Abstract
OBJECTIVE: Bax protein is a key mediator of apoptosis, and it might be related to chemosensitivity. The purpose of this study was to evaluate the prognostic role of Bax in patients with advanced gastric cancer treated with triplet chemotherapy COI regimen (capecitabine, oxaliplatin, and irinotecan). METHODS: Pretreatment tissue blocks were available for 23 consecutive patients, selected for good performance status (ECOG ≤ 1) and consenting for treatment with first-line COI at a single institution. Bax levels were classified as positive or negative by immunohistochemistry (bax N20; Santa Cruz Biotechnology) and related to outcome in terms of response rate, progression-free survival, and overall survival. RESULTS: Bax-negative and -positive samples were 26% and 74%, respectively. Bax expression was associated with significantly higher response rate (87% vs 33%), progression-free survival (8.7 vs 4.9 months, P = .016), and overall survival (23.8 vs 12.7 months, P = .025). In multivariate analysis including Bax and performance status, low Bax independently predicted worse outcome, along with suboptimal performance status. CONCLUSIONS: In advanced gastric cancer, Bax expression was related to clinical benefit with COI regimen. Whether Bax is a prognostic or mixed prognostic/predictive factor warrants prospective confirmation. It is to be defined if Bax predicts sensitivity to platinum analogs or to whatever chemotherapy regimen.
Introduction
Despite decreasing incidence, gastric cancer is still the second most common cause of cancer-related death in the world [1]. Even if radical resection is the mainstay of curative treatment of gastric cancer, disease recurrence is very common. Outcome of unresectable or metastatic gastric cancer is still extremely poor, although chemotherapy demonstrated to confer a benefit in terms of survival and quality of life [2]. There is no standard first-line chemotherapeutic regimen, although cisplatin and fluoropirimidine combination is the backbone of treatment, with or without other agents such as epirubicin or docetaxel [3]. Recently, oxaliplatin and capecitabine are replacing cisplatin and 5-fluorouracil, with equivalent efficacy and significantly less acute toxicity [4]; irinotecan has proven activity, and clinical outcome has certainly improved by combination of the most active drugs and with the addition of the targeted agent trastuzumab for a subset of patients with HER2-positive disease [5].
We previously conducted a phase 1/2 study of capecitabine in combination with oxaliplatin and irinotecan (COI regimen) to find the maximum tolerated dose and to evaluate the activity of the triplet in patients with advanced colorectal cancer [6]. The COI regimen was administered every other week and was designed to maximize the dose of irinotecan and sequence-dependent synergistic interactions among the three drugs, while giving capecitabine and oxaliplatin at fixed, clinically relevant doses. On the basis of our preliminary data, we conducted a phase 2 study with COI as first-line treatment in patients with advanced gastric cancer selected for good performance status, demonstrating the safety and promising activity of this triplet regimens [7].
Apoptosis-regulating proteins and DNA-repairing enzymes are ideal candidates for clinical and pathologic studies investigating predictive and prognostic biomarkers. Preclinical studies documented that exposure of cells to a variety of cytotoxic agents including platinum analogs is associated with the typical morphologic changes of apoptosis [8]. Thus, it was hypothesized that an intact apoptotic pathway is necessary for chemotherapy-induced cell death and, conversely, that abnormalities in the ability of a cell to undergo apoptosis may lead to the development of chemoresistance. The apoptotic cell death is regulated by a genetic program involving both effectors and repressors. Bax is a proapoptotic Bcl-2 family member and is induced by functional p53 to counter the death repressor activity of both Bcl-2 and Bcl-XL, with the consequence to accelerate cell death induced by growth factors withdrawal and chemotherapy drugs [8–10]. Apoptosis depends on the relative amount of Bcl-2 and Bax homodimers and heterodimers present in the cell, and sensitivity to cytotoxic agents such as platinum analogs is favored when increased mitochondrial concentration of Bax/Bax homodimers occurs [11,12]. This observation raises the possibility that reduced levels of downstream molecules such as Bax may render cancer cells resistant to chemotherapy-induced apoptosis, even in a p53-independent manner.
