Abstract
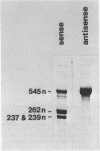
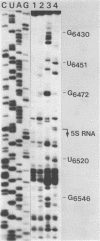
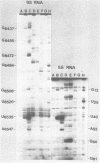
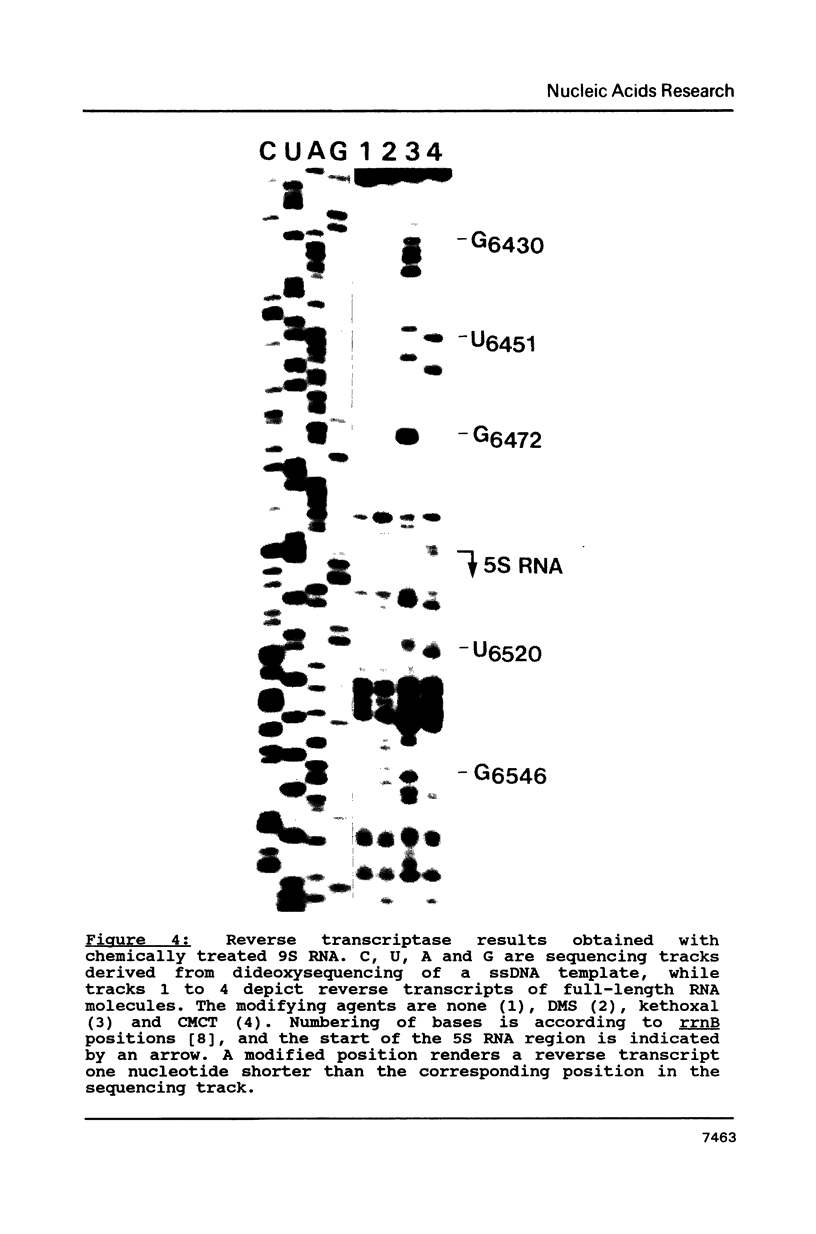
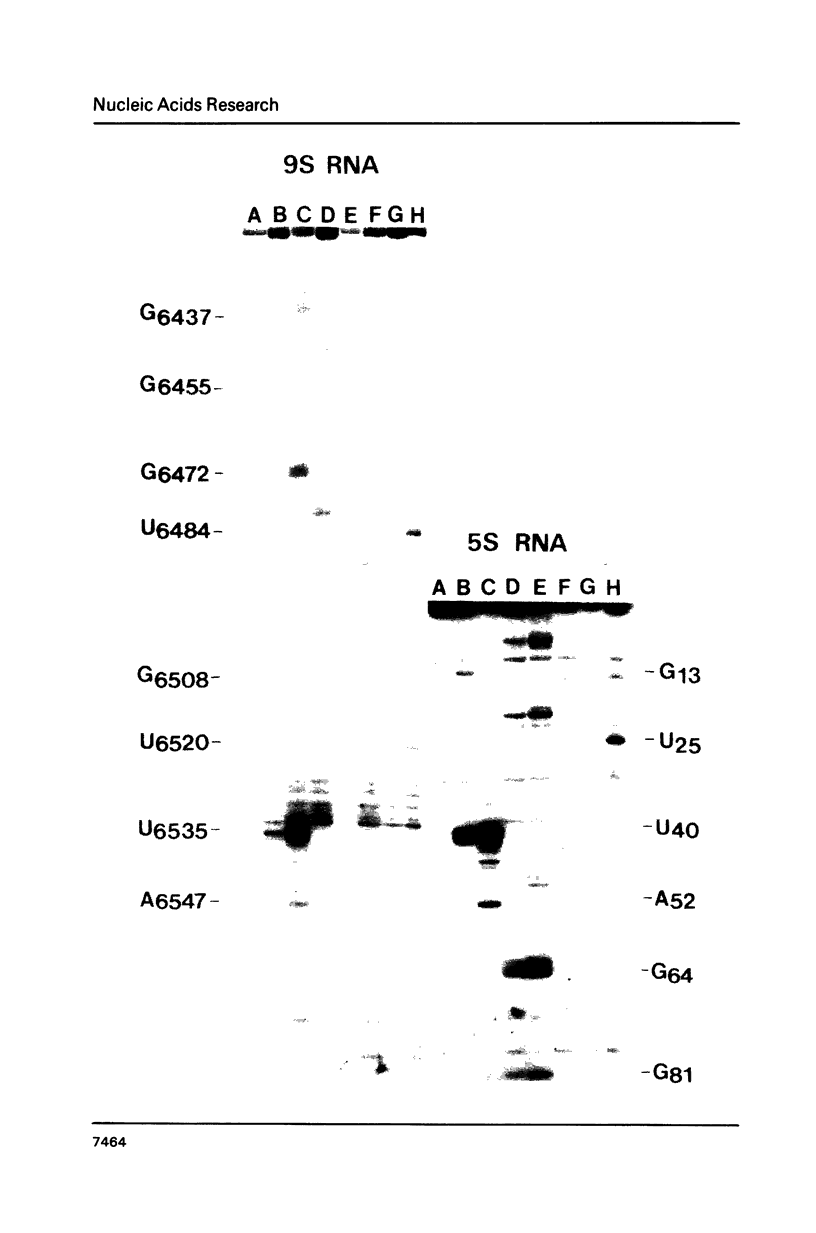
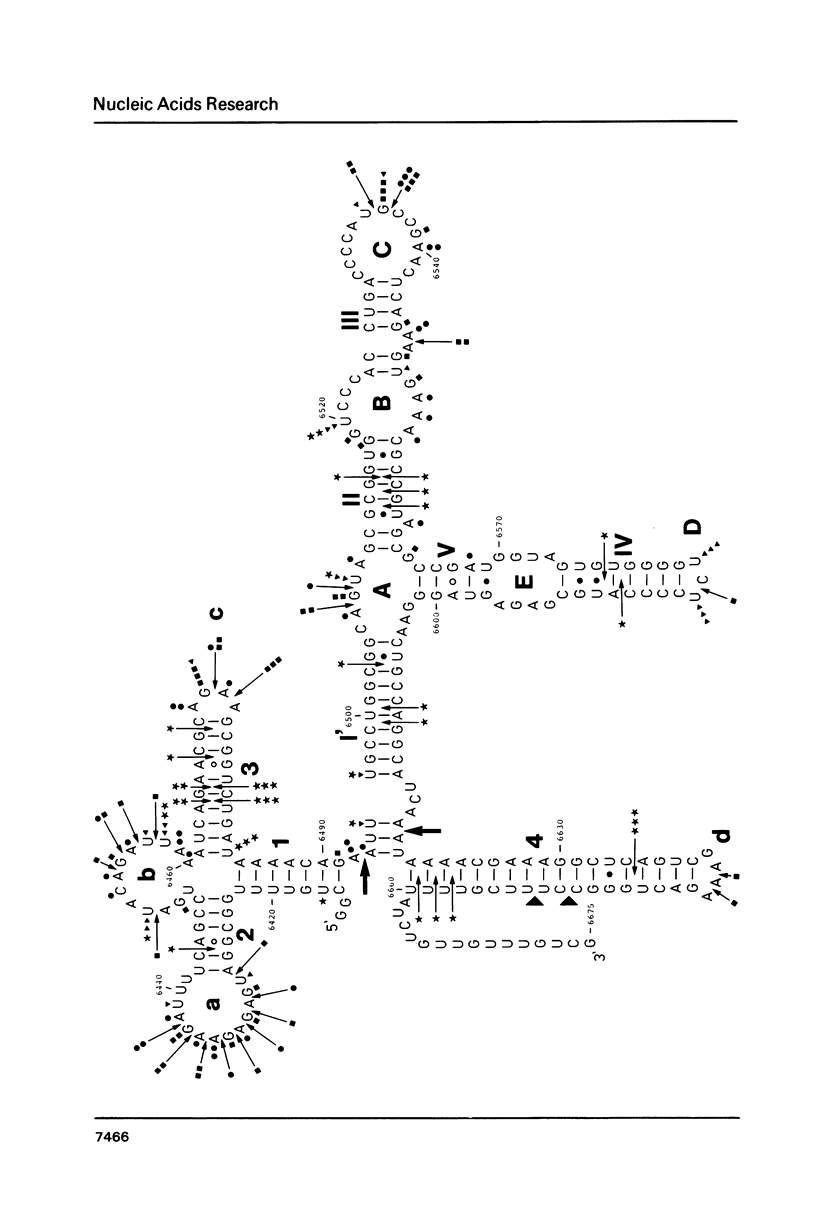
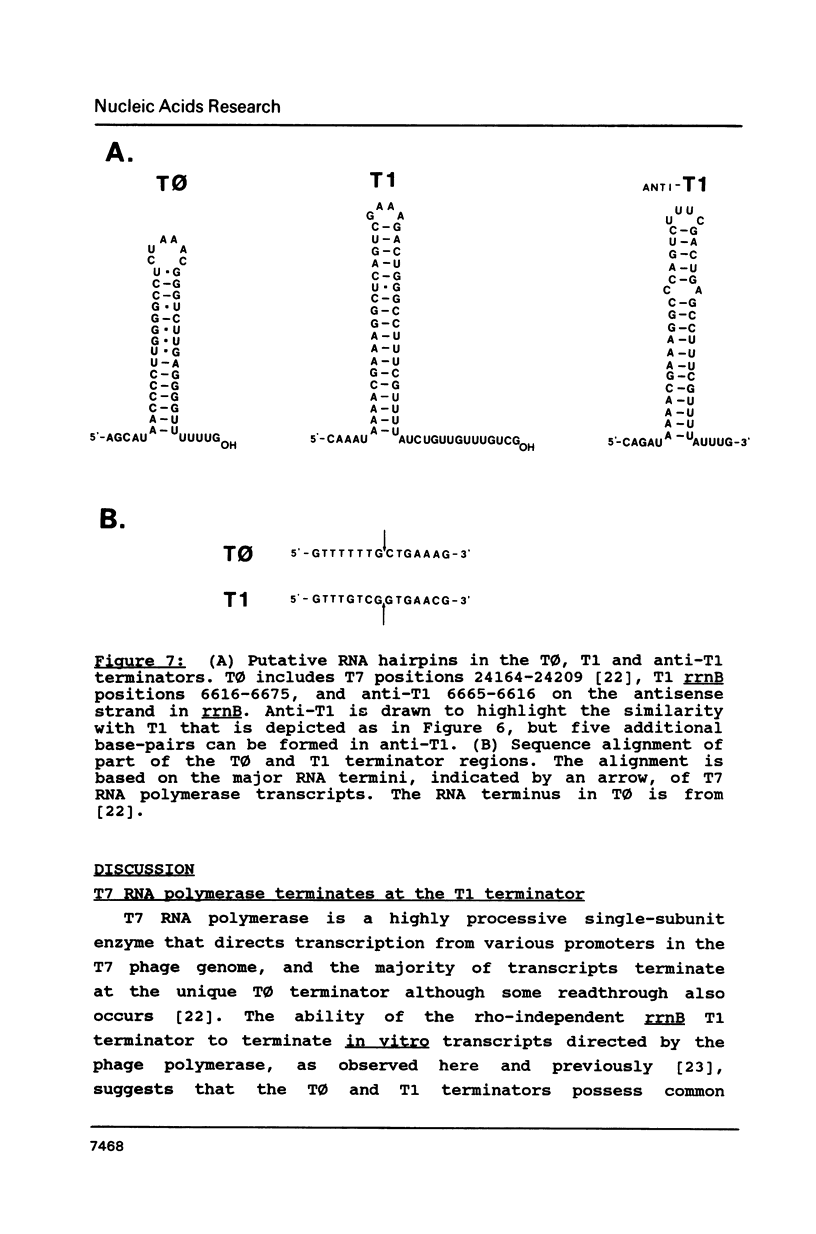
The secondary structure of the 9S RNA precursor to ribosomal 5S RNA in Escherichia coli has been determined using chemical reagents and ribonucleases in combination with a reverse transcription procedure. The 9S RNA precursor was generated in vitro by T7 RNA polymerase, and the rrnB operon terminator, T1, was able to terminate the in vitro transcript. The secondary structure model exhibits three structural domains corresponding to a 5' region, a mature region and a terminator region. The mature domain is structurally identical to 5S RNA, and the ribosomal proteins L18 and L25 are able to bind to the precursor. The processing endoribonuclease RNase E cleaves between the structural domains. Moreover, an intramolecular refolding of the nascent transcript must take place if the current view of RNase III processing stems is correct.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W., Barker R. System for DNA sequencing with resolution of up to 600 base pairs. J Biochem Biophys Methods. 1984 Mar;9(1):33–47. doi: 10.1016/0165-022x(84)90064-2. [DOI] [PubMed] [Google Scholar]
- Bachellerie J. P., Hearst J. E. Specificity of the photoreaction of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen with ribonucleic acid. Identification of reactive sites in Escherichia coli phenylalanine-accepting transfer ribonucleic acid. Biochemistry. 1982 Mar 16;21(6):1357–1363. doi: 10.1021/bi00535a039. [DOI] [PubMed] [Google Scholar]
