Abstract
Social status can have striking effects on health in humans and other animals, but the causes often are unknown. In male vertebrates, status-related differences in health may be influenced by correlates of male social status that suppress immune responses. Immunosuppressive correlates of low social status may include chronic social stress, poor physical condition, and old age; the immunosuppressive correlates of high status may include high testosterone and energetic costs of reproduction. Here we test whether these correlates could create status-related differences in immune function by measuring the incidence of illness and injury and then examining healing rates in a 27-y data set of natural injuries and illnesses in wild baboon males. We found no evidence that the high testosterone and intense reproductive effort associated with high rank suppress immune responses. Instead, high-ranking males were less likely to become ill, and they recovered more quickly than low-ranking males, even controlling for differences in age. Notably, alpha males, who experience high glucocorticoids, as well as the highest testosterone and reproductive effort, healed significantly faster than other males, even other high-ranking males. We discuss why alpha males seem to escape from the immunosuppressive costs of glucocorticoids but low-ranking males do not, including the idea that glucocorticoids' effects depend on an individual's physiological and social context.
Keywords: dominance rank, glucocorticoid hormones, Papio cynocephalus, Savanna baboon, ecoimmunology
During the last few decades there has been major scientific interest in how social hierarchies influence individual health (1–3). In humans, for instance, socioeconomic status predicts differences in disease risk and longevity that cannot be explained entirely by differences in education or access to resources (3, 4). Similar patterns occur in animal hierarchies (3, 5). Understanding the relationships between social status and health is important because it has implications for human well-being (3), individual disease risk (6, 7), the evolution of social behavior (8), and the spread of infectious disease (9, 10).
However, despite the importance of social status, there is currently no consensus on what factors cause status-related differences in health. One leading hypothesis proposes that differences in social status predict differences in exposure to chronic stress, which has negative impacts on health (3, 11). Specifically, members of some social ranks may be more likely to experience negative, unpredictable events or are less able to cope with these events, which in turn activate glucocorticoids, a major component of the stress response. Although the stress response has many short-term benefits, long-term exposure to glucocorticoids can compromise metabolic, cardiovascular, and immune function (3, 11). In humans, chronic stress is thought to be higher in individuals with low socioeconomic status (1, 12); in other animals the relationships between stress and social status vary across species and populations (13, 14).
In male nonhuman vertebrates, other factors besides chronic social stress may cause status-related differences in health. For instance, in societies where male reproductive success is determined by dominance rank, the energetic costs of achieving and maintaining high rank, as well as the energetic costs of reproductive effort (e.g., muscle anabolism, male–male competition, mate guarding, parental care), which are likely to be higher for high-ranking males, may limit the energy available for immune responses (15–17). Indeed, several studies of vertebrates have documented a major life history tradeoff between male reproductive effort and health (18–20). This tradeoff is thought to be mediated by testosterone and sometimes by glucocorticoids, which help direct energetic resources toward reproduction and away from tasks associated with long-term survival, such as immune function (15, 17, 21–23). In support of this notion, experimental increases in testosterone, within normal physiological limits, suppress immune function and increase parasite loads in several species (24, 25). In addition, some studies in wild vertebrates have found that high-ranking males have high testosterone and/or glucocorticoids and poor health (18, 21, 26, 27). Hence, in addition to the social stress associated with low rank, differences in reproductive effort also may cause status-related differences in health.
However, despite experimental evidence that testosterone or glucocorticoids can suppress immune function (25, 28, 29), attempts to understand the relative roles of these hormones in natural populations are rare. Such studies are complicated by the fact that testosterone and glucocorticoids often are correlated also with differences in age and physical condition. All four of these correlates may influence status-related differences in health (5), and the causal connections between these correlates and immune responses are complex and difficult to disentangle. The complexity of these relationships may partly explain why, when researchers evaluate the effects of hormones on immune function in the wild, they often find conflicting results (15, 23, 30). For instance, in many wild vertebrates, researchers find either no relationship or a positive relationship between testosterone and immune function (22, 30–32). Relationships between stress and immune function also are complex (33, 34); short-term stress can enhance immune responses (34), and researchers do not always find evidence for the immunosuppressive effects of glucocorticoids (6, 22). These results are important because they support the idea that there may be considerable flexibility in the relationships between testosterone, glucocorticoids, and immune function, and plasticity in these connections may buffer individuals against the potentially adverse effects of glucocorticoids or testosterone (22, 23, 35).
Here we investigate several mechanisms that could explain rank-related differences in immune function. We do so using healing rates from a 27-y data set of naturally occurring injuries and illnesses in wild baboons living in the Amboseli ecosystem, Kenya. Wound healing is a common and powerful measure of immune function in captive animals, and it provides an integrative measure of immune function that combines the inflammatory and cell-mediated processes of innate immunity (28, 29, 36, 37). It can be difficult to study wound healing in natural populations, especially in wild primates where researchers are usually constrained to noninvasive methods. However, healing rates have important functional significance for wild animals; if animals fail to recover efficiently from injuries and illnesses, they are likely to experience negative consequences, such as poor foraging efficiency, higher predation risk, and problems acquiring mates and raising offspring (38, 39).
