Abstract
The mature optic nerve cannot regenerate when injured, leaving victims of traumatic nerve damage or diseases such as glaucoma with irreversible visual losses. Recent studies have identified ways to stimulate retinal ganglion cells to regenerate axons part-way through the optic nerve, but it remains unknown whether mature axons can reenter the brain, navigate to appropriate target areas, or restore vision. We show here that with adequate stimulation, retinal ganglion cells are able to regenerate axons the full length of the visual pathway and on into the lateral geniculate nucleus, superior colliculus, and other visual centers. Regeneration partially restores the optomotor response, depth perception, and circadian photoentrainment, demonstrating the feasibility of reconstructing central circuitry for vision after optic nerve damage in mature mammals.
Keywords: oncomodulin, retina, pathfinding, gene therapy
Injuries to the mature CNS result in functional losses that persist throughout life. By virtue of its functional significance, accessibility, and well-defined anatomy, the optic nerve has been studied intensively for insights into factors that limit or promote axon regeneration in the CNS. In the past decade, several strategies have been found to enable retinal ganglion cells (RGCs), the projection neurons of the eye, to regenerate axons part-way through the injured optic nerve (1, 2). However, it is not known whether large numbers of regenerating axons can extend into the brain, reinnervate appropriate target areas, and restore function. We show here that a combination of methods that synergistically activate RGCs’ intrinsic growth state enables these cells to regenerate axons the entire length of the optic nerve, across the optic chiasm, and into the brain in mature mice. Axons innervate the dorsal lateral geniculate nucleus (DLG), superior colliculus (SC), and other visual target areas, leading to a partial recovery of depth perception, the optomotor response (OMR), and circadian photoentrainment. Our results demonstrate the feasibility of reconstructing the central circuitry for vision after optic nerve damage, and more generally, the possibility that long-distance axon regeneration can lead to functional improvements in the mature mammalian CNS.
Results
Long-Distance Regeneration and RGC Survival.
We induced regeneration by combining three treatments with partial effects. Injecting Zymosan into the eye causes an influx of inflammatory cells that secrete oncomodulin (Ocm) and other growth factors that cause RGCs to revert to an active growth state (3–5). Ocm binds to a high-affinity receptor on RGCs in a cAMP-dependent manner and, as shown by gain- and loss-of-function studies, plays a central role in mediating the effects of inflammation on optic nerve regeneration (3–5). Coinjecting the cAMP analog 4-(chlorophenylthio)adenosine (CPT)-cAMP with Zymosan prolongs the binding of Ocm to RGCs and augments regeneration (5). Deleting the gene for PTEN (phosphatase and tensin homolog) in RGCs de-represses signaling through the phosphatidylinositol-3 kinase pathway (6) and enhances the effects of Zymosan and CPT-cAMP (5). To investigate whether these treatments enable RGCs to regenerate axons into appropriate central target areas, we injected adeno-associated viruses carrying the gene for either Cre recombinase (AAV2-Cre: Group I, n = 35) or, as a control, GFP (AAV2-GFP: Group II, n = 19), into the left eyes of mature mice bearing a floxed allele of the pten gene. Because of the tropism of AAV2 for RGCs (7–9), Cre expression causes the pten gene to be excised from RGCs with high specificity and efficiency (5, 6). After allowing 2 wk for Cre-lox recombination to occur, we crushed the optic nerve and injected Zymosan plus CPT-cAMP immediately afterward and again 3 and 6 wk later in both Groups I and II. Mice were tested for visual function over the next 10–12 wk, then injected intraocularly with cholera toxin B fragment (CTB) to trace retinal projections (Fig. S1).
