Abstract
Analyses of datasets throughout the temperate midlatitude regions show a widespread tendency for species to advance their springtime phenology, consistent with warming trends over the past 20–50 y. Within these general trends toward earlier spring, however, are species that either have insignificant trends or have delayed their timing. Various explanations have been offered to explain this apparent nonresponsiveness to warming, including the influence of other abiotic cues (e.g., photoperiod) or reductions in fall/winter chilling (vernalization). Few studies, however, have explicitly attributed the historical trends of nonresponding species to any specific factor. Here, we analyzed long-term data on phenology and seasonal temperatures from 490 species on two continents and demonstrate that (i) apparent nonresponders are indeed responding to warming, but their responses to fall/winter and spring warming are opposite in sign and of similar magnitude; (ii) observed trends in first flowering date depend strongly on the magnitude of a given species’ response to fall/winter vs. spring warming; and (iii) inclusion of fall/winter temperature cues strongly improves hindcast model predictions of long-term flowering trends compared with models with spring warming only. With a few notable exceptions, climate change research has focused on the overall mean trend toward phenological advance, minimizing discussion of apparently nonresponding species. Our results illuminate an understudied source of complexity in wild species responses and support the need for models incorporating diverse environmental cues to improve predictability of community level responses to anthropogenic climate change.
Keywords: growing season, ecological forecasting
Within general trends toward earlier spring (1–7), observed cases of species and ecosystems that have not advanced their phenology, or have even delayed it, appear paradoxical, especially when made in temperate regions experiencing significant warming (2–5, 8–10). The typical interpretation of this pattern has been that nonresponders are relatively insensitive to spring warming, whereas species showing delays are often viewed as statistical noise or evidence for unknown confounding factors at play. However, physiological work on model species has shown that the timing of flowering is controlled by multiple, complex pathways related to temperature forcing at different times during the plant life cycle (11). One important pathway is vernalization–chilling requirements that must be met before a plant is able to respond to spring warming (12, 13). These experimental studies suggest that warm temperatures during the vernalization period (typically fall and winter) can delay dormancy or the fulfillment of chilling requirements (14), thereby delaying spring events, such as flowering (13–18). Modeling and laboratory studies further suggest that this effect could temper phenological advances projected from climate warming (19, 20).
The importance of vernalization for explaining negligible or delayed phenological trends in observed wild populations and communities over the past 50 y, however, is highly uncertain. Although one study has suggested a link (21), most vernalization research is focused on model and crop species (11). Extensions to wild communities have been relatively rare and generally confined to large-scale ecosystem metrics, such as the timing of spring “green-up” measured from satellite data (10, 22), or a handful of woody species. The one study that hypothesized vernalization requirements could explain observed divergent trends of species’ phenology in natural communities (21) quantified the divergent species’ sensitivities to fall and winter vs. spring warmth but did not explicitly test whether these divergent temperature responses could explain the observed long-term trends. Thus, our goal was to test the extent to which observed trends in wild species’ flowering times over the past 50 y can be attributed to species’ vernalization vs. spring warming responses.
Results
We analyzed phenological changes at the species level in relation to local climate data using two long-term, species-rich datasets of temperate plants from the United Kingdom and eastern United States. The first was a 47-y record (1954–2000) of first flowering dates (FFDs) from 384 species at Chinnor, United Kingdom (2); the second was a shorter (1970–2009) FFD dataset of 106 species from Washington, DC (23). We then used a model-building approach to classify each species in terms of its relative sensitivity to fall/winter vernalization and spring warming. Using 3-mo moving windows beginning the previous fall and ending at the end of the concurrent year, mean daily temperatures were compiled into seasonal growing degree day (GDD) summations and standardized to Z-score values (zero mean, unit SD) (Materials and Methods and Datasets S1 and S2). Our Z-score predictors represent normalized indices of accumulated warmth, with positive values indicating warmer than normal conditions and negative values indicating cooler than normal conditions. The motivation behind using these standardized predictors, rather than raw temperatures, was to remove the possible influence of the higher interannual temperature variance during the winter and early spring. From these climate predictors, we selected, for each species, significant climate associations using a liberal cutoff of P ≤ 0.10 for maximum inclusion of species categorized as sensitive to yearly climate variability. First, the best indicator of spring sensitivity was identified as the most significant climate predictor, if present, with a negative regression coefficient (i.e., earlier FFD associated with increased warming). We then identified whether inclusion of fall/winter sensitivity (i.e., evidence of dependence on vernalization) was supported as an additional predictor in the regression model, with the restriction that vernalization sensitivity (i) needed to occur before spring sensitivity, (ii) must have a significant positive regression coefficient (i.e., delayed FFD with increased warming), and (iii) must be significant at P ≤ 0.10 (P value method). As an alternative, we also based selection of the vernalization sensitivity on the Akaike Information Criterion corrected for small sample sizes (AICc). The two methods gave similar results, as did other analyses using a more stringent significance threshold (P ≤ 0.05) or alternative GDD criteria (SI Text and Figs. S1–S4). Although overall patterns were similar between the two datasets, the more southern site (Washington, DC) had too few species with significant vernalization sensitivities and too short a time series for complex analyses. We therefore conducted basic analyses on both datasets but confined more sophisticated analyses to the Chinnor data.
