Abstract
Dendritic cells (DCs) and B cells present antigen-derived peptides bound to MHC class II (MHC II) molecules for recognition by CD4-positive T lymphocytes. DCs control the intracellular traffic of peptide–MHC II complexes by regulating the ubiquitination of MHC II. In resting or “immature” DCs, ubiquitinated MHC II molecules are targeted to lysosomes, but upon pathogen-induced “maturation,” ubiquitination is down-regulated and MHC II can accumulate on the plasma membrane of mature DCs. Although B cells constitutively ubiquitinate their MHC II, it unexpectedly remains at the surface. We find that DCs and B cells differ in MHC II-conjugated ubiquitin (Ub) chain length: four to six Ub in immature DCs vs. two to three in B cells. In both cell types, experimentally increasing Ub chain length led to efficient lysosomal transport of MHC II, whereas MHC II with fewer than two Ubs did not reach lysosomes. Thus, Ub chain length plays a crucial role in regulating the intracellular fate and function of MHC II in DCs and B cells.
Dendritic cells (DCs) and B lymphocytes are professional antigen-presenting cells (APCs) capable of stimulating efficient T-cell responses (1, 2). However, their approaches to antigen presentation differ in important respects. Whereas DCs are highly endocytic and internalize a wide variety of antigens, B cells take up and process only the single antigen recognized by their B-cell receptor. DCs are also distinguished by their ability to regulate antigen processing and presentation by “maturation” (3, 4). Immature DCs, found in peripheral tissues, are adept at endocytic uptake of antigen but do not efficiently generate peptide–MHC class II (MHC II) complexes or express them stably on the cell surface. In part, this is because MHC II in immature DCs is ubiquitinated on a single conserved lysine in the cytoplasmic domain of the β-chain (5, 6) by E3 ligases of the membrane-associated RING-CH (MARCH) family (7, 8). Like other ubiquitinated membrane proteins (9), ubiquitinated MHC II molecules are targeted to and sequestered in multivesicular late endosomes and lysosomes. Upon receiving a maturation stimulus (e.g., Toll-like receptor agonist), however, ubiquitination ceases (5, 6) and peptide–MHC II complexes are translocated to and accumulate at the plasma membrane (10–13). In B cells, MHC II surface expression is always high despite also being ubiquitinated by MARCH ligases in naïve B cells (8).
Internalization and down-regulation of receptor tyrosine kinases by ubiquitination is well known. Ligand binding activates the kinase, resulting in autophosophorylation and subsequent recruitment of soluble E3 ligases (e.g., Cbl) that ubiquitinate one or more acceptor lysines. The ubiquitin (Ub) moieties are recognized by Ub-interaction motif (UIM)-containing adapter molecules (e.g., epsins, eps15) that associate with clathrin-coated pits, leading to receptor internalization (14–18). Upon delivery to early endosomes, Ub is recognized by members of the endosomal sorting complex required for transport (ESCRT) complexes 0–III, which prevent receptor recycling by facilitating entry of ubiquitinated cargo into nascent invaginations of the endosomal membrane (19). It is not known whether clathrin-coated pits and the ESCRT machinery recognize Ub similarly, or whether recognition requires a single Ub added to a single lysine, multiple lysines, or chains of Ub added to one or more sites (20–24). Nor is it known why ubiquitinated MHC II in naïve B cells remains on the surface, whereas in immature DCs it is sequestered in late endocytic compartments. Here, we show that differences in MHC II trafficking between DCs and B cells are a consequence of differences in Ub chain length, not cell type.
Results
MHC II Ubiquitination, Localization, and Endocytosis Differ Between DCs and B Cells.
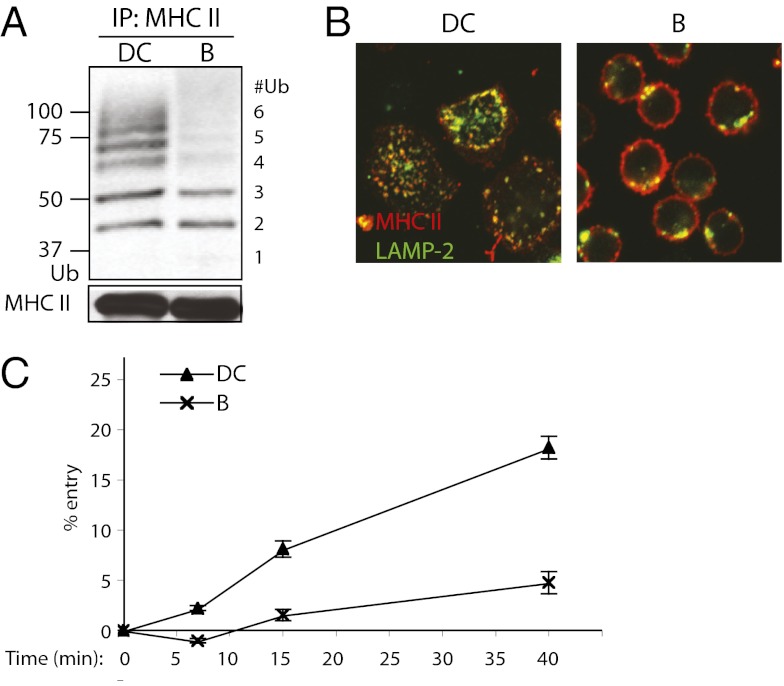
Given the different fates of ubiquitinated MHC II in DCs and B cells, we first asked whether the two cell types exhibited quantitative or qualitative differences in ubiquitination. Remarkably, Ub chain lengths were quite different, with up to six Ub monomers conjugated to MHC II in primary mouse bone marrow-derived DCs (BMDCs) but only two to three in splenic B cells (Fig. 1A). The difference in Ub chain length correlated with a difference in MHC II localization. In immature DCs, MHC II was predominantly in late endosomes and lysosomes, whereas in resting B cells it was primarily on the cell surface (Fig. 1B).
Fig. 1.
