Abstract
Castor oil is one of the oldest drugs. When given orally, it has a laxative effect and induces labor in pregnant females. The effects of castor oil are mediated by ricinoleic acid, a hydroxylated fatty acid released from castor oil by intestinal lipases. Despite the wide-spread use of castor oil in conventional and folk medicine, the molecular mechanism by which ricinoleic acid acts remains unknown. Here we show that the EP3 prostanoid receptor is specifically activated by ricinoleic acid and that it mediates the pharmacological effects of castor oil. In mice lacking EP3 receptors, the laxative effect and the uterus contraction induced via ricinoleic acid are absent. Although a conditional deletion of the EP3 receptor gene in intestinal epithelial cells did not affect castor oil-induced diarrhea, mice lacking EP3 receptors only in smooth-muscle cells were unresponsive to this drug. Thus, the castor oil metabolite ricinoleic acid activates intestinal and uterine smooth-muscle cells via EP3 prostanoid receptors. These findings identify the cellular and molecular mechanism underlying the pharmacological effects of castor oil and indicate a role of the EP3 receptor as a target to induce laxative effects.
Keywords: G-protein coupled receptor, peristalsis, Ricinus communis, PGE2
Castor oil, also known as Oleum Palmae Christi, is obtained from the seeds of Ricinus communis and has been used therapeutically for centuries (1, 2), being first described in the Ebers papyrus of ancient Egypt more than 3,500 y ago (3). Castor oil is a triglyceride characterized by a high content of the hydroxylated unsaturated fatty acid ricinoleic acid [(9Z,12R)-12-hydroxyoctadec-9-enoic acid] (4). After oral ingestion of castor oil, ricinoleic acid is released by lipases in the intestinal lumen, and considerable amounts of ricinoleic acid are absorbed in the intestine (5, 6). The released ricinoleic acid induces a strong laxative effect (5, 7). There is also a well-documented labor-inducing effect of castor oil in pregnant females at term; however, use of this drug for labor induction is not recommended because of unwanted effects, such as nausea (8).
The mechanisms underlying the pharmacological effects of ricinoleic acid remain elusive. Castor oil is regarded as a stimulant and irritant laxative without known mechanism of action (9). Several studies have shown that relatively high concentrations of ricinoleic acid can cause ultrastructural alterations in the villous tips of the intestinal mucosa (10, 11). Given the high concentrations of ricinoleic acid used in these experiments, it is, however, not clear whether these unspecific morphological effects are relevant for the laxative effect of castor oil. In part, conflicting data have been published with regard to the ability of ricinoleic acid to induce procontractile effects on intestinal smooth muscle and to alter intestinal ion transport and water flux. Although some groups observed an inhibition of water and electrolyte absorption (12–14), others found an activation of ion secretory processes by ricinoleid acid (15). In addition to effects of ricinoleic acid on intestinal ion transport and water flux, evidence has been provided that ricinoleic acid can directly affect intestinal motility (16–19). Whether these effects are mediated by the enteric nervous system or are direct effects on intestinal smooth muscle remained unclear.
The present study was undertaken to elucidate the molecular mechanism underlying the biological effect of castor oil-derived ricinoleic acid. Based on cellular signaling studies and an siRNA screening approach, we identified prostaglandin E2 receptors as targets of ricinoleic acid and show that the EP3 receptor mediates the effects of castor oil on the motility of the uterus and the intestine.
Results
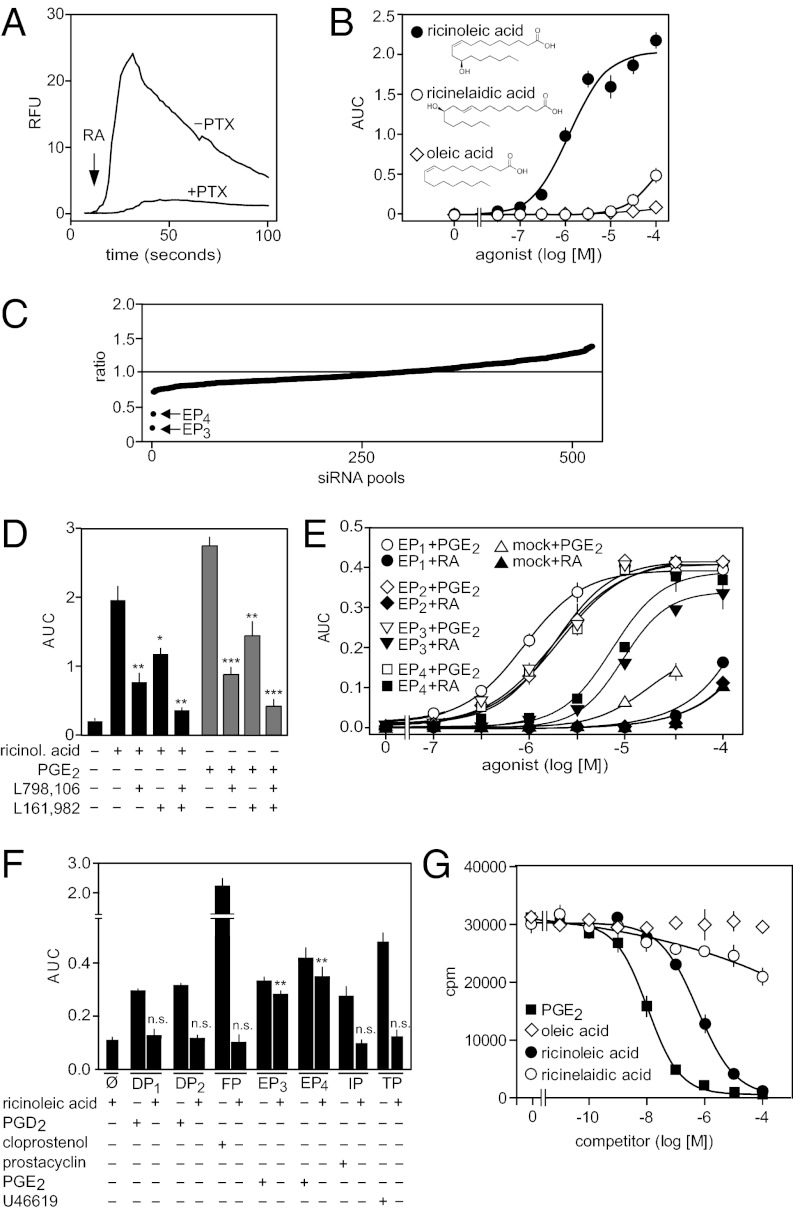