The aim of this study was to investigate the prognostic and predictive significance of Bax in a series of patients homogeneously treated with the triplet regimen consisting of irinotecan added to standard fluoropyrimidine and oxaliplatin-based chemotherapy.
Materials and Methods
Patients and Treatment
The investigation was conducted as a mono-institutional, prospective phase 2 study at Istituto Nazionale dei Tumori of Milan. Patients with chemonaive advanced gastric cancer were treated with COI regimen (irinotecan 180 mg/m2 infused during 90 minutes on day 1, followed by oxaliplatin 85 mg/m2 in a 3-hour infusion on day 2 and capecitabine 1000 mg/m2 per day orally twice daily from days 2 to 6 of a biweekly schedule) [13]. Treatment was continued until disease progression or unacceptable toxicity, for a maximum of up to eight cycles. Each patient provided a written informed consent before being enrolled in the study. Eligible patients had histologically confirmed inoperable or metastatic adenocarcinoma of the stomach or gastroesophageal junction, aged between 18 and 70 years, an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 1, and a life expectancy of at least 3 months. Other inclusion criteria were measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) and adequate hematologic, renal, and hepatic functions. No prior chemotherapy was allowed, except adjuvant chemotherapy, which had to be completed at least 6 months before treatment. Patients were excluded from study if they had any other active malignancy with the exception of nonmelanoma skin cancer or in situ cervical cancer or had documented brain metastases, uncontrolled cardiac disease, or other clinically significant, uncontrolled coexisting illness.
Clinical response was assessed every 8 weeks with radiologic examination (computed tomodensitometry or magnetic resonance imaging). The Response Evaluation Criteria in Solid Tumors (RECIST) were adopted for evaluation and objective tumor response was classified into partial response, stable disease, and progressive disease (PD) [14]. Patients with stable disease or progressive disease were defined as nonresponders. Response to therapy was also evaluated retrospectively by independent radiologists.
Bax Immunohistochemical Analysis
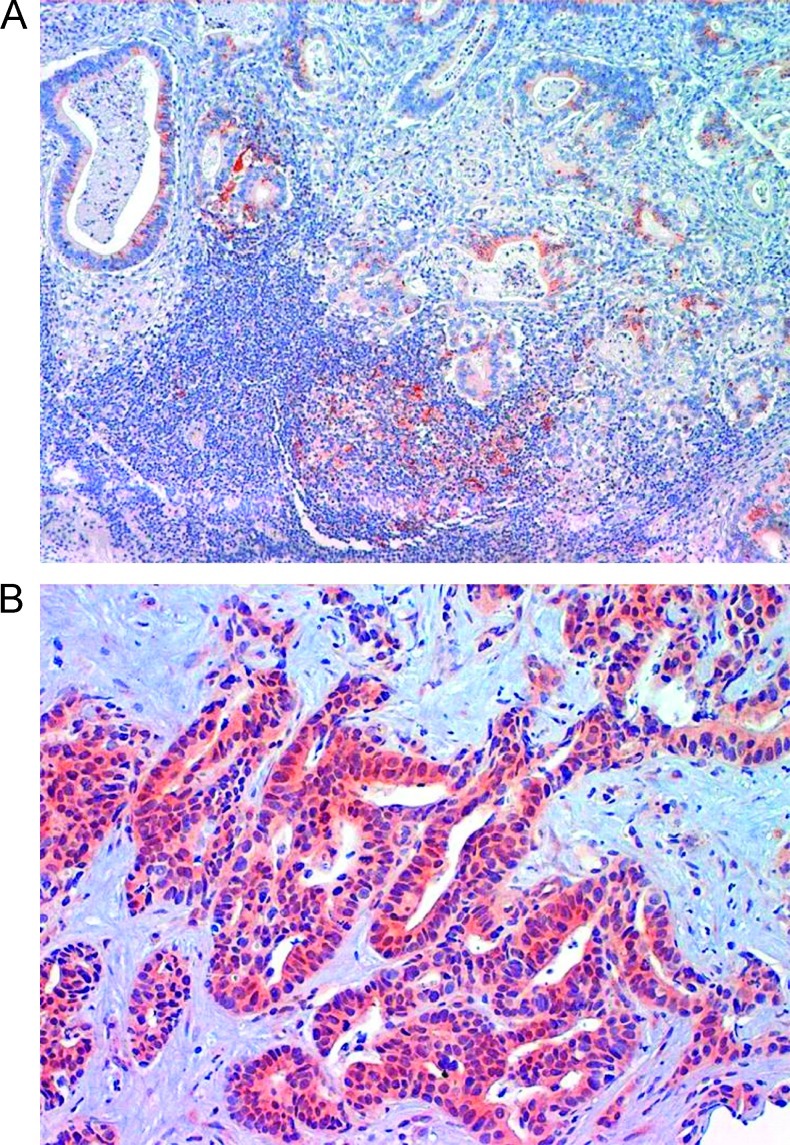
Immunohistochemical staining of formalin-fixed, paraffin-embedded tissue was performed for primary tumor obtained by endoscopic biopsy or gastrectomy. In patients with recurrent disease, tumor tissue from metastatic lesions was preferentially used for staining, if it was not available. Bax expression was assessed by immunohistochemistry (IHC) with a rabbit polyclonal antiserum specific for the amino-terminal of Bax (bax N-20; Santa Cruz