Abstract
Whereas short-term (minutes) facilitation at Aplysia sensory–motor neuron synapses is presynaptic, long-term (days) facilitation involves synaptic growth, which requires both presynaptic and postsynaptic mechanisms. How are the postsynaptic mechanisms recruited, and when does that process begin? We have been investigating the possible role of spontaneous transmitter release from the presynaptic neuron. In the previous paper, we found that spontaneous release is critical for the induction of long-term facilitation, and this process begins during an intermediate-term stage of facilitation that is the first stage to involve postsynaptic as well as presynaptic mechanisms. We now report that increased spontaneous release during the short-term stage acts as an orthograde signal to recruit postsynaptic mechanisms of intermediate-term facilitation including increased IP3, Ca2+, and membrane insertion and recruitment of clusters of AMPA-like receptors, which may be first steps in synaptic growth during long-term facilitation. These results suggest that the different stages of facilitation involve a cascade of pre- and postsynaptic mechanisms, which is initiated by spontaneous release and may culminate in synaptic growth.
Keywords: synaptic plasticity, metabotropic glutamate receptor, octopamine, latrotoxin, botulinum toxin
Whereas short-term (minutes) facilitation in Aplysia involves covalent modifications restricted to the presynaptic neuron, long-term (days) facilitation is accompanied by the growth of new synapses, which involves coordinated pre- and postsynaptic structural changes (1–4). Those findings raise two questions that are applicable to other forms of plasticity that can involve synaptic growth, such as the late phase of long-term potentiation (LTP) in the hippocampus (5–9) and other brain areas: (i) What signaling mechanisms coordinate the pre- and postsynaptic changes, and (ii) when is that signaling first engaged?
To address these questions, we have been investigating the possible role of spontaneous transmitter release from the presynaptic neuron in recruiting the postsynaptic mechanisms of long-term facilitation in Aplysia. We previously found that spontaneous transmitter release is critical for the induction of long-term facilitation (10) (in this issue of PNAS). In addition, we found that this signaling is engaged quite early, during an intermediate-term stage that incorporates elements of both short- and long-term plasticity (11, 12), and therefore might form part of a bridge or cascade connecting them. The intermediate stage is also the first stage to involve both presynaptic molecular mechanisms and postsynaptic mechanisms including IP3, Ca2+, protein synthesis, and membrane insertion of AMPA-like receptors (1, 2, 13, 14). Postsynaptic Ca2+ and protein synthesis are in turn necessary for long-term facilitation, in part through retrograde signaling (15).
We have now investigated mechanisms downstream of spontaneous release, and have found that it plays a critical role in recruitment of the postsynaptic mechanisms that contribute to long-term and intermediate-term facilitation. That is, increased spontaneous release during the short-term stage acts through metabotropic glutamate receptors to increase postsynaptic IP3, Ca2+, and membrane insertion and recruitment of clusters of AMPA-like receptors during the intermediate-term stage. These may in turn be early steps in a cascade that can culminate in synaptic growth during long-term facilitation.
Results
Long-Term and Intermediate-Term Facilitation Involve Postsynaptic Metabotropic Glutamate Receptors Linked to the Production of IP3, Which Depends on Presynaptic Spontaneous Release.
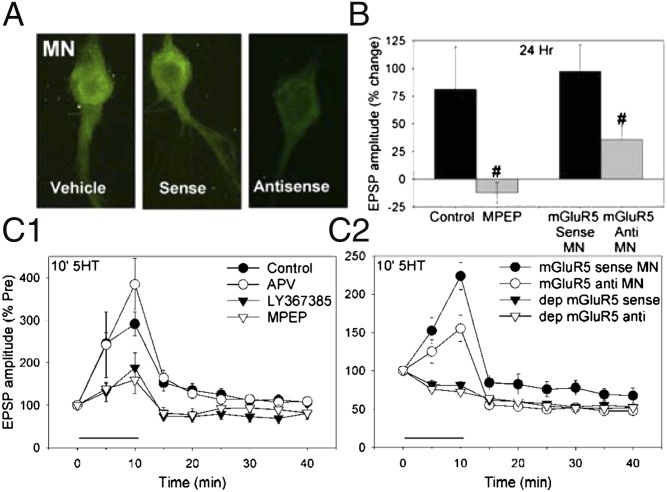
We first investigated postsynaptic receptors that are activated by spontaneous transmitter release during the induction of long-term facilitation. The sensory–motor neuron synapses are glutamatergic (16–18), and spontaneous release might affect the postsynaptic neuron by stimulating one or more types of glutamate receptors including NMDA, AMPA, and metabotropic receptors. Fluorescent in situ hybridization revealed that postsynaptic motor neurons express an Aplysia homolog of the group I metabotropic receptor mGluR5 (Fig. 1A). An inhibitor of these receptors, 2-methyl-6(phenylethynyl)pyridine (MPEP; 50 μM), blocked long-term facilitation produced by five pulses of serotonin (5HT) (10 μM) each 5 min in duration with a 15-min interstimulus interval (5× 5-min 5HT) (F[1,18] = 5.71, P < 0.05 compared with control) (Fig. 1B). In these experiments, MPEP was washed out with the 5HT, suggesting that it blocks induction rather than expression of the facilitation. Likewise, postsynaptic injection of an antisense oligonucleotide for the Aplysia mGluR5 (50 μg/mL in the electrode) reduced long-term facilitation by 5× 5-min 5HT (F[1,37] = 5.12, P < 0.05 compared with injection of the sense oligonucleotide). Postsynaptic injection of the antisense oligonucleotide also reduced the level of mGluR5 mRNA in the motor neuron measured either with real-time PCR (antisense = 48 ± 19% of sense, t[8] = 2.41, P < 0.05) or with in situ hybridization (F[1,18] = 8.72, P < 0.01 comparing sense and antisense) (Fig. 1A).
Fig. 1.
