Abstract
Tumor cells grow in nutrient- and oxygen-deprived microenvironments and adapt to the suboptimal growth conditions by altering their metabolic pathways. This adaptation process commonly results in a tumor phenotype that displays a high rate of aerobic glycolysis and aggressive tumor characteristics. The glucose regulatory molecule, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), is a bifunctional enzyme that is central to glycolytic flux and is downstream of the metabolic stress sensor AMP-activated protein kinase (AMPK), which has been suggested to modulate glycolysis and possibly activate isoforms of PFKFB, specifically PFKFB3 expressed in tumor cells. Our results demonstrated that long-term low pH exposure induced AMPK activation, which resulted in the up-regulation of PFKFB3 and an increase in its serine residue phosphorylation. Pharmacologic activation of AMPK resulted in an increase in PFKFB3 as well as an increase in glucose consumption, whereas in contrast, inhibition of AMPK resulted in the down-regulation of PFKFB3 and decreased glycolysis. PFKFB3 overexpression in DB-1 tumor cells induced a high rate of glycolysis and inhibited oxygen consumption, confirming its role in controlling glycolytic flux. These results show that low pH is a physiological stress that can promote a glycolytic phenotype commonly associated with tumorigenesis. The implications are that the tumor microenviroment contributes to tumor growth and treatment resistance.
Introduction
Tumors experience dynamic microenvironmental changes that require them to adapt to harsh nonphysiological conditions for continued survival. Tumor cells adapt because their energy metabolism is altered to survive mircoenvironmental changes, although the mechanisms of these metabolic modifications are not well characterized. A hallmark characteristic of altered tumor metabolism is a shift toward high-glucose flux or the Warburg effect, which is characterized by an increase in aerobic glycolysis [1]. Tumor cells do not only display an enhanced glycolytic rate but also exhibit roughly a 10-fold higher rate of conversion of glucose into lactate compared with normal cells independent of oxygen status. Glycolysis provides significantly less ATP in comparison to oxidative phosphorylation. However, glycolytic ATP generation is more than 100 times more rapid than oxidative phosphorylation, which ultimately can sustain high proliferation rates of some tumors [2]. It has been proposed that this energetically unfavorable environment is a stress that may provide cells a selective advantage [3]. Normal cells surrounding the tumor may become nutrient starved and ultimately undergo apoptosis, allowing the tumor more space to support growth [3]. The high rates of glycolysis are positively correlated with the aggressive nature of the tumor and demonstrate resistance to therapies [4].
The microenvironment of the tumor can be acidic, which is a direct consequence of poor perfusion and the high rate of lactate production [5]. The increase in glucose catabolism increases pyruvate accumulation where it is oxidized to enhance ATP production. Instead of being shuttled into the mitochondria for oxidation, pyruvate is converted into lactate, which cannot undergo mitochondrial oxidation and must be extruded. This outward flux of lactate leads to a decreased extracellular pH and creates an acidic environment where the metabolites cannot be cleared due to poorly developed vasculature. As a result, the tumor cells are chronically exposed to acidic conditions and develop a phenotype that is associated with increased malignancy, increased rates of metastasis, and chemotherapeutic resistance [6,7]. Tumor regulation of this malignant survival pathway likely involves modulation of the expression and activity of key glycolytic enzymes. Therefore, understanding the initiation of adaptation or the processes and pathways involved could lead to the development of intervention or prevention methods.
One potential molecule that could act as a sensor and initiate metabolic change is the ATP sensor AMP-activated protein kinase (AMPK). AMPK functions as a cellular energy meter and its activity increases when the ratio of AMP/ATP increases. In times of cellular stress, AMPK signaling drives glycolytic flux in an effort to return to energy homeostasis and replete cellular ATP levels through glucose catabolism [8]. If a cell is under metabolic stress, AMPK works to halt the anabolic processes that require ATP, such as protein synthesis and cell proliferation. In addition, this protein works to increase pathways that produce ATP, such as glycolysis [9]. Because of the inefficient nature of the increased glycolytic phenotype seen in cancer cells, there is a dysregulation of ATP production leading to the activation of AMPK [10].