In a screen for potential receptor-mediated effects using a library of biologically active lipids, we observed a Ca2+ transient after exposure of various cell types to ricinoleic acid. The response was strongest in the human megakaryocyte leukemia cell line MEG-01 (Fig. 1A). This effect was dose-dependent with an EC50 of 5 μM (Fig. 1B) and could be blocked by pretreatment of cells with pertussis toxin (Fig. 1A). The biologically inactive trans isomer of ricinoleic acid, ricinelaidic acid [(9E,12R)-12-hydroxyoctadec-9-enoic acid], as well as the nonhydroxylated homolog, oleic acid [(9Z)-octadec-9-enoic acid], were without effect (Fig. 1B). These data suggest that ricinoleic acid can specifically activate a G protein-coupled receptor (GPCR). To identify a putative GPCR activated by ricinoleic acid, we screened a small interfering RNA (siRNA) library targeting all known and predicted nonolfactory human GPCRs for its ability to interfere with activation of MEG-01 cells by ricinoleic acid. Fig. 1C shows that siRNA pools directed against mRNAs encoding EP3 and EP4 (20–22) strongly reduced ricinoleic acid effects in MEG-01 cells. We verified that EP3 and EP4 receptors are expressed in MEG-01 cells and that prostaglandin E2 (PGE2) has effects comparable to ricinoleic acid in these cells (Fig. S1). Consistent with a role of EP3 and EP4 receptors in mediating cellular effects of ricinoleic acid, the selective antagonists of EP3 and EP4 receptors, L-798,106 and L-161,982, respectively, at maximally active concentrations inhibited ricinoleic acid- and PGE2-induced calcium mobilization in MEG-01 cells (Fig. 1D). EP3/EP4-mediated effects of ricinoleic acid were not because of formation of PGE2 in response to ricinoleic acid (Fig. S2 A and B). Consistent with this finding, ricinoleic acid effects were not affected by inhibition of cyclooxygenase (COX)-1 and COX-2 (Fig. S2C).
Fig. 1.
Identification of EP3 and EP4 as ricinoleic acid receptors. (A) Effect of 100 μM ricinoleic acid on [Ca2+]i in untreated (−PTX) or pertussis toxin-treated (100 ng/mL for 16 h; +PTX) MEG-01 cells. Shown is a representative experiment. The arrow indicates the time point of addition of ricinoleic acid (RA). (B) Effect of increasing concentrations of ricinoleic acid and indicated related compounds on [Ca2+]i in MEG-01 cells. (C) siRNA screen to identify GPCRs mediating ricinioleic acid-induced increases in [Ca2+]i in MEG-01 cells. Shown is the ratio of the ricinoleic acid effect on [Ca2+]i in cells transfected with an siRNA pool against a particular human GPCR and cells transfected with control siRNA. The plot shows the ranked average ratios of three independent experiments performed with 514 siRNA pools. (D) Effect of the EP3 receptor antagonist L-798,106 (1 μM) and the EP4 receptor antagonist L-161,982 (1 μM) on ricinoleic acid (30 μM) and PGE2 (3 μM)-induced increases in [Ca2+]i in MEG-01 cells. (E) Effect of increasing concentrations of ricinoleic acid (closed symbols, RA) and PGE2 (open symbols) on [Ca2+]i in CHO-K1 cells transfected with cDNAs encoding human EP1, EP2, EP3, EP4, or transfected with an empty vector (mock) together with a Ca2+-sensitive bioluminescent fusion protein and the promiscuous Gα15. (F) Effect of the indicated stimuli at concentrations of 3 μM or 30 μM (ricinoleic acid) on [Ca2+]i in CHO-K1 cells expressing no receptor (ø) or DP1, DP2, FP, EP3, EP4, IP, or TP together with a promiscuous G protein α-subunit. (G) Effect of PGE2, ricinoleic acid, ricinelaidic acid, and oleic acid at the indicated concentrations on binding of 3H-PGE2 to CHO cells expressing the human EP3 receptor. Shown are mean values ± SEM, n ≥ 3. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; n.s., not significant compared with RA or PGE2 alone (D) or compared with nontransfected cells (ø; F).
To further characterize the effects of ricinoleic acid on prostanoid receptors, we heterologously expressed prostanoid receptors together with the promiscuous G protein α-subunit Gα15 (23) in CHO cells expressing a bioluminescent Ca2+-sensor (24). Among the four human PGE2 receptors (EP1–EP4), only EP3 and EP4 responded to ricinoleic acid (Fig. 1E). PGE2 activated all four receptors, indicating that EP1 and EP2 were functionally expressed (Fig. 1E). The biologically inactive ricinoleic acid isomer ricinelaidic acid was inactive (Fig. S3A). Whereas ricinoleic acid was about one order-of-magnitude less potent than PGE2, the efficacy of ricinoleic acid to activate EP3 and EP4 receptors was comparable with that of PGE2 (Fig. 1E). Ricinoleic acid also activated murine EP3 and EP4 receptors (Fig. S3B). None of the other prostanoid receptors, including IP, DP1, DP2, FP, and TP were activated by ricinoleic acid (Fig. 1F). In contrast to oleic acid, ricinoleic acid was able to displace 3H-PGE2 from EP3 receptors expressed in CHO cells with an IC50 of 500 nM, but ricinelaidic acid hardly competed with PGE2 for binding (Fig. 1G). Taken together, these data show that ricinoleic acid is a selective agonist of EP3 and EP4 receptors.