- Bram R. J., Young R. A., Steitz J. A. The ribonuclease III site flanking 23S sequences in the 30S ribosomal precursor RNA of E. coli. Cell. 1980 Feb;19(2):393–401. doi: 10.1016/0092-8674(80)90513-9. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Schiltz E., Chen R. The primary structure of the 5S RNA binding protein L18 from Escherichia coli ribosomes. FEBS Lett. 1975 Aug 15;56(2):359–361. doi: 10.1016/0014-5793(75)81127-6. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen J., Brown R. S., Sproat B. S., Garrett R. A. Xenopus transcription factor IIIA binds primarily at junctions between double helical stems and internal loops in oocyte 5S RNA. EMBO J. 1987 Feb;6(2):453–460. doi: 10.1002/j.1460-2075.1987.tb04775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. L., Holmes W. M. The distal end of the ribosomal RNA operon rrnD of Escherichia coli contains a tRNA1thr gene, two 5s rRNA genes and a transcription terminator. Nucleic Acids Res. 1980 Sep 11;8(17):3793–3807. doi: 10.1093/nar/8.17.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Leffers H., Christensen A., Andersen H., Garrett R. A. Structure and accessibility of domain I of Escherichia coli 23 S RNA in free RNA, in the L24-RNA complex and in 50 S subunits. Implications for ribosomal assembly. J Mol Biol. 1987 Jul 5;196(1):125–136. doi: 10.1016/0022-2836(87)90515-8. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Bjerring P., Buchardt O., Ebbesen P., Kanstrup A., Karup G., Knudsen P. H., Nielsen P. E., Nordén B., Ygge B. Psoralenamines. 3. Synthesis, pharmacological behavior, and DNA binding of 5-(aminomethyl)-8-methoxy-, 5-[[(3-aminopropyl)oxy]methyl]-, and 8-[(3-aminopropyl)oxy]psoralen derivatives. J Med Chem. 1985 Aug;28(8):1001–1010. doi: 10.1021/jm00146a006. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. Isolation of proteins from 50S ribosomal subunits of Escherichia coli. Eur J Biochem. 