Downstream of AMPK is the glucose regulatory molecule, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB). PFKFB3 is a bifunctional enzyme with four tissue dependent isoforms encoded by four genes (PFKFB1-4). The phosphofructo-2-kinase (PFK) domain functions to promote glycolysis by increasing levels of fructose-2,6-bisphosphate through phosphorylation of fructose-6-phosphate and the fructose-2,6-biphosphatase domain functions to catalyze the reverse reaction again producing fructose-6-phosphate [11]. AMPK has been shown to activate an isoform of this bifunctional enzyme, PFKFB2, through phosphorylation of serine 466 in heart tissue after an acute ischemic event [12,13]. This is of particular interest because of the mounting data indicating a role of the tumor PFKB isoform PFKFB3 in adaptation to the hypoxic tumor environment. This enzymatic stimulation of glycolysis leads to increased production of both glycolytic ATP and lactate [14].
We propose that there is an AMPK-PFKFB3 interaction during adaptation to low pH and that pathway is key in the up-regulation of glycolysis resulting in the development of glycolytic and potentially chemoresistant phenotypes, specifically the acidic pH tumor microenvironment. We report a novel metabolic adaptation response induced by low pH adaptation and describe mechanisms in vitro that would predict tumorigenesis and treatment resistance as seen in clinical settings. We describe the relationship between low pH and glycolytic flux, which involves activation of AMPK and the up-regulation of PFKFB3 leading to an increase in glucose consumption.
Materials and Methods
Cell Culture
Early-passage DB-1 melanoma cells, U87 glioblastoma cells, and SK-MEL-5 melanoma cells were grown as monolayers at 37°C in humidified 5% CO2 in α-minimum essential medium (fs) supplemented with 10% fetal bovine serum, 12 mM glucose, 10 ml/L non-essential amino acids solution, and 2.9 g/L l-glutamine (α-MEM+). Low pH medium was prepared at pH 6.7 as described [15]. Cells were grown at pH 7.3 or in low pH conditions at pH 6.7. The 6.7 grown cells were adapted to growth at pH 6.7 for at least 14 days, but no more than 60 days before they were used in experiments. Immediate acidification was for 48 hours. Cell phenotypes after adaptation to low pH have been described previously [15].
Transfections
Cells were transfected with a pcDNA3 5.4-kb vector (Invitrogen, Carlsbad, CA) containing PFKFB3. PFKFB3 complementary DNA (cDNA) was amplified using a forward primer (5′-CGGGATCCATGCCGTTGGAACTGACG-3′) and a reverse primer (5′-CCGCTCGAGTCAGTGTTTCCTGGAGGAG-3′). In the forward sequence, the BamHI domain is underlined, and in the reverse sequence, the XhoI domain is underlined. The FLAG sequence, Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys, was used as the transfection marker.
AMPK Activation and Inhibition
To activate AMPK, DB-1 cells were treated with 10 mM metformin (Calbiochem, Darmstadt, Germany) for 72 hours. DB-1 melanoma cells and U87 glioblastoma cells were treated with 1 mM AICAR (Sigma, St Louis, MO) for 8 hours to activate AMPK. For AMPK inhibition, DB-1 cells were treated with Compound C (Calbiochem) for 8 hours at 10 µM.
Rate of Glucose Consumption
A glucose detection kit (GHKB-2; Sigma), based on the enzymatic conversion of glucose-6-phosphate and NAD+ to 6-phosphogluconate and NADH, measured the amount of glucose present in the samples. Cells were grown on T-25 culture flasks. When the cells were near confluence, the medium was removed and replaced with new 7.3 α-MEM+ without 12 mM glucose or 6.7 α-MEM+. Enough 1-M glucose solution was added to each flask to bring the final glucose concentration to 20 mM. Exactly 6 or 24 hours later, 50-µl aliquots of medium were removed from each flask. Subsequently, each aliquot was then stored in an individual well of 96-well plate. The 96-well plate was then wrapped with Parafilm (Thermo Fisher Scientific, Waltham, MA) and placed in the freezer. To determine the rate of glucose consumption, the samples were successively diluted: 1) 10 µl of thawed sample/90 µl of deionized H2O and 2) 10 µl from a dilution of 1:100 µl glucose assay reagent (G2020; Sigma). A 96-well plate spectrophotometer was then used to determine the absorbance at 340 nm. A mean absorbance was calculated based on the individual absorbance readings from each of the aliquots removed.