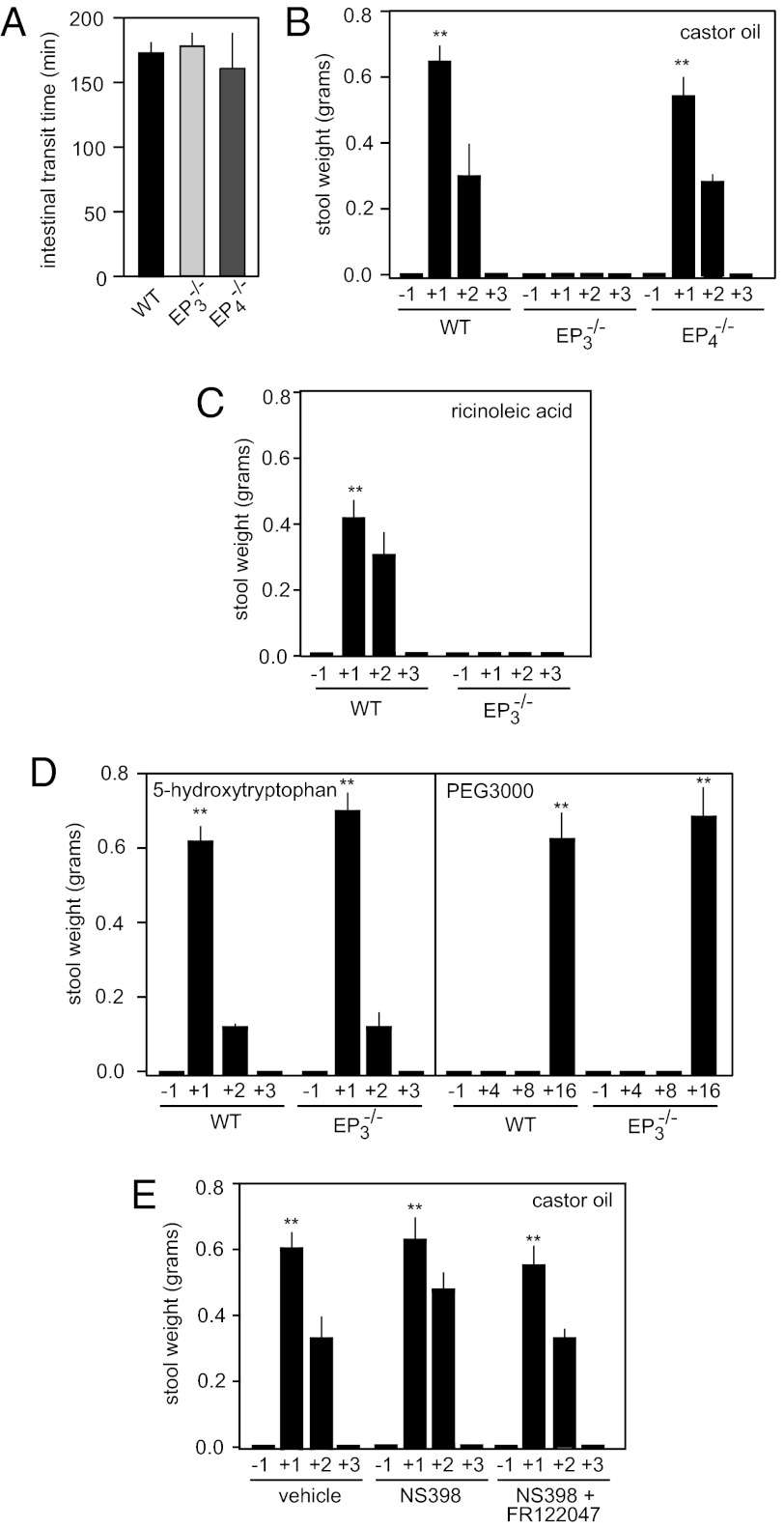
We then analyzed mice lacking EP3 or EP4 receptors (25–27) to test whether these receptors play a role in ricinoleic acid-induced pharmacological effects in vivo. Mice lacking either EP3 (Ptger3−/−; EP3−/−) or EP4 (Ptger4−/−; EP4−/−) showed normal intestinal transit time (Fig. 2A). When given castor oil, wild-type mice responded with a strong diarrhea, starting about 30 min after application. The laxative effect lasted for about 2 h. Interestingly, EP3 receptor-deficient mice were completely unresponsive, whereas mice lacking EP4 receptors responded like wild-type mice (Fig. 2B). Similarly, ricinoleic acid given orally also induced a strong laxative effect, which was abrogated in mice lacking the EP3 receptor (Fig. 2C). Mice lacking EP3 receptors were, however, indistinguishable from wild-type mice with regard to the effect of other laxatives, such as 5-hydroxytryptophan or polyethylene glycol (PEG3000) (Fig. 2D). Because ricinoleic acid has been reported to induce formation of PGE2 in the mammalian intestine (28), we tested the effect of COX inhibition on ricinoleic acid-induced diarrhea. Mice treated with COX-1 and COX-2 inhibitors responded normally to castor oil (Fig. 2E). Thus, ricinoleic acid-induced laxative effects are not a result of formation of prostanoids but are the result of a direct activation of EP3 receptors by ricinoleic acid.
Fig. 2.
EP3 mediates laxative effects of castor oil in vivo. (A) Intestinal transit time of wild-type mice (WT) and mice lacking EP3 (EP3−/−) or EP4 receptors (EP4−/−). (B) Effect of castor oil on feces formation in wild-type (WT), EP3-deficient (EP3−/−), or EP4-deficient mice (EP4−/−). Shown is the feces formation 1 h before (−1) as well as within the first, second, and third hour (+1, +2 +3) after oral application of 8 μL/g body weight castor oil. (C) Effect of ricinoleic acid (7.14 mg/g body weight) on stool formation in wild-type (WT) and EP3-deficient mice (EP3−/−) within the indicated period (h) before and after application of ricinoleic acid. (D) Effect of 5-hydroxytryptophan (1 μg/g body weight intraperitoneally) and polyethylene glycol (PEG3000) (10 μL/g body weight of a 17% wt/vol solution) on feces formation. Stool weight was determined within the indicated periods (h) before and after the treatment. (E) Effect of COX-1 and COX-2 inhibition on castor oil-induced diarrhea. Mice were pretreated with vehicle or 10 μg/g body weight NS398 or NS398 plus 5 μg/g body weight FR122047; 45 min later, castor oil (8 μL/g body weight) was given orally and the stool weight was determined within the indicated periods before and after castor oil application. Shown are mean values ± SEM, n ≥ 5. **P ≤ 0.01 (compared with time period −1).