Biotechnology, Santa Cruz, CA); all blocks were fixed in 10% formalin and embedded in paraffin, and serial sections were cut from each block in 4 µm, stained with hematoxylin and eosin, and confirmed pathologically. Immunohistochemical staining for Bax protein was performed using a streptavidin-peroxidase technique with the following procedure: tissue sections were deparaffinized in xylene and hydrated in serial alcohol solutions, respectively; endogenous peroxidase was blocked by incubation in 0.3% hydrogen peroxide in methanol for 20 to 30 minutes. Specimens were subjected to a 10-mM sodium citrate buffer, pH 6.0 for antigen retrieval (pH 6.0), and heated in an autoclave for 6 minutes (95°C). Slides were incubated with a 1:200 dilution of the primary rabbit antihuman Bax polyclonal antibody (bax N-20; Santa Cruz Biotechnology) for 1 hour at room temperature. A biotin-streptavidin detection system was used with diaminobenzidine as the chromogen. After several rinses in phosphate-buffered saline (PBS), sections were incubated with the linking reagent (biotinylated goat secondary antibodies) at room temperature for 30 minutes, followed by rinsing in PBS and incubation for 30 minutes with the peroxidase-conjugated streptavidin. After rinsing in PBS, the slides were incubated in peroxidase substrate solution containing hydrogen peroxide and 3′,3′-diaminobenzidine for 2 minutes. With each batch of test samples, lymphocytes in the germinal center of normal lymph nodes were evaluated as positive control; negative controls were performed by the omission of the primary antibody during the process of staining. Bax cytoplasmic staining was examined by two independent and blinded senior pathologists and quantified using a visual grading system based on the extent of staining (by percentage of positive tumor cells graded on a scale of 0–3: 0 = none, 1 = 1%–10%, 2 = 11%–50%, 3 = 51%–100%) and the intensity of staining (graded on a scale of 0–1 for no or weak staining and 2–3 for moderate or strong staining). A semiquantitative was obtained by multiplying the grades of extent and intensity of staining and the median value was chosen as the cutoff for dichotomizing expression (Figure 1, A and B). Sections were examined at five selected non-overlapping high-power fields (magnification, x40) to assess the proportion of cells that had cytoplasmic reaction.
Figure 1.
Representative immunohistochemical detection of BAX in two gastric adenocarcinoma samples. (A) Bax cytoplasmic immunostaining in well-differentiated tubular gastric adenocarcinoma and in germinal center (positive control). Magnification, x10. (B) Bax cytoplasmic immunostaining in moderately differentiated metastatic tubular gastric adenocarcinoma. Magnification, x20.