Our analyses identified a set of species showing simple and significant associations between phenological change and spring temperatures with no sensitivity to temperatures at other times of the year, which we term spring-only responders. A second set, divergent responders, showed significant but opposite associations between phenology and both spring and fall/winter temperatures (i.e., spring warming advanced FFD and fall/winter warming delayed it). At Chinnor, we found 275 spring-only responders (72% of species) and 70 divergent responders (18%). At Washington, DC, we identified 77 spring-only responders (73%) and 11 divergent responders (10%). Of the remaining species, 14 at Chinnor and 4 at Washington, DC did not advance in response to spring warming but did delay FFD with warmer fall/winter temperatures (vernalization only), whereas 25 species at Chinnor and 14 at Washington, DC had no significant response to temperatures during any season (nonresponders). Spring warming and vernalization sensitivities for each species, including SEs, are summarized in Datasets S1 and S2 for Chinnor and Washington, DC, respectively.
If vernalization requirements delay flowering with warmer falls and winters but also occur in species that respond to spring warmth by advancing flowering, we expect significant sensitivity to winter warming to be most prevalent among species showing no overall change or delayed spring flowering. In contrast, we expect species showing significant spring advancement to be primarily spring-only responders. For example, matching predictions, Acer campestre, a spring-only responder, significantly advanced its FFD by −0.27 d⋅y−1, a stark contrast to Clematis vitalba, a divergent responder with a near-zero trend in FFD (+0.02 d⋅y−1).
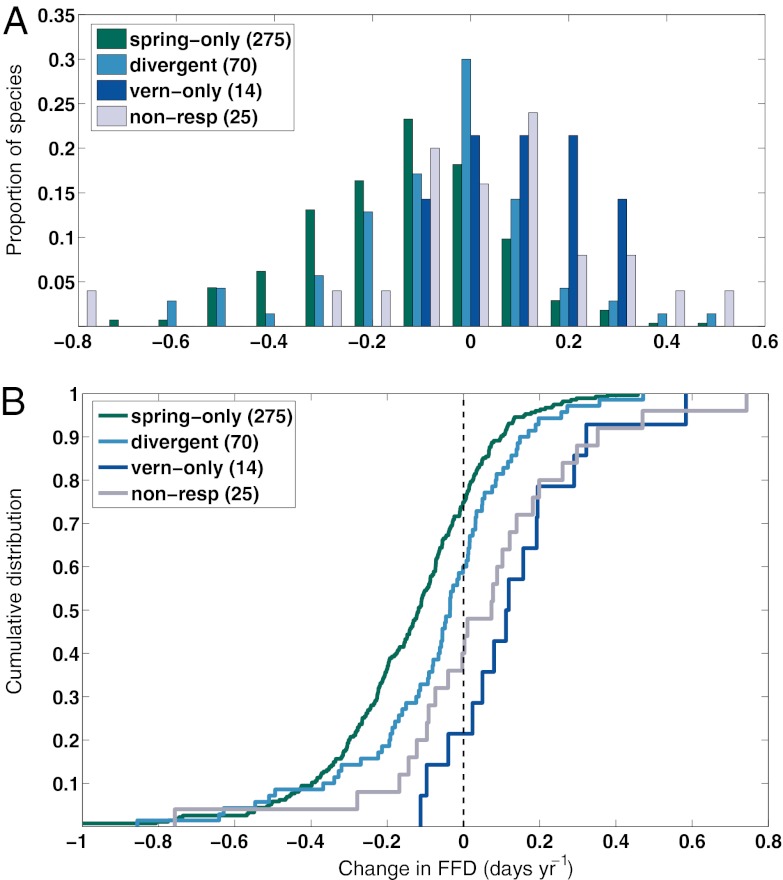
These predictions were further supported considering the full community of species. Vernalization sensitivity was most common among species with negligible or delayed flowering trends, species previously considered nonresponsive to warming trends over the past 50 y (Fig. 1). Divergent responders showed only a slight advance in FFD over time (mean trend in FFD: −0.07 d⋅y−1 at Chinnor and −0.09 d⋅y−1 at Washington, DC). Trends toward earlier flowering were significant (P ≤ 0.10) in only 17.1% and 36.4% of the divergent responders at Chinnor and Washington, DC, respectively. By contrast, spring-only responders advanced significantly more than divergent responders, and at nearly twice the mean rate (Table S1; −0.14 d⋅y−1 at Chinnor and −0.15 d⋅y−1 at Washington, DC).