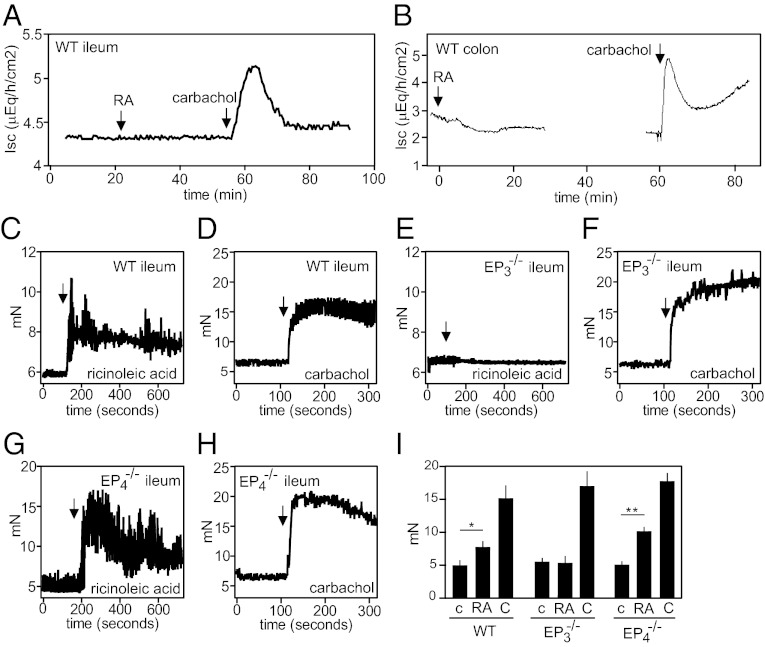
To further analyze the role of EP3 receptors in the pharmacological effects of castor oil, we performed in vitro experiments on isolated small intestine. In flux measurements on intestinal mucosa using the Ussing chamber, we did not observe any effect of ricinoleic acid, but carbachol induced a strong secretory effect (Fig. 3 A and B). However, myographic analysis of ileal segments of the small intestine from wild-type mice showed an increase in contractile activity in response to ricinoleic acid (Fig. 3 C and I), an effect insensitive to inhibition of COX-1 and COX-2 (Fig. S4) and also seen in segments from EP4−/− mice (Fig. 3 G and I). In contrast to wild-type and EP4-deficient ileal segments, the small intestine of EP3−/− mice did not respond to ricinoleic acid (Fig. 3 E and I) but showed a similar response as intestine from wild-type and EP4−/− mice to the muscarinic receptor agonist carbachol (Fig. 3 D, F, H, and I).
Fig. 3.
Ricinoleic acid effects on the intestine in vitro. (A and B) Effect of 100 μM ricinoleic acid (RA) or 100 μM carbachol on short-circuit current (Isc) in the ileum (A) or the distal colon (B) of wild-type mice. (C–I) Effect of 100 μM ricinoleic acid (C, E, G, I) or 100 μM carbachol (D, F, H, I) on the contractile activity of a segment of the ileum from wild-type (C, D, I), EP3−/− (E, F, I), or EP4−/− mice (G, H, I). Panel I shows the average tension during 5 min after addition of vehicle (c), ricinoleic acid (RA), or carbachol (C) to the ileum. Shown are mean values ± SEM *P ≤ 0.05, **P ≤ 0.01.
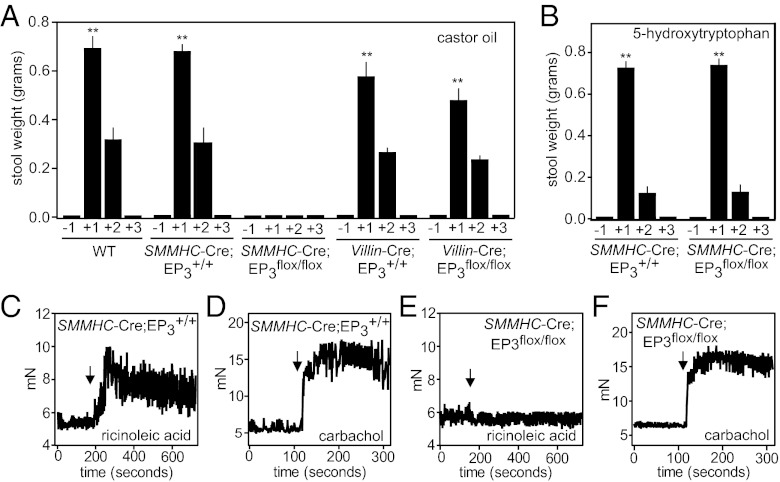
Expression of EP3 receptors has been reported in the intestine as well as in the uterus, the major sites of ricinoleic acid effects (29). In the mammalian intestine, EP3 receptors have been shown to be expressed in epithelial cells, enteric ganglia cells, immune cells, as well as in longitudinal but not circular smooth-muscle layers (22, 29–31). Using EP3 receptor-deficient mice that express β-galactosidase instead of EP3 (25), we could verify expression of EP3 in some epithelial cells, as well as in the longitudinal smooth-muscle layer of the intestine (Fig. S5). To test which cell type in the intestine mediates EP3 receptor-dependent laxative effects of ricinoleic acid, we mated mice carrying a conditional allele of the EP3 receptor (Ptger3flox) (27) with mice expressing the recombinase Cre, either under the control of the villin promoter (32) (villin-Cre) or the smooth-muscle myosin heavy-chain promoter (SMMHC-CreERT2) (33) to specifically induce EP3 receptor-deficiency in epithelial cells or in smooth-muscle cells, respectively. The response to castor oil given orally was indistinguishable between wild-type, SMMHC-CreERT2, villin-Cre, and villin-Cre;Ptger3flox/flox mice (villin-Cre;EP3flox/flox) (Fig. 4A). In contrast, mice in whom EP3 deficiency was induced specifically in smooth-muscle cells (SMMHC-CreERT2;EP3flox/flox) were completely unresponsive (Fig. 4A). The laxative effects of other stimuli, like 5-hydroxytryptophan and PEG3000, were not affected by smooth muscle-specific EP3 deficiency, indicating that these animals were responsive to diarrhea-inducing agents (Fig. 4B and Fig. S6). In addition, we verified absence of EP3 encoding mRNA in the intestinal smooth-muscle layer of smooth muscle-specific EP3 knockout (Fig. S7A), and we found no difference in basal intestinal function of smooth muscle-specific EP3 receptor-deficient mice compared with wild-type animals (Fig. S7B). As expected from the in vivo experiments, intestinal segments from smooth muscle-specific EP3 receptor-deficient mice were not contracted by ricinoleic acid but still responded to carbachol (Fig. 4 C–F). Thus, EP3 receptors on intestinal smooth-muscle cells mediate the laxative effects of ricinoleic acid released from castor oil.