Long-term and intermediate-term facilitation involve postsynaptic metabotropic glutamate receptors. (A) Examples of in situ hybridization for Aplysia mGluR5 in L7 motor neurons 6 h after injection of vehicle, sense oligonucleotide, or antisense oligonucleotide. The mGluR5 mRNA level was reduced by injection of the antisense oligonucleotide. (B) Long-term facilitation by 5× 5-min 5HT was reduced by the mGluR5 antagonist MPEP (n = 10), compared with control (n = 10). The facilitation was also reduced by injection of antisense oligonucleotide for Aplysia mGluR5 into the motor neuron (MN) (n = 19), compared with injection of sense oligonucleotide (n = 20). The average pretest value was 14 mV. (C1) Intermediate-term facilitation by 10-min 5HT was reduced by MPEP (n = 9) or the group I metabotropic glutamate receptor antagonist LY367385 (n = 7) but was not reduced by the NMDA receptor antagonist APV (n = 7), compared with control (n = 20). There was a significant overall effect of group (F[3,39] = 6.66, P < 0.01). The average pretest value was 11.2 mV. (C2) Facilitation by 10-min 5HT was also reduced by injection of antisense oligonucleotide for Aplysia mGluR5 into the motor neuron (n = 12), compared with injection of sense oligonucleotide (n = 9). Antisense mGluR5 had no significant effect on test-alone depression (n = 8), compared with sense (n = 7). The average pretest value was 16.5 mV, not significantly different between sense and antisense. In this and subsequent figures, the error bars indicate SEMs; *P < 0.05, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. no inhibitor.
Because the previous paper suggested that spontaneous transmitter release begins to contribute during an intermediate stage (10), we also investigated postsynaptic receptors that are activated by spontaneous release during intermediate-term facilitation. Similar to long-term facilitation, MPEP (50 μM) reduced intermediate-term facilitation by 10-min 5HT (20 μM, F[1,39] = 10.29, P < 0.01), and a general inhibitor of group I metabotropic glutamate receptors, LY367385 (500 μM), also reduced the facilitation (F = 10.34, P < 0.01 compared with control) (Fig. 1C). Likewise, postsynaptic injection of the antisense oligonucleotide for mGluR5 reduced the facilitation produced by 10-min 5HT (F[1,32] = 5.35, P < 0.05 for the interaction of sense/antisense–5HT), with no significant effect on the pretest excitatory postsynaptic potential (EPSP) or test-alone homosynaptic depression. By contrast, an inhibitor of NMDA receptors 2-amino-5-phosphonovaleric acid (APV; 100 μM) did not reduce intermediate-term facilitation by 10-min 5HT, and an inhibitor of AMPA receptors 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX; 50 μM) also did not reduce the early part of the facilitation (Fig. 4B). These results suggest that activation of postsynaptic mGluR5 receptors is critical for the induction of intermediate-term as well as long-term facilitation, supporting the idea that events during the intermediate stage could be first steps in a sequence that can lead to long-term facilitation (10). We therefore decided to focus our analysis on those early events.
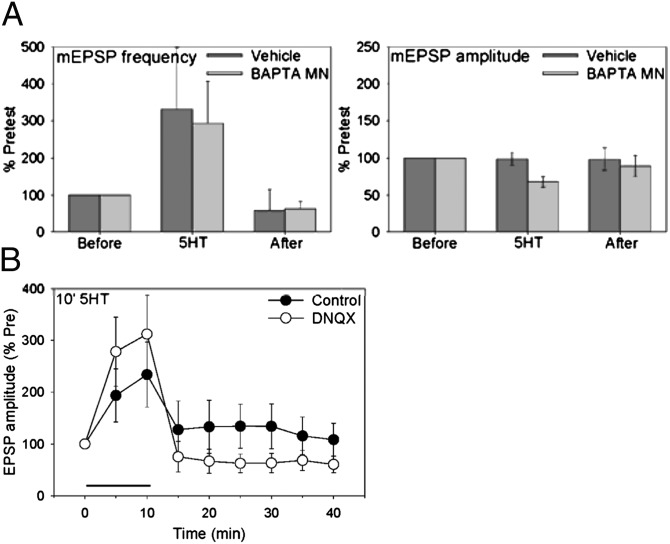
Fig. 4.
Expression of short-term facilitation involves a presynaptic increase in transmitter release, and expression of intermediate-term facilitation may also involve a postsynaptic increase in AMPA-like receptors. (A) Ten-minute 5HT produced an increase in the frequency of mEPSPs, with little change in their amplitude. Injection of BAPTA into the motor neuron (n = 6) did not have a significant effect on the percent increase in frequency or amplitude of mEPSPs, compared with vehicle control (n = 6). The average pretest values were 2.2 min−1 for mEPSP frequency and 0.15 mV for mEPSP amplitude, not significantly different between BAPTA and vehicle. (B) Facilitation after but not during 10-min 5HT was reduced by the AMPA receptor antagonist DNQX (n = 9), compared with control (n = 8). There was a significant drug–time interaction (F[7,98] = 3.85, P < 0.01) in a two-way analysis of covariance, with the pretest as covariate to adjust for differences in the pretest values. The average pretests were 1.6 mV for DNQX and 14.4 mV for control.
