreased the percentage of BrdU+ cells in precancerous areas away from tumors by 28% (P = 0.01), in keratoacanthomas by 24% (P < 0.01), and in squamous cell carcinomas by 41% (P = 0.07), compared with the sham-operated control mice (Fig. 2B). Overall, partial lipectomy decreased the proliferation index (ratio of BrdU+ cells/caspase-3+ cells) by 42% in precancerous areas away from tumors, by 51% in keratoacanthomas, and by 88% in squamous cell carcinomas compared with the sham-operated control mice (Fig. 2C).
Fig. 2.
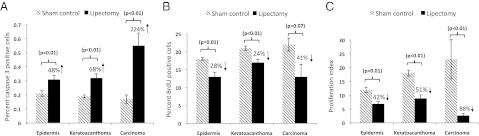
Surgical removal of the parametrial fat pads stimulates apoptosis and inhibits cell proliferation in tumors and in areas away from the tumors in mice treated with UVB and fed a high-fat diet. Mice described for the carcinogenesis study in Fig. 1A were killed at 24 h after the last dose of UVB, and all tumors were characterized by histopathology. (A) Caspase 3 (active form) positive cells and (B) BrdU+ cells were determined immunohistochemically. (C) Proliferation index (the ratio of BrdU+/caspase-3+ cells) was calculated from an area away from tumors and from tumors from each mouse. Each value is the mean ± SE.
mRNA Expression for Adipokines Is Higher in the Parametrial Fat Pads of Sham-Operated Control Mice than That in Compensatory Fat That Appears After Removal of the Parametrial Fat Pads.
Although the 40%-kcal fat diet increased body weight of the mice related to the low-fat chow diet (Fig. 1 A and B), the average percentage of the parametrial fat pads compared with total body fat between these groups was almost the same (accounting for ∼15% of total body fat) as assayed by dual-energy X-ray absorptiometry (DXA). We found that partially lipectomized mice restored their body fat very quickly, and the compensatory fat that returned after removal of the parametrial fat pads was redistributed to other places. The parametrial fat pads showed no signs of regeneration. The parametrial fat pads in sham-operated mice and compensatory fat that returned after partial lipectomy at the end of the tumorigenesis study described in Fig. 1A are shown in Fig. S1A. Because MCP1 (monocyte chemoattractant protein-1), SerpinE1 (Serpin peptidase inhibitor, clade E, member 1), and TIMP1 (tissue inhibitor of metalloproteinase-1) are adipokines that were reported to have antiapoptotic or proinflammatory effects, and high levels were found in UVB-induced tumors (discussed later), we evaluated the levels of mRNAs for MCP1, SerpinE1, and TIMP1 in the parametrial fat pads from sham-operated control mice and in the compensatory fat from partially lipectomized mice by real-time PCR at the end of the tumor study described in Fig. 1A. Our data indicated that the mRNA levels for MCP1, SerpinE1, and TIMP1 in the parametrial fat pads of sham-operated control mice were 54-, 83-, and 63-fold higher, respectively, than the levels in the compensatory fat of partially lipectomized animals (Fig. S1B). In a second study where mice fed a 60 kcal very-high-fat diet using a similar protocol as described in Fig. 1, the mRNA levels for MCP1, SerpinE1, and TIMP1 in the parametrial fat pads of sham-operated control mice were 29-, 43-, and 23-fold higher, respectively, than the levels in the compensatory fat of partially lipectomized animals.
Measurement of Adipokines in the Epidermis, Tumors, and Serum During a Carcinogenesis Study.