Statistical Analysis
Descriptive statistical methodology was used to analyze the results. All patients who had received at least one cycle of the study treatment were included in analyses of response, safety, and survival on an intention-to-treat basis. Results were reported with 95% confidence intervals (CIs). Progression-free survival (PFS) was measured from date of treatment until date of progression or death from any cause, and overall survival (OS) was measured from the date of starting treatment to the time of death from any cause.
Associations between Bax status and clinical response (by RECIST criteria) was assessed by two-tailed Fisher exact test. Survival curves were plotted by the Kaplan-Meier method and compared by log-rank test. For ordinal variables, a log-rank test of trend was applied. Cox regression model with a backward stepwise procedure was done for multivariate survival analysis [14].
Results
Patients' Characteristics
From January 2009 to November 2010, 41 consecutive patients with advanced gastric cancer were considered eligible for intensive triplet chemotherapy and received first-line COI at a single Institution. Tissue blocks were available for 23 patients, whose characteristics are shown in Table 1. Twenty-one subjects had measurable disease according to RECIST criteria, whereas only two subjects had evaluable nonmeasurable lesions and were not included in response assessment. Only four patients with recurrent disease received prior adjuvant chemotherapy (5-fluorouracil monotherapy in one, cisplatinand fluoropyrimidine-based in three cases); the remaining 19 subjects presented with metastatic disease at the time of diagnosis. Among the patients with initially metastatic disease, 4 of 17 underwent palliative gastrectomy, whereas 2 of 6 patients with recurrence received resection of metastatic lesions.
Table 1.
Patients' Characteristics.
| Main Characteristics | |
| Age (year) | |
| Median | 56 |
| Range | (41–70) |
| Sex, n (%) | |
| Male | 13 (57) |
| Female | 10 (43) |
| ECOG performance status, n (%) | |
| 0 | 16 (70) |
| 1 | 7 (30) |
| Disease status, n (%) | |
| Primary metastatic | 17 (74) |
| Recurrent | 6 (26) |
| Location of tumor, n (%) | |
| Proximal | 8 (45) |
| Mid/distal | 15 (65) |
| Lauren classification, n (%) | |
| Diffuse | 11 (48) |
| Intestinal | 12 (52) |
| Differentiation, n (%) | |
| Well/moderate | 6 (26) |
| Poor | 17 (74) |
| Adjuvant chemotherapy, n (%) | |
| Yes | 4 (17) |
| No | 19 (83) |
Correlation of Bax with Clinical Outcome
Among all 23 evaluated tumor specimens, 6 (26%) were Bax negative and 17 (74%) were considered as Bax positive. In the univariate analysis, there was no statistically significant association between Bax-negative expression and age (≤65 vs >65 years, 26% vs 25%; P = 1), sex (male vs female, 15% vs 40%; P = .34), and histologic subtype (intestinal vs diffuse type, 16% vs 36%; P = .37); interestingly, all patients with low Bax expression were affected by high-grade or undifferentiated gastric cancers.
The relationship between Bax expression and clinical outcome was evaluated in terms of objective tumor response, PFS, and OS. Overall, 15 (71%) of 21 evaluable patients responded to COI regimen (in particular, 11 partial responses and 4 complete responses were observed). Only two (33%) of six patients in the Bax-negative tumors group showed a response, and consequently, the response rate in patients with Bax-positive expression was 87% (P = .03). Therefore, low Bax expression was significantly associated with the lack of response to chemotherapy.