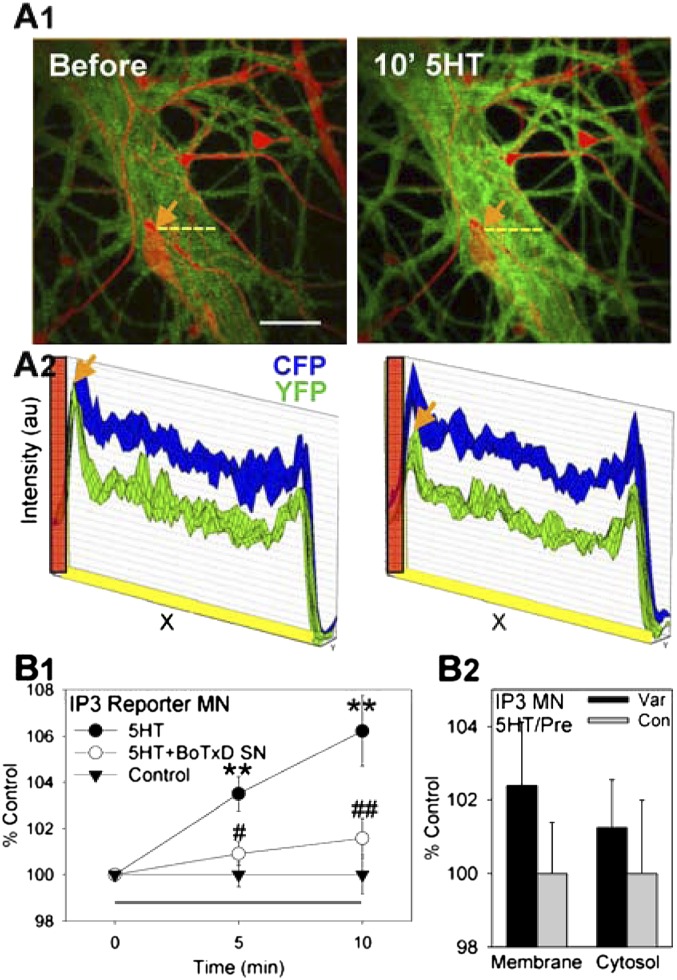
We next investigated postsynaptic mechanisms that may be recruited by activation of mGluR5 receptors, which are usually linked to the production of IP3. To examine the role of IP3, we used a fluorescent reporter of IP3 production, cyan/yellow PH domain reporter (CYPHR) that relies on intermolecular fluorescence resonance energy transfer (FRET). This reporter detects translocation from the membrane to the cytosol of the PH domain of phospholipase C when PIP2 is converted to IP3 (19). A decrease in FRET is indicative of an increase in IP3 production. We used the pNEX system (20) to express CYPHR in the motor neuron of sensory–motor neuron cocultures, and examined CFP/YFP FRET in the synaptic region (initial segment) before and during 10-min 5HT. We found that 10-min application of 5HT produced a decrease in FRET in the motor neuron indicative of increased IP3 production (F[1,20] = 17.12, P < 0.01 compared with a no-5HT control overall) (Fig. 2 A1 and B1). Presynaptic injection of the light chain of botulinum toxin D (BoTx D), which decreases spontaneous release (10), reduced this postsynaptic change (F = 9.45, P < 0.01 compared with vehicle injection). Furthermore, the increase in IP3 occurred throughout the initial segment of the motor neuron but appeared to be larger at sites adjacent to presynaptic varicosities of the sensory neuron (Fig. 2 A2 and B2), consistent with presynaptic induction. These results suggest that increased spontaneous transmitter release from the presynaptic neuron during 10-min 5HT activates metabotropic glutamate receptors, which stimulate production of IP3 in the postsynaptic neuron.
Fig. 2.
Intermediate-term facilitation is accompanied by an increase in postsynaptic IP3 production, which depends on spontaneous transmitter release from the presynaptic neuron. (A1) Examples of FRET of a fluorescent reporter of IP3 production (CYPHR) in the motor neuron before and after 10-min application of 5HT. IP3 production, which is accompanied by a decrease in CFP/YFP intermolecular FRET, is indicated by an increase in the intensity of green (which is proportional to the difference between CFP and YFP fluorescence) in these images. The sensory neuron was injected with the red fluorescent dye Alexa-568 to visualize points of contact with the motor neuron. (Scale bar, 20 μm.) (A2) The intensity of CFP and YFP fluorescence scanned across the dashed yellow lines in A1. There was a decrease in CFP/YFP FRET (evident as a decrease in YFP intensity) throughout the cytoplasm, but the decrease was larger at sites adjacent to a presynaptic varicosity of the sensory neuron (arrows). The location of the edge of the varicosity is indicated in red. (B1) Average results from experiments like the one shown in A1. Ten-minute 5HT (n = 7) produced a gradual increase in the CFP:YFP ratio indicative of IP3 production in the initial segment of the motor neuron, compared with the test-alone control (n = 8). This increase was reduced by injecting BoTx D into the sensory neuron (SN) (n = 8). (B2) Average results from experiments like the one shown in A2. There were trends for the increase in IP3 production to be larger at sites adjacent to sensory-neuron varicosities (Var, n = 9) than at other control sites on the initial segment of the motor neuron (Con, n = 10), and for that effect to be greater near the motor-neuron membrane than deeper in the cytosol.
Manipulations That Increase Spontaneous Release Increase Postsynaptic Ca2+.
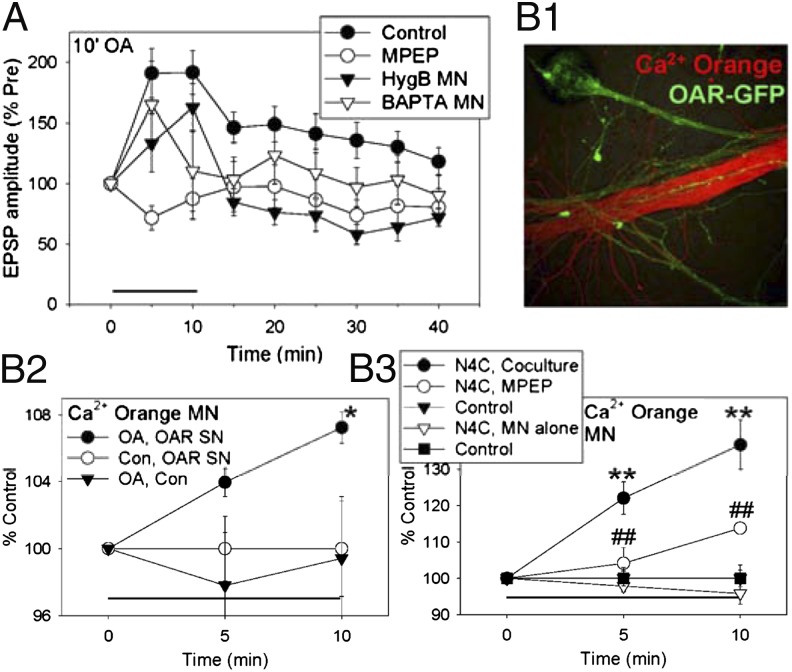
Because IP3 often stimulates Ca2+ release from intracellular stores, we next investigated changes in postsynaptic Ca2+. To examine whether these changes are recruited by spontaneous transmitter release from the presynaptic neuron, we also used two manipulations that act more selectively than 5HT does (10). First, 5HT can stimulate receptors on the postsynaptic as well as presynaptic neuron (3, 14). To enhance spontaneous release without directly affecting the postsynaptic neuron, we expressed in the sensory neuron an Aplysia octopamine receptor (OAR) that is not normally expressed in those neurons. Ten-minute application of octopamine (20 μM) to cocultures with OAR-expressing sensory neurons enhanced spontaneous release and produced intermediate-term facilitation of the evoked EPSP (10) (Fig. 3A). Like facilitation by 10-min 5HT (Fig. 1C), the facilitation by 10-min octopamine was significantly reduced by the mGluR5 antagonist MPEP (F[1,33] = 6.18, P < 0.05 compared with control). Also like facilitation by 10-min 5HT (2, 13), the facilitation by octopamine was reduced by injecting the protein synthesis inhibitor hygromycin B (200 mg/mL in the electrode) into the postsynaptic motor neuron (F = 5.10, P < 0.05 compared with vehicle) or by injecting the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; 200 mM in the electrode) into the postsynaptic neuron (F = 3.71, P < 0.05 one-tail test).