An antibody array was used to measure the levels of 38 adipokines in normal epidermis without UVB exposure as well as in the epidermis of nontumor-bearing animals and in squamous cell carcinomas from mice fed a low-fat chow diet and treated with UVB for 33 wk (samples from Fig. 1B). Only a small number of adipokines was observed in normal epidermis (no UVB treatment) from low-fat chow-fed mice (Fig. S2A). They were adiponectin, DPPIV, fetuin A, ICAM1, lipocalin-2 and RBP4, and other adipokines were either not present or were only present in small amounts. An increased number and level of adipokines were observed in the epidermis of nontumor-bearing or tumor-bearing animals and in tumors of mice treated with UVB for 33 wk. Among 38 adipokines examined, substantial amounts of three adipokines were present in tumors but not in the normal epidermis. They were: MCP1, SerpinE1, and TIMP1. In addition to these three adipokines, many other cytokines were increased in the epidermis of nontumor-bearing animals treated with UVB for 33 wk and in tumors compared with the normal epidermis. Compared with the epidermis, serum had more and higher levels of adipokines (Fig. S2 A vs. B). The serum from tumor-bearing mice had higher levels of adipokines than normal serum or serum from nontumor-bearing mice treated with UVB for 33 wk. TIMP1 was especially prominent in tumors and in the serum from tumor-bearing animals (Fig. S2 A and B). Western blot analysis confirmed that tumors had higher levels of MCP1, SerpinE1, and TIMP1 than the normal epidermis (Fig. S2C).
Surgical Removal of the Parametrial Fat Pads Decreases TIMP1 and Other Adipokines in the Epidermis and in the Serum of Mice Fed High-Fat Diets.
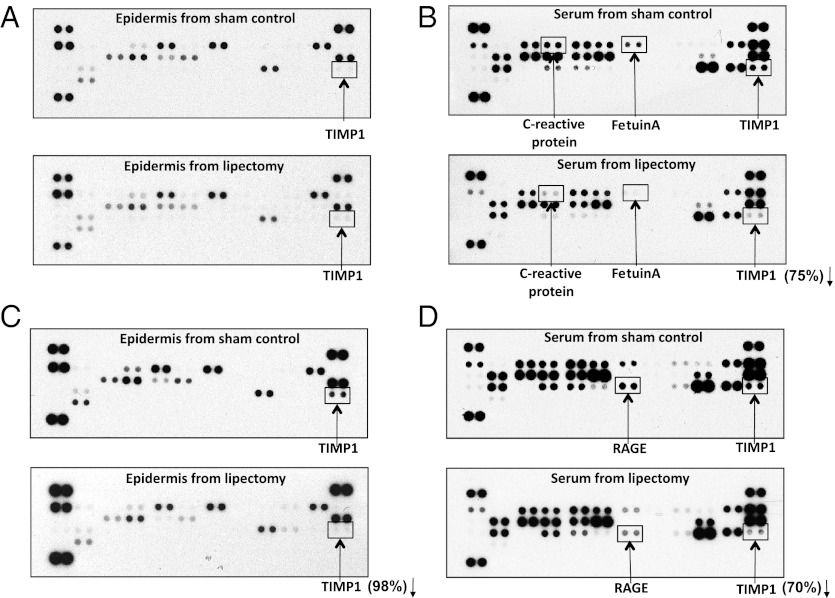
Because removal of the parametrial fat pads inhibited UVB-induced carcinogenesis in mice fed a high-fat diet, we evaluated the effects of removing the parametrial fat pads on the profile of adipokines in the epidermis and serum of mice fed a 40%-kcal or a 60%-kcal high-fat diet for 2 wk. Feeding SKH-1 mice either a 40%-kcal high-fat diet or a 60%-kcal very-high-fat diet for 2 wk resulted in a modest increase in adipokines in the epidermis (compare normal epidermis in Fig. S2A with Fig. 3 A and C) and large increases of adipokines in the serum compared with mice fed the low-fat chow diet (compare normal serum in Fig. S2B with Fig. 3 B and D). Although removal of the parametrial fat pads had a small inhibitory effect on the epidermal levels of adipokines in mice fed a 40%-kcal high-fat diet (Fig. 3A), densitometry analysis indicated that partial lipectomy decreased the serum levels of TIMP1 by 75%, and C-reactive protein and fetuin A were decreased by 78 and 79%, respectively, compared with the sham-operated control mice (Fig. 3B). Switching mice from a 40%-kcal high-fat diet to a 60%-kcal very-high-fat diet substantially increased the levels of several adipokines, especially TIMP1 in the epidermis (Fig. 3 A vs. C), and TIMP1 and RAGE (receptor for advanced glycation end products) in the serum (Fig. 3 B vs. D). Removal of the parametrial fat pads from mice fed a 60%-kcal very-high-fat diet resulted in a 98% decrease in epidermal TIMP1 (Fig. 3C), a 70% decrease in the serum levels of TIMP1, and a 59% decrease in the serum level of RAGE (Fig. 3D).