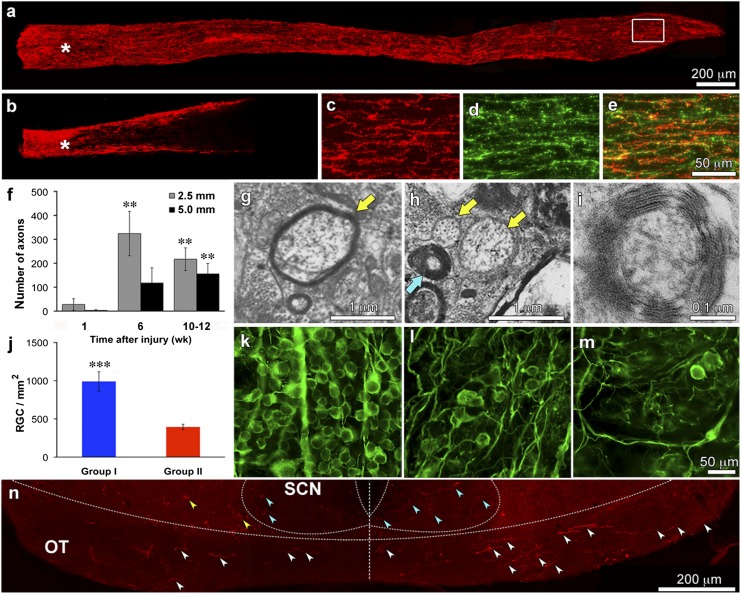
After 10–12 wk, Group I mice had numerous CTB+ axons extending the full length of the optic nerve (Fig. 1A), whereas Group II showed much less growth (Fig. 1B). Some CTB+ axons in Group I showed positive staining for the growth-associated protein GAP-43 (Fig. 1 C–E), suggesting that they are still in an active growth state. A time-course study showed few axons half-way (2.5 mm) or in the distal optic nerve (5.0 mm) of Group I mice 1 wk after nerve injury (28 ± 24 and 3 ± 3 axons, respectively) but many more at 6 (325 ± 93 and 118 ± 63 axons, respectively) and 10 wk (202 ± 43 and 131 ± 38 axons, respectively) (Fig. 1F) (difference significant at P < 0.01). To further evaluate the completeness of our lesions, we also examined the optic nerves of ptenflx/flx mice with no treatment at all and in Group II mice 1 wk after nerve crush. Unlike mice in Group I, which showed a moderate number of axons just beyond the injury site after 1 wk (Fig. S2A), mice in Group II showed many fewer axons (Fig. S2B) and untreated mice showed almost none (Fig. S2C). These results are similar to those of an earlier study from our laboratory, which showed a near-complete absence of regeneration after 2 wk in mice with optic nerve crush and no further treatment, and stronger regeneration in mice treated with Zymosan + CPT-cAMP and the pten gene deleted compared with similarly treated mice but with the pten gene remaining (5). We therefore conclude that our surgery does not spare an appreciable number of axons, that regeneration is optimal after combining inflammation, cAMP elevation and pten gene deletion, and that axons extend progressively over time.
Fig. 1.
Optic nerve regeneration. (A and B) Ten weeks after optic nerve injury, CTB+ axons (red) extend the full length of the optic nerve in Group I mice (A) but only part-way in Group II (B). (C–E) Region in the white rectangle of A double-labeled for CTB and GAP-43. (F) Time-course of axon regeneration in Group I mice as visualized by CTB-labeling. Bars show numbers of CTB+ axons counted 2.5 mm (gray) and 5 mm (black) from the injury site at 1, 6, and 10 wk after nerve injury. Note progressive increase in lengthy axons over time. (G–I) High-power EM images through optic nerves of Group I mice show axons that appear to be undergoing remyelination (G, arrow), unmyelinated axons (yellow arrows), and axons with thick myelin (blue arrow, H) that are seen at higher magnification to contain multiple lamellae of tightly-packed myelin (I). (J–M) RGC survival. (J) Average number of viable RGCs counted in the retinas of mice in Groups I and II. (K–M) RGC survival visualized by immunostaining for βIII-tubulin in uninjured (K), Group I (l), and Group II (M) mice. (N) CTB+ axons in the optic chiasm. Note axons coursing in the optic tract on the right side (contralateral to the regenerating optic nerve) and a smaller number on the left side (white arrowheads). Some CTB+ profiles are seen within the SCN bilaterally (blue arrowheads) and some are outside this area (yellow arrowheads). Error bars in F and J show SEM. **P < 0.01; ***P < 0.001. (Scale bars: A and B, 200 μm; C–E, 50 μm; K–M, 50 μm. All others as indicated.)
Electron microscopy revealed varying levels of myelination in Group I after 10 wk of regeneration. Some axons were enwrapped by thin myelin sheaths (Fig. 1G), some showed none (Fig. 1H, yellow arrows), and some had thick myelin (Fig. 1H, blue arrow) with multiple, well-packed lamellae (Fig. 1I). Optic nerves in all groups also contained numerous pathological axons with electron-dense cytoplasm surrounded by myelin at various stages of degeneration. It is possible that some of the profiles visualized by EM represent branches arising from single RGCs.
Combinatorial treatment increased RGC survival. On average, 36% of the original number of RGCs survived after 10 wk in Group I mice (989 ± 128 cells/mm2), and 16% of the original number survived in Group II mice (394 ± 35) (Fig. 1 J–M) (difference significant at P < 0.001). In the absence of treatment, only ∼5% of RGCs survive 8 wk after nerve damage (10).