Differences in Ub chain length on MHC II of splenic DCs and splenic B cells correlate with differences in MHC II endocytosis. (A) Western blot of splenic DC and splenic B-cell (B) MHC II immunoprecipitates. MHC II was immunoprecipitated (IP) with TIB120 antibody; IPs were immunoblotted (IB) with anti-Ub antibody P4D1 and MHC II β-chain antibody Thorax. (B) Confocal microscopy of wild-type splenic DCs and splenic B cells. Cells were bound to coverslips, fixed in paraformaldehyde (PFA) and labeled with anti-MHC II antibody TIB120 (red) and lysosomal marker LAMP-2 (green). (C) Endocytosis of surface MHC II in splenic DCs and B cells. Cells were bound to anti-MHC II antibody TIB120 on ice and washed. This was followed by incubating the cells at 37 °C for the various times indicated to allow internalization of antibody-bound surface MHC II. An acidic wash (pH 3.0) was then used to strip the surface of remaining antibody. Internalized MHC II was protected from the acid stripping and detected by flow cytometry. Mean fluorescence intensity (MFI) values of MHC II and SEM were determined, and internalization is expressed as percentages of control MFI levels. Control cells were treated identically except for substituting a PBS wash for the acidic wash.
The difference also correlated with the ability of both cell types to internalize MHC II. We monitored endocytosis by binding fluorescent anti-MHC II monoclonal antibody to DCs or B cells on ice, then warming the cells to 37 °C for various times. Surface-bound antibody was removed by incubation at pH 3.0, and acid-resistant, internalized antibody was quantified by flow cytometry. DCs internalized antibody more efficiently than B cells, even from the first time point (7 min) (Fig. 1C). By 40 min, DCs had internalized ∼20% of surface MHC II, whereas B cells internalized <5%.
MHC II Trafficking Is Altered by Variations in Ubiquitination in DCs.
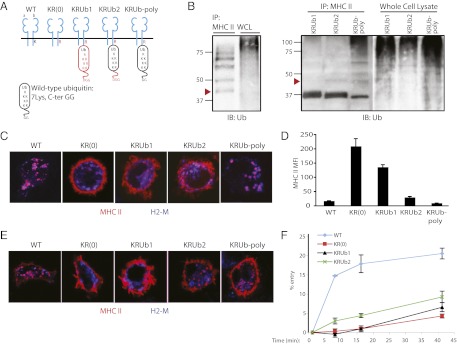
Because the difference between DCs and B cells might reflect cell type-specific differences in endocytosis rather than Ub chain length, we first reduced the number of Ubs added to MHC II in DCs. For this purpose, we constructed a ubiquitination-incompetent MHC II β-chain mutant whose single cytoplasmic lysine was converted to arginine (KR), and to which was fused a cassette encoding a single Ub at the C terminus of the β-chain. The Ub fusion provided two opportunities for further ubiquitination: (i) as a Ub donor via an isopeptide bond between its two C-terminal glycines and a substrate’s lysine, and (ii) as a Ub acceptor for E3 ligases via its own internal lysine residues (25). We therefore prepared two additional KR fusions using mutant Ub cDNAs in which the di-glycine motif was deleted alone or with K > R mutations of all seven internal Ub lysines (Fig. 2A). The control and mutant MHC II β-chain–Ub (KRUb) constructs were retrovirally expressed in DCs from MHC II β-chain−/− mice, as described previously (5, 12).
Fig. 2.
Expression and endocytosis of MHC II–Ub fusion proteins in DCs differ depending on Ub extension. (A) Diagram of MHC II: wild-type and Ub fusion constructs. Mutations (red) include a K > R mutation in MHC II β-chain and variations in fused monoubiquitin, including C-terminal di-glycine deletion and seven K > R mutations. (B) Western blots of MHC II IPs and whole-cell lysate (WCL). MHC II β-chain−/− BMDC cultures were retrovirally transduced to express wild-type MHC II (Left) or MHC II–Ub fusion constructs (Right). MHC II was immunoprecipitated from cell lysates with anti-MHC II antibody TIB120. IPs and WCLs were immunoblotted with anti-Ub antibody P4D1. Arrowheads approximate band migration of di-ubiquitinated MHC II. (C) Confocal microscopy of transduced BMDCs. Cells were bound to coverslips, fixed in PFA, and labeled with TIB120 (red) and lysosomal marker H2-M (blue). (D) MFI of surface-bound MHC II. Data are expressed as mean and SEM. (E) Confocal microscopy of surface MHC II uptake. Transduced BMDCs were incubated with TIB120 for 1 h at 37 °C for uptake, extensively washed, fixed, and stained to detect TIB120 (red) and H2-M (blue). (F) Endocytosis of surface MHC II. Acid wash protocol was as described in Fig. 1C.
Western blot for Ub after an immunoprecipitation of MHC II revealed two (red arrowhead) to six Ubs on control MHC II (Fig. 2B, Left). KR fused to wild-type Ub not only exhibited the two to six Ub species but also the 37-kDa monoubiquitinated MHC II input and higher-order poly-ubiquitinated species (designated KRUb-poly) (Fig. 2B, Right, right lane). Although the predominant species in KRUb constructs fused to the lysine and glycine Ub mutants (KRUb1, KRUb2) was the mono-Ub β-chain input, the KRUb with only the di-glycine deletion (KRUb2) also contained a small amount of di-ubiquitinated β-chain [Fig. 2B, KRUb2 (red arrowhead)]. Although imperfect, this panel of constructs enabled expression of MHC II bearing one, two, or multiple Ubs: KRUb1, KRUb2, and KRUb-poly. An entirely Ub-deficient MHC II was provided by expressing the KR mutant without fused Ub: KR(0).