Fig. 3.
Surgical removal of the parametrial fat pads decreases high-fat-diet–induced TIMP1 and other adipokines in the epidermis and in the serum. Female SKH-1 mice (10 mice per group) were given either a 40%-kcal high-fat diet or a 60%-kcal very-high-fat diet for 2 wk. Half of mice had their parametrial fat pads removed and the other half of mice was a sham-operated control. Two weeks after the surgery, the mice were killed, and the epidermis and serum were collected, combined separately, and subjected to antibody array for adipokines. Representative arrays are presented. (A) Epidermis from sham-operated control mice and epidermis from lipectomized mice fed a 40%-kcal high-fat diet. (B) Serum from sham-operated control mice and serum from lipectomized mice fed a 40%-kcal high-fat diet. (C) Epidermis from sham-operated control mice and epidermis from lipectomized mice fed a 60%-kcal very-high-fat diet. (D) Serum from sham-operated control mice and serum from lipectomized mice fed a 60%-kcal very-high-fat diet.
Discussion
More than 60 y ago, Baumann and Rusch were the first to demonstrate a stimulatory effect of a high-fat diet on UV-induced skin carcinogenesis (13). Subsequently, Black and his colleagues confirmed the stimulatory effect of a high fat diet on UV-induced formation of skin tumors in mice, and they provided evidence for an association of high-fat diets with actinic keratosis and skin cancer in humans (14–17). Recently, prospective studies indicated that high-fat diets increased the risk of squamous cell carcinomas, and individuals with a high body mass index were at a higher risk of contracting skin cancer than individuals with a low body mass index (18, 19). It is well known that tissue fat is metabolically active. In addition to storing calories, tissue fat secretes a large numbers of adipokines (20). Several studies have shown that obesity is associated with elevated plasma free fatty acid levels (21) and a significant rise in proinflammatory adipokines/cytokines, which may play a tumor-promoting role in the development of cancer (22–26). Iyengar et al. suggested that adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of antiapoptotic transcriptional programs and protooncogene stabilization (27). However, the molecular mechanisms underlying the effect of excess adipose tissue from a high-fat diet on UV-induced carcinogenesis have not been explored. Therefore, identification of the source of the production and secretion of proinflammatory adipokines in adipose tissue may provide a novel mechanistic link between specific local tissue fat and associated tumor formation. In addition, although there are several reports indicating that drug administration, low-fat diets, calorie restriction, or physical exercise are recommended and used widely for treating obesity, relevant models for lowering the formation and release of adipokines with possible tumor-promoting activities by surgical removal of specific tissue fat leading to alterations of skin cancer formation have not been reported. Our present study showed that mice with partial lipectomy had inhibition of UVB-induced skin tumorigenesis compared with their sham-operated controls. These results suggest that the parametrial fat pads have an important role for UVB-induced skin cancer formation, and surgical removal of these fat pads provides a unique approach for studying the direct effect of a decrease in tissue fat on UVB-induced skin tumor formation. This research opens a unique approach for studying how obesity contributes to cancer formation that will help determine molecular biomarkers of risk from tissue fat that can be used for designing future mechanistic studies. We expect that the possible utility of reducing tissue fat by partial lipectomy is not only cancer preventive for skin cancer, but may also be preventive or therapeutic for other cancers. It will be of interest to determine whether partial lipectomy inhibits the formation and growth of more lethal obesity-associated cancers such as cancers of the colon, prostate, and pancreas.