1971 Nov 11;23(1):12–16. doi: 10.1111/j.1432-1033.1971.tb01585.x. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Forget B. G., Monier R. A low molecular weight ribonucleic acid synthesized by Escherichia coli in the presence of chloramphenicol: characterization and relation to normally synthesized 5 s ribonucleic acid. J Mol Biol. 1971 Feb 14;55(3):407–421. doi: 10.1016/0022-2836(71)90326-3. [DOI] [PubMed] [Google Scholar]
- King T. C., Schlessinger D. S1 nuclease mapping analysis of ribosomal RNA processing in wild type and processing deficient Escherichia coli. J Biol Chem. 1983 Oct 10;258(19):12034–12042. [PubMed] [Google Scholar]
- King T. C., Sirdeshmukh R., Schlessinger D. RNase III cleavage is obligate for maturation but not for function of Escherichia coli pre-23S rRNA. Proc Natl Acad Sci U S A. 1984 Jan;81(1):185–188. doi: 10.1073/pnas.81.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. C., Sirdeskmukh R., Schlessinger D. Nucleolytic processing of ribonucleic acid transcripts in procaryotes. Microbiol Rev. 1986 Dec;50(4):428–451. doi: 10.1128/mr.50.4.428-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R. Secondary structure formation during RNA synthesis. Nucleic Acids Res. 1981 Oct 10;9(19):5109–5124. doi: 10.1093/nar/9.19.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liarakos C. D., Reinhart G., Kopper R. A., Maddox R. P. Characterization of the effects on ovalbumin mRNA of aminomethyl-trimethylpsoralen photoreaction with hen oviduct mRNA. Nucleic Acids Res. 1987 Oct 26;15(20):8417–8438. doi: 10.1093/nar/15.20.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebke H., Hatfull G. The sequence of the distal end of the E. coli ribosomal RNA rrnE operon indicates conserved features are shared by rrn operons. Nucleic Acids Res. 1985 Aug 12;13(15):5515–5525. doi: 10.1093/nar/13.15.