Abstract
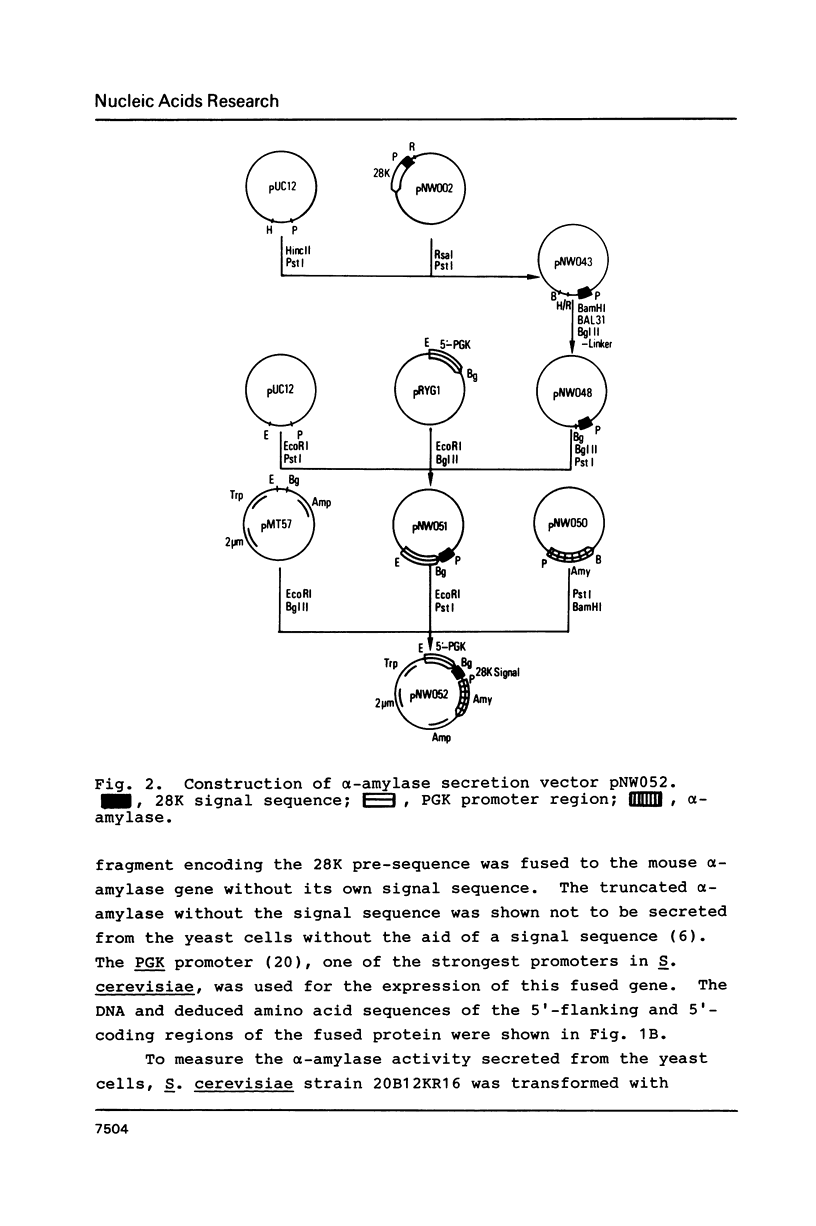
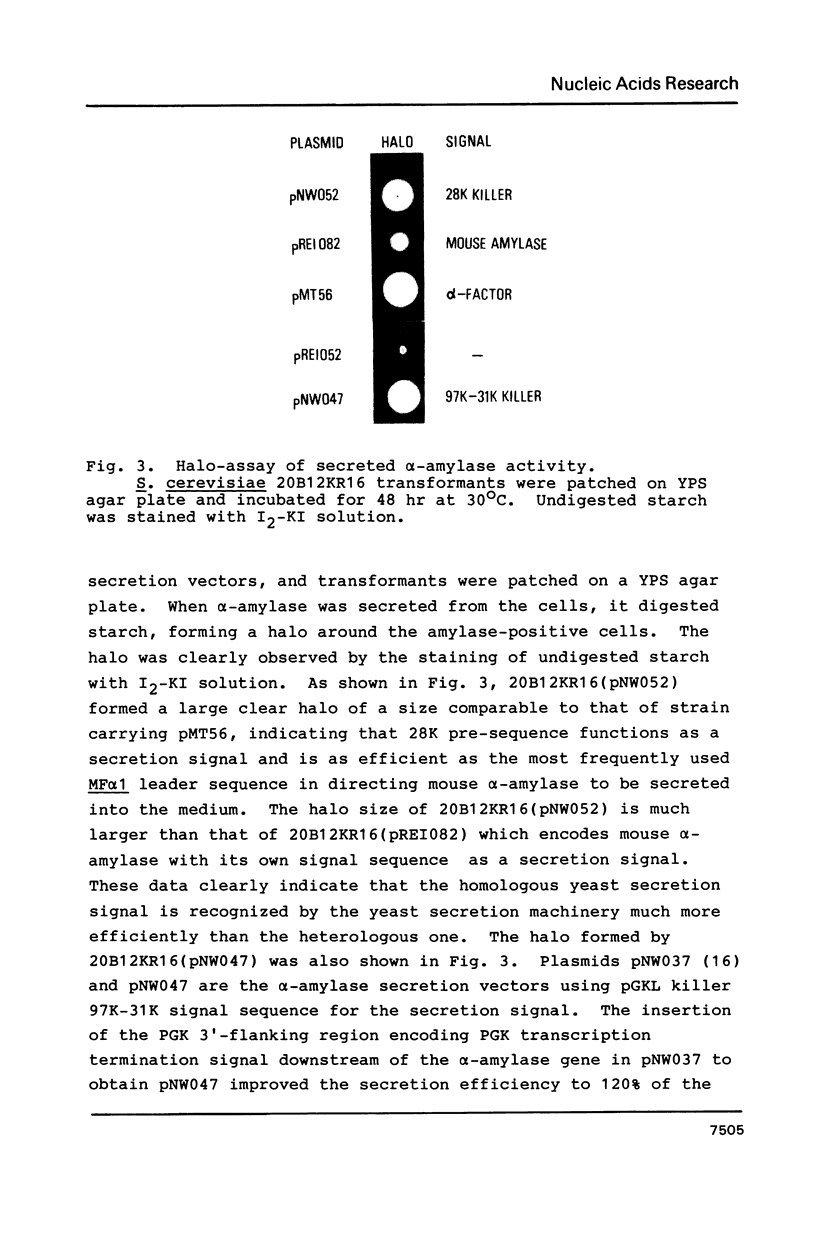
Saccharomyces cerevisiae harboring linear dsDNA plasmids, pGKL1 and pGKL2, secretes a killer toxin consisting of 97, 31 and 28 kilodalton subunits (Nucleic Acids Res., 15, 1031-1046, 1987). We isolated the DNA encoding the N-terminal pre-sequence of the 28K precursor protein and constructed a new secretion vector in S. cerevisiae. Mouse alpha-amylase fused to the 28K signal sequence was secreted into the culture medium with a high efficiency similar to those fused to the mating factor alpha and 97K-31K killer signal sequences. This data clearly indicates that 28K presequence functions as a secretion signal. Glycosylated and nonglycosylated alpha-amylase molecules were detected in the culture medium. The secretion of alpha-amylase was blocked by sec18-1 mutation. The secreted alpha-amylase recovered from the medium was found to migrate faster in SDS-polyacrylamide gel than the precursor form of alpha-amylase synthesized in vitro. These lines of evidence suggest that mouse alpha-amylase fused to 28K killer signal sequence was processed, glycosylated and secreted through the normal secretion pathway of the yeast.
Full text
PDF




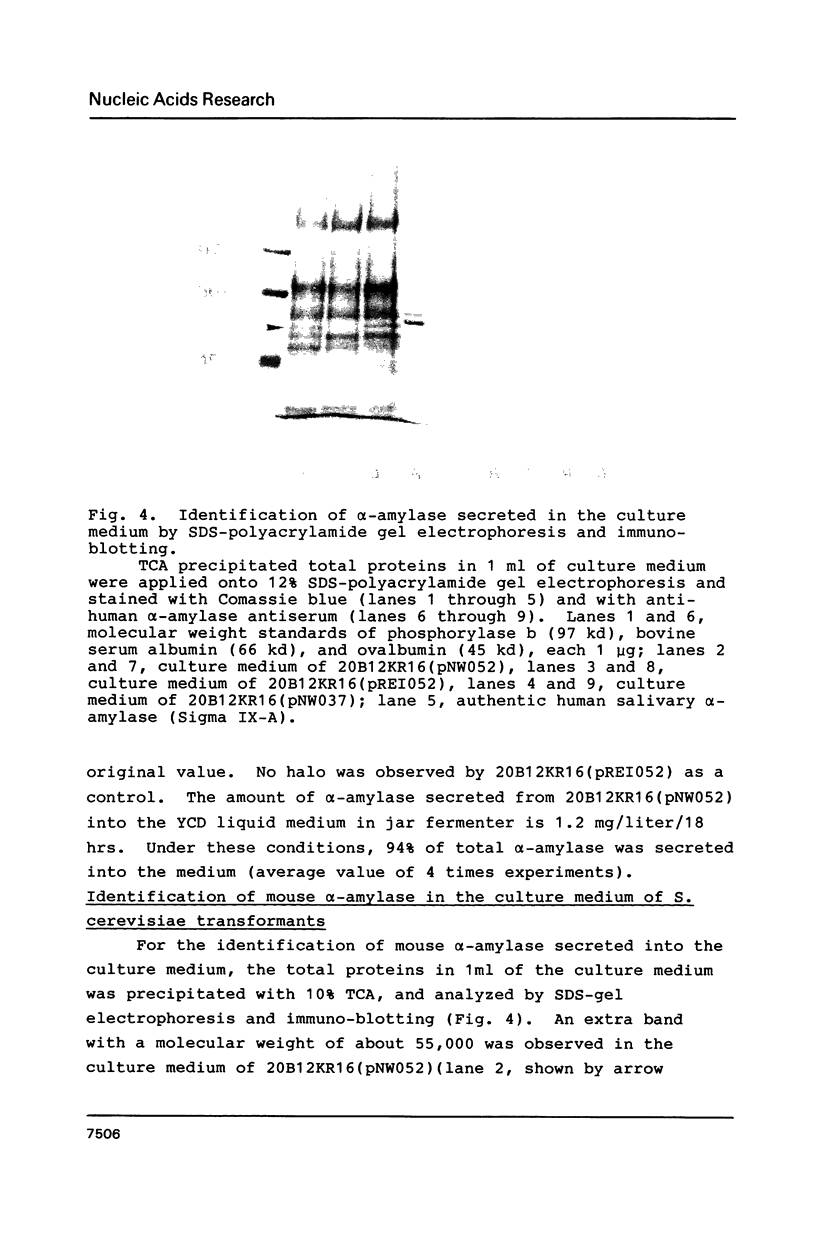





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitter G. A., Chen K. K., Banks A. R., Lai P. H. Secretion of foreign proteins from Saccharomyces cerevisiae directed by alpha-factor gene fusions. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5330–5334. doi: 10.1073/pnas.81.17.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Hansen W., Schauer I., Schekman R. Genes required for completion of import of proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1984 Jan;98(1):44–53. doi: 10.1083/jcb.98.