Our mechanistic studies with immunohistochemical methodology in the present paper indicated that partial lipectomy decreased UVB-induced skin tumor formation, which was associated with an increase in apoptosis and a decrease in cell proliferation in precancerous areas of the epidermis away from tumors and in tumors as measured by an increase in the percentage of caspase 3 (active form) positive cells and a decrease in the percentage of BrdU+ cells. Our antibody array data indicated that high-fat diets increased the levels of several adipokines, especially TIMP1, and removal of the parametrial fat pads resulted in a dramatic decrease of TIMP1 in the epidermis and in serum (Fig. 3). The serum level of RAGE was also decreased (Fig. 3). TIMP1 is a glycoprotein that is an inhibitor of the matrix metalloproteinases (a group of peptidases involved in degradation of the extracellular matrix). TIMP1 inhibits apoptosis (28–31) and protects human breast epithelial cells against both intrinsic and extrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathways, and thus may be oncogenic (32). Clinical studies using primary breast cancer cells revealed an association between high levels of TIMP1 mRNA or protein and a poor prognosis for patients with breast cancer (33–35). In addition, TIMP1 gene deficiency increased tumor cell sensitivity to chemotherapy-induced apoptosis (36). TIMP1 also has growth-promoting activity and promotes cell proliferation in a wide range of cell types (37, 38). These data imply a significant role for the antiapoptotic effect of TIMP1 in chemoresistance. TIMP1 is an adipocyte-secreted protein that is up-regulated in obesity and promotes adipose tissue development (39). Overexpression of TIMP1 in transgenic mice increased the rate of adipocyte differentiation (40), whereas TIMP1-deficient mice were protected from nutritionally induced obesity (41). TIMP1-deficient mice treated with a high-fat diet had significantly lower body weight and significantly less s.c. fat than wild-type mice, and these differences were much less pronounced for mice on a low-fat diet (41). TIMP1 was also reported to play an important role in the pathogenesis of nonmelanoma skin cancer and is a useful indicator of cutaneous cancer invasion and progression (42). However, the effect of lowering the level of TIMP1 by removal of a specific tissue fat to prevent UVB-induced skin cancer formation has not been reported. Recent studies showed that RAGE is a multifunctional receptor with multiple ligands that is known to play a key role in several diseases including cancer, and overexpression of RAGE is associated with enhanced autophagy, diminished apoptosis, and greater tumor cell viability (43). MCP1 is overexpressed in obese mice compared with their lean controls, and white adipose tissue is a major source of MCP1 (44). MCP1 is a chemokine that regulates the recruitment of monocytes into sites of inflammation and cancer, and neutralization of MCP1 reduces the growth of prostate, breast, and lung cancer in mice. MCP1 was reported to inhibit apoptosis, and treatment with MCP1 led to significant up-regulation of VEGF and MMP-9 and down-regulation of caspase 3 mRNA and protein compared with an untreated control, whereas MCP1 siRNA-mediated knockdown reversed these changes (45). Thus, MCP1 is proposed as a molecular target for cancer treatment (46–48). SerpinE1 plays a crucial role in invasion of malignant cells across the basal lamina, and increased levels are present in a number of cancers as well as in obesity (49). Thus, our results suggest that (i) tissue fat secretes antiapoptotic adipokines that inhibit apoptosis in DNA-damaged precancerous cells and in cancer cells, which may help explain why obese individuals have an increased risk of cancer, and (ii) partial lipectomy lowers the serum levels of TIMP1 and other proinflammatory adipokines, which may help explain why partial lipectomy has an inhibitory effect on UVB-induced skin cancer formation. It should be noted that although the antibody array included 38 adipokines, other important adipokines/inflammatory factors such as IL-18 and IFN-γ that were reported to previously be linked with fat-caused inflammation (50, 51) were not included. We will evaluate these factors in future studies.