Total glucose consumption after AMPK inhibition with Compound C (Sigma) and AMPK activation by AICAR (Sigma) was measured using an ACCU-CHECK glucometer. Normal pH U87 cells and chronically acidified DB-1 cells were cultured in six-well plates and treated with Compound C or AICAR, respectively. Medium samples were taken in 1-ml aliquots before and after Compound C and AICAR treatment and were stored at -20°C until analyzed. Samples were thawed at 37°C and read three times each.
Lactate Production
A lactate detection kit (826-UV; Sigma) based on the enzymatic conversion of lactate and NAD+ to pyruvate and NADH was used to measure the amount of lactate present in the samples. To determine the rate of lactate production, NAD (826-3; Sigma) was reconstituted as directed in the protocol (826-UV; Sigma). A 10:100 dilution of sample (obtained from the glucose consumption assay 96-well plate)/NAD solution was prepared, and the absorbance at 340 nm was measured using a 96-well plate spectrophotometer. A mean absorbance was calculated from the individual absorbance readings from each of the aliquots removed.
Western Blot Analysis
Western blot analysis was carried out with a total of 25 µg of each sample in 7% and NUPAGE gels (Invitrogen). Protein determination was done using the Protein dotMETRIC Assay (G-Biosciences, Maryland Heights, MO). Antibodies to p-AMPK Thr 172, total AMPK (Abcam, Cambridge, MA), phosphorylated p53 Ser 392, total p53 (Cell Signaling Technology, Boston, MA), and PFKFB3 (Abnova, Taipei, Taiwan) were used at a dilution of 1:1000. The phosphorylated serine antibody was used at a dilution of 2 µg/ml (Abcam), and an antirabbit secondary was used at a dilution of 1:10000 was used for all Western blot analyses. Western detection was carried out using CDP star from Tropix (Applied Biosystems, Chicago, IL). PFKFB3 overexpression was determined by Western blot using anti-FLAG antibody (Sigma Aldrich).
Immunoprecipitations
Chronically acidified and pH 7.3 SK-MEL-5 cells grown in culture were washed twice with ice-cold PBS and then were lysed with ice-cold NP-40 buffer substituted with 1% Triton X, 150 mM NaCl, and 50 mM Tris-HCl. Protease inhibitor cocktail, NaF, and NaV were added immediately before use. Lysates were immunoprecipitated with 10 µl of anti-PFKFB3 (Abnova) antibody overnight at 4°C with gentle agitation. Before adding to lysates, 75 µl of Protein A Magnetic Beads (Abnova) was washed twice with cold immunoprecipitation (IP) buffer. Lysates were then added to beads and incubated overnight at 4°C on a rocking platform. Beads were then pelleted by centrifugation and washed three times with buffer. Lithium dodecyl sulfate (LDS) sample buffer was then added to beads, samples were boiled for 10 minutes at 100°C, and the supernatant was removed.
Oxygen Consumption
The rate of oxygen consumption was measured at 37°C using a Clark electrode and amplifier (Yellow Springs Instrument Co, Yellow Springs, OH) as previously described [15]. A water bath was used to maintain a constant temperature, and cells were stirred continuously. MLA Brand pipettes (Fisher) were used to add cells and solutions to the measurement chamber. MLA Brand pipettes were chosen because of their inability to expel excess air into the chamber by overdepressing the plunger. For each experimental trial, 30 µl of cell suspension was placed in the oxygen consumption chamber, and a basal rate of oxygen consumption was measured for 5 minutes before addition of 5 µl of either HEPES respiration buffer [15], 16.7 mM glucose, or 100 µM dinitrophenol (DNP). The addition of DNP allows for the measurement of maximum oxygen consumption because DNP uncouples oxidative phosphorylation, leading to oxygen consumption without ATP production. (The basal rate is defined as the rate of oxygen consumption of cells in buffer before an addition of another solution.) The rate of oxygen consumption was then measured for another 5 minutes after addition of an agent. A 100-µl aliquot of the cell solution was removed from the chamber after the second interval and stored at -20°C before protein determination.