At the level of the optic chiasm in Group I, many fibers were seen coursing in the optic tract contralateral to the regenerating nerve, and a smaller number were seen ipsilaterally (Fig. 1N, white arrowheads). Summing over all sections through the chiasm, the number of CTB-labeled axons that cross the midline and continue on in the contralateral optic tract was, on average, 42.5 ± 15.9% (mean ± SEM) of the CTB+ axons counted in the distal optic nerve.
Regenerating Axons Reinnervate Visual Target Areas.
We next investigated whether regenerating axons can navigate to their proper destinations in the di- and mesencephalon. Of 15 Group I mice examined histologically, all but 2 had regenerating axons in appropriate target areas, and in some cases, reinnervation was dense (Table S1). Twelve of the 13 Group I mice with central reinnervation had axons in the contralateral suprachiasmatic nucleus (SCN) and in some mice this projection was bilateral (Fig. 1N, blue arrowheads). Fibers were sometimes seen outside the SCN (Fig. 1N, yellow arrowheads). Whether the latter fibers are destined for the SCN or are mistargeted is uncertain.
Eleven of the 13 Group I mice with central reinnervation showed axons within the DLG contralateral to the regenerating nerve (Fig. 2 A–D). The extent of the DLG can be visualized by the pale NeuN+ staining of its cells (Fig. 2D). CTB+ fibers were never seen in the ipsilatateral DLG (Fig. 2E) nor in the DLG of Group II mice (Fig. 2F). In addition to the DLG, some Group I mice showed many CTB+ profiles in the contralateral ventral lateral geniculate nucleus (VLG) (Fig. 2G).
Fig. 2.
Reinnervation of the lateral geniculate nucleus. CTB+ fibers at the rostral (A), middle (B), and caudal (C) levels of the DLG contralateral to the regenerating optic nerve in a Group I mouse. Counterstaining for the neuronal protein NeuN shows CTB+ fibers to be confined to the neuropil of the DLG (dotted line). (E) CTB+ axons are absent in the DLG ipsilateral to the regenerating nerve in the same mouse shown in A–D. (F) No CTB+ fibers are seen in the DLG of any Group II mice. (G–I) Reinnervation of the VLG contralateral to the regenerating optic nerve. CTB+ axons (G) show intense labeling for the RGC-specific marker ERRβ (H and I), and colocalize with the presynaptic marker Vglut2 (J). (K and L) Distribution of the postsynaptic marker PSD-95 at low (K) and high magnification (L). L is a single confocal plane showing apposition of CTB+ terminals and PSD-95+ structures. Side panels show z-stacks of images in the orthogonal planes. (Scale bars: A–I, 100 μm; all others, 20 μm.)
CTB-labeling coincided with labeling for estrogen-related receptor-β (ERRβ), a selective marker for RGCs (11) that labels more axons (Fig. 2H, blue arrowheads) than CTB itself (Fig. 2 G–I). CTB staining also overlapped with that of vesicular glutamate transporter-2 (Vglut2), a presynaptic marker for retinal terminals (12) (Fig. 2J), and was in close apposition to elements staining for the postsynaptic density protein PSD-95 (Fig. 2 K and L). Labeled varicosities in the lateral geniculate nucleus varied considerably in size (compare Fig. 2 J and K). In Group II mice, the side of the brain contralateral to the regenerating optic nerve showed no immunostaining for CTB or ERRβ, and lower levels of Vglut2 and PSD-95 (Fig. S3), presumably associated with nonretinal synapses.
Group I mice showed variable degrees of innervation in the contralateral SC. The reinnervation of the SC, as with the DLG, was seen at multiple anterior-posterior levels and did not show an obvious laminar distribution pattern (Fig. 3 A–D). Little reinnervation was seen in the SC ipsilateral to the regenerating nerve (Fig. 3E) or in the contralateral SC of Group II mice (Fig. 3F). Half the mice in Group I showed innervation of the olivary pretectal nucleus (OPT) (Fig. 3G) and slightly fewer mice had fibers in the medial terminal nucleus (MTN) (Fig. 3J) contralateral to the regenerating nerve. Innervation was generally weak or absent in the same areas ipsilateral to the regenerating nerve (Fig. 3 H and K), and absent in the same areas of Group II mice (Fig. 3 I and L).
Fig. 3.
Reinnervation of other target areas. (A–D, F, and G) Superior colliculus. CTB+ axons are seen at the rostral (A), middle (B), and caudal (C) levels of the SC of a Group I mouse. (D) Counterstaining with an antibody to NeuN shows that CTB+ axons are within the neuropil of the SC. Dotted line indicates approximate ventral extent of the SC. Absence of CTB+ axons in the SC across from the side shown in A–D (E) and in a Group II mouse (F). G–L Reinnervation of the OPT and MTN. CTB+ fibers are abundant on the side contralateral to the regenerating optic nerve (G and J) but are absent from the ipsilateral side (H and K) and from either side in Group II mice I and L. (Scale bars: 100 μm. Bar in C is for panels A–C, E, and F; bar in I is for G–I; bar in L is for J–L).