Fig. 1.
Change (temporal trend) in FFD within four response categories at Chinnor. Plant species at Chinnor fall into four categories of observed seasonal temperature responses that covary with the species’ long-term trends in FFD over time: species that respond to spring warming only (spring-only responders, n = 275 species), species with both significant spring warming and fall/winter vernalization sensitivities (divergent responders, n = 70 species), species with fall/winter vernalization sensitivity only (vern-only, n = 14 species), and species with no significant climate sensitivity (non-resp, n = 25 species). Observed changes in FFD over time (x axis, trend in d⋅y−1) are negative for species that have advanced their spring FFD and positive for species that have delayed their spring FFD. (A) Normalized histogram for each response category. (B) Empirical cumulative distribution function for each response category.
Greater advance over time of spring-only responders compared with divergent responders could be caused by either or both of two effects. First, species that do not have significant vernalization sensitivities could have stronger responses to spring warming (i.e., higher spring sensitivities). Second, vernalization responses may be counteracting spring warming responses that would otherwise advance FFD.
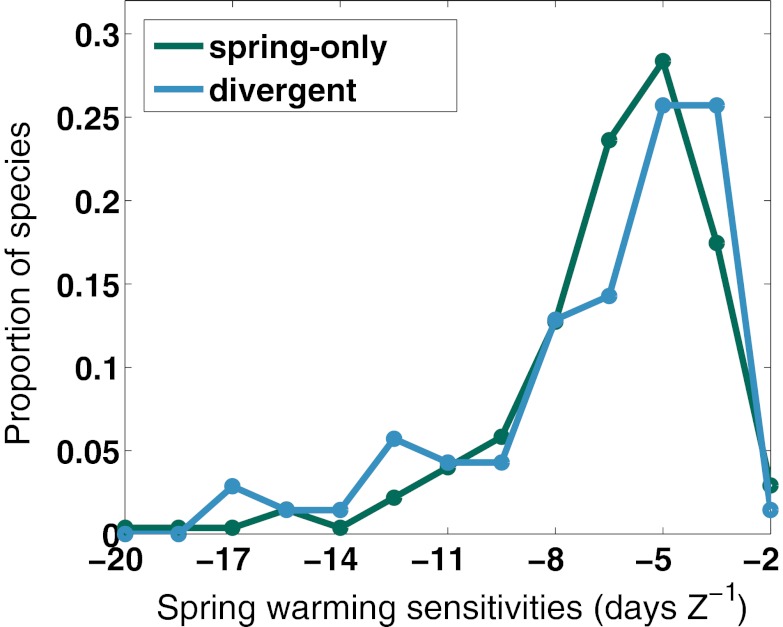
We tested for these effects in turn, using the longer, more speciose Chinnor dataset. We found that species in both groups were equally responsive to external warming in spring (i.e., their average estimated spring warming sensitivities were almost identical) (Fig. 2 and Table S2; Chinnor: −6.4 d⋅Z−1 in spring-only responders vs. −6.7 d⋅Z−1 in divergent responders; Student t test, P = 0.51). Indeed, our two example species, A. campestre (spring-only responder) and C. vitalba (divergent responder), have similar spring warming sensitivities (−5.4 d⋅Z−1 and −5.1 d⋅Z−1, respectively), despite widely divergent FFD trends. We can therefore reject our first hypothesis, and we note that the differences in long-term trends between spring-only and divergent responders are likely not attributable to different sensitivities to spring temperature forcing.
Fig. 2.
Spring warming sensitivities for spring-only (green) and divergent (blue) responders. Normalized histograms compare the magnitude of spring warming sensitivities between the spring-only (green line) and divergent (blue line) responders. Because the climate predictors are standardized to zero mean and unit SD (Z-score), sensitivity units are in days per Z-score deviation. Mean spring warming sensitivities are not significantly different (P = 0.51; Table S2) between the spring-only and divergent species: −6.4 d⋅Z−1 for spring-only responders and −6.7 d⋅Z−1 for divergent responders. Results indicate that spring-only and divergent responders are equally sensitive and responsive to spring warmth.