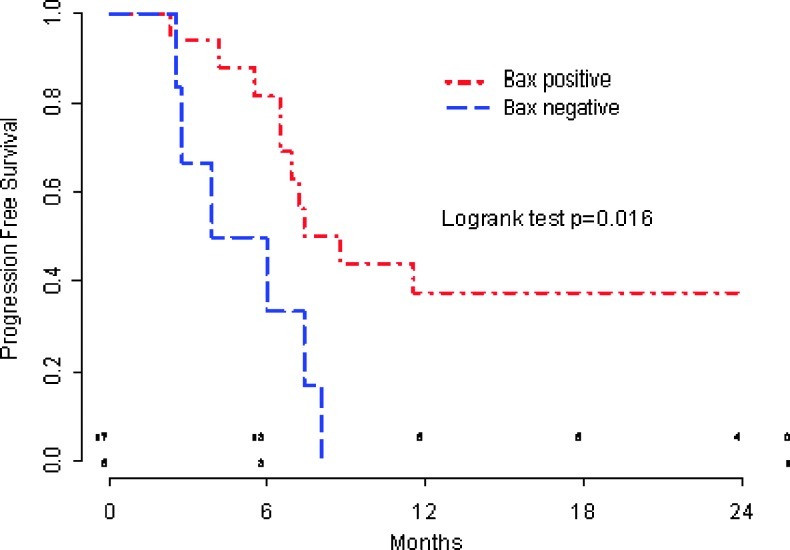
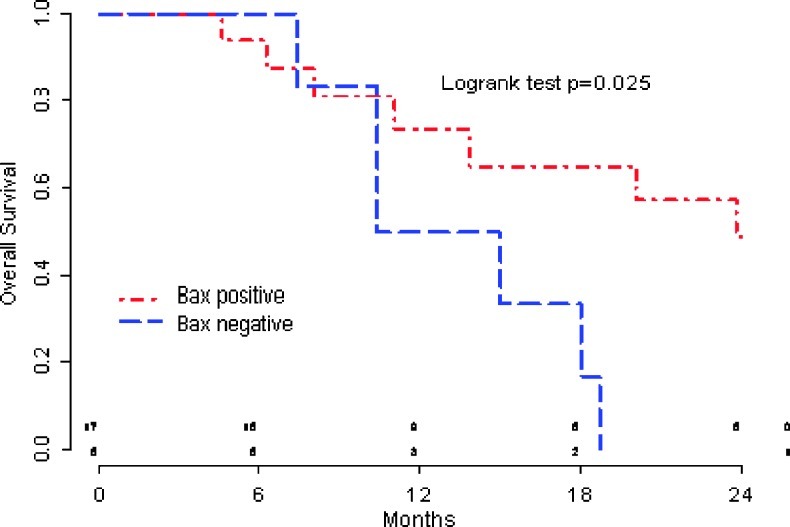
With a median follow-up of 24 months, COI regimen produced a 7-month median PFS and a promising 18-month median OS in good-intermediate performance status advanced gastric cancer patients. By Cox univariate analysis of survival, we showed that patients with Bax-negative tumors had a worse clinical outcome in terms of PFS compared with Bax-positive tumors (P = .016; median, 4.9 vs 8.7 months; hazard ratio [HR] = 3.40, CI = 1.17–9.93; Figure 2). Similarly, the OS of patients with low Bax expression was significantly shorter compared with those patients with a Bax-positive tumor (P = .025; median, 12.7 vs 23.8 months; Figure 3).
Figure 2.
PFS according to Bax status.
Figure 3.
OS according to Bax status.
In multivariate analysis including Bax and PS, significantly higher risks for progression (HR = 4.51, CI = 1.30–15.6, P = .02) and death (HR = 6.69, CI = 1.30–15.6, P = .01) were showed for Bax-negative tumors, and suboptimal PS (ECOG 1) was associated with a trend for worst overall survival (P = .08).
Discussion
The independent validation of predictive biomarkers is a significant issue for oncologists to treat patients who are more likely to obtain a benefit and avoid potential toxicity in case of refractoriness. At present, only clinical prognostic factors are currently available to drive the treatment decision making in advanced gastric cancer [15]; very little is available in terms of reliable prognostic indicators of response to chemotherapy and clinical outcome, whereas the most promising predictive factors still need to be validated in large and randomized trials.