As expected, wild-type MHC II was found intracellularly with relatively little surface expression, whereas KR(0) was largely at the plasma membrane (Fig. 2C). Surprisingly, KRUb1 localization was barely distinguishable from KR(0), suggesting that a single Ub is not a sufficient signal for MHC II internalization and/or lysosomal targeting, much like what has been seen in experiments using CD4, which internalizes at the same rate unmodified as monoubiquitinated (26). With one additional Ub in KRUb2, a small amount of MHC II accumulation in H2-M–positive late endocytic compartments was detected. In fact, KRUb2 expression in DCs was reminiscent of endogenous MHC II localization in B cells (Fig. 2C vs. Fig. 1B). Localization of KRUb-poly resembled that of endogenous oligoubiquitinated MHC II in immature DCs.
The surface expression of MHC II for each transductant was quantified by flow cytometry (Fig. 2D). The reduction in surface MHC II in KRUb2 or KRUb-poly was dramatic, with both exhibiting low levels of surface expression comparable to that found in cells expressing wild-type MHC II.
We next determined whether Ub chain length also affected internalization and lysosomal targeting of surface MHC II by monitoring the continuous uptake of anti-MHC II antibody (1 h at 37 °C). Similar to the results obtained for MHC II distribution at steady state (Fig. 2C), KR(0)- and KRUb1-expressing DCs bound but failed to internalize appreciable amounts of antibody (Fig. 2E). Significant internalization, however, was exhibited by KRUb2- and KRUb-poly–expressing DCs. These results were similar to antibody uptake by DCs expressing wild-type MHC II (Fig. 2E), although it was unclear whether antibody internalized by KRUb2-expressing cells was delivered efficiently to H2-M–positive late endosomes and lysosomes. We presume the nonlysosomal structures represent early endosomes, but staining with antibodies to endogenous early endosome markers (e.g., EEA1) was too inefficient to test colocalization reliably.
We next used flow cytometry to monitor the internalization of antibody prebound at 0 °C. As shown in Fig. 2F, relatively little endocytosis was observed for cells expressing KR(0) or KRUb1. KRUb2-expressing DCs did internalize a significantly greater amount of antibody at all time points, albeit less than in cells expressing wild-type MHC II. This may reflect enhanced efficiency of the internalization step or greater antibody accumulation by lysosomal targeting. Antibody uptake by KRUb-poly–expressing cells could not be detected owing to the low levels of antibody bound at 0 °C to surface MHC II in most cells (Fig. 2D) and a possible partial impairment of internalization of antibody bound at 37 °C in others (Fig. 2E). Similar data were obtained using monovalent anti-MHC II Fab fragments, indicating that the results were not influenced by cross-linking.
Taken together, these data demonstrate that monoubiquitination is insufficient for internalization and lysosomal delivery of surface MHC II in DCs. Each additional Ub facilitated increased MHC II endocytosis or lysosomal accumulation, although efficient lysosomal accumulation was favored with chain lengths greater than two Ubs. Most importantly, reducing the MHC II Ub chain length in immature DCs produced a localization pattern reminiscent of that in B lymphocytes: predominantly cell surface.
Ubiquitinated MHC II in B Cells Behaves Similarly to DCs.
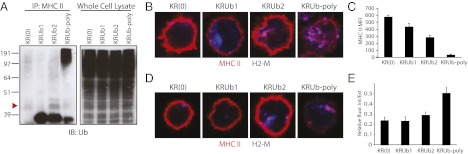
Using the same panel of KRUb constructs, we next asked whether increasing Ub chain length would enhance MHC II endocytosis and lysosomal delivery in B cells. We retrovirally infected MHC II β-chain−/− B cells activated by overnight incubation with LPS to stimulate proliferation, a necessary step to prepare cells for retroviral infection. We observed similar MHC II ubiquitination patterns as in DCs, although KRUb-poly produced fewer di- and triubiquitinated β-chains in favor of additional poly-ubiquitinated forms (Fig. 3A).
Fig. 3.
Expression and endocytosis of MHC II–Ub fusion proteins in splenic B blasts differ depending on Ub extension. (A) Western blots of MHC II IPs and WCLs of MHC II β-chain−/− splenic B blasts retrovirally transduced to express MHC II–Ub fusion constructs. MHC II was immunoprecipitated from cell lysates with anti-MHC II antibody Y3P. IPs and WCLs were immunoblotted with anti-Ub antibody (Biomol). Arrowhead approximates band migration of di-ubiquitinated MHC II. (B) Confocal microscopy of transduced B blasts. Cells were bound to coverslips, fixed in PFA, and labeled with TIB120 (red) and H2-M (blue). (C) MFI of surface-bound MHC II in transduced B blasts. Data are expressed as mean and SEM. (D) Confocal microscopy of surface MHC II uptake. Transduced B blasts were incubated with TIB120 for 30 min at 37 °C for uptake, extensively washed, fixed, and stained to detect TIB120 (red) and H2-M (blue). (E) Quantification of MHC II antibody internalization. The cell interior and exterior were identified on z axis sections at 2.0 ⌈m above the coverslip, and MHC II MFI was quantified for each region. Relative intensity of interior to exterior regions is shown as the mean and SEM.
By immunofluorescence, the steady-state distribution of KR(0) and KRUb1 was restricted to the plasma membrane. In KRUb2-expressing cells, most MHC II was on the surface, but a fraction could be detected intracellularly. KRUb-poly–expressing B cells, however, looked similar to immature DCs, with the bulk of MHC II in lysosomes and relatively little on the plasma membrane (Fig. 3B). These results were supported by flow cytometry, which showed that increasing Ub chain length to greater than two Ub decreased surface expression of MHC II (Fig. 3C).
Internalization of anti-MHC II antibody at 37 °C recapitulated the steady-state distribution pattern. Little, if any, internalized antibody was observed for KR(0) and KRUb1, a small amount for KRUb2, and a considerable amount for KRUb-poly (Fig. 3D). The antibody uptake results were quantified by determining the ratio of each cell’s interior to surface fluorescence intensity at a defined z axis section (5). KRUb-poly was consistently found to have the highest ratio, whereas KRUb2 exhibited a small but statistically significant increase in fractional internalization relative to KR(0) and KRUb1 (Fig. 3E). By expressing an MHC II β-chain conjugated to greater than two Ub in B cells, it was possible to enhance MHC II endocytosis and late endocytic delivery, producing a phenotype reminiscent of immature DCs. Thus, Ub chain length, not cell type, is responsible for the intracellular fate of MHC II.