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman H. B., Draper D. E. On the recognition of helical RNA by cobra venom V1 nuclease. J Biol Chem. 1986 Apr 25;261(12):5396–5403. [PubMed] [Google Scholar]
- Meyhack B., Pace N. R. Involvement of the mature domain in the in vitro maturation of Bacillus subtilis precursor 5S ribosomal RNA. Biochemistry. 1978 Dec 26;17(26):5804–5810. doi: 10.1021/bi00619a030. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., McDougall J., Van Ryk D. I. Structure and evolution of the 4.5-5S ribosomal RNA intergenic region from Glycine max (soya bean). Nucleic Acids Res. 1987 Sep 25;15(18):7593–7603. doi: 10.1093/nar/15.18.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Pace B., Stahl D. A., Pace N. R. The catalytic element of a ribosomal RNA-processing complex. J Biol Chem. 1984 Sep 25;259(18):11454–11458. [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Robertson H. D. Escherichia coli ribonuclease III cleavage sites. Cell. 1982 Oct;30(3):669–672. doi: 10.1016/0092-8674(82)90270-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Roy M. K., Singh B., Ray B. K., Apirion D. Maturation of 5-S rRNA: ribonuclease E cleavages and their dependence on precursor sequences. Eur J Biochem. 1983 Mar 1;131(1):119–127. doi: 10.1111/j.1432-1033.1983.tb07238.x. [DOI] [PubMed] [Google Scholar]
- Sarmientos P., Sylvester J. E., Contente S., Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivo in multicopy plasmids. Cell. 1983 Apr;32(4):1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- Sproat B. S., Brown D. M. A new linkage for solid phase synthesis of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Apr 25;13(8):2979–2987. doi: 10.1093/nar/13.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Pace B., Marsh T., Pace N. R. The ribonucleoprotein substrate for a ribosomal RNA-processing nuclease. J Biol Chem. 1984 Sep 25;259(18):11448–11453. [PubMed] [Google Scholar]