Return of Visually Guided Behaviors.
Mice were tested for several innate visual behaviors to evaluate the functional consequences of regeneration. Taking advantage of animals’ innate aversion to depth, we evaluated depth perception using a visual cliff apparatus (Fig. 4A). Untreated ptenflx/flx mice with bilateral nerve damage, as well as mice in Groups I and II, stepped off the shallow end of the visual cliff faster than intact ptenflx/flx mice (Fig. 4B, difference not significant). However, like the latter controls, Group I mice tended to return to the shallow end and spent 62.5% of their time there (75 ± 5 s of 120 s) (Fig. 4 B and C). In contrast, Group II mice spent 41% of their time on the shallow end, which was similar to untreated mice with bilateral optic nerve damage. This value is close to what would be expected by chance, because the shallow end represents 43% of the apparatus (between-group difference significant at P < 0.001) (Fig. 4B). For mice in Group I, the distribution of time spent on the shallow end was similar to that of normal controls, whereas the distribution for Group II mice was similar to that of blind animals (Fig. 4C).
Fig. 4.
Partial recovery of visually guided behaviors. (A) Top-down view of visual cliff. (B) Average latency to step off shallow end (Left) and total time spent on shallow end (Right). ***P < 0.01. (C) Population frequency histogram for time spent on shallow end. (D) Top-down view of apparatus used to evaluate OMR. (E) Average OMR (response threshold, cycles/degree) as a function of time. (F) Frequency distribution of the OMR. Note that y-axes in E and F are discontinuous. (G) Circadian photoentrainment: percentage of overall activity in 1-h bins for individual mice (Left) and group averages (Right). Mice were maintained on a continuous cycle of lights on at 7:00 AM and off at 7:00 PM before testing and for the first 2.5 d in the activity monitor. The light cycle was set back 6 h on day 3. Error bars represent SEM.
The OMR is an innate behavior that compensates for movements of the whole visual field (Fig. 4D). A clear OMR emerged in 28% of Group I mice 10 wk after optic nerve injury (difference between Groups I and II significant at P < 0.05) (Fig. 4E). Some movements were occasionally seen in Group II mice but are likely to be a result of random head movements that are also seen at times in blind controls (Fig. 4F). The average acuity of Group I mice remained well below normal (Movie S1).
Mice were also evaluated for entrainment of their circadian activity patterns. Unoperated ptenflx/flx mice maintained on a 7:00 AM lights-on/7:00 PM lights-off schedule showed peak activity levels between 5:00 PM and midnight (Fig. 4G), which persisted over the 5-d test period despite a 6-h setback in the light-dark cycle on day 3. Untreated mice, as well as mice in Groups I and II, showed circadian activity patterns with a normal periodicity. However, only the activity of Group I was entrained to the light-dark cycle. The activity peak for Group I occurred 8 h later than that of normal mice but showed a fixed relationship to the light-dark cycle, as demonstrated by the coherence in the average group activity (Fig. 4G). Like untreated mice with bilateral optic nerve injury, the behavior of Group II was not synchronized to the ambient light-dark cycle and there was no clear peak in the group average (Fig. 4G) (P < 0.01 for the difference in activity between Groups I and II from 11:00 PM to 3:00 AM).
The pupillary light reflex (PLR) was tested starting 1 wk after transecting the remaining intact optic nerve (week 8), at which point it was observed in 10% of Group I mice. By the end of the study, about one-third of Group I mice showed a clear PLR (Fig. S4 A–C), albeit slower and less complete than that of normal mice (Movie S2). This diminished response may be caused in part by a relatively weak retinal input or the intraocular inflammation and cataracts seen in many mice toward the end of the study (Fig. S4A). No Group II mice showed a clear PLR (P < 0.01, difference in average change in pupil diameter at weeks 10–11). However, the mouse iris contains melanopsin and can contract in response to bright light without neural input (13). Thus, the basis for the intergroup difference seen here is uncertain.
Discussion
Recent studies have demonstrated the potential for lengthy axon regeneration in the optic nerves of mature mice (5) but have left open the question of whether these axons can enter the brain in significant numbers, navigate to their proper destinations, and restore functional circuits. Our findings provide positive answers to these questions. We show that, with adequate stimulation, some RGCs are able to regenerate axons the full length of the visual pathway and into their correct target zones, leading to a partial recovery of vision.