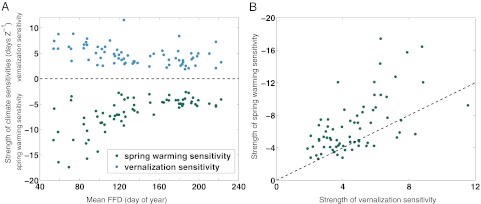
Instead, the data support our second hypothesis: Vernalization sensitivities partially or wholly compensate for spring warming responses in divergent responders. In the divergent responders, earlier flowering species tend to have larger magnitude sensitivities both to spring and fall/winter temperatures (Fig. 3A). The absolute magnitude of the spring warming sensitivity tends to be higher than the vernalization sensitivity (Fig. 3B); the ratio of vernalization to spring warming sensitivity, averaged across the divergent responders, is 0.77 (Fig. 3; mean spring warming sensitivity: −6.7 d⋅Z−1, mean vernalization sensitivity: +4.7 d⋅Z−1). Under uniform seasonal warming, vernalization sensitivities of these species can be expected to counter about three-quarters of the trend toward earlier FFD induced by spring warming.
Fig. 3.
Vernalization vs. spring warming sensitivities for divergent responders (n = 70 species categorized as divergent responders based on P value criteria in the Chinnor dataset). (A) Each species from the divergent responders group is represented by two dots: one blue dot for the magnitude of its vernalization sensitivity and one green dot for the magnitude of its spring warming sensitivity. Negative values indicate that warming advances FFD, and positive values indicate that warming delays FFD (y axis). Mean FFD for each species (day of year) is shown (x axis). The absolute magnitudes of the spring and vernalization (fall/winter) temperature sensitivities tend to decrease with later mean FFD, suggesting earlier flowering species are more sensitive to external climate forcing. (B) Estimated vernalization (x axis) and spring warming (y axis) sensitivities for each of the 70 divergent species. The dashed line indicates the 1:1 line, and the y axis is reversed. The preponderance of circles above the dashed line indicates that spring warming sensitivities generally are stronger (have greater absolute magnitude) than fall/winter vernalization sensitivities.
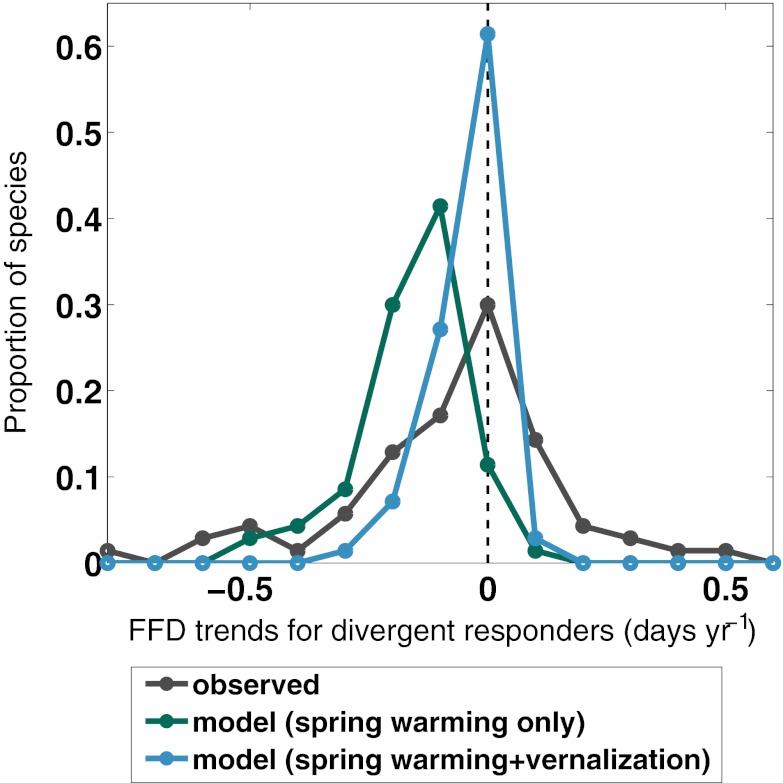
Using our regression models, we compared modeled FFD trends of the Chinnor divergent responders to see if including the vernalization term improved the match to observed trends. Although this is not an independent hindcast (i.e., we are forcing the model with the original calibration data), a comparison between the two statistically valid models for the divergent responders (spring warming only vs. spring warming + vernalization) may provide additional support for our hypothesis. A simple seasonal warming model (using only spring warming sensitivity) overpredicts advances for the 70 divergent responders [Fig. 4; model: −0.16 d⋅y−1, observed: −0.07 d⋅y−1], whereas the same class of model accurately captures trends toward earlier FFD for the 275 spring-only responders (Fig. S5 and Table S3; model: −0.16 d⋅y−1, observed: −0.14 d⋅y−1). By contrast, trends from the model incorporating both vernalization and spring warming sensitivities are consistent with, and statistically indistinguishable from, the observations (Fig. 4 and Table S4; model: −0.05 d⋅y−1). Even though this test is not a true independent validation, it does demonstrate that in the absence of any other forcing, warming trends during the spring would significantly advance flowering of the divergent responders, a response contrary to the observations. Only when we incorporate the compensating influence of the vernalization term do our predictions closely approximate the negligible observed trends for these species. This analysis helps to illustrate our central conclusion: Diminished trends in observed FFD for species with vernalization sensitivities are not attributable to nonresponsiveness to climate but are instead caused by opposing responses to similar temperature forcing (warming) in different seasons.