Fig. 4.
Effects of castor oil on the intestine are mediated by EP3 expressed by smooth-muscle cells. (A and B) Effect of 8 μL/g body weight castor oil (A) or 1 μg/g body weight 5-hydroxytryptophan intraperitoneally (B) on feces excretion in wild-type mice (WT), mice with smooth muscle-specific EP3 receptor deficiency (SMMHC-CreERT2;EP3flox/flox), mice with intestinal epithelial cell-specific EP3 receptor deficiency (villin-Cre;EP3flox/flox), or mice expressing Cre in smooth muscle cells or intestinal epithelial cells (SMMHC-CreERT2;EP3+/+ and villin-Cre;EP3+/+, respectively). Shown is the excretion of feces during the indicated periods before and after application of the diarrhea-inducing agents. Shown are mean values ± SEM, n ≥ 6. **P ≤ 0.01 (compared with time period −1). (C–F) Effect of 100 μM ricinoleic acid (C and E) or 100 μM carbachol (D and F) on the contractile activity of segments of the ileum prepared from mice expressing the Cre recombinase in smooth muscle cells (SMMHC-CreERT2;EP3+/+) (C and D) or mice with smooth muscle-specific EP3 receptor deficiency (SMMHC-CreERT2;EP3flox/flox) (E and F). Arrows indicate time points of stimuli application. All animals carrying the SMMHC-CreERT2 transgene had been induced by treatment with tamoxifen.
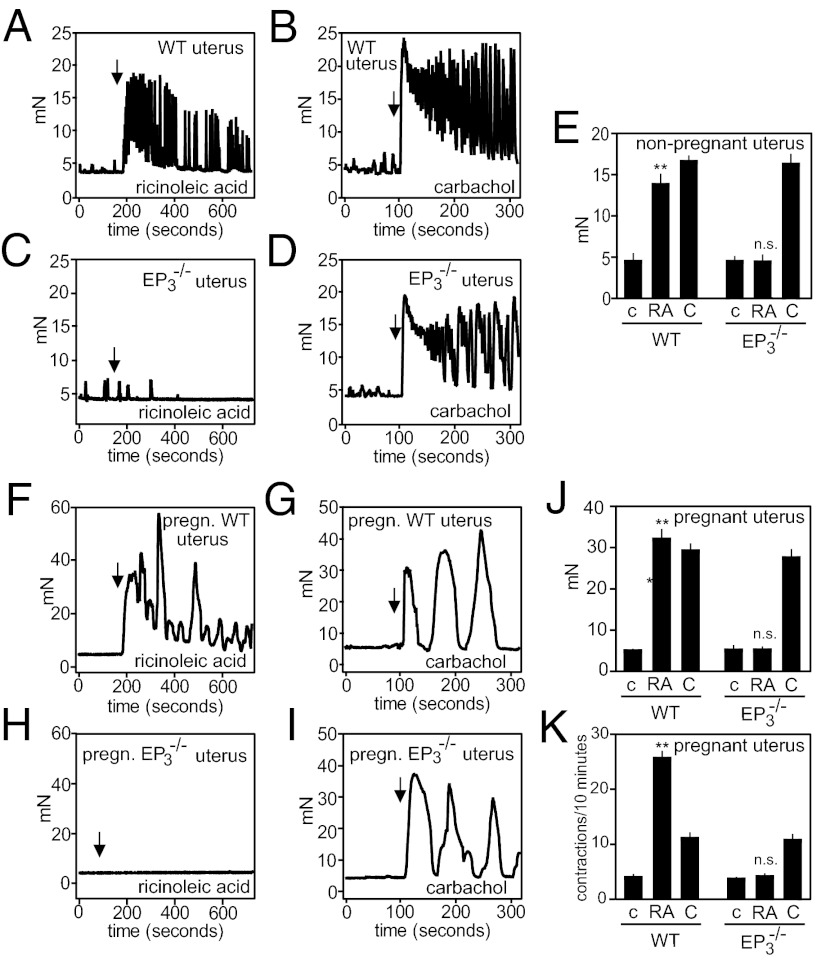
Given the labor-inducing effects of castor oil and the expression of EP3 in the myometrium (7, 8, 34, 35) (Fig. S5C), we measured the effect of ricinoleic acid on contractility in nonpregnant and pregnant uteri. Whereas nonpregnant and pregnant wild-type uteri showed a strong increase in magnitude and frequency of contractions when exposed to ricinoleic acid (Fig. 5 A, E, F, J, and K), uteri from EP3 receptor-deficient mice did not respond at all (Fig. 5 C, E, H, J, and K). Both wild-type and EP3-deficient uteri from nonpregnant and pregnant mice contracted when exposed to carbachol (Fig. 5 B, D, E, G, I, J, and K).
Fig. 5.