Male baboons are a good system for understanding the relationships between dominance rank and immune function because high male rank usually leads to high reproductive success (40, 41) and because we already know a good deal about the behavioral and physiological correlates of dominance rank in baboons (41–45). Baboons live in multimale, multifemale social groups characterized by linear dominance hierarchies. Male baboons disperse after maturity, and when they join a new group they often rise rapidly in rank. Once they obtain high rank, males experience several costs that could limit immune function. For instance, high-ranking males are more likely to engage in energetically expensive mate-guarding episodes (45, 46). These mate-guarding episodes, called “consortships,” may last from a few hours to a few days, during which time male foraging is compromised; indeed, consorting males have feeding bouts that are 10–15% shorter than those of nonconsorting males, and it is not uncommon for males to abandon consortships (46, 47). In addition, in Amboseli, high-ranking males have higher fecal testosterone levels than low-ranking males (45), both when the dominance hierarchy is unstable and when it is stable (45). Furthermore, alpha males (the highest-ranked male in each group) experience higher costs than other high-ranking (but nonalpha) males, including high testosterone, higher rates of conflict than beta males, and the most time spent in consortships (45). Finally, unlike other high-ranking males, alpha males experience high glucocorticoid levels—levels that are significantly higher than in beta males (45). Together, these correlates of high rank, especially alpha status, may suppress immune function. In support, previous research has found that high-ranking male baboons have higher parasite loads, and one highly aggressive alpha male had a low white blood cell count (48, 49).
However, although the costs of high rank may suppress immune function, there are several reasons to expect that correlates of low rank, such as old age, poor physical condition, and chronic stress, also are important in shaping male health and immune function. For instance, in baboons, dominance rank declines with age (40), and senescence alone might lead to poor immune function in low-ranking males (50). Body mass index also declines with age (43), and this effect, combined with the fact that low-ranking males are more likely than high-ranking males to be interrupted while feeding (51), might limit the energy low-ranking males can devote to immune function. Finally, glucocorticoid levels are higher in low-ranking males than in high-ranking males (other than the alpha male) (42, 45). Hence, if chronic stress has stronger negative effects on immune function than testosterone and reproductive effort, then low-ranking males should have poorer immune function than high-ranking males (with the possible exception of the alpha male). In support, prior studies found that low-ranking male baboons have a dysregulated stress response that often co-occurs with immunosuppression (42, 52), as well as suppressed insulin-like growth factor, a hormone that promotes cellular growth and wound healing (53).
Our objectives were to understand how rank and age predicted the incidence of injury and illness and to understand how rank-related differences in age and hormone levels predicted males’ recovery from injuries and illnesses. Our study was conducted in the Amboseli ecosystem, Kenya, where the Amboseli Baboon Research Project (ABRP) has conducted long-term, individual-based research on wild baboons since 1971. To maintain the animals’ habituation to human observers, the ABRP relies almost entirely on noninvasive observations; hence all records of injuries and illnesses were collected noninvasively by observing the animals at a distance of ∼3–5 m. If low social status suppresses immune responses, we expected that low-ranking males would face a greater incidence of illness than high-ranking males. We further expected that high-ranking males would face a higher incidence of injuries than low-ranking males, as has been reported in another wild baboon population (54). After analyzing incidence, we used univariate and multivariate approaches to understand how rank, age, and several other factors predicted male recovery from injuries and illnesses. Specifically, we tested whether high social status is associated with slower healing, as predicted by theories focused on the potentially immunosuppressive correlates of high rank, or whether high status instead is associated with more rapid healing, as predicted by theories focused on the potentially immunosuppressive correlates of low rank. Our results help clarify which mechanisms are important in creating rank-related differences in health in male nonhuman primates.
Results
From 1982 through 2009, we observed 633 cases of naturally occurring injuries and illnesses in 166 adult male baboons living in groups with 1–17 adult males (8–47 adults of both sexes). Injuries included linear cuts or slashes, puncture wounds, contusions, scrapes, limps, and one eye injury. Illnesses were characterized by digestive problems, persistent respiratory problems, or signs of weakness, lethargy, and cachexia. Observations of injuries were much more frequent than observations of illnesses. Indeed, males were more than 12 times as likely to be injured (0.88 observations per male per year) as they were to become visibly ill (0.07 cases per male per year).
Rank, Age, and the Incidence of Illness and Injury.
If the costs of high rank suppress immune function, high-ranking males might experience a higher incidence of illness than low-ranking males. However, we found no support for this hypothesis. Instead, both rank- and age-specific incidence of illness were highest for older, low-ranking males (Fig. S1). Specifically, the incidence of illness increased in older males and lower-ranking males, and the relationship between age and the incidence of illness was stronger than the relationship with rank (age and illness: r2 = 0.64, F = 20.59, P = 0.0011; rank and illness: r2 = 0.25, F = 5.38, P = 0.0388). Age and rank also influenced the incidence of injury. Age- and rank-specific incidences of injury were best fit by a second-order polynomial so that the highest incidence of injuries occurred in middle-aged, midranking males (Fig. S2; rank-specific incidence: r2 = 0.52, F = 8.18, P = 0.0067; age-specific incidence r2 = 0.64, F = 10.10, P = 0.0038).
Patterns of Healing.
To inform the construction of multivariate models of healing rates, we performed a series of univariate survival analyses to understand how single variables predicted male recovery from injuries and illnesses. We assessed healing rates for 448 injuries and illnesses in 144 adult males (Table S1). Males usually required a few weeks to recover from illnesses and injuries; the median time to heal was 25 d, although the fastest-healing 25% of injuries and illnesses healed in less than 14 d, and the slowest-healing 25% of injuries and illnesses required more than 40 d to heal. These recovery rates were similar to those in a prior study on wound healing in wild baboons (55).