ATP Assay
Intracellular ATP concentrations were assayed using a colorimetric ATP assay kit (ab83355; Abcam). Normal and chronically acidified DB-1 and SK-MEL-5 cells were lysed according to the manufacturer's instructions. Briefly, 1 x 106 cells were lysed in 100 µl of provided assay buffer. Samples were run through a 10-kDa filter (Abcam) to remove any interfering proteins before analysis. Samples were placed into a 96-well plate, and the reaction mixture was made according to the protocol provided by the manufacturer and read at 550 nm.
Statistical Analysis
All measurements represent the mean of three different experiments with three values of each ±SE or SD where indicated. Statistical significance was evaluated using a Student's t test, and significant values were noted as follows: *P < .05, **P < .01 to .001, and ***P < .001.
Results
Short-term and Long-term Acidification Activates AMPK and PFKFB3
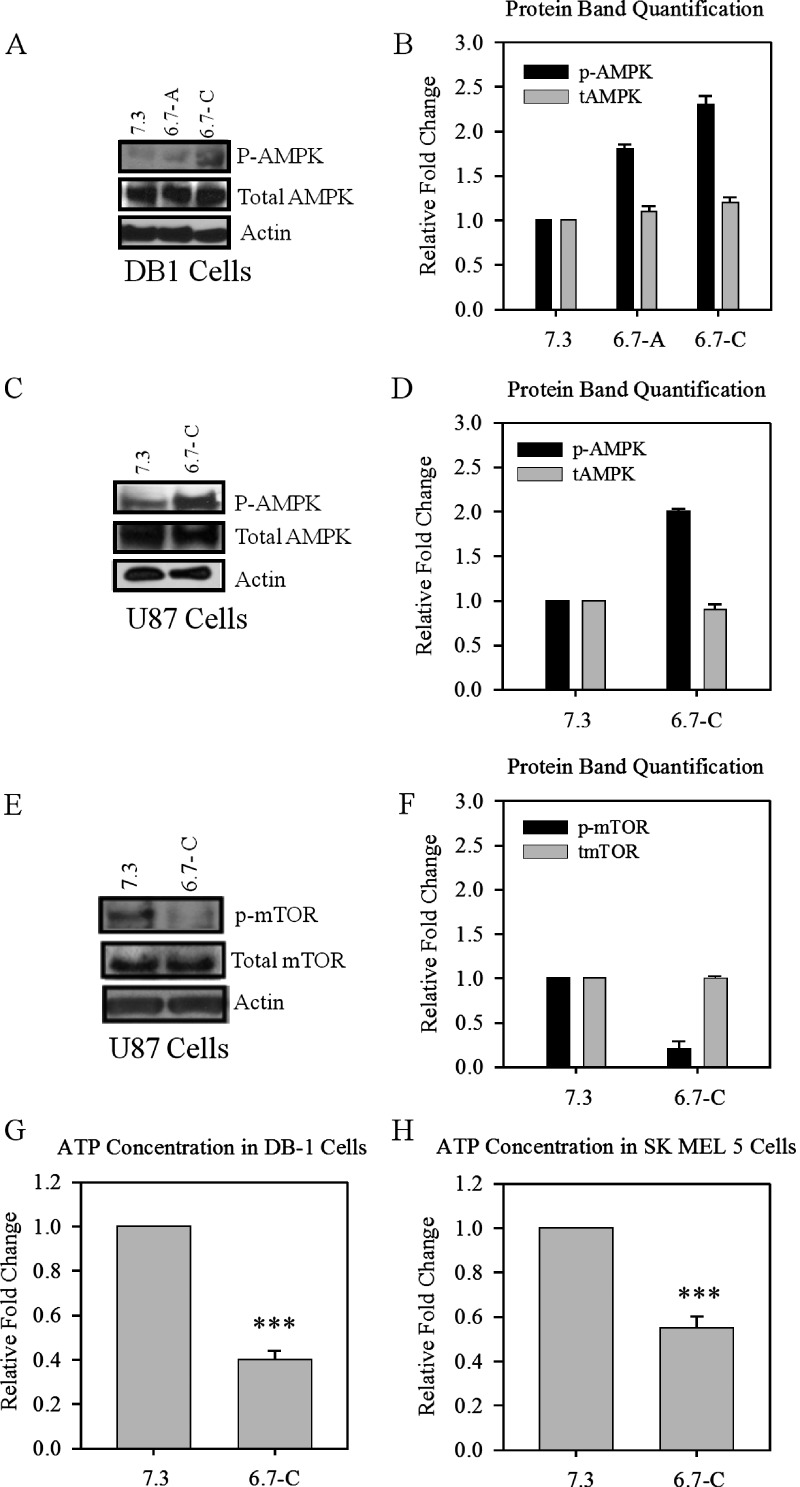
AMPK acts as a cellular fuel gauge and responds to changes in energy status by increasing glucose usage and by decreasing anabolic processes. Once activated, AMPK induces a series of downstream glycolytic events aimed at increasing energy production. Therefore, we investigated whether AMPK could act as a metabolic sensor during short-term and long-term acidification and increase the expression and activation of glycolytic proteins, a hallmark of tumors. After both short-term and long-term acidification of DB-1 cells, activation of AMPK was observed as measured by phosphorylation of the alpha subunit at Thr 172 (Figure 1, A and B). AMPK activation was also confirmed in chronically acidified U87 cells (Figure 1, C and D). AMPK activation has been shown to inhibit proliferative processes through the inhibition of the mammalian target of rapamycin pathway. The protein levels of p-mTOR were decreased after low pH adaptation and confirm AMPK activity (Figure 1, E and F).