The ability of RGC axons to reinnervate appropriate target areas suggests that signals required for axon guidance may persist in the adult brain. Projections to the DLG, SC, and other visual areas were largely restricted to the contralateral side of the brain, and we seldom observed axons in inappropriate areas. During development, the optic chiasm is a decision point for growing axons, with the vast majority projecting to the contralateral side of the brain, based on interactions involving ephrins, proteoglycans, cell-adhesion molecules, and semaphorins (14). In the present study, most regenerating fibers crossed the midline within 10 wk of nerve injury, whereas in our previous study, small but nearly equal numbers of axons were seen at short distances beyond the chiasm ipsi- and contralaterally after 6 wk (5). This difference suggests either that axons that project bilaterally, such as those destined for the SCN, are among the first to enter the brainstem; or that many of the ipsilaterally projecting axons that enter early are subsequently withdrawn. Beyond the chiasm, the formation of appropriate connections relies on a host of guidance signals that include ephrins, slits, Wnts, and cadherins (15–17). Several lines of evidence suggest that guidance signals that enable RGC axons to form a topographically organized map upon the SC are present in adult rats (18–20). In general, however, we do not know whether the same signals that guide retinal axons to their destinations during development govern the restoration of connections in adult mice.
The visual functions studied here rely on several distinct pathways. The optomotor response relies on the accessory optic system (AOS), which normally helps stabilize the retinal image during self-generated motion. Direction-sensitive ganglion cells (DSGCs) that respond to movements of the whole visual field project to the terminal nuclei of the AOS as well as to the DLG and SC (15, 21, 22). The tegmental MTN is innervated by DSGCs that respond to upward and downward motions (23), and was heavily innervated in some of our Group I mice. DSGCs that respond selectively to temporal-to-nasal motion project to other parts of the AOS as well as to the SC and DLG (22). The anatomical basis of depth avoidance, as evaluated using a visual cliff, does not appear to be established yet, but may require pattern vision mediated by the DLG or SC. One or both of these areas were innervated in every Group I mouse that showed successful regeneration (Table S1).
Photoentrainment of circadian activity involves input from intrinsically photosensitive RGCs to the SCN (24), and this projection was visualized in all but one Group I mouse. Unlike mice that received no treatment after optic nerve injury or Group II mice with incomplete regeneration, the circadian activity patterns of Group I mice were synchronized to the day-night cycle, although with a considerable delay compared with normal controls. The PLR involves projections from a specific subset of intrinsically photosensitive RGCs to the OPT (24). Retinal projections to the OPT were seen in half of our Group I mice. However, the relationship of the PLR to reinnervation of the OPT is difficult to evaluate in light of the fact that melanopsin signaling within the iris contributes to the mouse PLR (13).
Consistent with the observed behavioral improvements, regenerating axon terminals appear to form synapses, as suggested by their expression of the presynaptic marker VGlut2 and close apposition to PSD-95+ postsynaptic elements. Within the lateral geniculate nucleus we observed small and large varicosities, consistent with prior descriptions of terminals arising from axons of different calibers (25). Axons that arise from particular classes of RGCs terminate at characteristic depths within the mouse DLG (22, 26–29). Our results do not show any consistent laminar patterning within the DLG, perhaps because of insufficient time for segregation to occur, insufficient sampling, absence of targeting cues, or because multiple classes of RGCs are regenerating their axons. Further work will be required to determine whether particular classes of RGCs are better able to regenerate axons than others.
Although the methods used here cannot be applied in the clinic, they point to methods that might be. Induction of an inflammatory response in the eye could damage the lens and retina and would be unacceptable clinically, as would long-term deletion of pten, a tumor-suppressor gene. On the other hand, gene therapy is now a reality for other ocular disorders (30) and could be used to deliver genes for growth factors, such as Ocm, or shRNAs to decrease expression of growth inhibitors such as PTEN, under the control of regulatable promoters to turn off gene expression after regeneration is achieved. We believe that our findings lend encouragement to the possibility that, with improved methods, it might be possible to partially repair the optic nerve after traumatic injury or in degenerative diseases such as glaucoma.
Materials and Methods
Surgery.