Our results indicate that partially lipectomized mice restore their body fat very quickly, and the parametrial fat pads showed no signs of regeneration. Because high levels of adipokines that inhibit apoptosis and enhance inflammation were found in tumors and in the serum of tumor-bearing animals, we measured the levels of adipokines in the epidermis and serum following partial lipectomy. We found that high mRNA for TIMP1, MCP1, and SerpinE1 were present in the parametrial fat pads from sham-operated control mice, whereas the compensatory fat that appeared at a different anatomic location after surgical removal of the parametrial fat pads had a manyfold lower level of mRNA for TIMP1, MCP1, and SerpinE1, demonstrating large molecular differences between different sources of tissue fat. These results led us to hypothesize that high dietary fat caused the parametrial fat pads to secrete high levels of TIMP1 and other proinflammatory molecules that promote UVB-induced skin carcinogenesis. According to this concept, removal of the parametrial fat pads should decrease the levels of TIMP1 and other fat-cell–derived antiapoptotic and/or growth-promoting molecules that result in an inhibition of UVB-induced skin tumor formation. An additional explanation of differences between the parametrial fat pad and the compensatory fat may be related to cellular diversity in parametrial fat pads vs. compensatory fat; for example, macrophage infiltration is associated with obesity-related inflammation (52, 53) and extra cellular matrix turnover by metalloproteinases (54). These macrophages also form proinflammatory feedback loops with preadipocytes and adipocytes to produce MCP1, TNF-α, MIP-1α, etc (53). F4/80 and CD1/b immunostaining can be used to identify the macrophage content of fat (55). It is possible that the parametrial fat contains more activated macrophages compared with the compensatory fat.
Finally, it is important to indicate that the conclusions in this manuscript only apply to an animal model, and the possible effects of cosmetic lipectomy or other lipectomy procedures on cancer risk in humans are unknown.
In summary, our data indicated that surgical removal of the parametrial fat pads in mice fed a high-fat diet inhibited UVB-induced formation of skin tumors, and tumor size was also dramatically reduced. Lipectomy-induced inhibition of carcinogenesis was specifically effective in mice fed a high-fat diet but not in mice fed a low-fat chow diet. Mechanistic studies indicated that removal of the parametrial fat pads enhanced apoptosis and decreased cell proliferation in tumors as well as in precancerous areas of the epidermis away from the tumors. Antibody array data indicated that lipectomy in mice fed a high-fat diet decreased the level of TIMP1 as well as other adipokines. The mRNA level for TIMP1, MCP1, and SerpinE1 in the compensatory fat that appeared after surgery was dramatically decreased compared with the mRNA level for TIMP1, MCP1, and SerpinE1 in parametrial fat pads from sham-operated control mice. This is a unique demonstration that surgical removal of tissue fat can inhibit carcinogenesis in obese mice.
Materials and Methods
All mouse experiments were approved by the institutional animal care and use committee of Rutgers University. Detailed methods for animal treatments, diets, surgical removal of parametrial fat pads, histopathological analysis, and immunohistochemical staining for caspase-3+ and BrdU+ cells, body fat composition by dual-energy X-ray absorptiometry, adipokine antibody array, RT-PCR, and statistical analysis can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Ms. Annette Dionisio for her help in the preparation of the manuscript. The project described here was supported by Grants RO1CA128997 and RO1 CA130857 from the National Cancer Institute (NCI), and in part, by Training Grant ES007148 and Center Grant ES005022 from the National Institute of Environmental Health Sciences (NIEHS).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205810109/-/DCSupplemental.