Ricinoleic acid augments contraction of uterine smooth muscle via EP3. Effect of 100 μM ricinoleic acid (A, C, E, F, H, J, K) or 100 μM carbachol (B, D, E, G, I, J, K) on the contractile activity of uteri prepared from nonpregnant (A–E) or pregnant (embryonic day 17.5, F–K) wild-type (A, B, E, F, G, J, K) or EP3−/− mice (C–E, H–K). Arrows indicate the time point of addition of stimuli. E and J show the average tension during 5 min, and K shows the number of uterus contraction during 10 min after addition of vehicle (c), ricinoleic acid (RA), or carbachol (C) to uteri. Shown are mean values ± SEM **P ≤ 0.01; n.s., not significant [compared with respective control (c)].
Discussion
Castor oil, a natural triglyceride containing mainly ricinoleic acid, has a long history as a remedy because of its various biological effects, including an increase in propulsive intestinal motility. Based on the observation that ricinoleic acid, which is released from castor oil by intestinal lipases, induces calcium transients in various cells, and by using a siRNA screen against all nonolfactory GPCRs, we found that ricinoleic acid is a selective agonist of EP3 and EP4 receptors. Using mice with constitutive and conditional EP3 or EP4 receptor deficiency, we show that the pharmacological effects of castor oil are mediated by activation of EP3 receptors on smooth-muscle cells. This discovery provides the long-sought mechanism of action of one of the oldest drugs, which is still used in conventional, alternative, and folk medicine. The unexpected, highly specific mechanism of action may promote a reevaluation of the medical use of castor oil and suggests novel approaches to increase intestinal motility.
Consistent with a specific mechanism, the pharmacological activity of ricinoleic acid shows a strong dependency on the structure of the drug as the trans isomer ricinelaidic acid, as well as the nonhydroxylated fatty acid oleic acid are without effect (7). This structure-activity relationship can also be found with regard to the ability of ricinoleic acid and related fatty acids to activate the EP3 and EP4 receptor. Although both PGE2 and ricinoleic acid are unsaturated and hydroxylated fatty acids, the ability of ricinoleic acid to act as an EP3/EP4 receptor agonist was not foreseeable with the currently available tools. Given the high specificity of ricinoleic acid for EP3 and EP4 receptors, it may be of interest to further explore the potential of hydroxylated fatty acids to specifically activate prostanoid receptors.
PGE2 and EP receptors have been implicated in the regulation of intestinal and uterine functions (30, 34). Both, pharmacological and molecular biology studies showed the presence of EP3 receptors in the pregnant uterus, and activation of EP3 receptors has been demonstrated to evoke contraction of uterine smooth muscle (35, 36). In fact, the PGE1 and PGE2 analogs misoprostol and sulprostone can be used to induce labor (37). In the small intestine, EP3 receptors are expressed in smooth-muscle cells, neurons, as well as in some epithelial cells. Although the procontractile effect of PGE2 on isolated intestinal smooth muscle involves EP1, EP3, and FP receptors (38), it has been unclear which receptor is responsible for the increase in peristalsis induced by PGE2 (39). Based on in vitro studies using cells endogenously or heterologously expressing prostanoid receptors, as well as on the use of inducible and tissue-specific EP3 receptor-deficient mice, our data clearly indicate that the EP3 receptor on intestinal smooth-muscle cells mediates the laxative effect of ricinoleic acid and, therefore, is a major prostanoid receptor in the intestine mediating propulsive effects on gut motility.
The fact that ricinoleic acid has been shown to release prostanoids from intestinal tissue under in vitro conditions (28) raises the question whether PGE2 acting on EP3 receptors contributes to the pharmacological effects of castor oil-derived ricinoleic acid. In animals with blocked COX-1 and COX-2 activity, castor oil- and ricinoleic acid-induced effects were not affected. Thus, PGE2, which may be formed in response to ricinoleic acid, is not sufficient to mediate the effects, and ricinoleic acid acting on EP3 receptors is responsible for castor oil-induced laxative and labor-inducing effects.
The clinical use of castor oil as a laxative has been decreasing in conventional medicine, but castor oil is still used widely in alternative and folk medicine. Lack of knowledge regarding the mechanism of action of castor oil may have contributed to the reduced use of castor oil in academic medicine. Despite older reports that castor oil, when given in adequate doses, is a well-tolerated and safe laxative (40), more recently the general view has emphasized the unwanted effects of castor oil, such as unpleasant taste, cramps, and danger of fluid and electrolyte losses because of stimulation of water and electrolyte secretion (9). Regarding the basic mechanism of the laxative effect, we found no evidence for an effect of ricinoleic acid on electrolyte and water fluxes in the mucosa of the ileum and the colon. Instead, castor oil and ricinoleic acid induce contraction of intestinal smooth muscle, an effect that is consistent with the expression of the EP3 receptor in the longitudinal smooth-muscle layer of the intestinum. These data provide a mechanism underlying the laxative effects of castor oil, and they suggest that an orally available specific EP3 receptor agonist with high first-pass effect may be a relatively safe laxative that produces an efficient properistaltic effect without causing additional water and electrolyte losses because of prosecretory effects.
The fact that ricinoleic acid also acts on EP4 receptors raises the question whether some of the castor oil effects are also mediated by this prostanoid receptor subtype. In mice lacking EP4 receptors, castor oil-induced laxation was not affected, strongly indicating that this effect indeed requires EP3 but not EP4 receptors. Given the expression of EP4 receptor in some epithelial cells of the intestine, as well as in immune cells (31, 41), it may be interesting to test the effect of castor oil on regenerative and inflammatory processes involving the intestinal epithelium (42, 43).
Here we report on the mechanism by which castor oil exerts its effects on gut and uterus motility. We show that the active component of castor oil, ricinoleic acid, is a selective agonist of EP3 and EP4 receptors, and that the pharmacological effects of castor oil are mediated by activation of EP3 receptors on smooth-muscle cells. This remarkable specificity of the mechanism of action of castor oil is rather unexpected because castor oil has been regarded as an agent that exerts its effects through unspecific mechanisms. In addition, our data indicate that EP3 receptors are potential targets for drugs to induce laxation.
Methods
Reagents.