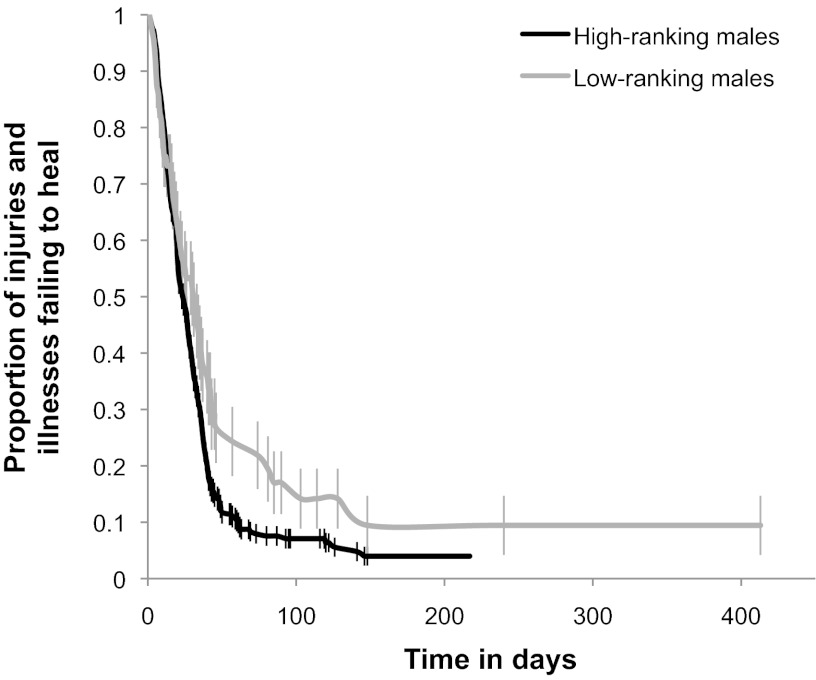
We tested seven univariate predictors of healing rates, including (i) illness and injury type; (ii) differences in methods of data collection before and after 1991; (iii) the male’s dominance rank at the time he became injured or ill; (iv) the male’s age at the time he became injured or ill; (v) the male’s access to human refuse as a food source (56, 57); (vi) the size of the male’s social group, as measured by the number of adult male and female members; and (vii) the season (wet or dry) at the time the male became injured or ill. The only significant univariate predictors of healing were injury and illness type [χ2 = 26.08, degrees of freedom (DF) = 8, P = 0.0010), study period (median days to heal during and before 1991 = 17 d; median days to heal after 1991 = 30 d; χ2 = 26.3429, DF = 1, P < 0.0001], and dominance rank (χ2 = 4.37, P = 0.0365). Fig. 1 shows a survival plot comparing healing rates of high-ranking males (ranks 1–8) and low-ranking males (ranks lower than 8). High-ranking males required a median of 25 d to heal, whereas low-ranking males required a median of 31 d to heal, ∼25% longer than high-ranking males.
Fig. 1.
Survival curves depicting the proportion of injuries and illnesses in adult male baboons failing to heal as a function of time in days. The black line indicates injuries and illnesses observed in high-ranking males (ranks 1–8; n = 380), the gray line indicates injuries and illnesses in low-ranking males (ranks lower than 8; n = 68). Error bars indicate SE. High-ranking males healed significantly more quickly than low-ranking males (log-rank test: χ2 = 4.37, P = 0.0365).
Multivariate Survival Analyses of Healing Rates.
We constructed proportional hazards models to examine whether dominance rank, age, group size, season, or feeding regime significantly predicted variation in healing rates among males, while controlling for differences in study period and the type of illness or injury. Again, we found no evidence that the costs of high rank slow the healing process. Instead, healing rates were slower for low-ranking males (Table 1). Indeed, at any given point in time, the top-ranked males were three times more likely than the lowest-ranked males to recover from an injury or illness. Because the immune processes involved in recovering from infectious disease differ from those involved in wound healing, we repeated this analysis using only the 423 injuries in our data. Here again, high-ranking males recovered from injuries more quickly than low-ranking males (Table S2).
Table 1.
The best-supported proportional hazards model of male healing rates as determined by likelihood ratio tests
| Source of variation | Hazard ratio | DF | χ2 | P |
| Time period (pre- or post-1991) | 1.80 | 1 | 25.08 | < 0.0001 |
| Injury or illness type | — | 8 | 17.29 | 0.0444 |
| Dominance rank | 3.07 | 1 | 14.44 | 0.0001 |
| Group size | 2.28 | 1 | 8.47 | 0.0036 |
n = 448; whole model χ2 = 59.45; DF = 11, P < 0.0001, log likelihood = 1854.311.
In male baboons, age and rank are correlated; therefore slower recovery rates in low-ranking males could be explained by natural, age-related declines in immune function (50). However, we found that rank was a stronger predictor of healing rates than age. Specifically, although healing rates declined significantly with age in a model without rank (Table S3), when we included both rank and age in the model, rank absorbed most of the variance explained by age, and age no longer was a significant predictor of healing rates (Table S4).
Our best model of male recovery rates (Table 1) indicated an effect of group size, such that males in large groups recovered more rapidly than males in small groups. The effect of dominance rank on healing rates was still significant after group size was removed from the model (χ2 = 8.16, P = 0.0043) (Table S5), but the reverse was not true; group size did not significantly predict healing rates in the absence of dominance rank (χ2 = 2.42, P = 0.1196) (Table S6). Consequently, we added an interaction term (dominance rank × group size) to the model. The interaction term was significant (χ2 = 4.81, P = 0.0283) (Table S7); High-ranking males experienced the same healing rates, regardless of group size, but low-ranking males healed faster in larger groups. However, the sample size of injuries and illnesses observed in low-ranking males in small groups was small (12 injuries and illnesses occurred in males occupying ranks lower than 8 in groups with fewer than 30 adults), and more work is needed to understand the effects of group size on healing rates.