Fig. 3.
Presynaptic manipulations that increase spontaneous release can produce facilitation that involves postsynaptic mechanisms, including increased postsynaptic Ca2+. (A) Ten-minute octopamine produced intermediate-term facilitation in cocultures with expression of octopamine receptors in the sensory neurons. The facilitation was reduced by the mGluR5 antagonist MPEP (n = 5) or by injection of the protein synthesis inhibitor hygromycin B (n = 5) or the Ca2+ chelator BAPTA (n = 13) into the motor neuron, compared with control (n = 14). There was a significant overall effect of group (F[3,33] = 3.13, P < 0.05). The average pretest value was 11.5 mV, not significantly different between the groups. (B1) Example of fluorescence of the Ca2+ indicator Calcium Orange injected into the motor neuron (red) and OAR-GFP expressed in the sensory neuron (green). (B2) On average, 10-min octopamine produced a gradual increase in the intensity of Calcium Orange fluorescence in the initial segment of the motor neuron in cocultures with OAR-expressing sensory neurons (OA, OAR SN, n = 6), compared with either no octopamine (Con, OAR SN, n = 6) or no OAR expression (OA, Con, n = 6). There was a significant group–time interaction (F[2,15] = 4.62, P < 0.05). The average pretest values were not significantly different between the groups. (B3) Ten-minute application of N4C mutant α-latrotoxin produced a gradual increase in the intensity of Calcium Orange fluorescence in the initial segment of the motor neuron in cocultures (n = 6), compared with controls without α-latrotoxin (n = 6). N4C α-latrotoxin did not produce an increase in Calcium Orange fluorescence in motor neurons alone (n = 8), compared with controls (n = 7). The increase in cocultures was reduced by the mGluR5 antagonist MPEP (n = 6). There was a significant overall effect of group (F[4,28] = 18.05, P < 0.01). The average pretest values were not significantly different between the groups, and the baseline controls were not significantly different between cocultures and motor neurons alone.
In cocultures with OAR-expressing sensory neurons, octopamine also produced a gradual increase in Ca2+ in the synaptic region (initial segment) of the motor neuron (F[1,30] = 4.25, P < 0.05 after 10-min OA compared with a no-OA control for bleaching or other effects of the testing procedure) (Fig. 3B 1 and 2). By contrast, octopamine had no effect when OAR was not expressed in the sensory neuron (F = 4.97, P < 0.05 compared with OAR expression), suggesting that it does not stimulate endogenous receptors on the motor neuron. Collectively, these results suggest that activation of presynaptic octopamine receptors produces facilitation in part by increasing spontaneous release of transmitter, activating metabotropic glutamate receptors, and stimulating postsynaptic mechanisms including increased Ca2+ and protein synthesis.
As another way to enhance spontaneous release, we used α-latrotoxin (LaTx), which stimulates release of docked vesicles from presynaptic terminals (21). Ten-minute application of a mutant α-latrotoxin that does not form Ca2+ pores (LaTxN4C; 20 nM) (22, 23) produced a gradual increase in Ca2+ in the synaptic region of the motor neuron in cocultures (F[1,28] = 41.87, P < 0.01 compared with control), but no increase in isolated motor neurons (Fig. 3B3). Furthermore, the postsynaptic increase in Ca2+ in cocultures was reduced by the mGluR5 antagonist MPEP (50 μM, F = 20.28, P < 0.01), supporting the idea that it was due to spontaneous release of glutamate from the presynaptic neuron. We also obtained similar results with wild-type α-latrotoxin (1 nM) (F[1,38] = 9.61, P < 0.01 for the increase in Ca2+ after 10-min α-latrotoxin compared with control), although in that case, α-latrotoxin also produced a smaller increase in Ca2+ in isolated motor neurons (F = 3.36, P < 0.05 one-tail compared with cocultures). α-Latrotoxin and octopamine may each have additional effects, but one effect that they are known to have in common is increasing spontaneous release. These results thus support the idea that spontaneous release from the presynaptic neuron recruits postsynaptic mechanisms including increased Ca2+.
Expression of Intermediate-Term Facilitation Involves a Presynaptic Increase in Transmitter Release That Stimulates a Postsynaptic Increase in AMPA-like Receptors.
To this point, we have focused on mechanisms of induction of intermediate-term facilitation. We next examined mechanisms of expression of the facilitation. Facilitation by 10-min 5HT is accompanied by little or no increase in mini amplitude and a much larger increase in mini frequency (Fig. 4A), which is traditionally thought to reflect an increase in presynaptic transmitter release. However, the increase in mini frequency might also be due to membrane insertion of AMPA-like glutamate receptors at postsynaptically silent synapses, or an increase in the number of synapses. We have obtained evidence for all three of these mechanisms, each of which contributes during a progressively later time window, suggesting that one mechanism could lead to another in a possible cascade.
We first investigated presynaptic transmitter release. Injection of BAPTA into the postsynaptic motor neuron had very little effect on the increase in miniature (m)EPSP frequency or amplitude during or after 10-min 5HT (Fig. 4A). Similarly, injection of BoTx D into the sensory neuron, which reduces spontaneous release and also reduces the postsynaptic increase in IP3 during 10-min 5HT (Fig. 2B), had very little effect on the percent increase in mEPSP frequency (10). These results suggest that the early increase in mEPSP frequency does not depend on postsynaptic mechanisms, and therefore reflects a presynaptic increase in release. Consistent with this idea, stimulation of presynaptic octopamine receptors also produced an early increase in mEPSP frequency (10).