Figure 1.
Protein levels of phosphorylated AMPK in acutely and chronically acidified DB-1 and U87 cells and intracellular ATP levels in chronically acidified SK-MEL-5 cells. (A) Western blot of p-AMPK and total AMPK in acutely and chronically acidified DB-1 melanoma cells and (B) protein quantification. (C) Protein levels of chronically acidified U87 glioblastoma cells and (D) protein quantification. (E) Western blot of p-mTOR and total mTOR in chronically adapted U87 cells and (F) protein quantification. Relative fold change in ATP levels after long-term adaptation in (G) DB-1 and (H) SK-MEL-5 cells. Blots and ATP assay are representative of at least three independent experiments. Bars are mean ± SE. ***P < .001 when compared with control.
To explore a possible mechanism of AMPK activation by low pH, we assayed intracellular ATP concentrations in normal and chronically acidified low-pH DB-1 cells and observed that the low-pH cells had roughly two-fold less ATP compared with normal-pH cells (Figure 1G). The decrease in ATP concentration after acidification was confirmed in SK-MEL-5 cells (Figure 1H). The decreased ATP levels after adaptation to low pH are indicative of low energy levels, which could contribute to the activation of AMPK after long-term acidification.
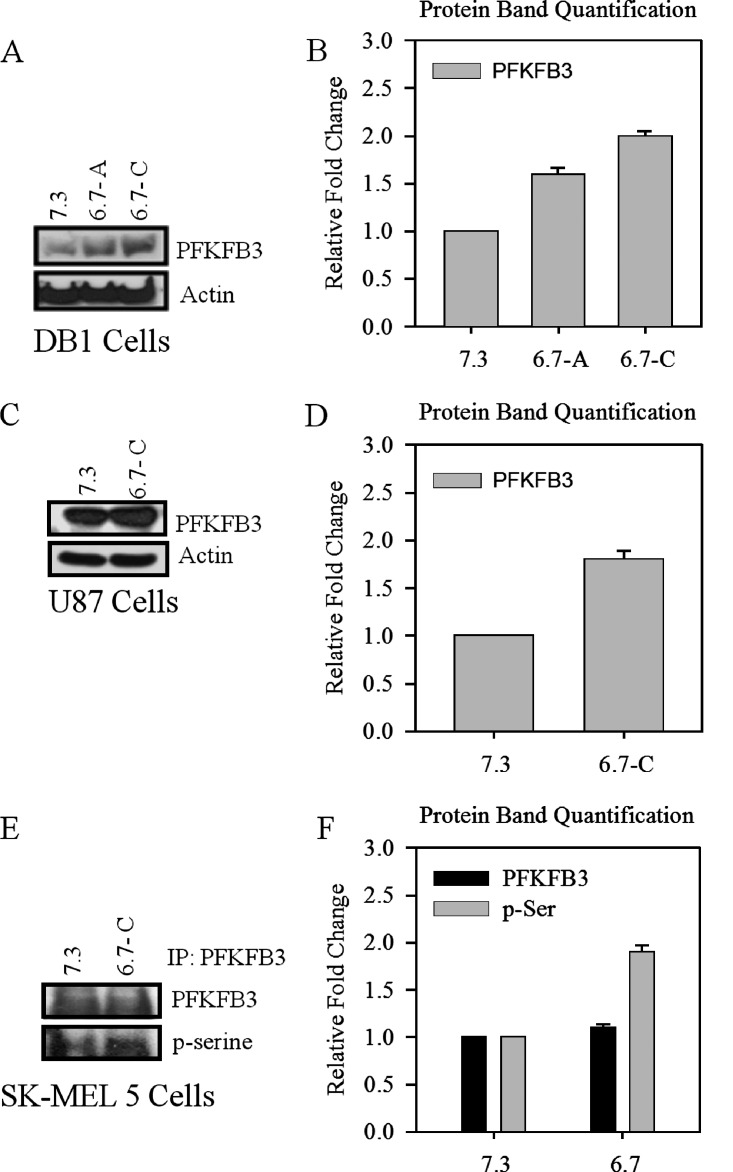
Because AMPK can increase and activate glycolytic enzymes, we investigated the effect of acidification on the level of PFKFB3, a major glucose regulatory protein central to glyolytic metabolism. After acutely acidifying DB-1 cells to pH 6.7 for 48 hours and chronically (>14 days), we observed an increase in protein levels of PFKFB3 (Figure 2, A and B) and confirmed this increase further in chronically acidified U87 glioblastoma cells (Figure 2, C and D).
Figure 2.