Surface Expression of MHC II Decreases with Increasing Ub Chain Length in DCs and B Cells.
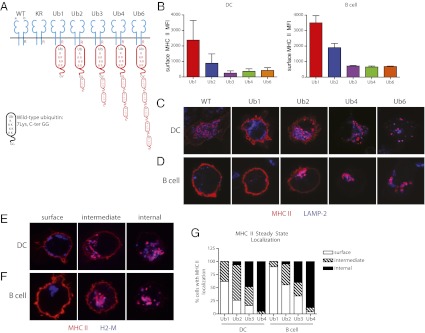
Any conclusions concerning the precise number of Ubs required for lysosomal transport were limited by the fact that the Ub fused to MHC II β-chain was subject to further modification by Ub ligases in the cell, yielding chains of indeterminate length, conjugation site, and branching. Ub addition was also likely to be dynamic, with a small fraction of total MHC II additionally ubiquitinated and for variable lengths of time. We therefore synthesized a series of KR molecules fused to tandem lysine-mutant Ub chains. Linear chains occur naturally and are synthesized by the LUBAC E3 ligase. They have been shown to interact with Ub-binding proteins (e.g., NEMO) with affinities comparable to K63-linked Ub chains, and can direct proteasomal degradation (27). Although there is no evidence that tandem repeats play a role in endocytosis, they are structurally similar to lysine 63-linked Ub chains (28). Thus, we constructed MHC II K > R fusions (KR-Ub) encoding stable chains of one, two, three, four, or six Ub monomers (Fig. 4A).
Fig. 4.
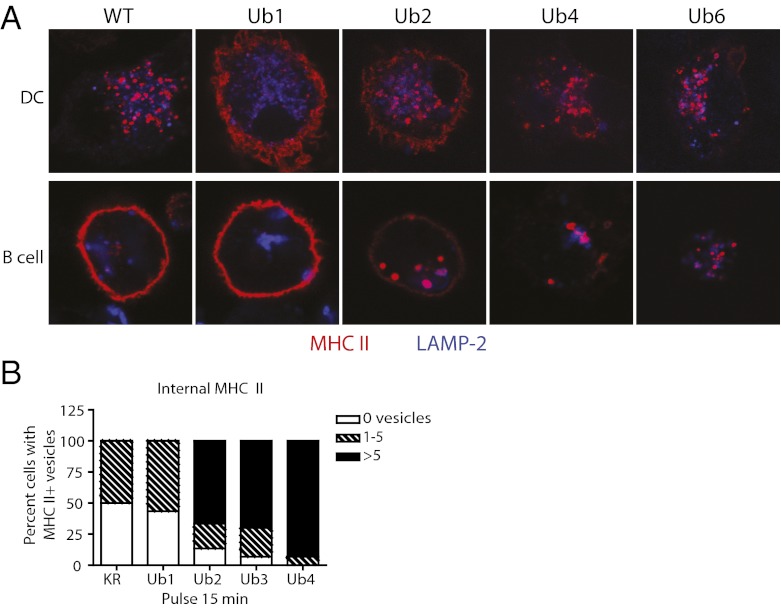
Surface expression and localization of MHC II synthetic Ub chains in DCs and splenic B blasts decrease with increasing Ub chain length. (A) Diagram of MHC II: wild-type and Ub chain conjugates. Mutations (red) include a K > R mutation in MHC II β-chain, seven K > R mutations in Ub, and a C-terminal G > V mutation in Ub. (B) MFI of surface-bound MHC II in transduced MHC II β-chain−/− BMDCs (Left) and splenic B blasts (Right). Data are expressed as mean and SEM. (C–F) Confocal microscopy of transduced BMDCs (C and E) and B blasts (D and F). (C and D) Steady-state localization of MHC II in BMDCs and B cells expressing wild type, Ub1, Ub2, Ub4, and Ub6. (E and F) Ub3-expressing BMDCs and B cells exhibit a heterogeneous population of MHC II localization: all surface, all internal, and intermediate between the two extremes. Cells were bound to coverslips, fixed in PFA, and labeled with anti-MHC II antibody TIB120 (red) and lysosomal marker LAMP-1 or H2-M (blue). (G) Quantification of MHC II localization. Transduced BMDCs and B cells were stained with TIB120 and H2-M, then scored for MHC II localization: all surface, both surface and internal, or all internal. n = 50.
Expression of the KR-Ub tandem repeats in DCs and B cells clearly illustrated a relationship between Ub chain length and MHC II trafficking. Consistent with the KRUb fusion proteins (Figs. 2 and 3), surface expression of KR-Ub in MHC II β-chain−/− DCs and B cells decreased with increasing Ub monomers (Fig. 4B). Similarly, steady-state localization of MHC II in both DCs and B cells correlated with Ub chain length. Whereas Ub1 was largely restricted to the plasma membrane, both surface and intracellular MHC II were observed in Ub2-expressing cells, and Ub4 and Ub6 were detected largely in intracellular compartments (Fig. 4 C and D).
Surprisingly, despite its low surface expression of MHC II by FACS, Ub3 by immunofluorescence exists as a heterogeneous population at steady state, resembling Ub2 more than Ub4 and Ub6. DCs and B cells can be found with all surface MHC II, all internal MHC II, and both surface and internal MHC II (Fig. 4 E and F). To more accurately measure the effect of Ub chain length on MHC II localization, DCs and B cells expressing Ub1, Ub2, Ub3, and Ub4 (comparable to Ub6) were scored according to the cellular localization of MHC II: all surface, all internal, or intermediate. The data reveal that with each additional Ub attached to MHC II, an increasing fraction of total MHC II can be found intracellularly in both DCs and B cells (Fig. 4G). From Ub2 to Ub3, there are fewer “all surface” and “intermediate” cells, in favor of more “all internal” cells.