We next investigated possible postsynaptic mechanisms of expression. An inhibitor of AMPA receptors (DNQX; 50 μM) strongly reduced the pretest EPSP (t[15] = 5.21, P < 0.01) but did not reduce the early part of facilitation by 10-min 5HT (Fig. 4B). However, DNQX significantly reduced facilitation after washout of the 5HT (F[1,112] = 5.29, P < 0.05 compared with control), suggesting that expression of the later part of facilitation may involve up-regulation of AMPA-like receptors (13). To examine this mechanism more directly, we expressed in the motor neuron the Aplysia homolog of the AMPA receptor subunit GluR1, ApGluR1 (GenBank accession no. DQ222204). ApGluR1 is 80% identical to mammalian GluR1 in the ligand-binding domain and 95% identical in the pore region, suggesting that Aplysia and mammalian GluR1 molecules may have similar functions. Supporting this idea, ApGluR1 in motor neurons is distributed in puncta that partially colocalize with puncta of both the presynaptic protein synapsin and the postsynaptic protein ApNR1 (4).
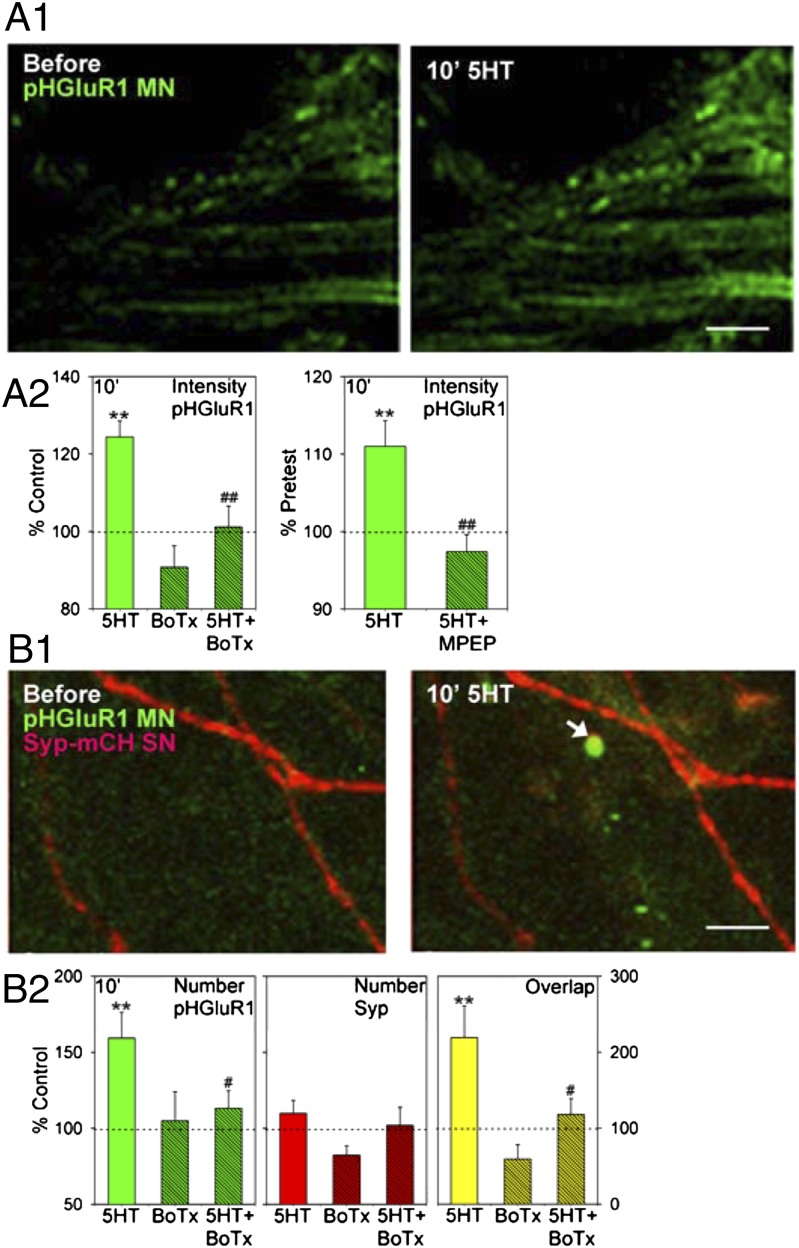
To monitor membrane insertion, we tagged ApGluR1 with a pH-dependent variant of GFP (pHluorin) that increases its fluorescence intensity following fusion of transport vesicles with the surface membrane, which exposes the protein to the extracellular medium (24). Ten-minute application of 5HT produced an increase in the fluorescence intensity of puncta of ApGluR1-pHluorin indicative of membrane insertion in the synaptic region (initial segment) of the motor neuron (F[1,30] = 21.30, P < 0.01 compared with a no-5HT control) (Fig. 5A). This postsynaptic change was reduced by presynaptic injection of BoTx D (F = 9.11, P < 0.01 compared with vehicle injection), whereas BoTx D alone did not have a significant effect. The increase in intensity was also reduced by the mGluR5 antagonist MPEP (50 μM) (t[24] = 3.57, P < 0.01). These results suggest that the increase in postsynaptic ApGluR1-pHluorin intensity is caused at least in part by spontaneous release of glutamate from the presynaptic neuron.
Fig. 5.