Fig. 4.
Observed and modeled FFD trends (d⋅y−1) for the 70 divergent responders at Chinnor. Observed trends in FFD are colored gray, modeled FFD trends using only spring warming sensitivities are colored green, and modeled FFD trends using spring warming and vernalization sensitivities are colored blue (n = 70 species). The observed trends are centered on zero, and trends for the models incorporating vernalization sensitivity are similarly centered. If only the spring warming predictors are considered for these species, modeled trends are biased negative, predicting a general advance in the timing of FFD that is at odds with the observations. Including vernalization sensitivity as well as spring warming sensitivity improves the ability of the model to reproduce the observed temporal FFD trends.
Discussion
Although phenological advancement with spring warming is near-ubiquitous in temperate ecosystems (found for 89.3% and 83.0% of species at Chinnor and Washington, DC), our results suggest that focusing only on spring warming sensitivities will lead to inaccurate interpretations and predictions for species that may rely both on fall/winter chilling and spring forcing. For these species, vernalization plays a major role in what is often abstracted into a two-phase system leading to spring phenology. In the first phase, cool autumn and winter temperatures are needed to release dormancy in a plant (“chilling”), and in the second phase, warm spring temperatures then promote cell growth (“forcing”) (19, 24). The best predictions of spring phenology for species with vernalization will thus require models that accurately estimate the break of dormancy—a process that has continually proven difficult to measure (24) and may be controlled by complex interactions of multiple cues (24, 25).
Accounting for vernalization sensitivities in future projections will be important for understanding community level responses to warming, especially if warming trends are not uniform across the growing season (e.g., if winter warming outpaces spring warming). For example, species with spring-only responses may continue to show overall spring advancement, whereas divergent responders may exhibit small or negligible trends. This process could explain the emergence of novel gaps between flowering periods of sympatric species (26), with potentially large cascading effects on pollinators that rely on continual flowering (27, 28).
We identified significant vernalization sensitivity in nearly one in five of the species analyzed from Chinnor. We also found a lower prevalence of species with significant vernalization sensitivity in Washington, DC (only 1 in 10) compared with Chinnor. This pattern is expected, based on physiological work indicating that temperate species show a latitudinal cline in vernalization requirements, with sensitivities to fall and winter temperature being smaller at lower latitude (e.g., Washington, DC at 38.4° N) compared with higher latitude (e.g., Chinnor at 51.4° N) sites (29). Alternatively, the fewer divergent responders in the Washington, DC dataset may be attributable to differences in the two datasets themselves, specifically the shorter time period and fewer number of sampled species in Washington, DC compared with Chinnor. If divergent responders comprise a small fraction of total species in a community, as this analysis seems to indicate, detection of these species will be difficult in datasets that do not holistically sample the community.
Interestingly, our analyses extend to fall/winter responders a pattern previously recognized in spring responders. Prior studies documented that responses to spring temperature have been strongest in early-flowering species, declining for species that normally flower later in spring and summer (30, 31). We document this same pattern with respect to both spring and fall/winter sensitivities in divergent responders: The response to temperature is greatest for early spring bloomers and declines for species that are late spring/summer bloomers (Fig. 3A).
The datasets we use document first observed flower rather than mean population flowering dates, a distinction that could bias our results or complicate our interpretations. For example, if increased warming in spring leads to earlier bud break and increased risk for exposure to damaging frost events, advances in flowering may be underestimated because the total number of buds flowering, and the opportunity to observe the earliest flower accurately, will be reduced. We believe this issue is unlikely to be a major concern within the Chinnor dataset, however. First, many of our species flower in late spring and early summer (May and later), when the risk for exposure to frost events is minimal. Second, frost dates over much of Europe and the United Kingdom have generally advanced in step with earlier spring, mitigating the potential impact of these frost events on species phenology (32, 33).