MHC II Internalization Efficiency Increases with Increasing Ub Chain Length in DCs and B Cells.
Endocytosis of anti-MHC II antibody recapitulated the steady-state distribution. Whereas Ub1 was poorly internalized in DCs and B cells, Ub4 and Ub6 mediated efficient transport to late endosomes and lysosomes, and Ub2 exhibited an intermediate degree of internalization (Fig. 5A). Again, Ub3 displayed heterogeneity, with no clearly representative degree of MHC II internalization. In all KR-Ub–expressing cells, although we would expect the ratio of internal-to-external MHC II to mirror that seen in Fig. 3E, with the ratio inversely proportional to Ub number, this value could not be reliably measured, because total protein levels were also inversely proportional to Ub number: although immunofluorescence clearly shows that chain lengths of four or more Ub enable efficient MHC II internalization, it also follows that those species are efficiently degraded.
Fig. 5.
MHC II internalization is enhanced with increasing numbers of Ub. (A) Confocal microscopy of surface MHC II uptake. Transduced MHC II β-chain−/− BMDCs (Top) and B blasts (Bottom) were incubated with TIB120 for 1 h at 37°C for continuous uptake, extensively washed, fixed, and stained to detect TIB120 (red) and lysosomal marker LAMP-1 (blue). (B) Quantification of MHC II antibody uptake. Transduced BMDCs were incubated with TIB120 at 37 °C for 15 min, washed extensively, and fixed immediately. Surface TIB120 was blocked, cells were permeabilized, internalized TIB120 was stained, and cells were scored by number of internal MHC II vesicles. n = 30.
The extent of internalization of the Ub2 and Ub3 tandem constructs seemed greater than observed for endogenously generated di- and triubiquitinated MHC II molecules in B cells. This is not surprising given that these tandem repeats are stably expressed, whereas endogenous chains are transient and comprise only a small fraction of total MHC II at any one time. There are likely more opportunities for internalization of even poorly recognized but uniformly expressed di- and triubiquitinated molecules. Nevertheless, the internalization data also suggested that the stably expressed Ub1 was no better at mediating antibody uptake than nonubiquitinated MHC II (KR(0); Figs. 2E and 3D). To examine this possibility quantitatively, as well as to quantify MHC II internalization in Ub3-expressing cells, we scored anti-MHC II antibody internalization in DCs expressing KR, Ub1, Ub2, Ub3, and Ub4 by scoring the number of internal vesicles labeled after 15 min at 37 °C: zero, one to five, or more than five MHC II-positive vesicles. Given the low frequency (<10%) of productively infected cells, biochemical measurements were not possible.
Even though roughly half of Ub1-expressing DCs had internalized antibody after 15 min, all these cells displayed fewer than five vesicles per cell (Fig. 5B). These values were indistinguishable from antibody uptake in KR-expressing cells, confirming that a single Ub does not detectably enhance MHC II endocytosis. Increasing the Ub tandem repeat from one to two markedly increased internalization efficiency: 87% of DCs internalized Ub2 in 15 min, with 77% of those containing more than five vesicles. Ub3 was similar to Ub2, with a slight increase in MHC II internalization efficiency: 93% of DCs internalized Ub3 in 15 min, with 75% of those containing more than five vesicles. As expected, 100% of Ub4-expressing DCs internalized MHC II antibody, with the vast majority (93%) containing more than five vesicles. These data suggest that DCs use a mechanism for inefficient, ubiquitin-independent internalization of MHC II at steady state, but upon the addition of at least two Ubs, internalization is rendered more efficient, and each subsequent Ub enhances internalization further still, similar to wild-type MHC II in DCs (Figs. 4C and 5A).
Enhanced Ubiquitination of MHC II in B Cells Results in More Intracellular MHC II.
Finally, we used a strategy to increase Ub chain length in B cells without relying on synthetic Ub constructs. MARCH-I has been shown to ubiquitinate a variety of membrane proteins, notably MHC II in APCs (7, 8). Normally expressed at low levels in B cells and DCs, MARCH-I overexpression has been shown to decrease the surface expression of MHC II in B cells (8); Ub chain length was not evaluated in these studies, however.