Intermediate-term facilitation is accompanied by an increase in postsynaptic AMPA-like receptors that depends on presynaptic release. (A1) Examples of ApGluR1-pHluorin (pHGluR1) fluorescence in the initial segment of the motor neuron before and after 10-min application of 5HT. (Scale bar, 20 μm.) (A2) Average results from experiments like the one shown in A1, in which we measured changes in the intensity of pHGluR1 puncta that were present at both times. 5HT (n = 13 cultures) produced an increase in fluorescence intensity indicative of membrane insertion of pHGluR1, compared with the test-alone control (n = 13). This increase was reduced by injecting BoTx D into the sensory neuron (n = 4), whereas BoTx D alone (n = 4) did not have a significant effect, compared with vehicle control. There was a significant overall effect of group (F[3,30] = 10.46, P < 0.01). The average pretest values were not significantly different between BoTx D and vehicle. The increase in pHGluR1 intensity produced by 5HT (n = 11) was also reduced by the mGluR5 antagonist MPEP (n = 15). (B1) Examples of pHGluR1 (green) in the motor neuron and synaptophysin-mCherry (Syp-mCH; red) in the sensory neuron before and after 10-min 5HT. The arrow indicates a new punctum of pHGluR1 that overlapped a punctum of Syp-mCH (yellow). (Scale bar, 20 μm.) (B2) Average results from experiments like the one shown in B1, in which we measured changes in the number of puncta of pHGluR1, Syp-mCH, and their overlap. 5HT (n = 19) produced an increase in the number of puncta of pHGluR1, compared with the test-alone control (n = 14). This increase was reduced by injecting BoTx D into the sensory neuron (n = 9), whereas BoTx D alone (n = 6) did not have a significant effect, compared with vehicle control. There was a significant overall effect of group (F[3,44] = 3.61, P < 0.05). The average pretest value was 28, not significantly different between BoTx D and vehicle. Results were similar for the overlap of pHGluR1 and Syp-mCH (overall F[3,45] = 4.17, P < 0.05; average pretest value = 4.8%, not significantly different between BoTx D and vehicle). By contrast, there were no significant changes in the number of puncta of Syp-mCH (average pretest value = 28, not significantly different between BoTx and vehicle).
In addition to the increase in intensity, 10-min 5HT also stimulated the appearance of new puncta of ApGluR1-pHluorin, leading to an increase in the total number of puncta (F[1,44] = 8.87, P < 0.01 compared with control) (Fig. 5B). This increase was also reduced by presynaptic injection of BoTx D (F = 4.06, P < 0.05 compared with vehicle), whereas BoTx D alone did not have a significant effect. To examine whether these increases in ApGluR1 might contribute to a functional increase in synaptic transmission, we expressed in the sensory neuron the presynaptic protein synaptophysin tagged with mCherry. There was a significant increase in overlap of puncta of ApGluR1 and synaptophysin during 10-min 5HT (F[1,45] = 7.51, P < 0.01 compared with a no-5HT control), which could contribute to a rapid increase in synaptic transmission. This increase was reduced by presynaptic injection of BoTx D (F = 4.09, P < 0.05 compared with vehicle injection), whereas BoTx D alone did not have a significant effect. There was also an increase in puncta of ApGluR1 that did not overlap puncta of synaptophysin. However, there was not a significant increase in puncta of synaptophysin during 10-min 5HT, although there are increases at later times during intermediate- and long-term facilitation (12).
Collectively, these results suggest that an early increase in spontaneous transmitter release from the presynaptic neuron induces membrane insertion of postsynaptic ApGluR1 at existing puncta and the formation of new puncta. The increase in ApGluR1 puncta during intermediate-term facilitation precedes an increase in presynaptic synaptophysin puncta, and therefore may be a first step in a program that (with additional 5HT exposure) can lead to new synapse assembly during long-term facilitation.
Discussion
Synaptic plasticity has traditionally been divided along three dimensions: (i) modulatory vs. activity-dependent forms, (ii) pre- vs. postsynaptic forms, and (iii) short- vs. long-term forms. In addition, these basic types of plasticity can be combined in various ways to make hybrids with different functional characteristics. Our results have identified a combination in which the modulatory transmitter 5HT enhances spontaneous release of glutamate from the presynaptic neuron. Glutamate then acts on postsynaptic receptors to recruit mechanisms of intermediate-term facilitation including IP3, Ca2+, protein synthesis, and membrane insertion of AMPA-like receptors, which may be first steps in synaptic growth during long-term facilitation. The postsynaptic mechanisms of intermediate-term facilitation are similar to those involved in an activity-dependent form of plasticity at these synapses, homosynaptic potentiation (2, 25). This variation of plasticity thus links short-term presynaptic modulatory effects with postsynaptic mechanisms of intermediate-term “activity-dependent” plasticity in the absence of activity. In addition, it provides a function for spontaneous release as an orthograde signal that recruits postsynaptic mechanisms contributing to intermediate- and long-term plasticity.
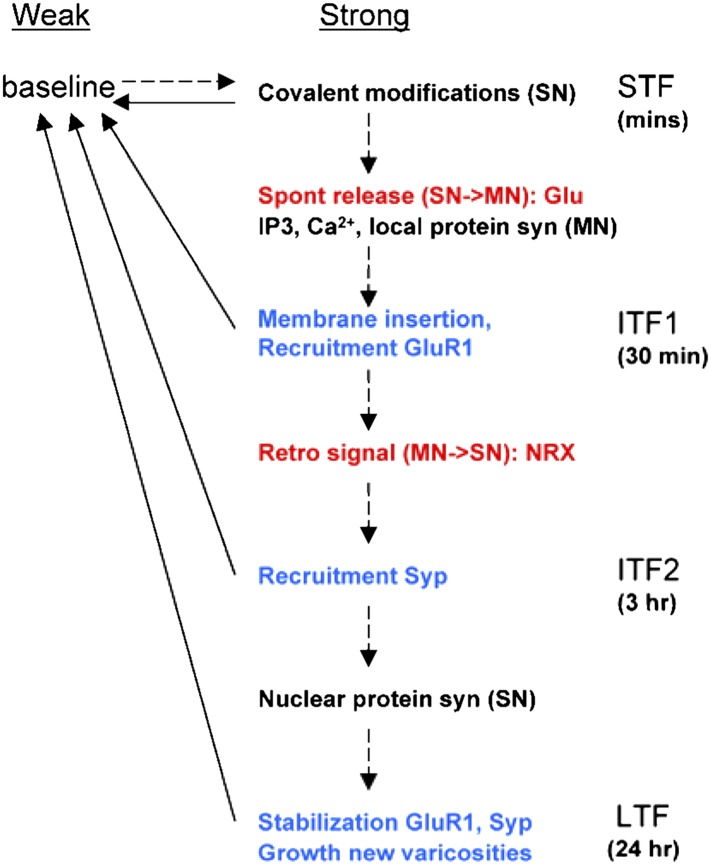
Our results also suggest that plasticity has similarities with synaptic growth. Thus, like synapse formation (26), synaptic plasticity may have a number of stages involving different pre- and postsynaptic mechanisms coordinated by back-and-forth signaling in a chain or cascade that can culminate in growth (Fig. 6). Consistent with this idea, we have found that spontaneous transmitter release from the presynaptic neuron during short-term facilitation recruits postsynaptic molecular mechanisms of intermediate-term facilitation including IP3, Ca2+, and formation of clusters of AMPA-like glutamate receptors. Postsynaptic Ca2+ is in turn necessary for long-term facilitation, perhaps through retrograde signaling (15, 27).