Up to now, the role of Bax seems more prognostic than predictive. As matter of fact, it was mainly studied in patients who underwent curative surgery for gastric cancer and was correlated to pathologic characteristic and risk of disease relapse and death. In a surgical series of 57 resected gastric cancer specimens, a strong relationship between decreased Bax expression and diffuse-type gastric or poorly differentiated morphology was identified [16]. Bax-negative expression was associated with dedifferentiation, lymph node metastases, and poor clinical prognosis in a different series of 47 resected gastric cancer [17]. Moreover, the role of the low expression of Bax as a poor prognostic factor has been reported in several malignancies treated with chemotherapy or chemoradiotherapy, such as breast, ovarian, and head and neck cancer [18–20]. At present, few data have been published so far on the prognostic and/or predictive role of Bax as a biomarker in advanced gastroesophageal cancers treated with chemotherapy. Low Bax expression assessed by IHC was identified as an independent prognostic factor of poor outcome in locally advanced esophageal cancer patients treated with cisplatin-and 5-fluorouracil-based concurrent chemoradiotherapy [21]. Although previous analyses showed inconsistent results about the role of Bax in gastric cancer [22,23], a recent retrospective study in 72 patients treated with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX-4 regimen) for advanced disease finally documented a significant association between low Bax expression and poor overall survival in terms of prognosis, in both univariate and multivariate analysis; nevertheless, Bax expression failed to show a predictive role in terms of response rate in patients with measurable disease [24]. In the present study, we evaluated the role of Bax as potential mediator of chemoresistance in gastric cancer treated with a highly active first-line triplet regimen. In fact, low Bax expression could be associated with more aggressive clinical evolution of the disease and possibly lack of benefit from palliative chemotherapy. Our data suggest that the Bax-positive status might identify patients who are likely to respond to such combination of chemotherapy and to have a longer PFS and OS. Thus, low expression of Bax was independently associated with poor PFS and OS, as well as performance status, which has been suggested as an important prognostic factor in gastric cancer [15,25]. To our knowledge, this is the first report to explore the prognostic significance of Bax patients with gastric cancer treated with a three-drug regimen, in which irinotecan was added to standard platinum-and fluoropyrimidine-based chemotherapy.
The main limitations of the present study are constituted by the small sample size and the retrospective, nonrandomized nature. Technically, specimens were obtained from primary resected tumors in five of six patients with distant recurrence, and in addition, Bax expression in the small biopsies may not have been similar to the real results eventually obtained with surgical specimens. Even if this study leaves open the possibility that Bax is a prognostic rather than a predictive biomarker of chemotherapy benefit, the correlation with objective response end point is by itself provocative because the response to third-generation regimens, such as irinotecan-and oxaliplatin-based combination, was moderately correlated with PFS but not OS in a published comprehensive review [26].
Conclusions
In patients with advanced gastric cancer selected for good-intermediate performance status, Bax expression evaluated by IHC is associated with a higher likelihood of clinical response to first-line combination with COI regimen. The outcome of patients with Bax-expressing tumors and treated with an intensive triplet regimen was promising, with a median PFS of 8.77 months and a median OS of 23.8 months. Conversely, Bax-negative tumors showed an extremely poor clinical outcome in terms of response and survival.
The results of the current study suggest that patients with gastric cancer and high expression of Bax might benefit from intensive triplet chemotherapy COI regimen. Nevertheless, the value of Bax as prognostic factor needs further confirmation by larger and prospective studies. Above all, its predictive significance should be independently validated with randomized trials including a control and possibly platinum-free arm of chemotherapy. In fact, Bax could predict a large-spectrum chemoresistance or, more realistically, insensitivity to DNA-damaging agents, such as platinum derivatives.
Footnotes
All authors have no conflicts of interest.