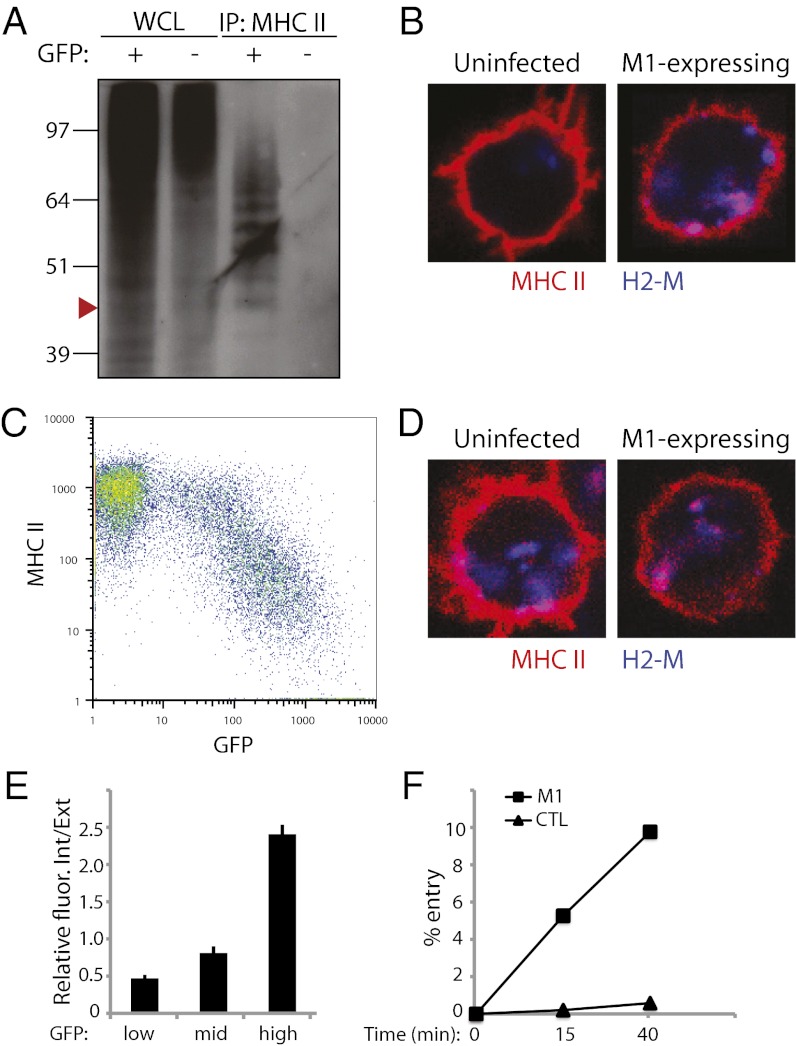
As for the above experiments, splenic B cells were activated by overnight LPS incubation, then retrovirally transduced with a MARCH-1 construct. Although MHC II in freshly isolated splenic B cells was found to be di- and triubiquitinated (Fig. 1A), B blasts activated by LPS stimulation lost this ubiquitination (Fig. 6A, GFP−), much like in DCs. Nevertheless, ubiquitination was restored by overexpression of MARCH-I (Fig. 6A, GFP+). Furthermore, rather than reproducing the typical B-cell di- and triubiquitination, the MHC II–Ub pattern resembled that normally found in immature DCs, with up to seven distinct monomers (Fig. 6A).
Fig. 6.
Increased efficiency of MHC II endocytosis in MARCH-I–overexpressing B cells. (A) Western blot of WCL and MHC II IPs of wild-type splenic B blasts retrovirally transduced to express MARCH-I (M1) Ub ligase. GFP(+)-transduced cells were sorted, with GFP(-) cells as control. MHC II was immunoprecipitated from cell lysates with anti-MHC II TIB120 antibody. IPs and WCLs were immunoblotted with anti-Ub antibody P4D1. Arrowhead approximates band migration of di-ubiquitinated MHC II. (B) Confocal microscopy of M1-expressing B blasts. Cells were bound to coverslips, fixed in PFA, and labeled with TIB120 (red) and lysosomal marker H2-M (blue). (C) FACS analysis of surface-bound MHC in unsorted M1-expressing B blasts. Cells were stained with PE-conjugated TIB120. (D) Confocal microscopy of surface MHC II uptake in M1-expressing B blasts. B blasts were incubated with TIB120 for 30 min at 37 °C for uptake, extensively washed, fixed, and stained to detect TIB120 (red) and H2-M (blue). (E) Quantification of TIB120 internalization as described in Fig. 3E for cells with low-, mid-, and high-intensity GFP. Data are expressed as mean and SEM. (F) Endocytosis of surface MHC II in M1-expressing B cells. Acid wash protocol on sorted GFP(+) (“M1”) and GFP(-) (“CTL”) cells as described in Fig. 1C.
Further characterization of MHC II in MARCH-I–expressing B cells revealed that MHC II localized to both the cell surface and lysosomal compartments at steady state (Fig. 6B). Consistent with this observation, surface MHC II steadily decreased with increasing MARCH-I expression (indicated by increasing GFP intensity) (Fig. 6C). Increased MHC II internalization was again seen as correlating with the efficiency of MARCH-I expression (Fig. 6 D and E).
By overexpressing MARCH-I, we not only restored but also enhanced MHC II ubiquitination in wild-type B blasts and observed the predicted changes in MHC II localization and internalization. As a final quantitative measure, an acid-wash assay showed that the endocytic rate of MHC II in MARCH-I–expressing B cells was significantly increased relative to nonexpressing cells (Fig. 6F).
Discussion
Although the difference in Ub chain length between DCs and B cells does not seem profound, by manipulating Ub addition in a physiological setting we found that chain lengths of two to three (B cells) vs. four to six (DCs) were wholly responsible for determining the localization of MHC II in both cell types. As such, two important questions are answered.
First, the sole reason immature DCs accumulate MHC II in late endosomes and lysosomes, whereas B cells retain MHC II at the plasma membrane, can be attributed to the longer Ub chains conjugated to MHC II in DCs. The ability of DCs to regulate tightly the intracellular distribution of MHC II is likely to reflect the specialized features of DCs as APCs. Immature DCs survey peripheral tissues, where they encounter incoming pathogens or self-antigens and capture them by nonspecific endocytosis. DCs then process and load internalized antigens onto MHC II molecules during their 24- to 48-h trek to lymphoid organs. Having antigen and MHC II colocalized in antigen processing compartments likely contributes to the exquisite efficiency that characterizes DCs as APCs (1). B cells, on the other hand, can achieve efficient antigen presentation by using a highly specific receptor-mediated mechanism of antigen uptake via the B-cell receptor (2). Although the data clearly demonstrate the importance of degree of MHC II ubiquitination, they do not indicate how each cell type regulates chain length—by ligase, deubiquitinase, or other factors.