Protein levels of PFKFB3 and phosphorylated serine residues after short-term and long-term acidification of DB-1, U87, and SK-MEL-5 cells. (A) Western blot of PFKFB3 protein levels in acutely and chronically acidified DB-1 cells and (B) protein quantification. (C) Protein levels of PFKFB3 in chronically acidified U87 cells and (D) protein quantification. Immunoprecipitation of PFKFB3 in chronically adapted low-pH SK-MEL-5 cells and (E)Western blot of PFKFB3 and pan serine phosphorylation and (F) protein quantification. Blots are representative of at least three independent experiments.
Previous data demonstrated that when AMPK was activated because of an ischemic event in heart tissue, there was a subsequent phosphorylation of a serine residue on the heart PFKFB isoform PFKFB2, promoting an oxygen-sparing means of energy production [16]. PFKFB3 is a bifunctional enzyme with both a kinase component and a phosphatase component, which are regulated posttranslationally. The kinase portion of PFKFB3 becomes phosphorylated at Ser 461 and in turn increases glycolysis [17]. We hypothesized that, after adaptation of tumor cells, in addition to an increase in protein levels, there would also be a phosphorylation event on PFKFB3, enhancing its kinase activity, thus promoting glycolysis. Owing to the lack of a commercially available antiphosphorylated PFKFB3 at Ser 461, we used a pan antiserine antibody to detect changes in PFKFB3 phosphorylation on serine residues followed by long-term acidification. We immunoprecipitated PFKFB3 from normal and chronically adapted SK-MEL-5 melanoma cells and then analyzed phosphorylation levels using Western blot. We observed a marked increase in total serine phosphorylation after adaptation to low pH compared with cells grown at normal pH (Figure 2, E and F).