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hishinuma F., Nakamura K., Hirai K., Nishizawa R., Gunge N., Maeda T. Cloning and nucleotide sequences of the linear DNA killer plasmids from yeast. Nucleic Acids Res. 1984 Oct 11;12(19):7581–7597. doi: 10.1093/nar/12.19.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzeman R. A., Leung D. W., Perry L. J., Kohr W. J., Levine H. L., Goeddel D. V. Secretion of human interferons by yeast. Science. 1983 Feb 11;219(4585):620–625. doi: 10.1126/science.6186023. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Holland M. J., McCabe P. C., Cole G. E., Wittman V. P., Tal R., Watt K. W., Gelfand D. H., Holland J. P., Meade J. H. Expression, Glycosylation, and Secretion of an Aspergillus Glucoamylase by Saccharomyces cerevisiae. Science. 1985 Apr 5;228(4695):21–26. doi: 10.1126/science.228.4695.21. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Bond M. W., Otsu K., Arai K., Arai N. Secretion of mature mouse interleukin-2 by Saccharomyces cerevisiae: use of a general secretion vector containing promoter and leader sequences of the mating pheromone alpha-factor. Gene. 1985;37(1-3):155–161. doi: 10.1016/0378-1119(85)90268-9. [DOI] [PubMed] [Google Scholar]
- Nishide T., Nakamura Y., Emi M., Yamamoto T., Ogawa M., Mori T., Matsubara K. Primary structure of human salivary alpha-amylase gene. Gene. 1986;41(2-3):299–304. doi: 10.1016/0378-1119(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Singh A., Lugovoy J. M., Kohr W. J., Perry L. J. Synthesis, secretion and processing of alpha-factor-interferon fusion proteins in yeast. Nucleic Acids Res. 1984 Dec 11;12(23):8927–8938. doi: 10.1093/nar/12.23.