All procedures were carried out with the approval of the Animal Care and Use Committee of Children’s Hospital Boston in conformity with National Institutes of Health guidelines. Intraocular injections and optic nerve surgery were performed as previously described (4, 5). To delete the pten gene, adeno-associated virus (seroform 2: AAV2, 1012 GC/mL) expressing Cre recombinase (AAV2-Cre; Vector Laboratories) was injected intraocularly (3 μL) in ptenflx/flx mice, 6–8 wk of age, avoiding injury to the lens (Group I, n = 35). As a control, AAV2-expressing GFP (3 μL, Vector Laboratories) was injected instead (AAV2-GFP: Group II, n = 19). Two weeks after virus injections, mice were reanesthetized and the left optic nerve was exposed and crushed with fine forceps (Dumont; WPI) 1 mm behind the eye for 5 s. To augment regeneration, we induced a limited inflammatory reaction in the eye by injecting Zymosan (Sigma; 12.5 μg/μL, sterilized before use) along with the cAMP analog CPT-cAMP (Sigma; 50 μM, 3 μL) into the posterior chamber of the eye immediately after surgery in both Groups I and II. For mice with long survival times, we injected Zymosan at half the original dose plus CPT-cAMP at the original dose again 3 and 6 wk later. To verify that visual behaviors were mediated by the regenerating optic nerve, we reanesthetized mice in Groups I and II at 7 wk and transected the previously intact right optic nerve. Five days before the end of the study, we injected CTB (3 μL, 1%; List Biological) as an anterograde tracer to visualize axons and nerve terminals originating in RGCs. Most mice in the behavioral study survived for 10 wk but a few survived 12 wk. In addition to the groups described above, we generated a no-treatment group by injuring the optic nerves bilaterally in ptenflx/flx mice 6–8 wk of age (n = 8) and did not administer Zymosan or CPT-cAMP. To investigate the completeness of our surgeries and the progression of regeneration, some animals in all groups were examined 1 wk after optic nerve damage.
Anatomical Studies.
Mice were given an overdose of anesthesia at the completion of the study and were perfused transcardially with normal saline and phosphate-buffered paraformaldehyde (PFA). Retinas, optic nerves, and brains were dissected and immersion-fixed in 4% (wt/vol) PFA for 24 h. Optic nerves and brains were transferred to 30% (wt/vol) sucrose (12–24 h), embedded in OCT Tissue Tek Medium (Sakura Finetek), and frozen. To analyze CTB+ fibers by light microscopy, optic nerves were Cryostat-sectioned longitudinally at 14 μm, mounted on coated glass slides, and immunostained with primary antibodies to CTB (made in rabbit; GenWay, 1:500) and growth-associated protein GAP-43, followed by fluorescently labeled secondary antibodies (4). Axons were counted at fixed distances from the injury site in ≥ 8 longitudinal sections per nerve to estimate the total number of regenerating axons (5).
RGC Survival.
RGC survival was evaluated by immunostaining whole retinas with a rabbit anti–βIII-tubulin antibody (Abcam; 1:500) followed by an Alexa 594-conjugated antibody to rabbit IgG made in goat. Within the ganglion cell layer, βIII tubulin is expressed only in RGCs (31). Immuno-positive cells were counted under fluorescent illumination (400×) in 16 prespecified areas and averaged to estimate RGC survival per square millimeter (mean ± SEM) in five to eight mice per group (5, 31).
Electron Microscopy.
Additional animals in Groups I and II were created to investigate regeneration at the EM level 10 wk after optic nerve injury. Following fixation with 4% (wt/vol) paraformaldehyde/2% (vol/vol) glutaraldehyde, 1-mm-thick segments of the optic nerve 2.5-mm distal to the lesion site were immersion-fixed in 2.5% glutaraldehyde for 2 h, washed in 0.1 M phosphate buffer (pH 7.4), followed by 0.1 M cacodylate buffer (pH 7.4), and postfixed for 1 h in 1% (wt/vol) osmium tetroxide containing 0.8% potassium ferrocyanide, and 5 nM calcium chloride in 0.1 M cacodylate buffer (pH 7.4). Segments were washed in 0.1 M cacodylate buffer (pH 7.4) and distilled water and stained in 1% (wt/vol) uranyl acetate overnight, dehydrated in graded acetone, infiltrated with Poly/Bed 812 resin (Polysciences), and polymerized at 60° for 48 h. Semithin cross-sections (500 nm) were stained with Toluidine blue for light microscopy analysis. Ultrathin cross-sections (70 nm) were collected on copper grids and contrasted in uranyl acetate and lead citrate. Microscopy was carried out using a Zeiss 900 transmission electron microscope operated at 80 Kv. Several electron micrographs were recorded digitally at 3,000× for each ultrathin nerve section. EM was carried out on optic nerves from eight mice in Group I, four in Group II, and eight mice with no treatment after nerve injury.
Central Projections.