Alpha Males Compared with Other High- and Low-Ranking Males.
Among high-ranking males, alpha males arguably experience the highest energetic and endocrine costs of high rank. Glucocorticoid levels are significantly higher in alpha males than in beta (second-ranked) males and instead resemble those of low-ranking males (males occupying ranks lower than 8, in groups with 9–17 adult males) (45). Moreover, alpha males spend the most time in energetically costly consortships, compared with beta males and other high-ranking males, and also should experience high costs of aggressive conflict (45). Given these potential costs of alpha status, we tested whether alpha males experienced suppressed immune function relative to beta males, all other high-ranking males (ranks 2–8), and low-ranking males (ranks lower than 8). We found no evidence for this hypothesis; although there were no significant differences in healing rates between alpha and beta males, alpha males healed significantly faster than all other high-ranking males and than low-ranking males (Table 2). This result indicates that the correlates of alpha status—high testosterone, high reproductive effort, and high glucocorticoids—did not meaningfully suppress immune function in alpha males and that alpha males, in fact, may have had enhanced immune function relative to other males.
Table 2.
Proportional hazards models of healing rates for three comparisons of male rank
| Source of variation | Hazard ratio | DF | χ2 | P |
| Alpha males vs. beta males (n = 100; whole model χ2 = 13.74; P = 0.0560) | ||||
| Time period (pre- or post-1991) | 1.49 | 1 | 2.65 | 0.1035 |
| Injury or illness type | 4 | 8.45 | 0.0764 | |
| Group size (number of adults) | 1.46 | 1 | 0.62 | 0.4318 |
| Alpha males vs. beta males | 1.15 | 1 | 0.4 | 0.5294 |
| Alpha males vs. other high-ranking males (n = 380; whole model χ2 = 66.75; P < 0.0001) | ||||
| Time period (pre- or post-1991) | 1.93 | 1 | 26.02 | < 0.0001 |
| Injury or illness type | — | 8 | 29.05 | 0.0003 |
| Group size (number of adults) | 2.3 | 1 | 7.59 | 0.0059 |
| Alpha males vs. other high-ranking males | 1.55 | 1 | 6.65 | 0.0099 |
| Alpha males vs. low-ranking males (n = 120; whole model χ2 = 20.56; P = 0.0244) | ||||
| Time period (pre- or post-1991) | 1.55 | 1 | 3.49 | 0.0617 |
| Injury or illness type | — | 7 | 5.09 | 0.6487 |
| Group size (number of adults) | 2.76 | 1 | 3.76 | 0.0525 |
| Alpha males vs. low ranking males | 2.49 | 1 | 11.34 | 0.0008 |
Alpha males, rank 1; beta males, rank 2; other high-ranking males, ranks 2–8; low-ranking males, ranks lower than 8.
Discussion
Social status can have striking effects on mortality and disease risk, and discovering which factors influence status-related differences in health is of major scientific interest (1, 12, 58). In male vertebrates, several factors could explain status-related differences in health and immune function, including differences in physical condition, stress, reproductive effort, testosterone, and age (5). Although there is strong evidence to support each of these factors individually (3, 5, 15, 50, 59), their relative importance in wild populations is not well understood. We found no evidence that immunosuppressive correlates of high rank in male baboons, such as high reproductive effort and testosterone (43, 45, 60), increased the incidence of illness or slowed recovery from illnesses or injuries. Instead, high-ranking male baboons were less likely to exhibit signs of illness and recovered more rapidly from injuries and illnesses compared with low-ranking males. Moreover, alpha males, who experience the highest testosterone and energetic costs, as well as high glucocorticoids (45), healed more quickly than other high-ranking and low-ranking males. These results support the idea that there is evolutionary flexibility in the relationships between hormones and important traits such as immune function or reproduction (22, 23, 35). Moreover, they help clarify how social status influences health in nonhuman primates: Correlates of low-rank, such as age, chronic stress, and poor physical condition, are associated with higher disease risk and slow healing, whereas the elevated testosterone and intense reproductive effort linked to high rank are associated with lower disease risk and rapid recovery.
These results are somewhat surprising in the context of other studies on status-related differences in health in male vertebrates, including research on baboons in Amboseli (48, 49). In several species, testosterone has been proposed to mediate the tradeoff between reproductive effort, survival, and immune function (17, 61). Indeed there is strong evidence from laboratory-based studies that testosterone suppresses aspects of immune function, including wound healing (62). Wild studies also support the immunosuppressive effects of testosterone, but the results vary across species, populations, and measures of immune function (15, 23, 30). One explanation for these mixed results is evolutionary flexibility in the strength of the connection between testosterone and immune function (23). Relevant to baboons, differences in life histories, especially in the pace of life, might predict variation in the tradeoff between reproductive effort, testosterone, and immune function (16, 63–65). Indeed, the strongest evidence for this tradeoff comes from short-lived, seasonally breeding species of birds, reptiles, and rodents (21, 66). Short-lived species are expected to favor reproduction over survival; hence, they might forgo immune defense in the face of increased testosterone and reproductive effort. In contrast, long-lived, nonseasonally breeding species might favor survival and immune function over reproductive effort and might not experience strong immunosuppressive effects of testosterone. However, research on the relationships between reproductive effort, testosterone, and immune responses in long-lived, year-round breeding species is rare, except in humans, for which a few studies find support for the immunosuppressive effects of testosterone (20), but others do not (67, 68). More work is needed on a range of species to understand the relationships between the pace of life histories and the immunosuppressive effects of testosterone.