This study demonstrates, in a long-term empirical analysis, that the interplay between vernalization and spring warming sensitivities to temperature explains much of the apparently paradoxical behavior of wild species that are showing later spring events or failing to change their timing despite local warming. It directly supports, but scales up to the community level for both herbaceous and woody species, previous work on process-based modeling that included vernalization of dominant tree species (19, 24). Our results highlight the dominant role of temperature in controlling plant phenology (34) and suggest that plant responses to spring warming may be more universal than suggested by trend-based studies; many species in Chinnor and Washington, DC respond significantly to spring temperature forcing, regardless of their trend over time. Other abiotic cues may also significantly influence flowering phenology depending on species and location, including photoperiod, irradiance, and soil moisture (35–38). However, the exact roles and impacts of these other factors are unclear (39–41), and molecular and physiological studies suggest temperature can trump such cues (17). Clearly, more laboratory and field studies are needed to understand how these diverse pathways interact to influence flowering in wild communities. Our results lend field-based, community-level support for the numerous experimental- and physiological-based studies of vernalization that show how chilling requirements could retard species responses to spring warming. Importantly, our work suggests that long-term observations of wild plants, coupled with further physiological studies, can be used to improve model predictions of the diversity of species’ phenological responses to climate change.
Materials and Methods
All FFD for the analyses came from datasets located at Chinnor, United Kingdom (2) and Washington, DC (23). Daily minimum and maximum temperature data for Chinnor were taken from the Global Historical Climatology Network station at Oxford (GHCN ID UK000056225, located at 51.77° N, 1.27° W), with temporal coverage of the years 1854–2001. For the Washington, DC site, temperature data were taken from GHCN station USW00013721 (38.30° N, 76.42° W), covering the years 1945–2010. We converted the daily maximum and minimum temperatures to daily mean temperatures and built our suite of predictor variables using 3-mo moving window GDD summations, with a base temperature of 0 °C. We used GDD summations, rather than mean temperatures, in recognition of the well-established concept that phenology responds primarily to integrated climate forcing (42, 43). Once each seasonal GDD summation was calculated, we standardized to zero mean and unit SD. The seasonal summations began with August-September-October of the previous year, continuing with September-October-November of the previous year and on in that fashion until October-November-December of the current year.
Because we have no a priori knowledge of either the timing or magnitude of species sensitivities to climate forcing, we designed a model-building approach to identify the seasons of response objectively. Our methodology is based on reasonable expectations regarding how phenology responds to climate, drawing from research in the plant physiology and phenological literature. Specifically, these expectations are (i) spring warming is the primary control on flowering for most species at these temperature-limited sites, (ii) vernalization responses occur during months and seasons before the spring warming responses, and (iii) timing of flowering does not respond to temperature forcing that occurs in months or seasons after the emergence of flowering for most species (13, 34, 40). We filtered all FFD time series in both datasets by restricting our analyses to those FFD series with at least 8 overlapping years with the GHCN temperature data. We compared our FFD time series, using linear least-squares regression, against every GDD summation predictor with a mean date before the mean FFD for that species. Restricting the allowed predictors in this way limited the possibility of nonsensical results (e.g., FFD responding to GDD forcing that occurs after flowering). From the remaining predictors, we first selected for the most significant (smallest P value) predictor where the resulting regression equation had a negative regression coefficient, indicating earlier FFD with increased warmth. This became the spring warming sensitivity for that species. If the selection procedure did not identify a regression equation with a significant negative regression coefficient, the algorithm was then allowed to select any significant predictor if present. For those species with significant spring warming sensitivities, an additional step attempted to select for a significant predictor with a positive regression coefficient (delayed FFD with increased warming) that occurred in a season before the spring warming sensitivity. This was then flagged as a potential vernalization sensitivity, and the model for this species was refit with two (spring warming and vernalization) predictors rather than only the spring warming predictor. In the case of these divergent responders, the sign and season of the spring warming coefficient are unchanged.