Brains were sectioned in the coronal plane at 40 μm between the rostral diencephalon and caudal mesencephalon. Sections were immunostained with one or more of the following antibodies: anti-CTB (as above); anti- ERRβ (Sigma; E0156, 1:500); anti-Vglut2 (Millipore; AB2251, 1:2,000; courtesy of Beth Stevens, Children’s Hospital, Harvard Medical School, Boston, MA), and anti–PSD-95 (Cell Signaling). All secondary antibodies were from Molecular Probes and were used at a concentration of 1:500. Images were captured using either a Nikon E800 fluorescent microscope or by confocal microscopy (Zeiss LSM700). In some cases, CTB labeling was weak or absent in the optic nerve and brain, probably because of insufficient labeling in the eye. In these cases central projections were visualized by ERRβ immunostaining.
Visual Cliff.
Depth avoidance was evaluated on a visual cliff apparatus (32). One side of a transparent Plexiglas box 41 cm long × 10.1 cm wide × 20.6 cm high (inner dimensions) was positioned directly over an 18-cm-long checkerboard pattern with black and white squares measuring 2 cm × 2 cm (“shallow end”). The “deep end” was suspended 70 cm above a similar pattern measuring 60 cm × 60 cm. Mice were placed at the back of the shallow end and their behavior was recorded for 2 min. Because the floor of the apparatus is level and the apparatus is cleaned thoroughly between tests, mice have to rely on visual cues to distinguish the shallow end from the deep end. Videos were analyzed for (i) latency to step off the shallow end and (ii) total time spent on the shallow end. Behavior was evaluated by an investigator blind to the animals’ treatment in n = 4 unoperated ptenflx/flx mice, n = 25 mice in Group I, n = 17 for Group II, and n = 8 blinded ptenflx/flx mice with no further treatment.
Optomotor Response.
OMR was evaluated using an OptoMotry virtual cylinder apparatus (Cerebral Mechanics). Mice were allowed to move freely on a small elevated platform surrounded by a banked array of four liquid-crystal monitors, the displays of which simulated high-contrast stripes of variable spatial frequency rotating in either a clockwise or counterclockwise direction (33). Mirrors on the floor and ceiling of the apparatus further created a 3D appearance. The speed of rotation, spatial frequency, and contrast of the stimuli were controlled by the system software. Video recordings were taken over a 5-min interval and were evaluated by two blinded observers to determine the maximum spatial frequency at which mice showed a reliable tracking response. Acuity thresholds were determined using a staircase method with 100% contrast. To establish a criterion for a reliable response, we analyzed the level of spontaneous movements in blind mice. Because some of the mice were not examined histologically, it is possible that some of the Group I mice remaining in the study did not have central reinnervation. We excluded one mouse each from Groups I and II that showed high spontaneous activity when tested 3 wk after optic nerve surgery, and two Group I mice that were histologically verified as having no central reinnervation, leaving n = 32 mice in Group I and n = 18 mice in Group II.
Circadian Activity.
Mice were maintained on a continuous 12:12 light-dark cycle (lights on at 7:00 AM, off at 7:00 PM) before being placed individually in one of eight cages of an InfraMot Activity System (TSE Systems). Locomotor activity was detected using infrared sensors and was recorded in 20-min bins during the 5-d interval between receiving intraocular injections of the anterograde tracer CTB and being killed. On day 3, the light-dark cycle was set back 6 h (lights on at 1:00 AM and off at 1:00 PM). For intergroup comparisons, activity was integrated over 1-h intervals and normalized to the total activity over the 5-d test period. The study included 11 unoperated ptenflx/flx mice, 4 blind mice with bilateral optic nerve damage and no further treatment, 15 mice in Group I, and 9 in Group II.
Pupillary Light Reflex.
Awake, alert mice were hand-held 27 cm under the lamp of a surgical microscope and the pupillary response was video-recorded at ∼10× magnification. The light intensity at this distance was measured as 5,500 Lux. Unoperated ptenflx/flx mice showed some constriction in ambient light and full constriction under increased lighting within 5 s (n = 4). Mice with regenerating optic nerves showed little constriction in ambient light and often required 30–40 s to reach steady-state under bright light. A blinded investigator quantified the change in pupillary diameter over 40 s for n = 13 mice in group 1 and 13 in Group II. A 20% change in diameter was considered reliable to exclude the largest changes seen in one Group I mouse found to have no CTB+ axons in the thalamus and in one Group II mouse.
Statistics.
Group comparisons used unpaired Student t tests. Reported P values are all two-tailed.