References
- 1.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.Grimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Bajetta E, Celio L, Ferrario E, Di Bartolomeo M, Denaro A, Dotti K, Mancin M, Bajetta R, Colombo A, Pusceddu S. Capecitabine plus oxaliplatin and irinotecan regimen every other week: a phase I/II study in first-line treatment of metastatic colorectal cancer. Ann Oncol. 2007;18:1810–1816. doi: 10.1093/annonc/mdm347. [DOI] [PubMed] [Google Scholar]
- 7.Bajetta E, Verzoni E, Ferrario E, Dotti K, Gevorgyan A, Celio L. Feasibility study of biweekly capecitabine, oxaliplatin, and irinotecan in patients with untreated advanced gastric cancer. Tumori. 2009;95:43–47. doi: 10.1177/030089160909500108. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 9.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 10.Raffo AJ, Kim AL, Fine RL. Formation of nuclear bax/p53 complexes is associated with chemotherapy induced apoptosis. Oncogene. 2000;19:6216–6228. doi: 10.1038/sj.onc.1203995. [DOI] [PubMed] [Google Scholar]
- 11.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 12.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 13.Franceschelli LE, Celio L, Colonna V, Fossile E, Ferrario E, Valente M, Ducceschi M, Bajetta E. Triplet combination of capecitabine plus oxaliplatin and irinotecan in the treatment of patients with previously untreated advanced gastric cancer (AGC) ESMO Congress. 2010 Abstract 721P. [Google Scholar]
- 14.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 15.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 16.Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15–17. doi: 10.3748/wjg.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anagnostopoulos GK, Stefanou D, Arkoumani E, Chalkley L, Karagiannis J, Paraskeva K, Mathou N, Dellaporta E, Tsianos E, Agnantis NJ. Expression of Bax protein in gastric carcinomas. A clinicopathological and immunohistochemical study. Acta Gastroenterol Belg. 2007;70:285–289. [PubMed] [Google Scholar]
- 18.Kang SY, Oh YT, Han JH, Choi JH, Lim HY, Kim HI, Lee HW, Jang JH, Park JS, Kim HC, et al. Concurrent chemoradiotherapy in patients with nasopharyngeal cancer: prognostic significance of low expression of bax. Neoplasma. 2006;53:450–456. [PubMed] [Google Scholar]
- 19.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius VM, Niskanen E, Nordling S, Reed JC. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995;55:4471–4478. [PubMed] [Google Scholar]
- 20.Kupryjanczyk J, Szymanska T, Madry R, Timorek A, Stelmachów J, Karpi?ska G, Rembiszewska A, Ziółkowska I, Kraszewska E, Debniak J, et al. Evaluation of clinical significance of TP53, BCL-2, BAX and MEK1 expression in 229 ovarian carcinomas treated with platinum-based regimen. Br J Cancer. 2003;88:848–854. doi: 10.1038/sj.bjc.6600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang SY, Han JH, Lee KJ, Choi JH, Park JI, Kim HI, Lee HW, Jang JH, Park JS, Kim HC, et al. Low expression of Bax predicts poor prognosis in patients with locally advanced esophageal cancer treated with definitive chemoradiotherapy. Clin Cancer Res. 2007;13:4146–4153. doi: 10.1158/1078-0432.CCR-06-3063. [DOI] [PubMed] [Google Scholar]
- 22.Nakata B, Muguruma K, Hirakawa K, Chung YS, Yamashita Y, Inoue T, Matsuoka T, Onoda N, Kato Y, Sowa M. Predictive value of Bcl-2 and Bax protein expression for chemotherapeutic effect in gastric cancer: a pilot study. Oncology. 1998;55:543–547. doi: 10.1159/000011910. [DOI] [PubMed] [Google Scholar]
- 23.Muguruma K, Nakata B, Hirakawa K, Yamashita Y, Onoda N, Inoue T, Matsuoka T, Kato Y, Sowa M. p53 and Bax protein expression as predictor of chemotherapeutic effect in gastric carcinoma. Gan To Kagaku Ryoho. 1998;25(suppl 3):400–403. [PubMed] [Google Scholar]
- 24.Jeong SH, Han JH, Kim JH, Ahn MS, Hwang YH, Lee HW, Kang SY, Park JS, Choi JH, Lee KJ, et al. Bax predicts outcome in gastric cancer patients treated with 5-fluorouracil, leucovorin, and oxaliplatin palliative chemotherapy. Dig Dis Sci. 2011;56:131–138. doi: 10.1007/s10620-010-1280-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, Park JO, Park YS, Lim HY, Sohn TS, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;37:30–37. doi: 10.1093/annonc/mdl501. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa W, Sasaki Y. Correlation between tumor response to first-line chemotherapy and prognosis in advanced gastric cancer patients. Ann Oncol. 2006;17:1665–1672. doi: 10.1093/annonc/mdl174. [DOI] [PubMed] [Google Scholar]