We then used two criteria to determine the significance of the vernalization cue. The first is based on retaining the vernalization predictor if the P value for the associated regression coefficient passed a significance threshold of P ≤ 0.10 (P value method). As an alternative, we also based selection of the additional vernalization sensitivity on the AICc using a ΔAIC cutoff of 2 (“AICc method”) (44). Those species with a significant spring warming sensitivity only were labeled as spring-only responders, and those with significant spring and vernalization sensitivities were labeled as divergent responders. Vernalization and spring warming predictors used in the divergent responder models were almost uniformly uncorrelated. Of the 89 species initially identified as potential divergent responders at Chinnor, only 5 (Anisantha sterilia, Glycerian fluitans, Malva moschata, Ophrys apifera, and Vinca minor) had vernalization and spring warming predictors that were significantly correlated at the P ≤ 0.10 level. Of these, only M. moschata passed the primary test for inclusion of the vernalization predictor (P ≤ 0.10; 70 species). Autocorrelation is unlikely to have a significant impact on our regressions. At Chinnor, average lag-1 autocorrelation across the 384 species was r = 0.08. Of these species, only 45 (11.7%) had significant autocorrelation at the P ≤ 0.05 level and only 67 (17.5%) were significant at the less restrictive P ≤ 0.10 level. Seasons of response for the spring warming and vernalization predictors were fit separately for each species; once selected, these seasons were fixed from year to year. Thus, for example, a species with a spring warming response during March-April-May will respond, in the model, to temperature variability during this seasonal interval every year.
Supplementary Material
Acknowledgments
We thank R. S. R. Fitter and A. H. Fitter for previous work, including data and theories, which provided much of the background for this study. Additional support came from the National Phenology Network (United States). We thank three anonymous reviewers and the editor for providing comments and critiques that significantly improved the manuscript. This work was conducted as a part of the “Forecasting Phenology” Working Group supported by the National Center for Ecological Analysis and Synthesis, a center funded by National Science Foundation Grant EF-0553768; the University of California, Santa Barbara; and the State of California. Support for E.M.W. came from the National Science Foundation Postdoctoral Fellow program Grant DBI-0905806. This is publication ISEM-2012-050 of the Institut des Sciences de l'Evolution de Montpellier Lamont contribution #7548.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.B.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118364109/-/DCSupplemental.
References
- 1.Bradley NL, Leopold AC, Ross J, Huffaker W. Phenological changes reflect climate change in Wisconsin. Proc Natl Acad Sci USA. 1999;96:9701–9704. doi: 10.1073/pnas.96.17.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biology. 2007;13:1860–1872. [Google Scholar]
- 5.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 6.Rosenzweig C, et al. Attributing physical and biological impacts to anthropogenic climate change. Nature. 2008;453:353–357. doi: 10.1038/nature06937. [DOI] [PubMed] [Google Scholar]
- 7.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 8.Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- 9.Menzel A. Trends in phenological phases in Europe between 1951 and 1996. Int J Biometeorol. 2000;44:76–81. doi: 10.1007/s004840000054. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Tarpley D, Sullivan JT. Diverse responses of vegetation phenology to a warming climate. Geophys Res Lett. 2007 10.1029/2007GL031447. [Google Scholar]
- 11.Wilczek AM, et al. Effects of genetic perturbation on seasonal life history plasticity. Science. 2009;323:930–934. doi: 10.1126/science.1165826. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson D, Porter JR. Temperature, plant development and crop yields. Trends Plant Sci. 1996;1(4):119–124. [Google Scholar]
- 13.Chouard P. Vernalization and its relations to dormancy. Annu Rev Plant Physiol. 1960;11(1):191–238. [Google Scholar]
- 14.Murray MB, Cannell MGR, Smith RI. Date of budburst of fifteen tree species in Britain following climatic warming. J Appl Ecol. 1989;26:693–700. [Google Scholar]
- 15.Cannell MGR, Smith RI. Climatic warming, spring budburst and forest damage on trees. J Appl Ecol. 1986;23:177–191. [Google Scholar]
- 16.Lambers H, Chapin FS, III, Pons TL. Life cycles: Environmental influences and adaptations. In: Lambers H, Chapin FS III, Pons TL, editors. Plant Physiological Ecology. 2nd Ed. New York: Springer; 2008. pp. 375–402. [Google Scholar]
- 17.Mouradov A, Cremer F, Coupland G. Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell. 2002;14(Suppl):S111–S130. doi: 10.1105/tpc.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MD, Hanes JM. Continental-scale phenology: Warming and chilling. Int J Climatol. 2010;30:1595–1598. [Google Scholar]