References
- 1.Kripke ML. Sunlight and skin cancer. Tex Med. 1986;82:52–53. [PubMed] [Google Scholar]
- 2.Stern RS, Weinstein MC, Baker SG. Risk reduction for nonmelanoma skin cancer with childhood sunscreen use. Arch Dermatol. 1986;122:537–545. [PubMed] [Google Scholar]
- 3.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Rogers HW, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y-P, et al. Inhibitory effects of orally administered green tea, black tea, and caffeine on skin carcinogenesis in mice previously treated with ultraviolet B light (high-risk mice): Relationship to decreased tissue fat. Cancer Res. 2001;61:5002–5009. [PubMed] [Google Scholar]
- 6.Lou YR, Lu YP, Xie JG, Huang MT, Conney AH. Effects of oral administration of tea, decaffeinated tea, and caffeine on the formation and growth of tumors in high-risk SKH-1 mice previously treated with ultraviolet B light. Nutr Cancer. 1999;33:146–153. doi: 10.1207/S15327914NC330205. [DOI] [PubMed] [Google Scholar]
- 7.Michna L, Lu YP, Lou YR, Wagner GC, Conney AH. Stimulatory effect of oral administration of green tea and caffeine on locomotor activity in SKH-1 mice. Life Sci. 2003;73:1383–1392. doi: 10.1016/s0024-3205(03)00468-5. [DOI] [PubMed] [Google Scholar]
- 8.Michna L, et al. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 9.Lu YP, et al. Voluntary exercise together with oral caffeine markedly stimulates UVB light-induced apoptosis and decreases tissue fat in SKH-1 mice. Proc Natl Acad Sci USA. 2007;104:12936–12941. doi: 10.1073/pnas.0705839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y-P, et al. 2012 Oral caffeine during voluntary exercise markedly inhibits skin carcinogenesis and decreases cytokines associated with inflammation in UVB-treated mice. 103rd Annual Meeting of the American Association for Cancer Research, Chicago, Abstract 4286. [Google Scholar]
- 11.Lu YP, et al. Stimulatory effect of voluntary exercise or fat removal (partial lipectomy) on apoptosis in the skin of UVB light-irradiated mice. Proc Natl Acad Sci USA. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou YR, et al. Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2011;32:1078–1084. doi: 10.1093/carcin/bgr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumann CA, Rusch HP. Effect of diet on tumors induced by ultraviolet light. Am J Cancer. 1939;35:213–221. [Google Scholar]
- 14.Black HS, Lenger W, Phelps AW, Thornby JI. Influence of dietary lipid upon ultraviolet-light carcinogenesis. Nutr Cancer. 1983;5:59–68. doi: 10.1080/01635588309513780. [DOI] [PubMed] [Google Scholar]
- 15.Black HS. Influence of dietary factors on actinically-induced skin cancer. Mutat Res. 1998;422:185–190. doi: 10.1016/s0027-5107(98)00191-2. [DOI] [PubMed] [Google Scholar]
- 16.Black HS, et al. Effect of a low-fat diet on the incidence of actinic keratosis. N Engl J Med. 1994;330:1272–1275. doi: 10.1056/NEJM199405053301804. [DOI] [PubMed] [Google Scholar]
- 17.Black HS, Lenger WA, Gerguis J, Thornby JI. Relation of antioxidants and level of dietary lipid to epidermal lipid peroxidation and ultraviolet carcinogenesis. Cancer Res. 1985;45:6254–6259. [PubMed] [Google Scholar]
- 18.Ibiebele TI, et al. Dietary pattern in association with squamous cell carcinoma of the skin: A prospective study. Am J Clin Nutr. 2007;85:1401–1408. doi: 10.1093/ajcn/85.5.1401. [DOI] [PubMed] [Google Scholar]
- 19.Ibiebele TI, van der Pols JC, Hughes MC, Marks GC, Green AC. Dietary fat intake and risk of skin cancer: A prospective study in Australian adults. Int J Cancer. 2009;125:1678–1684. doi: 10.1002/ijc.24481. [DOI] [PubMed] [Google Scholar]
- 20.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: A regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, et al. Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:5412–5419. doi: 10.1210/jcem.86.11.8027. [DOI] [PubMed] [Google Scholar]
- 22.Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review) Int J Oncol. 2006;28:737–745. [PubMed] [Google Scholar]
- 23.Berstein LM. Hormones of adipose tissue (adipocytokines): Ontogenetic and oncologic aspects. Adv Gerontol. 2005;16:51–64. [PubMed] [Google Scholar]
- 24.Ogunwobi O, Mutungi G, Beales IL. Leptin stimulates proliferation and inhibits apoptosis in Barrett’s esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology. 2006;147:4505–4516. doi: 10.1210/en.2006-0224. [DOI] [PubMed] [Google Scholar]
