Abstract
A chimeric gene was constructed by fusing the DNA sequences containing the 5' flanking region of the mouse alpha 1(III) collagen gene to the coding sequence of the bacterial chloramphenicol acetyltransferase (CAT) gene. Transient transfection experiments indicated that the alpha 1(III) promoter is active in NIH 3T3 fibroblasts and BC3H1 smooth muscle cells. The activity of the alpha 1(III) collagen promoter-CAT plasmid is stimulated approximately ten fold by the presence of the SV40 enhancer element. Removing sequences upstream of -200 stimulates the activity of the chimeric gene eight fold. Further deletion analysis identified sequences located between -350 and -300 that were instrumental in repressing the activity of the promoter. This 50 bp region contains a direct repeat sequence that may be involved in the regulation of the mouse alpha 1(III) collagen gene. Truncating the alpha 1(III) promoter to -80 further stimulated expression. We propose that the positive regulatory elements of this gene appear to be located within the first 80 bp of the promoter, whereas elements located further upstream exert a negative effect on the expression of the gene. Regulation of the alpha 1(III) gene contrasts with that of the alpha 2(I) collagen gene, which appears to be regulated by several positive elements located in various regions of the promoter.
Full text
PDF


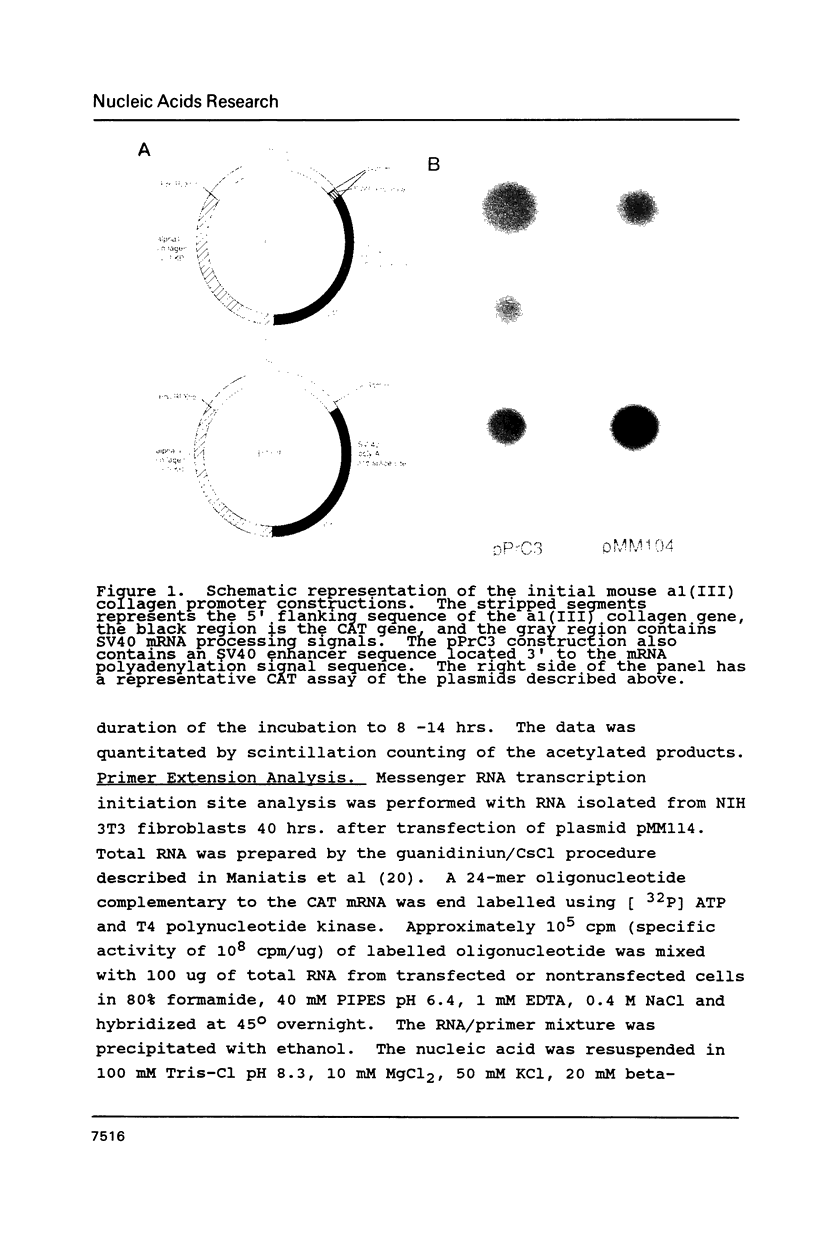










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ramirez F. Fine structural analysis of the human pro-alpha 1 (I) collagen gene. Promoter structure, AluI repeats, and polymorphic transcripts. J Biol Chem. 1985 Feb 25;260(4):2315–2320. [PubMed] [Google Scholar]
- Dickson L. A., de Wet W., Di Liberto M., Weil D., Ramirez F. Analysis of the promoter region and the N-propeptide domain of the human pro alpha 2(I) collagen gene. Nucleic Acids Res. 1985 May 24;13(10):3427–3438. doi: 10.1093/nar/13.10.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamochi A., Paterson B., de Crombrugghe B. Differential binding of a CCAAT DNA binding factor to the promoters of the mouse alpha 2(I) and alpha 1(III) collagen genes. J Biol Chem. 1986 Aug 25;261(24):11310–11314. [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Liau G., Mudryj M., de Crombrugghe B. Identification of the promoter and first exon of the mouse alpha 1 (III) collagen gene. J Biol Chem. 1985 Mar 25;260(6):3773–3777. [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., McKeon C., de Crombrugghe B., Pastan I. Regulation of the expression of genes encoding types I, II, and III collagen during chick embryonic development. J Biol Chem. 1983 Aug 25;258(16):10041–10048. [PubMed] [Google Scholar]
- Ohno S., Taniguchi T. Inducer-responsive expression of the cloned human interferon beta 1 gene introduced into cultured mouse cells. Nucleic Acids Res. 1982 Feb 11;10(3):967–977. doi: 10.1093/nar/10.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Vuorio E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J Cell Biol. 1987 Apr;104(4):1077–1084. doi: 10.1083/jcb.104.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Setoyama C., de Crombrugghe B. Regulation of a collagen gene promoter by the product of viral mos oncogene. Nature. 1985 Mar 21;314(6008):286–289. doi: 10.1038/314286a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Yamada Y., de Crombrugghe B. DNA sequence comparison of the regulatory signals at the 5' end of the mouse and chick alpha 2 type I collagen genes. J Biol Chem. 1984 Jun 25;259(12):7411–7415. [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Eldridge J. D., Paterson B. M. Expression and regulation of chicken actin genes introduced into mouse myogenic and nonmyogenic cells. Proc Natl Acad Sci U S A. 1984 May;81(10):2980–2984. doi: 10.1073/pnas.81.10.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Yamada Y., Mudryj M., de Crombrugghe B. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J Biol Chem. 1983 Dec 25;258(24):14914–14919. [PubMed] [Google Scholar]
- Yu Y. T., Manley J. L. Structure and function of the S1 nuclease-sensitive site in the adenovirus late promoter. Cell. 1986 Jun 6;45(5):743–751. doi: 10.1016/0092-8674(86)90788-9. [DOI] [PubMed] [Google Scholar]