Fig. 6.
Cascade model of synaptic plasticity in Aplysia. In cascade models (30), synapses have two levels of strength (weak and strong) and several increasingly long-lasting states. In Aplysia, relatively weak stimulation produces short-term facilitation (STF) that lasts minutes, stronger stimulation produces intermediate-term facilitation (ITF) that lasts minutes to hours, and even stronger stimulation produces long-term facilitation (LTF) that lasts days. The different stages of facilitation may involve a series or cascade of pre- and postsynaptic mechanisms that is initiated by spontaneous transmitter release during STF, progresses through two stages of ITF, and can culminate in synaptic growth during LTF. The mechanisms in this growth cascade are a subset of all mechanisms involved in facilitation, and some other mechanisms may act in parallel and contribute only to specific stages. Thus, the stages per se do not necessarily form a cascade. Dashed lines, transitions that are initiated by different durations or patterns of 5HT; solid lines, spontaneous transitions; red, extracellular signaling molecules; blue, structural modifications; NRX, neurexin; syn, synthesis; Syp, synaptophysin. See Discussion for details.
The AMPA-like receptors may also contribute to that signaling (28, 29). Some of the new clusters of AMPA-like receptors colocalize with clusters of the presynaptic protein synaptophysin and therefore could form functional synapses. However, there is also an increase in clusters of AMPA-like receptors that do not colocalize. Because that increase precedes an increase in synaptophysin clusters, it may be analogous to “prepatterning” of acetylcholine (ACh) receptors on muscles before they are innervated (30). As has been proposed for prepatterning of ACh receptors, the new clusters of AMPA-like receptors may then feed back to recruit clusters of synaptophysin during a later stage of intermediate-term facilitation, and synaptic growth during long-term facilitation (12).
These results are similar to theoretical “cascade” models of memory storage (31). Such models progress through a series of increasingly stable states and are thus able to exhibit both plasticity and long-term stability, which are essential features of memory but tend to be mutually exclusive in simpler models. This advantage of a cascade of mechanisms might also explain why plasticity involves so many different mechanisms, and why they make different contributions under different experimental conditions. These features of learning-related plasticity have been observed in a variety of species and brain areas including Aplysia and hippocampus (3). Furthermore, recent evidence suggests that long-term potentiation in hippocampus involves a cascade of mechanisms roughly similar to the one we have proposed for facilitation in Aplysia (9, 32). Thus, long-term plasticity may have properties of cascade models more generally.
Materials and Methods
Cell culture, electrophysiological methods, and statistical analysis were generally the same as described previously (2, 10, 25).
Expression of Constructs for Fluorescent Fusion Proteins and Image Analysis.
The DNA construct for CYPHR was a gift from A. C. Newton (University of California San Diego, San Diego, CA), the OAR construct was obtained from B. K. Kaang (Seoul National University, Seoul, Korea), ApGluR1 is described in ref. 4, and synaptophysin is described in ref. 12. Constructs for fluorescent fusion proteins (CYPHR, OAR-GFP, ApGluR1-pHluorin, and synaptophysin-mCherry) were cloned into the Aplysia expression vector pNEX3 (20). Purified plasmid DNAs were microinjected into the sensory neuron or motor neuron, which was examined 1 d later with an Olympus FV1000 scanning unit coupled to an IX81 inverted microscope. The laser intensity and gain were not changed throughout the experiments.
In experiments with expression of CYPHR in the motor neuron, the sensory neuron was also labeled by injecting it with the red fluorescent dye Alexa-568 to identify points of contact between the two neurons. Measurements of CFP/YFP FRET (33) were then taken at those points and other control points in the synaptic region (initial segment) of the motor neuron, and also near the motor-neuron membrane and deeper in the cytoplasm, before and during 10-min application of 5HT. A Z series consisting of 15 or 20 optical sections (with Kalman averages of two or three scans for each section) was collected for the entire thickness of the tissue and stacked to make a projected image. The data are presented as mean percentage change in corrected FRET (34).
The Ca2+ indicator Calcium Orange (Molecular Probes) was injected into the motor neuron 2 h before the start of an experiment, and fluorescence intensity was measured in the synaptic region (initial segment) of the neuron. The intensity and number of puncta of ApGluR1-pHluorin and synaptophysin-mCherry and their overlap were quantified with MetaMorph software.
In Situ Hybridization.
Sequence information for the Aplysia ortholog of mGluR5 was obtained from the neuronal EST database (35). The predicted amino acid sequence is 57% identical and 79% similar to human and mouse mGluR5. Sense and antisense RNA probes labeled with digoxigenin (DIG) were constructed and used for in situ hybridization in isolated L7 motor-neuron cultures as described previously (36) (SI Materials and Methods). Staining was much more intense with the antisense probe (Fig. 1A) than with the sense probe. Six hours after injection of vehicle or sense or antisense oligonucleotide into the motor neuron, the cultures were fixed with 4% (wt/vol) paraformaldehyde in artificial seawater and visualized with a Fluorescent Antibody Enhancer Kit (Roche) for DIG detection.
Reagents.
5HT and octopamine were applied through the perfusion system. Drugs were added to the perfusion 30 min before the start of an experiment and were present for the remainder of experiments with 10-min 5HT, or until washout of 5× 5-min 5HT. Because there was variability between culture batches, drug and control treatments were always compared in the same batch, usually on the same day. LY367385, MPEP (EMD Bioscience), 5HT, octopamine, APV, DNQX (Sigma), wild-type α-latrotoxin (Alomone Labs), and the N4C mutant (a gift from Thomas Sudhof, Stanford University, Stanford, CA) were prepared as stock solutions in water and diluted in artificial seawater before use. In experiments with wild-type α-latrotoxin and their controls, LaCl3 (20 μM) was added to attempt to block α-latrotoxin pores (22). In some experiments, inhibitors were injected intracellularly into the sensory or motor neuron 30 min (or 4 h for mGluR5 oligonucleotides) before the start of an experiment. Because the injection procedure itself may have had some effects, inhibitor injections were always compared with vehicle injections. BAPTA, hygromycin B (Calbiochem), the light chain of botulinum toxin D (List Biological Laboratories), and sense and antisense oligonucleotides were diluted in the vehicle solution (0.5 M KAc with 10 mM Tris⋅HCl, pH 7.4, and 0.2% Fast Green to visualize the injection). The oligonucleotides were designed based on the mGluR5 sequence in the Aplysia EST database. The sense oligonucleotide (5′-TAT GGG ATA CAG AGA GTT GAG-3′) and antisense oligonucleotide (5′-CTC AAC TCT CTG TAT CCC ATA-3′) were synthesized and PAGE-purified by Sigma-Genosys.