- 25.Ogunwobi OO, Beales IL. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept. 2006;134:105–113. doi: 10.1016/j.regpep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Ogunwobi OO, Beales IL. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colorectal Dis. 2007;22:401–409. doi: 10.1007/s00384-006-0181-y. [DOI] [PubMed] [Google Scholar]
- 27.Iyengar P, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 28.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 29.Guedez L, et al. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59:6267–6275. [PubMed] [Google Scholar]
- 31.Murphy F, et al. N-Cadherin cleavage during activated hepatic stellate cell apoptosis is inhibited by tissue inhibitor of metalloproteinase-1. Comp Hepatol. 2004;3(Suppl 1):S8. doi: 10.1186/1476-5926-2-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XW, et al. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: A potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 2005;65:898–906. [PubMed] [Google Scholar]
- 33.Nakopoulou L, et al. Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol. 2002;197:307–313. doi: 10.1002/path.1129. [DOI] [PubMed] [Google Scholar]
- 34.Würtz SO, Schrohl AS, Mouridsen H, Brünner N. TIMP-1 as a tumor marker in breast cancer—an update. Acta Oncol. 2008;47:580–590. doi: 10.1080/02841860802022976. [DOI] [PubMed] [Google Scholar]
- 35.Wu ZS, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008;122:2050–2056. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 36.Davidsen ML, et al. TIMP-1 gene deficiency increases tumour cell sensitivity to chemotherapy-induced apoptosis. Br J Cancer. 2006;95:1114–1120. doi: 10.1038/sj.bjc.6603378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 38.Luparello C, Avanzato G, Carella C, Pucci-Minafra I. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res Treat. 1999;54:235–244. doi: 10.1023/a:1006121129382. [DOI] [PubMed] [Google Scholar]
- 39.Chavey C, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 40.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lijnen HR, Demeulemeester D, Van Hoef B, Collen D, Maquoi E. Deficiency of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) impairs nutritionally induced obesity in mice. Thromb Haemost. 2003;89:249–255. [PubMed] [Google Scholar]
- 42.O’Grady A, et al. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: Implications for tumour progression. Histopathology. 2007;51:793–804. doi: 10.1111/j.1365-2559.2007.02885.x. [DOI] [PubMed] [Google Scholar]
- 43.Kang R, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi CL, et al. Monocyte chemoattractant protein-1 modulates invasion and apoptosis of PC-3M prostate cancer cells via regulating expression of VEGF, MMP9 and caspase-3. Asian Pac J Cancer Prev. 2011;12:555–559. [PubMed] [Google Scholar]
- 46.Fujimoto H, et al. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–1284. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 47.Cai Z, et al. Monocyte chemotactic protein 1 promotes lung cancer-induced bone resorptive lesions in vivo. Neoplasia. 2009;11:228–236. doi: 10.1593/neo.81282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Y, et al. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metastasis. 2009;26:161–169. doi: 10.1007/s10585-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 49.Gao S, et al. Epigenetic alterations of the SERPINE1 gene in oral squamous cell carcinomas and normal oral mucosa. Genes Chromosomes Cancer. 2010;49:526–538. doi: 10.1002/gcc.20762. [DOI] [PubMed] [Google Scholar]
- 50.Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun. 2005;337:422–429. doi: 10.1016/j.bbrc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 51.Rocha VZ, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- 55.de Meijer VE, Sverdlov DY, Le HD, Popov Y, Puder M. Tissue-specific differences in inflammatory infiltrate and matrix metalloproteinase expression in adipose tissue and liver of mice with diet-induced obesity. Hepatol Res. 2012 doi: 10.1111/j.1872-034X.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 56.Reddy BS, Patlolla JM, Simi B, Wang SH, Rao CV. Prevention of colon cancer by low doses of celecoxib, a cyclooxygenase inhibitor, administered in diet rich in omega-3 polyunsaturated fatty acids. Cancer Res. 2005;65:8022–8027. doi: 10.1158/0008-5472.CAN-05-0212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.