Manipulation of AMPK Activation Modulates PFKFB3 Activity and Alters Glucose Consumption
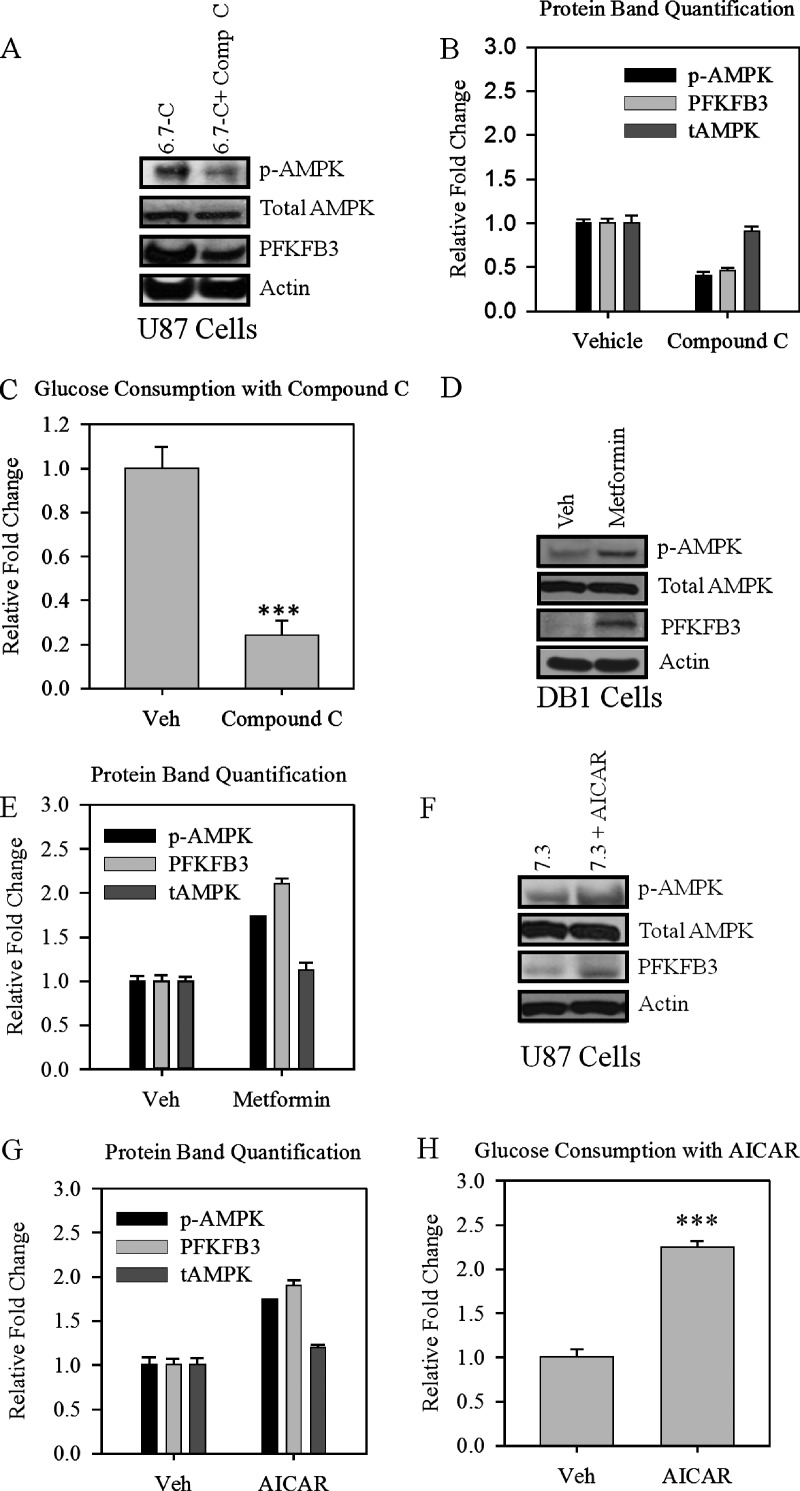
To confirm that AMPK is the upstream sensor that regulates glycolytic flux in our low-pH model, we inhibited AMPK with the ATP competitive inhibitor Compound C in chronically acidified U87 glioblastoma cells. After 24 hours of treatment with Compound C, we observed a decrease in AMPK phosphorylation and a subsequent decrease in PFKFB3 protein levels (Figure 3, A and B). A functional decrease in glucose consumption was also observed in chronically adapted U87 cells after treatment with Compound C (Figure 3C).
Figure 3.
Manipulation of AMPK decreases PFKFB3 expression and glycolysis. (A) Western blot of p-AMPK, total AMPK, and PFKFB3; (B) protein quantification in chronically acidified U87 cells; and (C) rate of glycolysis in U87 after 8 hours of treatment with Compound C expressed in a relative fold decrease. (D) Western blot of p-AMPK, total AMPK, and PFKFB3 and (E) protein quantification after 72 hours of treatment with Metformin. (F) Western blot of p-AMPK, total AMPK, and PFKFB3; (G) protein quantification; and (H) rate of glucose consumption after AMPK activation with 1 mM AICAR in pH 7.3. U87 cells were expressed as a relative fold increase. Bars are mean ± SE. ***P < .001 when compared with control. Experiments in this figure were repeated twice.