- Stark M. J., Gourse R. L., Jemiolo D. K., Dahlberg A. E. A mutation in an Escherichia coli ribosomal RNA operon that blocks the production of precursor 23 S ribosomal RNA by RNase III in vivo and in vitro. J Mol Biol. 1985 Mar 20;182(2):205–216. doi: 10.1016/0022-2836(85)90339-0. [DOI] [PubMed] [Google Scholar]
- Steen R., Dahlberg A. E., Lade B. N., Studier F. W., Dunn J. J. T7 RNA polymerase directed expression of the Escherichia coli rrnB operon. EMBO J. 1986 May;5(5):1099–1103. doi: 10.1002/j.1460-2075.1986.tb04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Szeberényi J., Roy M. K., Apirion D. Precursor nucleotides at the 5' end are not required for processing by RNase E at the 3' end of 5-S rRNA. Eur J Biochem. 1983 Nov 2;136(2):321–326. doi: 10.1111/j.1432-1033.1983.tb07744.x. [DOI] [PubMed] [Google Scholar]
- Szeberényi J., Roy M. K., Vaidya H. C., Apirion D. 7S RNA, containing 5S ribosomal RNA and the termination stem, is a specific substrate for the two RNA processing enzymes RNase III and RNase E. Biochemistry. 1984 Jun 19;23(13):2952–2957. doi: 10.1021/bi00308a016. [DOI] [PubMed] [Google Scholar]
- Young R. A., Steitz J. A. Complementary sequences 1700 nucleotides apart form a ribonuclease III cleavage site in Escherichia coli ribosomal precursor RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3593–3597. doi: 10.1073/pnas.75.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Sequencing psoralen photochemically reactive sites in Escherichia coli 16 S rRNA. Anal Biochem. 1982 Jan 1;119(1):86–89. doi: 10.1016/0003-2697(82)90669-8. [DOI] [PubMed] [Google Scholar]