Our results also differ from some prior studies on status-related patterns of parasitism in wild, nonseasonally breeding primates. For instance, early work in Amboseli found that high-ranking male baboons have higher burdens of nematode parasites than low-ranking males (48). In addition, in chimpanzees, high-ranking males have higher fecal testosterone levels than low-ranking males, and male chimpanzees with high testosterone and high glucocorticoids were infected with a richer community of intestinal parasites than were with males with low levels of both of these hormones (15, 60, 69, 70). Although these studies support the hypothesis that high testosterone and reproductive effort suppress immune function, there are at least two reasons why our results might differ from these prior studies. First, because patterns of parasite infection are influenced by both susceptibility and exposure to infectious agents, parasite burdens do not necessarily reflect differences in immune function and instead might reflect differences in parasite exposure.
Second, our results might differ from prior studies on social rank and parasitism because the tradeoffs between reproductive effort and immune function and the effects of hormones on immune responses might be complex. For instance, testosterone might suppress some types of immune response and not others, and high-ranking male baboons might be selected to prioritize wound healing over defense against gastrointestinal parasites. Wound healing involves cell-mediated innate immunity and inflammatory processes, whereas defense against intestinal parasites relies largely on the helper T cell type 2 component of adaptive immunity; both these immune processes are energetically costly (62, 71, 72). Consequently, males might not maintain both responses at a high level. If the evolutionary costs of poor wound healing are worse for males than the costs of poor macroparasite defense, males might maintain wound healing in the face of high testosterone despite its costs. That said, research on how different arms of the immune system vary with male life histories and hormones is in its infancy (73), and more work is needed to understand the effects of testosterone on different arms of immune function.
Instead of implicating high rank as a costly social status, our results support the hypothesis that correlates of low rank lead to reduced immune function in low-ranking males (3, 11). Immunosuppressive correlates of low rank include senescence, poor physical condition, and elevated glucocorticoids (42, 43, 45). All these correlates of low rank, and interactions between them, may explain why we observed slower recovery rates in low-ranking males. Low-ranking males tend to be older (40); aging has important negative effects on both physical condition and immune function, and these effects might slow wound healing (50). Although rank was a stronger predictor of immune responses than age, males may senesce at different rates, and dominance rank may be a strong predictor of physical (as opposed to chronological) aging. Low-ranking male baboons also exhibit evidence of chronic stress, characterized by elevated glucocorticoids and dexamethasone resistance (42, 45). Such chronic stress seems to have particularly strong effects on wound healing; for instance, experimental stressors caused by negative social interactions or restraint have been shown to slow the process of wound healing in mice and humans (28, 29, 74). Finally, aging can worsen the negative effects of the stress response (75, 76); hence, old age might exacerbate the effects of stress in low-ranking male baboons.
Although our results support the hypothesis that glucocorticoids suppress immune function in low-ranking males, we also found evidence for flexibility in the relationships between glucocorticoids and immune function in baboons. In Amboseli, alpha males experience high glucocorticoid levels (45), but they recover from injuries and illnesses significantly faster than other males, even faster than other high-ranking males, who do not experience elevated glucocorticoids. These results echo studies that find that under some conditions males can escape from the adverse consequences of glucocorticoids on reproduction (35). At least three factors might contribute to the finding that glucocorticoids do not suppress wound healing in alpha males. First, alpha males’ relative youth might mitigate the costs of glucocorticoids (75). Second, alpha and low-ranking males seem to experience elevated glucocorticoids as a result of different stressors and over different periods of time; such differences may alter the immunosuppressive effects of stress. Specifically, high glucocorticoids in alpha males probably are caused primarily by energetic stress, whereas high glucocorticoids in low-ranking males probably are caused largely by social stressors (e.g., high rates of received aggression, a lack of a sense of control, and few coping mechanisms) (45). Different types of stressors can have different effects on immune response; for instance, chronic stress can suppress immune function, whereas acute stress can enhance immune responses (34). Hence, the stress response in alpha males might allow them to respond to the energetic demands of alpha status and might even promote wound healing. In addition, males might not hold alpha status long enough to experience the negative effects of chronic stress. The median number of contiguous months that males hold alpha status in Amboseli is 4 mo (range 1–56 mo), and the median total number of months at alpha status is 9 mo; this amount of time might not be long enough for males to experience chronic and dysregulated stress. Third, alpha males may experience higher levels of social support, especially grooming, than low-ranking males, and it is possible that hormonal correlates of social support (i.e., oxytocin) or the hygienic benefits of grooming may mitigate the immunosuppressive effects of glucocorticoids on wound healing (36). In support of this suggestion, alpha males spend more time than low-ranking males in consortships with females, and grooming is frequent among consort partners (45, 77). In addition, adult female baboons often form close social relationships with adult males (77, 78), and top-ranked males may experience stronger friendships than low-ranking males (79).
In addition to the effects of male rank on wound healing, we also found that low-ranking males in larger groups seemed to recover from injuries and illnesses more quickly than those in small groups. We do not yet understand why group size influences healing biology so that low-status males in larger groups heal more rapidly. These males may experience benefits relative to those in small groups that we have not yet identified, such as lower nutritional stress, but a full explanation awaits a study of multiple aspects of male life in groups of different sizes.