Supplementary Material
Acknowledgments
We thank the Intellectual and Developmental Disabilities Research Center of the Children’s Hospital (National Institutes of Health Grant P30 HD018655) for use of the Histology, Image Analysis, and Animal Behavior Cores; Paul Rosenberg and Mustafa Sahin (Children’s Hospital, Harvard Medical School) for comments on the manuscript; Jerry Silver (Case Western Reserve) for suggestions; David Zurakowski (Children’s Hospital) for advice on statistics; and Nathalia Amado for assistance with editing videos. This study was supported in part by National Eye Institute Grant EY05690 (to L.B.); Congressionally Directed Medical Research Program/Department of Defense Grant DM102446 (to L.B.); The Dr. Miriam and Sheldon Adelson Medical Research Foundation (L.B.); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (S.d.L.); Institutional Program for Young Researcher Overseas Visit Program of the Japan Society for the Promotion of Science (Y.K.); the Kawasaki Medical School Alumni Association Fund for Foreign Study (T.K.); and China Scholarship Council Grant 2010638086 (to Y.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119449109/-/DCSupplemental.
References
- 1.Benowitz LI, Yin Y. Optic nerve regeneration. Arch Ophthalmol. 2010;128:1059–1064. doi: 10.1001/archophthalmol.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun F, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 4.Yin Y, et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci USA. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurimoto T, et al. Long-distance axon regeneration in the mature optic nerve: Contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010;30:15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey AR, et al. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors. Mol Cell Neurosci. 2002;21:141–157. doi: 10.1006/mcne.2002.1168. [DOI] [PubMed] [Google Scholar]
- 9.Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 10.Cenni MC, et al. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci. 1996;8:1735–1745. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 11.Real MA, Heredia R, Dávila JC, Guirado S. Efferent retinal projections visualized by immunohistochemical detection of the estrogen-related receptor beta in the postnatal and adult mouse brain. Neurosci Lett. 2008;438:48–53. doi: 10.1016/j.neulet.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Fujiyama F, et al. Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. J Comp Neurol. 2003;465:234–249. doi: 10.1002/cne.10848. [DOI] [PubMed] [Google Scholar]
- 13.Xue T, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petros TJ, Rebsam A, Mason CA. Retinal axon growth at the optic chiasm: To cross or not to cross. Annu Rev Neurosci. 2008;31:295–315. doi: 10.1146/annurev.neuro.31.060407.125609. [DOI] [PubMed] [Google Scholar]
- 15.Osterhout JA, et al. Cadherin-6 mediates axon-target matching in a non-image-forming visual circuit. Neuron. 2011;71:632–639. doi: 10.1016/j.neuron.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada T, Harada C, Parada LF. Molecular regulation of visual system development: More than meets the eye. Genes Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt AM, et al. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 18.Wizenmann A, Thies E, Klostermann S, Bonhoeffer F, Bähr M. Appearance of target-specific guidance information for regenerating axons after CNS lesions. Neuron. 1993;11:975–983. doi: 10.1016/0896-6273(93)90126-c. [DOI] [PubMed] [Google Scholar]
- 19.Knöll B, et al. Graded expression patterns of ephrin-As in the superior colliculus after lesion of the adult mouse optic nerve. Mech Dev. 2001;106:119–127. doi: 10.1016/s0925-4773(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 20.Vidal-Sanz M, Avilés-Trigueros M, Whiteley SJ, Sauvé Y, Lund RD. Reinnervation of the pretectum in adult rats by regenerated retinal ganglion cell axons: Anatomical and functional studies. Prog Brain Res. 2002;137:443–452. doi: 10.1016/s0079-6123(02)37035-3. [DOI] [PubMed] [Google Scholar]
- 21.Rivlin-Etzion M, et al. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci. 2011;31:8760–8769. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huberman AD, et al. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonehara K, et al. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS One. 2008;3:e1533. doi: 10.1371/journal.pone.0001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter DA, Jhaveri S. Retino-geniculate axons regenerating in adult hamsters are able to form morphologically distinct terminals. Exp Neurol. 1997;146:315–322. doi: 10.1006/exnr.1997.6495. [DOI] [PubMed] [Google Scholar]
- 26.Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21:228–237. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krahe TE, El-Danaf RN, Dilger EK, Henderson SC, Guido W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J Neurosci. 2011;31:17437–17448. doi: 10.1523/JNEUROSCI.4370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huberman AD, et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung DC, Lee V, Maguire AM. Recent advances in ocular gene therapy. Curr Opin Ophthalmol. 2009;20:377–381. doi: 10.1097/ICU.0b013e32832f802a. [DOI] [PubMed] [Google Scholar]
- 31.Cui Q, Yip HK, Zhao RC, So KF, Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 32.Glynn D, Bortnick RA, Morton AJ. Complexin II is essential for normal neurological function in mice. Hum Mol Genet. 2003;12:2431–2448. doi: 10.1093/hmg/ddg249. [DOI] [PubMed] [Google Scholar]
- 33.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.