Second, it is now clear that Ub chain length plays a key role in determining the intracellular fate of ubiquitinated membrane proteins. To date, the significance of oligo-Ub chains vs. Ub monomers (conjugated to a single or multiple lysine acceptors) has been unclear (20–24), although the K63 linkage is well known to play a role in multivesicular body (MVB) sorting and lysosomal targeting (29, 30). Although previous studies have attributed a role for Ub number in the regulation of chimeric proteins [e.g., CD4 (26)], the use of a biologically relevant example here clearly demonstrates the physiological importance of Ub chain length as a critical determinant of intracellular fate. One possible explanation for this feature is facilitating lysosomal sorting efficiency. Because the affinity of a single Ub for a single UIM is low (31), longer chains would exert greater avidity for such interactions, as recently shown in vitro for the MVB sorting adapter Hrs, which, incidentally, contains two UIMs (32). This is further evidenced by the observations here that each additional Ub conjugated to MHC II subsequently promotes more endocytosis and intracellular localization of MHC II. These observations, then, reveal lysosomal sorting to be a nonstochastic, probabilistic event, dependent upon the strength of interactions between ubiquitinated cargo and sorting machinery. This is consistent with our previous finding that even in immature DCs, only ∼10% of the total MHC II is ubiquitinated at any one time (5). Because Ubs are typically removed from membrane proteins upon sequestration into MVBs, the transient presence of Ub chains of different length is sufficient to control the final fate of MHC II molecules.
Although endocytosis was not observed for monoubiquitinated MHC II, we cannot eliminate the possibility of continuous internalization and recycling of a small pool of MHC II, which cannot be easily detected by antibody uptake and even less so by cell surface biotinylation. Nor do the data distinguish between MHC II ubiquitination preceding or succeeding plasma membrane trafficking. Overexpression studies of MARCH-1 localize the ligase to endosomal membranes (33, 34), where it may be poised to ubiquitinate MHC II during its translocation to the cell surface or along the endocytic route. The data also suggest that Ub conjugated to MHC II may not exert its function until at least MHC II reaches the cell surface, or later. The MHC II antibody internalization assay in Fig. 5 required MHC II delivery to the cell surface, and both Ub4 and Ub6 were clearly tagged by extracellularly added antibody, despite being largely intracellular at the steady state. Thus, at least a fraction of the oligoubiquitinated MHC II can still reach the surface, after which point Ub chains signal MHC II internalization.
Despite extensive progress in understanding the relationship between ubiquitination and membrane traffic, the role of oligoubiquitination has remained murky both because most membrane proteins have multiple potential lysine conjugation sites and because it has been difficult to control the length of Ub chains. By identifying two physiologically significant examples in which chain length on a single acceptor lysine was shown to regulate cargo transport to lysosomes, our data demonstrate that chain length is indeed an important factor in determining the fate of ubiquitinated membrane proteins.
Materials and Methods
Cells.
BMDCs from C57/BL6 mice (Jackson Laboratory) and MHC II β-chain−/− mice (Taconic) were prepared by depleting bone marrow cells of erythrocytes, T cells, B cells, granulocytes, and MHC II-positive cells, and subsequently cultured in DC growth medium [RPMI 1640 (Invitrogen) supplemented with 5% (vol/vol) FBS, 2 mM l-glutamine, 20 μg/mL gentamicin, 50 μM β-mercaptoethanol, and recombinant mouse GM-CSF (produced as culture supernatant from J558L cells transfected with mouse GM-CSF cDNA)]. Cells were incubated at 37 °C in 7% CO2, and medium was changed every 2 d. Splenic DCs from C57/BL6 mice were isolated using CD11c+ cell isolation beads (Miltenyi), and the purity was determined by FACS analysis (80–95%). Splenic B cells were isolated from C57/BL6 mice and MHC II β-chain−/− mice using a B-cell Isolation Kit (Miltenyi). B cells were cultured in B-cell medium [IMDM (Gibco) supplemented with 10% FBS, 4 mM l-glutamine, and 50 μM β-mercaptoethanol].
Plasmids and Retroviral Transduction.
The cDNA of wild type MHC II IAb was cloned into the LZRS-pBMN plasmid (a gift from Gary Nolan, Stanford University, Palo Alto, CA) using the HindIII and NotI sites. The KR(0) mutant construct was generated by site-directed mutagenesis. The KRUb-poly construct was generated by PCR, eliminating the stop codon of the KR(0) mutant and fusing it in-frame with cDNA encoding Ub, a gift from Pietro De Camilli [Yale University, New Haven, CT (27)]. The KRUb2 mutant was generated by eliminating the two terminal glycine residues of the fused Ub in the KRUb-poly construct. The KRUb1 mutant was generated by site-directed mutagenesis of the seven internal lysines of the Ub of the KRUb2 mutant. The cDNA of wild-type murine MARCH-I was cloned into the LZRS-pBMN plasmid using the HindIII and NotI sites. IRES-EGFP, obtained by PCR using pIRES-EGFP2 (Clontech) as a template, was inserted using the NotI sites of the LZRS vectors. All KR-Ub tandem repeat plasmids were synthesized by Blue Heron. Retrovirus was generated by transfection of plasmid vectors into phoenix-ecotropic cells using Fugene (Roche). Stable transfectants were selected in puromycin, and virus was collected in DC growth medium or B-cell medium supplemented with polybrene (American Bioanalytical). Virus was added to DC or B-cell cultures, and tissue culture plates were spun in a tabletop centrifuge at 32 °C, 1,200 × g for 2 h. Virus was removed, and fresh medium was added. Expression was assayed 24 h (B cells) or 48 h (DCs) after transduction. In each experiment, viral transduction efficiency was monitored by measuring GFP expression by flow cytometry in each experiment; this approach was preferable to measuring MHC II β-chains because their rates of degradation would be expected to vary as a function of the numbers of ubiquitins attached. Moreover, for each MHC II–Ub construct used, the relative amount of MHC II on the surface was independent of levels of GFP expressed, as was the steady-state intracellular distribution.
Western Blot.
MHC II expression was determined by Western blot. KR-Ub transductants were sorted on GFP and CD11c (for DCs) or B220 (for B cells) double-positive cells. Sorted cells were lysed, denatured in sample buffer, and run for SDS/PAGE. MHC II expression was detected using Bett anti-MHC II antibody.
Antibodies.