Finally, although our results suggest that the stress, age, and poor physical condition associated with low rank influence rank-related differences in immune function, we cannot attribute causality to these correlations. Hence, it is not clear whether (i) low dominance rank causes poor immune function because low rank leads to stress and poor physical condition or (ii) age and declining health cause both poor immune function and poor competitive ability and hence low dominance rank. Because the maintenance of high rank seems to be energetically costly and to depend on physical strength and condition, males who are not in peak physical condition are unlikely to be able to maintain high rank. In support, in our population, severe injury or illness sometimes precedes a decline in rank. That said, these two hypotheses—that health drives rank, or that rank drives health—are not mutually exclusive, and it is likely that both forces interact to shape differences in health and immune function in male baboons. In the future, longitudinal measures of health and dominance rank in males may be useful to elucidate causality by helping to reveal whether changes in physical condition or immune function precede or follow changes in rank.
Materials and Methods
A full description of the materials and methods is given in SI Materials and Methods. Briefly, study subjects were wild, male baboons studied by the ABRP, Kenya. The ABRP has collected observations of the incidence and healing of naturally occurring injuries and illnesses in all study animals since 1982. Observations were not clinical diagnoses and were collected noninvasively by observing the animals at a distance of 3–5 m. Before 1991, healing was monitored every few days until the injury or illness healed. After 1991, injuries and illnesses were monitored less frequently (average time between observations = 14 d).
In addition to monitoring injuries and illnesses, observers collected a variety of life history and behavioral information that might influence healing, including (i) dominance ranks (80, 81); (ii) births, which were used to calculate age for 48% of the males in our study [for the remaining 52% of males, age was estimated to within 1 or 2 y using well-defined metrics (82)]; (iii) group membership, used to calculate group size or determine whether the male became injured or became ill in a wild-feeding group or in the group that foraged part-time at the refuse pit of a nearby tourist lodge; and (iv) the season (wet or dry) during which the male became injured.
To understand how male rank and age predicted the incidence of injury and illness, we calculated age and rank-specific incidences of injury and illness as the rate of injury or illness per male-year. To investigate predictors of healing, we first performed univariate survival analyses to test whether study period (before or after 1991), injury and illness type (Table S1), or any of the predictor variables listed above (i.e., dominance rank, age, group size, group type, and season) predicted significant differences in healing rates. We then used multivariate proportional hazards models to test whether any of our predictor variables significantly predicted healing rates, controlling for differences in study period or injury and illness type. Finally, we tested whether observer bias or differences in injury severity could explain differences in healing across males. We found no evidence that these biases could explain our results (see SI Materials and Methods for full details). All statistical tests were performed using JMP software (version 9.0.2). Best-fitting models were identified via likelihood ratio tests.
All protocols were noninvasive and adhered to the laws and guidelines of Kenya (Kenya Research Permit nos. NCST RRI/12/1/SS011/1543 to E.A.A. and NCST 5/002/R/777 to S.C.A. and NCST 5/002/R/776 to J.A.). All protocols were approved by the Animal Care and Use Committees at the University of Notre Dame (13-030), Duke University (A0840903), and Princeton University (1689).
Supplementary Material
Acknowledgments
We thank the Chicago Zoological Society; the L. S. B. Leakey Foundation; the Max Planck Institute for Demography; the National Geographic Society; the Office of the President of the Republic of Kenya; the Kenya Wildlife Service and its Amboseli staff and wardens; and members of the Amboseli-Longido pastoralist communities for their cooperation and assistance in Kenya. We thank the Amboseli Baboon Project long-term field team (R. S. Mututua, S. Sayialel, and J. K. Warutere), V. Somen, and T. Wango for their untiring assistance in Amboseli and Nairobi. Several people contributed to the long-term data collection and management over the years; in particular, we are grateful to the late G. Hausfater, who established the protocol for this dataset, and to N. Learn and L. Opkala, who prepared the database component for analyses. We thank Randy Nelson and Robert Sapolsky for thoughtful reviews of our manuscript. We further acknowledge the support of the National Science Foundation for the majority of the data represented here; in the past decade, in particular, this work was supported by Grants IOS 1053461, IBN 9985910, IBN 0322613, IBN 0322781, BCS 0323553, BCS 0323596, DEB 0846286, DEB 0846286, DEB 0846532, and IOS 0919200. Support also came from the National Institute of Aging Grants R01AG034513-01 and P01AG031719 and from the Princeton Center for the Demography of Aging Grant P30AG024361. E.A.A. was supported by funds from the Clare Boothe Luce Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206391109/-/DCSupplemental.