Ricinoleic acid, ricinelaidic acid, oleic acid, carbachol, 5-hydroxytryptophan, PEG3000, and pertussis toxin were from Sigma-Aldrich. PGE2, PGD2, NS398, L161,982, cloprostenol, prostacyclin and U46619 were from Cayman Chemical. FR122047 and l-798,106 were from Tocris. Fura-2/AM was from Invitrogen.
Cell Transfection and Determination of [Ca2+]i.
MEG-01 cells were seeded on a 96-well plate and loaded with 5 μM Fura-2/AM in calcium-free HBSS containing 10 mM Hepes, pH 7.4, for 1 h at 37 °C. Cells were washed in HBSS containing 1.8 mM Ca2+ and were then transferred to an automated fluorescent plate reader (Flexstation-3; Molecular Devices). After ligand stimulation, calcium transients were recorded as relative fluorescence units (RFU) for 2 min, and the integrated area under the calcium transients was quantified by using SoftMaxPro (Molecular Devices) and expressed as area under the curve (AUC).
For studies of heterologously expressed receptors, CHO-K1 cells stably expressing a calcium-sensitive bioluminescent fusion protein consisting of aequorin and green fluorescent protein (24) were seeded in 96-well plates and transfected with indicated cDNAs or control DNA (50 ng/well) using FuGENE6 reagent (Roche Diagnostics), as previously described (44). Two days after transfection, cells were loaded with 5 μM coelenterazine h (Invitrogen) in calcium-free HBSS containing 10 mM Hepes, pH 7.4, for 3.5 h at 37 °C. Forty-five minutes before experiments, the buffer was replaced with HBSS containing 1.8 mM CaCl2. Measurements were performed by using a luminometer plate reader (Luminoskan Ascent; Thermo Electron). The area under each calcium transient (measured for 1 min) has been calculated using Ascent software (Thermo Electron) and expressed as AUC.
siRNA Screening.
Four separate siRNAs of a siRNA library directed against 514 genes including 407 nonolfactory human GPCRs and 86 olfactory human GPCRs (Qiagen) targeting the same mRNA were pooled. MEG-01 cells were reverse-transfected with siRNA pools at a final concentration of 5 nM for each siRNA. Seventy-two hours later, cells were loaded with Fura-2/AM and ricinoleic acid-induced calcium transients were recorded and analyzed as described above. Ratios of AUC of ricinoleic acid induced calcium transients in cells transfected with siRNA pools targeting a particular GPCR and AUC of effects in cells transfected with scrambled siRNA were determined.
RT-PCR.
RNA was isolated from MEG-01 cells with the RNeasy Mini Kit (Qiagen). For reverse-transcription reaction, 1 μg total RNA was reverse-transcribed. cDNA synthesis was monitored by PCR of a 401-bp fragment of glyceraldehyde-3-phosphate dehydrogenase.
Genetic Mouse Models.
EP3- and EP4-deficient mice have been described previously (25, 26) and were kept in the animal house of the Institute for Clinical Pharmacology of the J. W. Goethe University, Frankfurt. Ptger3flox/flox mice (Jackson Laboratories) were used for the conditional deletion experiment and also served as a source for global EP3 receptor-deficient mice after crossing with EIIa-Cre (45) animals. The inducible SMMHC-CreERT2 line has been described previously (33), and villin-Cre mice were from Jackson Laboratories. Cre activity was induced by intraperitoneal administration of tamoxifen (50 μL of a 20 mg/mL solution in miglyol per mouse) for 5 consecutive days. Experiments were performed at least 7 d after the last injections. All animal experiments were approved by the Regierungspräsidia Karlsruhe and Darmstadt.
Determination of Laxative Effects.
To measure the laxative effects of different substances, mice were starved 16 h before the experiments. On the day of the experiment, mice were treated with the indicated substances, and the bottom of the cages was covered with white tissue paper. The weight of shapeless and watery stools was determined hourly.
Determination of Intestinal Transit Time.
Total intestinal transit time was measured as described previously (46). Briefly, mice were given carmine red solution orally (150 μL tap water containing 3 mg carmine red). Mice were returned to individual cages covered with white paper. The time taken until the excretion of red feces was measured.
Myography.
See SI Methods.
Ussing Chamber Experiments.
See SI Methods.
Radioligand Binding Assay.
See SI Methods.
Statistics.
Unless indicated, data are expressed as means ± SEM. Statistical analyses of differences between two groups were performed by nonparametric, unpaired, two-tailed Mann–Whitney test. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors wish to thank Svea Hümmer for excellent secretarial help. This work was supported by the German Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201627109/-/DCSupplemental.