“Bett” or “Thorax” (rabbit polyclonal antibodies against IAb) was used for Western blot. TIB120 (anti-MHC II) (BD Biosciences) was used for immunoprecipitation, immunofluorescence microscopy, and FACS analysis. Y3P (anti-MHC II) (ATCC) was used for immunoprecipitation. CD11c mAb, biotin-CD86 mAb, FITC-CD86 mAb, PerCP/Cy5.5-CD86 mAb, APC-CD86 mAb, PE-CD80 mAb, PE-TIB120 mAb, and Pacific Blue-B220 mAb were purchased from BD Biosciences and used for FACS analysis. P4D1 (anti-Ub mAb; Covance) or rabbit polyclonal anti-Ub antibody (Biomol) was used for Western blot. “Ulm” [rabbit anti-H2-M antibody (28)], anti-LAMP-2 (grown from hybridomas in the laboratory), and anti-LAMP-1 (BioLegend) were used for immunofluorescence microscopy.
Immunoprecipitation.
BMDCs, splenic DCs, or B cells (1 × 107) were solubilized in Triton-lysis buffer [1% Triton X-100, 10 mM Tris·HCl (pH 7.6), 100 mM NaCl, and 5 mM MgCl2] supplemented with 20 mM N-ethylmaleimide and protease inhibitor mixtures. The soluble cell lysates were mixed with 5 μg of MHC II antibody (TIB120 or Y3P) and rocked for 1 h. Protein G-Sepharose beads were added and rocked for 1 more hour. The beads were washed three times with Triton lysis buffer and run for SDS/PAGE.
Endocytosis of MHC II and Quantitation.
BMDCs cultured from MHC II β-chain−/− mice were retrovirally transduced on day 2 to express wild-type or MHC II–Ub fusion proteins. On day 5, cells were seeded on Alcian blue-coated coverslips and treated with MHC II antibody (20 μg/mL, TIB120) for 30 min to 1 h at 37 °C. Subsequently, the cells were washed several times and fixed with 4% paraformaldehyde. Cells were then permeabilized and stained to detect cell-associated anti-MHC II antibody and lysosomal H2-M. For quantitation of internalized MHC II antibody, DCs were treated with antibody for 15 min at 37 °C, washed, and fixed with 4% paraformaldehyde. Surface antibody was blocked with unconjugated anti-rat IgG (Jackson ImmunoResearch), cells were permealized, internalized antibody stained with fluorescent anti-rat IgG, and antibody-positive vesicles counted. Splenic B blasts generated from MHC II β-chain−/− mice were retrovirally transduced to express wild-type MHC II, MHC II–Ub fusion proteins, or MARCH-I after overnight culture with 25 μg/mL of LPS (Sigma). The day after transduction, B cells were seeded on coverslips and treated as described above for the BMDCs. For quantitation, B cells were imaged on a Leica SP5 confocal microscope. Images of z-sections taken 2.0 mm from the coverslip were used for quantitative analysis. To distinguish surface-bound from internalized anti-MHC II antibody, we defined the outer edge of each cell by applying a global intensity threshold to the MHC II image, then smoothening the resulting binary mask to remove any small protrusions. The “interior” was defined by eroding the outer edge of each cell a distance of seven pixels. The “surface” was defined as the region between the interior and the outer edge of each cell. These manipulations and the integrated red or green fluorescence intensity of each area were analyzed in the Metamorph software package (MDS Analytical Technologies).
Cell Sorting.
After retroviral transduction with MARCH-1, B cells were washed and resuspended in PBS supplemented with 0.5% BSA and 2 mM EDTA. Cells were passed through a 70-μm cell strainer and sorted using a BD FACSAria. Transduced B cells were gated on B220+GFP+ cells. Nontransduced B cells were gated on B220+GFP− cells.
Acknowledgments
We thank members of the I.M. laboratory for their support, Vishva Dixit for excellent advice and discussion, and Ivan Dikic for kindly supplying cDNA constructs. This paper is dedicated to our dear friend and mentor Ralph Steinman who passed just too early to learn he had been awarded the Nobel Prize for discoveries on which this paper, and our careers, are based.
Footnotes
The authors declare no conflict of interest.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2011.
See Profile on page 8790.
References
- 1.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 2.Vascotto F, et al. Antigen presentation by B lymphocytes: How receptor signaling directs membrane trafficking. Curr Opin Immunol. 2007;19:93–98. doi: 10.1016/j.coi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 5.Shin JS, et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 6.van Niel G, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.De Gassart A, et al. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci USA. 2008;105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuki Y, et al. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 10.Boes M, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 11.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 12.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 13.Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 15.Polo S, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 16.Confalonieri S, Di Fiore PP. The Eps15 homology (EH) domain. FEBS Lett. 2002;513:24–29. doi: 10.1016/s0014-5793(01)03241-0. [DOI] [PubMed] [Google Scholar]
- 17.Hurley JH, Wendland B. Endocytosis: Driving membranes around the bend. Cell. 2002;111:143–146. doi: 10.1016/s0092-8674(02)01044-9. [DOI] [PubMed] [Google Scholar]
- 18.Traub LM. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 19.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barriere H, et al. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 21.Haglund K, et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 22.Hawryluk MJ, et al. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Pickart CM, Fushman D. Polyubiquitin chains: Polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Barriere H, Nemes C, Du K, Lukacs GL. Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery. Mol Biol Cell. 2007;18:3952–3965. doi: 10.1091/mbc.E07-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahighi S, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136:1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Komander D, et al. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauwers E, Jacob C, André B. K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J Cell Biol. 2009;185:493–502. doi: 10.1083/jcb.200810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan LM, et al. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains—from structures to functions. Nat Rev Mol Cell Biol. 2009;10:659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren X, Hurley JH. VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J. 2010;29:1045–1054. doi: 10.1038/emboj.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Früh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol. 2004;78:1109–1120. doi: 10.1128/JVI.78.3.1109-1120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jabbour M, Campbell EM, Fares H, Lybarger L. Discrete domains of MARCH1 mediate its localization, functional interactions, and posttranscriptional control of expression. J Immunol. 2009;183:6500–6512. doi: 10.4049/jimmunol.0901521. [DOI] [PMC free article] [PubMed] [Google Scholar]