References
- 1.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapolsky RM. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418. [Google Scholar]
- 4.Adler NE, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Fairbanks B, Hawley DM. Interactions between host social behavior, physiology, and disease susceptibility. In: Demas GE, Nelson RJ, editors. Ecoimmunology. New York: Oxford Univ Press; 2012. pp. 440–467. [Google Scholar]
- 6.Cohen S, et al. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom Med. 1997;59:213–221. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lindstrom KM. Social status in relation to Sindbis virus infection clearance in greenfinches. Behav Ecol Sociobiol. 2004;55:236–241. [Google Scholar]
- 8.Nunn CL, Altizer S. Infectious Diseases in Primates: Behavior, Ecology, and Evolution. Oxford, UK: Oxford Univserity Press; 2006. [Google Scholar]
- 9.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson RS, Marion G, Hutchings MR. Effects of host social hierarchy on disease persistence. J Theor Biol. 2008;253:424–433. doi: 10.1016/j.jtbi.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 13.Creel S. Social dominance and stress hormones. Trends Ecol Evol. 2001;16:491–497. [Google Scholar]
- 14.Abbott DH, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 15.Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- 16.Stearns SC. The Evolution of Life Histories. Oxford, UK: Oxford Univ Press; 1992. [Google Scholar]
- 17.Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139:603–622. [Google Scholar]
- 18.Mills SC, et al. Fitness trade-offs mediated by immunosuppression costs in a small mammal. Evolution. 2010;64:166–179. doi: 10.1111/j.1558-5646.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 19.Norris K, Anwar M, Read AF. Reproductive effort influences the prevalence of haematozoan parasites in great tits. J Anim Ecol. 1994;63:601–610. [Google Scholar]
- 20.Muehlenbein MP, Alger J, Cogswell F, James M, Krogstad D. The reproductive endocrine response to Plasmodium vivax infection in Hondurans. Am J Trop Med Hyg. 2005;73:178–187. [PubMed] [Google Scholar]
- 21.Mills SC, et al. Testosterone-mediated effects on fitness-related phenotypic traits and fitness. Am Nat. 2009;173:475–487. doi: 10.1086/597222. [DOI] [PubMed] [Google Scholar]
- 22.Roberts ML, Buchanan KL, Hasselquist D, Evans MR. Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm Behav. 2007;51:126–134. doi: 10.1016/j.yhbeh.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Hau M. Regulation of male traits by testosterone: Implications for the evolution of vertebrate life histories. Bioessays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- 24.Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 25.Casto JM, Nolan V, Jr, Ketterson ED. Steroid hormones and immune function: Experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) Am Nat. 2001;157:408–420. doi: 10.1086/319318. [DOI] [PubMed] [Google Scholar]
- 26.Negro SS, Caudron AK, Dubois M, Delahaut P, Gemmell NJ. Correlation between male social status, testosterone levels, and parasitism in a dimorphic polygynous mammal. PLoS ONE. 2010;5:e12507. doi: 10.1371/journal.pone.0012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts ML, Buchanan KL, Evans MR, Marin RH, Satterlee DG. The effects of testosterone on immune function in quail selected for divergent plasma corticosterone response. J Exp Biol. 2009;212:3125–3131. doi: 10.1242/jeb.030726. [DOI] [PubMed] [Google Scholar]
- 28.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 29.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 30.Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: A review of the evidence. Anim Behav. 2004;68:227–239. [Google Scholar]
- 31.Nunn CL, Lindenfors P, Pursall ER, Rolff J. On sexual dimorphism in immune function. Philos Trans R Soc Lond B Biol Sci. 2009;364:61–69. doi: 10.1098/rstb.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duckworth RA, Mendonça MT, Hill GE. A condition dependent link between testosterone and disease resistance in the house finch. Proc Biol Sci. 2001;268:2467–2472. doi: 10.1098/rspb.2001.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 34.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: When and how. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 36.Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS. Beyond phytohaemagglutinin: Assessing vertebrate immune function across ecological contexts. J Anim Ecol. 2011;80:710–730. doi: 10.1111/j.1365-2656.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- 38.Hudson PJ, Dobson AP, Newborn D. Do parasites make prey vulnerable to predation? Red grouse and parasites. J Anim Ecol. 1992;61:681–692. [Google Scholar]
- 39.Wilson BS. Tail injuries increase the risk of mortality in free-living lizards (Uta stansburiana) Oecologia. 1992;92:145–152. doi: 10.1007/BF00317275. [DOI] [PubMed] [Google Scholar]
- 40.Alberts SC, Watts HE, Altmann J. Queuing and queue-jumping: Long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim Behav. 2003;65:821–840. [Google Scholar]
- 41.Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: From mating opportunities to paternity success. Anim Behav. 2006;72:1177–1196. [Google Scholar]
- 42.Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54:1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- 43.Altmann J, Gesquiere L, Galbany J, Onyango PO, Alberts SC. Life history context of reproductive aging in a wild primate model. Ann N Y Acad Sci. 2010;1204:127–138. doi: 10.1111/j.1749-6632.2010.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gesquiere LR, Onyango PO, Alberts SC, Altmann J. Endocrinology of year-round reproduction in a highly seasonal habitat: Environmental variability in testosterone and glucocorticoids in baboon males. Am J Phys Anthropol. 2011;144:169–176. doi: 10.1002/ajpa.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gesquiere L, et al. Life at the top: Energetic and psychological stress in wild male primates. Science. 2011;333:357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen KLR. Changes in the activity budgets of yellow baboons (Papio cynocephalus) during sexual consortships. Behav Ecol Sociobiol. 1985;17:161–170. [Google Scholar]