References
- 1.Gaginella TS, Phillips SF. Ricinoleic acid: Current view of an ancient oil. Am J Dig Dis. 1975;20:1171–1177. doi: 10.1007/BF01070759. [DOI] [PubMed] [Google Scholar]
- 2.Scarpa A, Guerci A. Various uses of the castor oil plant (Ricinus communis L.). A review. J Ethnopharmacol. 1982;5:117–137. doi: 10.1016/0378-8741(82)90038-1. [DOI] [PubMed] [Google Scholar]
- 3.Nunn J. Ancient Egyptian Medicine. London: The British Museum Press; 1996. [Google Scholar]
- 4.Saalmüller L. Ueber die fetten Säuren des Ricinusöls. [On the fatty acids of castor oil.] Justus Liebigs Ann Chem. 1848;64:108–126. German. [Google Scholar]
- 5.Meyer H. Ueber den wirksamen Bestandtheil des Ricinusöls. [On the active component of castor oil.] Arch Exp Path Pharmak. 1890;28:145–152. German. [Google Scholar]
- 6.Watson WC, Gordon RS., Jr Studies on the digestion, absorption and metabolism of castor oil. Biochem Pharmacol. 1962;11:229–236. doi: 10.1016/0006-2952(62)90078-3. [DOI] [PubMed] [Google Scholar]
- 7.Burdock GA, Carabin IG, Griffiths JC. Toxicology and pharmacology of sodium ricinoleate. Food Chem Toxicol. 2006;44:1689–1698. doi: 10.1016/j.fct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Garry D, Figueroa R, Guillaume J, Cucco V. Use of castor oil in pregnancies at term. Altern Ther Health Med. 2000;6:77–79. [PubMed] [Google Scholar]
- 9.Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux; anti-emetics; agents used in biliary and pancreatic disease. In: Brunton LL, editor. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12 Ed. New York: McGraw Hill; 2011. pp. 1323–1349. [Google Scholar]
- 10.Cline WS, Lorenzsonn V, Benz L, Bass P, Olsen WA. The effects of sodium ricinoleate on small intestinal function and structure. J Clin Invest. 1976;58:380–390. doi: 10.1172/JCI108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaginella TS, Haddad AC, Go VL, Phillips SF. Cytotoxicity of ricinoleic acid (castor oil) and other intestinal secretagogues on isolated intestinal epithelial cells. J Pharmacol Exp Ther. 1977;201:259–266. [PubMed] [Google Scholar]
- 12.Bright-Asare P, Binder HJ. Stimulation of colonic secretion of water and electrolytes by hydroxy fatty acids. Gastroenterology. 1973;64:81–88. [PubMed] [Google Scholar]
- 13.Ammon HV, Phillips SF. Inhibition of ileal water absorption by intraluminal fatty acids. Influence of chain length, hydroxylation, and conjugation of fatty acids. J Clin Invest. 1974;53:205–210. doi: 10.1172/JCI107539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammon HV, Thomas PJ, Phillips SF. Effects of oleic and ricinoleic acids on net jejunal water and electrolyte movement. Perfusion studies in man. J Clin Invest. 1974;53:374–379. doi: 10.1172/JCI107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racusen LC, Binder HJ. Ricinoleic acid stimulation of active anion secretion in colonic mucosa of the rat. J Clin Invest. 1979;63:743–749. doi: 10.1172/JCI109358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwao I, Terada Y. On the mechanism of diarrhea due to castor oil. Jpn J Pharmacol. 1962;12:137–145. doi: 10.1254/jjp.12.137. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JJ, Gaginella TS, Bass P. Actions of ricinoleic acid and structurally related fatty acids of the gastrointestinal tract. I. Effects on smooth muscle contractility in vitro. J Pharmacol Exp Ther. 1975;195:347–354. [PubMed] [Google Scholar]
- 18.Izzo AA, Mascolo N, Viola P, Capasso F. Inhibitors of nitric oxide synthase enhance rat ileum contractions induced by ricinoleic acid in vitro. Eur J Pharmacol. 1993;243:87–90. doi: 10.1016/0014-2999(93)90172-e. [DOI] [PubMed] [Google Scholar]
- 19.Mathias JR, Martin JL, Burns TW, Carlson GM, Shields RP. Ricinoleic acid effect on the electrical activity of the small intestine in rabbits. J Clin Invest. 1978;61:640–644. doi: 10.1172/JCI108975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: Subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 21.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 23.Offermanns S, Simon MI. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- 24.Baubet V, et al. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc Natl Acad Sci USA. 2000;97:7260–7265. doi: 10.1073/pnas.97.13.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ushikubi F, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 26.Segi E, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 27.Lazarus M, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 28.Capasso F, Tavares IA, Tsang R, Rennie JA, Bennett A. Eicosanoid formation by mammalian intestine. Effects of some intestinal secretagogues. Eur J Pharmacol. 1987;138:107–113. doi: 10.1016/0014-2999(87)90344-x. [DOI] [PubMed] [Google Scholar]
- 29.Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology. LXXXIII: Classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev. 2011;63:471–538. doi: 10.1124/pr.110.003517. [DOI] [PubMed] [Google Scholar]
- 30.Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto K, et al. Cellular localization of mRNAs for prostaglandin E receptor subtypes in mouse gastrointestinal tract. Am J Physiol. 1997;272:G681–G687. doi: 10.1152/ajpgi.1997.272.3.G681. [DOI] [PubMed] [Google Scholar]
- 32.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 33.Wirth A, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 34.Myatt L, Lye SJ. Expression, localization and function of prostaglandin receptors in myometrium. Prostaglandins Leukot Essent Fatty Acids. 2004;70:137–148. doi: 10.1016/j.plefa.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Senior J, Marshall K, Sangha R, Clayton JK. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br J Pharmacol. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotani M, et al. Multiple signal transduction pathways through two prostaglandin E receptor EP3 subtype isoforms expressed in human uterus. J Clin Endocrinol Metab. 2000;85:4315–4322. doi: 10.1210/jcem.85.11.6989. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg AB, Greenberg MB, Darney PD. Misoprostol and pregnancy. N Engl J Med. 2001;344:38–47. doi: 10.1056/NEJM200101043440107. [DOI] [PubMed] [Google Scholar]
- 38.Okada Y, et al. Characterization of prostanoid receptors mediating contraction of the gastric fundus and ileum: Studies using mice deficient in prostanoid receptors. Br J Pharmacol. 2000;131:745–755. doi: 10.1038/sj.bjp.0703627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahbazian A, Heinemann A, Peskar BA, Holzer P. Differential peristaltic motor effects of prostanoid (DP, EP, IP, TP) and leukotriene receptor agonists in the guinea-pig isolated small intestine. Br J Pharmacol. 2002;137:1047–1054. doi: 10.1038/sj.bjp.0704958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fingl E. Laxatives and cathartics. In: Goodman LS, Gilman A, editors. The Pharmacological Basis of Therapeutics. 5 Ed. New York: MacMillan; 1975. pp. 976–986. [Google Scholar]
- 41.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 42.Kabashima K, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang GL, et al. The prevention of colitis by E Prostanoid receptor 4 agonist through enhancement of epithelium survival and regeneration. J Pharmacol Exp Ther. 2007;320:22–28. doi: 10.1124/jpet.106.111146. [DOI] [PubMed] [Google Scholar]
- 44.Tunaru S, Lättig J, Kero J, Krause G, Offermanns S. Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G) Mol Pharmacol. 2005;68:1271–1280. doi: 10.1124/mol.105.015750. [DOI] [PubMed] [Google Scholar]
- 45.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA. 2007;104:7699–7704. doi: 10.1073/pnas.0609778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.