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Duncan M. J., Moir D. T. Heterologous protein secretion from yeast. Science. 1985 Sep 20;229(4719):1219–1224. doi: 10.1126/science.3939723. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Boyd A. The killer toxin of Kluyveromyces lactis: characterization of the toxin subunits and identification of the genes which encode them. EMBO J. 1986 Aug;5(8):1995–2002. doi: 10.1002/j.1460-2075.1986.tb04455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Mileham A. J., Romanos M. A., Boyd A. Nucleotide sequence and transcription analysis of a linear DNA plasmid associated with the killer character of the yeast Kluyveromyces lactis. Nucleic Acids Res. 1984 Aug 10;12(15):6011–6030. doi: 10.1093/nar/12.15.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J. Resolution of sequence discrepancies in the ORF1 region of the Kluyveromyces lactis plasmid k1. Nucleic Acids Res. 1988 Jan 25;16(2):771–771. doi: 10.1093/nar/16.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Wada N., Hishinuma F. A novel yeast secretion vector utilizing secretion signal of killer toxin encoded on the yeast linear DNA plasmid pGKL1. Biochem Biophys Res Commun. 1987 Apr 29;144(2):613–619. doi: 10.1016/s0006-291x(87)80010-4. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Wada N., Hishinuma F. Expression and identification of immunity determinants on linear DNA killer plasmids pGKL1 and pGKL2 in Kluyveromyces lactis. Nucleic Acids Res. 1987 Feb 11;15(3):1031–1046. doi: 10.1093/nar/15.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]