- 47.Alberts SC, Altmann J, Wilson ML. Mate guarding constrains foraging activity of male baboons. Anim Behav. 1996;51:1269–1277. [Google Scholar]
- 48.Hausfater G, Watson DF. Social and reproductive correlates of parasite ova emissions by babonns. Nature. 1976;262:688–689. doi: 10.1038/262688a0. [DOI] [PubMed] [Google Scholar]
- 49.Alberts SC, Sapolsky RM, Altmann J. Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Horm Behav. 1992;26:167–178. doi: 10.1016/0018-506x(92)90040-3. [DOI] [PubMed] [Google Scholar]
- 50.McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. J Gerontol A Biol Sci Med Sci. 2011;66:1309–1317. doi: 10.1093/gerona/glr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Post DG, Hausfater G, McCuskey SA. Feeding behavior of yellow baboons (Papio cynocephalus): Relationship to age, gender and dominance rank. Folia Primatol (Basel) 1980;34:170–195. doi: 10.1159/000155954. [DOI] [PubMed] [Google Scholar]
- 52.Sapolsky RM. Endocrinology alfresco: Psychoendocrine studies of wild baboons. Recent Prog Horm Res. 1993;48:437–468. doi: 10.1016/b978-0-12-571148-7.50020-8. [DOI] [PubMed] [Google Scholar]
- 53.Sapolsky RM, Spencer EM. Insulin-like growth factor I is suppressed in socially subordinate male baboons. Am J Physiol. 1997;273:R1346–R1351. doi: 10.1152/ajpregu.1997.273.4.R1346. [DOI] [PubMed] [Google Scholar]
- 54.MacCormick HA, et al. Male and female aggression: Lessons from sex, rank, age, and injruy in olive baboons. Behav Ecol. 23:684–691. [Google Scholar]
- 55.Drews C. Contexts and patterns of injuries in free-ranging male baboons (Papio cynocephalus) Behaviour. 1996;133:443–474. [Google Scholar]
- 56.Banks WA, Altmann J, Sapolsky RM, Phillips-Conroy JE, Morley JE. Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. J Clin Endocrinol Metab. 2003;88:1234–1240. doi: 10.1210/jc.2002-021695. [DOI] [PubMed] [Google Scholar]
- 57.Altmann J, Muruthi P. Differences in daily life between semi-provisioned and wild-feeding baboons. Am J Primatol. 1988;15:213–222. doi: 10.1002/ajp.1350150304. [DOI] [PubMed] [Google Scholar]
- 58.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Norris K, Evans MR. Ecological immunology: Life history trade-offs and immune defense in birds. Behav Ecol. 2000;11:19–26. [Google Scholar]
- 60.Muehlenbein MP, Watts DP, Whitten PL. Dominance rank and fecal testosterone levels in adult male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am J Primatol. 2004;64:71–82. doi: 10.1002/ajp.20062. [DOI] [PubMed] [Google Scholar]
- 61.Wedekind C, Folstad I. Adaptive or nonadaptive immunosuppression by sex hormones. Am Nat. 1994;143:936–938. [Google Scholar]
- 62.Gilliver SC, Ashworth JJ, Ashcroft GS. The hormonal regulation of cutaneous wound healing. Clin Dermatol. 2007;25:56–62. doi: 10.1016/j.clindermatol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 63.Martin LB, 2nd, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin LB, 2nd, Hasselquist D, Wikelski M. Investment in immune defense is linked to pace of life in house sparrows. Oecologia. 2006;147:565–575. doi: 10.1007/s00442-005-0314-y. [DOI] [PubMed] [Google Scholar]
- 65.Sheldon BC, Verhulst S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 66.Olsson M, Wapstra E, Madsen T, Silverin B. Testosterone, ticks and travels: A test of the immunocompetence-handicap hypothesis in free-ranging male sand lizards. Proc Biol Sci. 2000;267:2339–2343. doi: 10.1098/rspb.2000.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Granger DA, Booth A, Johnson DR. Human aggression and enumerative measures of immunity. Psychosom Med. 2000;62:583–590. doi: 10.1097/00006842-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 68.Singh AB, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 69.Muehlenbein MP. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. Am J Phys Anthropol. 2006;130:546–550. doi: 10.1002/ajpa.20391. [DOI] [PubMed] [Google Scholar]
- 70.Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: A test of the 'challenge hypothesis'. Anim Behav. 2004;67:113–123. [Google Scholar]
- 71.Martin LB, Weil ZM, Kuhlman JR, Nelson RJ. Trade-offs within the immune systems of female White-footed Mice, Peromyscus leucopus. Funct Ecol. 2006;20:630–636. [Google Scholar]
- 72.Koski KG, Su Z, Scott ME. Energy deficits suppress both systemic and gut immunity during infection. Biochem Biophys Res Commun. 1999;264:796–801. doi: 10.1006/bbrc.1999.1596. [DOI] [PubMed] [Google Scholar]
- 73.Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- 74.Kiecolt-Glaser JK, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 75.Bauer ME, Jeckel CMM, Luz C. The role of stress factors during aging of the immune system. Ann N Y Acad Sci. 2009;1153:139–152. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman CL, et al. Immune function and HPA axis activity in free-ranging rhesus macaques. Physiol Behav. 2011;104:507–514. doi: 10.1016/j.physbeh.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smuts BB. Sex and Friendship in Baboons. New York: Aldine Transaction; 1985. [Google Scholar]
- 78.Palombit RA, Seyfarth RM, Cheney DL. The adaptive value of ‘friendships’ to female baboons: Experimental and observational evidence. Anim Behav. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- 79.Manson JH. Mating patterns, mate choice, and birth season heterosexual relationships in free-ranging rhesus macaques. Primates. 1994;35:417–433. [Google Scholar]
- 80.Hausfater G. Dominance and Reproduction in Baboons: A Quantitative Analysis. Basel: S. Karger; 1975. [Google Scholar]
- 81.Alberts SC, Altmann J. Preparation and activation: Determinants of age at reproductive maturity in male baboons. Behav Ecol Sociobiol. 1995;36:397–406. [Google Scholar]
- 82.Alberts SC, Altmann J. Balancing costs and opportunities: Dispersal in male baboons. Am Nat. 1995;145:279–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.