- 19.Morin X, et al. Leaf phenology in 22 North American tree species during the 21st century. Glob Change Biol. 2009;15:961–975. [Google Scholar]
- 20.Morin X, Roy J, Sonié L, Chuine I. Changes in leaf phenology of three European oak species in response to experimental climate change. New Phytol. 2010;186:900–910. doi: 10.1111/j.1469-8137.2010.03252.x. [DOI] [PubMed] [Google Scholar]
- 21.Fitter AH, Fitter RSR, Harris ITB, Williamson MH. Relationships between first flowering date and temperature in the flora of a locality in central England. Funct Ecol. 1995;9(1):55–60. [Google Scholar]
- 22.Yu H, Luedeling E, Xu J. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc Natl Acad Sci USA. 2010;107:22151–22156. doi: 10.1073/pnas.1012490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Asab MS, Peterson PM, Shetler SG, Orli SS. Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodivers Conserv. 2001;10:597–612. [Google Scholar]
- 24.Caffarra A, Donnelly A, Chuine I. Modelling the timing of Betula pubescens budburst. II. Integrating complex effects of photoperiod into process-based models. Clim Res. 2011;46(2):159–170. [Google Scholar]
- 25.Heide OM, Prestrud AK. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 2005;25:109–114. doi: 10.1093/treephys/25.1.109. [DOI] [PubMed] [Google Scholar]
- 26.Sherry RA, et al. Divergence of reproductive phenology under climate warming. Proc Natl Acad Sci USA. 2007;104:198–202. doi: 10.1073/pnas.0605642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moeller DA. Facilitative interactions among plants via shared pollinators. Ecology. 2004;85:3289–3301. [Google Scholar]
- 28.Palmer TM, Stanton ML, Young TP. Competition and coexistence: Exploring mechanisms that restrict and maintain diversity within mutualist guilds. Am Nat. 2003;162(4) Suppl:S63–S79. doi: 10.1086/378682. [DOI] [PubMed] [Google Scholar]
- 29.Linkosalo T, Häkkinen R, Hänninen H. Models of the spring phenology of boreal and temperate trees: Is there something missing? Tree Physiol. 2006;26:1165–1172. doi: 10.1093/treephys/26.9.1165. [DOI] [PubMed] [Google Scholar]
- 30.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Change Biol. 2006;12:1969–1976. [Google Scholar]
- 31.Willis CG, et al. Favorable climate change response explains non-native species’ success in Thoreau’s woods. PLoS ONE. 2010;5:e8878. doi: 10.1371/journal.pone.0008878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzel A, Jakobi G, Ahas R, Scheifinger H, Estrella N. Variations of the climatological growing season (1951–2000) in Germany compared with other countries. Int J Climatol. 2003;23:793–812. [Google Scholar]
- 33.Sunley RJ, Atkinson CJ, Jones HG. Chill unit models and recent changes in the occurrence of Winter chill and Spring frost in the United Kingdom. J Hortic Sci Biotechnol. 2006;81:949–958. [Google Scholar]
- 34.Chuine I, Morin X, Bugmann H. Warming, photoperiods, and tree phenology. Science. 2010;329:277–278, author reply 278. doi: 10.1126/science.329.5989.277-e. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman JK, Wright SJ, Calderón O, Pagan MA, Paton S. Flowering and fruiting phenologies of seasonal and aseasonal neotropical forests: The role of annual changes in irradiance. J Trop Ecol. 2007;23:231–251. [Google Scholar]
- 36.Körner C, Basler D. Plant science. Phenology under global warming. Science. 2010;327:1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- 37.Caffarra A, Donnelly A, Chuine I, Jones MB. Modelling the timing of betula pubescens budburst. I. Temperature and photoperiod: A conceptual model. Clim Res. 2011;46(2):147–157. [Google Scholar]
- 38.Luedeling E, Brown PH. Equivalence of winter chill models for fruit and nut trees around the world. Int J Biometeorol. 2011;55:411–421. doi: 10.1007/s00484-010-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanino KK, Kalcsits L, Silim S, Kendall E, Gray GR. Temperature-driven plasticity in growth cessation and dormancy development in deciduous woody plants: A working hypothesis suggesting how molecular and cellular function is affected by temperature during dormancy induction. Plant Mol Biol. 2010;73(1–2):49–65. doi: 10.1007/s11103-010-9610-y. [DOI] [PubMed] [Google Scholar]
- 40.Rohde A, Bastien C, Boerjan W, Thomas S. Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiol. 2011;31:472–482. doi: 10.1093/treephys/tpr038. [DOI] [PubMed] [Google Scholar]
- 41.Häkkinen H, Tanino K. Tree seasonality in a warming climate. Trends Plant Sci. 2011;16:412–416. doi: 10.1016/j.tplants.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Leon AJ, Lee M, Andrade FH. Quantitative trait loci for growing degree days to flowering and photoperiod response in sunflower (Helianthus annuus l.) Theor Appl Genet. 2001;102:497–503. [Google Scholar]
- 43.McMaster GS, Wilhelm WW. Growing degree-days: One equation, two interpretations. Agricultural and Forest Meteorology. 1997;87:291–300. [Google Scholar]
- 44.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd Ed. New York: Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.