Supplementary Material
Acknowledgments
We thank Tom Abrams, Kelsey Martin, and Steve Siegelbaum for their comments. This research was supported by Grants NS045108, MH045923, and GM097502 and the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206846109/-/DCSupplemental.
References
- 1.Antonov I, Kandel ER, Hawkins RD. Presynaptic and postsynaptic mechanisms of synaptic plasticity and metaplasticity during intermediate-term memory formation in Aplysia. J Neurosci. 2010;30:5781–5791. doi: 10.1523/JNEUROSCI.4947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin I, Kandel ER, Hawkins RD. Whereas short-term facilitation is presynaptic, intermediate-term facilitation involves both presynaptic and postsynaptic protein kinases and protein synthesis. Learn Mem. 2011;18(2):96–102. doi: 10.1101/lm.1949711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CH, Barco A, Hawkins RD, Kandel ER. Molecular studies of learning and memory in Aplysia and hippocampus: A comparative analysis of implicit and explicit memory storage. In: Byrne J, editor. Learning and Memory: A Comprehensive Reference. Oxford: Elsevier; 2008. pp. 11–29. [Google Scholar]
- 4.Li HL, et al. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Zablow L, Kandel ER, Siegelbaum SA. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nat Neurosci. 1999;2(1):24–30. doi: 10.1038/4525. [DOI] [PubMed] [Google Scholar]
- 6.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 7.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 8.De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008;6:e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonova I, Lu F-M, Zablow L, Udo H, Hawkins RD. Rapid and long-lasting increase in sites for synapse assembly during late-phase potentiation in rat hippocampal neurons. PLoS One. 2009;4:e7690. doi: 10.1371/journal.pone.0007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin I, et al. Spontaneous transmitter release is critical for the induction of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci USA. 2012;109:9131–9136. doi: 10.1073/pnas.1206914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim J-H, et al. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40(1):151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: Dependence on release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis, and modulation of postsynaptic AMPA receptor efficacy. J Neurosci. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villareal G, Li Q, Cai D, Glanzman DL. The role of rapid, local, postsynaptic protein synthesis in learning-related synaptic facilitation in Aplysia. Curr Biol. 2007;17:2073–2080. doi: 10.1016/j.cub.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai D, Chen S, Glanzman DL. Postsynaptic regulation of long-term facilitation in Aplysia. Curr Biol. 2008;18:920–925. doi: 10.1016/j.cub.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale N, Kandel ER. L-Glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci USA. 1993;90:7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trudeau LE, Castellucci VF. Excitatory amino acid neurotransmission at sensory-motor and interneuronal synapses of Aplysia californica. J Neurophysiol. 1993;70:1221–1230. doi: 10.1152/jn.1993.70.3.1221. [DOI] [PubMed] [Google Scholar]
- 18.Conrad P, Wu F, Schacher S. Changes in functional glutamate receptors on a postsynaptic neuron accompany formation and maturation of an identified synapse. J Neurobiol. 1999;39:237–248. [PubMed] [Google Scholar]
- 19.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaang BK. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett. 1996;221(1):29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- 21.Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashton AC, et al. α-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem. 2001;276:44695–44703. doi: 10.1074/jbc.M108088200. [DOI] [PubMed] [Google Scholar]
- 23.Volynski KE, et al. Mutant α-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation: This action does not require neurexins. J Biol Chem. 2003;278:31058–31066. doi: 10.1074/jbc.M210395200. [DOI] [PubMed] [Google Scholar]
- 24.Ashby MC, Ibaraki K, Henley JM. It’s green outside: Tracking cell surface proteins with pH-sensitive GFP. Trends Neurosci. 2004;27:257–261. doi: 10.1016/j.tins.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Jin I, Hawkins RD. Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at Aplysia sensory-motor neuron synapses. J Neurosci. 2003;23:7288–7297. doi: 10.1523/JNEUROSCI.23-19-07288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YB, et al. Neurexin-neuroligin transsynaptic interaction mediates learning-related synaptic remodeling and long-term facilitation in Aplysia. Neuron. 2011;70:468–481. doi: 10.1016/j.neuron.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ripley B, Otto S, Tiglio K, Williams ME, Ghosh A. Regulation of synaptic stability by AMPA receptor reverse signaling. Proc Natl Acad Sci USA. 2011;108:367–372. doi: 10.1073/pnas.1015163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, Uemura T, Yoshida T, Mishina M. GluRδ2 assembles four neurexins into trans-synaptic triad to trigger synapse formation. J Neurosci. 2012;32:4688–4701. doi: 10.1523/JNEUROSCI.5584-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Curr Opin Neurobiol. 2002;12(1):100–103. doi: 10.1016/s0959-4388(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 31.Fusi S, Drew PJ, Abbott LF. Cascade models of synaptically stored memories. Neuron. 2005;45:599–611. doi: 10.1016/j.neuron.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone VP, Raymond CR. A protein synthesis and nitric oxide-dependent presynaptic enhancement in persistent forms of long-term potentiation. Learn Mem. 2011;18:625–633. doi: 10.1101/lm.2245911. [DOI] [PubMed] [Google Scholar]
- 33.Udo H, et al. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron. 2005;45:887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Gordon GW, Berry G, Liang XH, Levine B, Herman B. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys J. 1998;74:2702–2713. doi: 10.1016/S0006-3495(98)77976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moroz LL, et al. Neuronal transcriptome of Aplysia: Neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giustetto M, et al. Axonal transport of eukaryotic translation elongation factor 1α mRNA couples transcription in the nucleus to long-term facilitation at the synapse. Proc Natl Acad Sci USA. 2003;100:13680–13685. doi: 10.1073/pnas.1835674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.