To demonstrate that AMPK could induce the low-pH-adapted phenotype, we pharmacologically activated AMPK using the antidiabetic drug metformin and the more specific activator AICAR in normal-pH DB-1 and U87 cells. After treatment with metformin for 72 hours (Figure 3, D and E) or AICAR for 8 hours (Figure 3, F and G), we observed phosphorylation of AMPK as well as an increase in PFKFB3 protein levels. Again, we assayed glucose consumption after activation of AMPK with AICAR and found that activation of AMPK in normal pH cells recapitulated the low-pH phenotype and caused an increase in glucose consumption (Figure 3H).
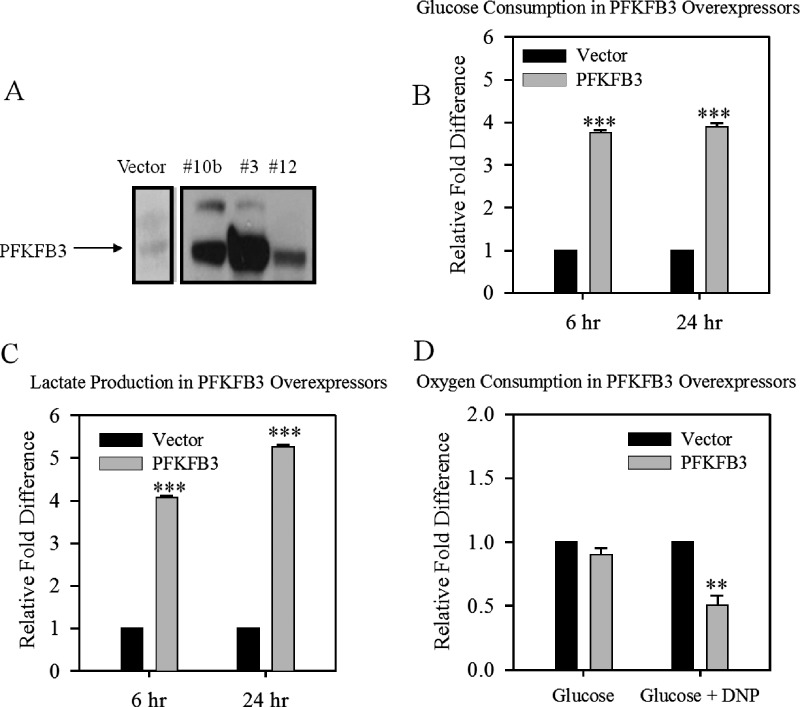
To demonstrate that PFKFB3 alone could induce the observed changes in glucose flux, we transfected DB-1 melanoma cells with the PFKFB3 gene. After overexpression, there was an increase in glucose consumption and lactate production (Figure 4, A–C) relative to vector controls. However, the basal rates of oxygen consumption of the PFKFB3 overexpression after the addition of glucose were the same compared with the vector (Figure 4D). Therefore, we determined the maximum rate of respiration in these cells using 100 µM DNP, which inhibits ATP production by uncoupling oxidative phosphorylation, leading to increased oxygen consumption. The PFKFB3 overexpressors had a lower maximum rate of respiration than the vector; this confirms a shift from oxidative respiration to glycolysis. Taken together, these results indicate that a low-pH microenvironment results in AMPK-mediated changes in glycolytic flux and PFKFB3 is a downstream mediator of the changes.
Figure 4.
Effect of PFKFB3 expression on glycolysis and oxygen consumption. Characterization of PFKFB3 overexpression in DB-1 cells grown at pH 7.3 by anti-FLAG detection by (A) Western blot, and (B) its effect on the rate of glucose consumption, (C) on lactate production, and (D) on oxygen consumption with and without DNP. Glucose and lactose are expressed as a relative fold difference to vector at 6 and 24 hours. Clark electrode oxygen consumption readings are representative, and numbers indicate the relative rate after addition (posttreatment rate / pretreatment rate). Bars are mean ± SE of three independent experiments. **P < .01 to .001, ***P < .001 when compared with control.
Discussion
We show here that extracellular pH has a direct affect on glycolytic flux in our tumor model. Exposure to low extracellular pH induced phosphorylation of AMPK and increased glucose uptake in chronically acidified cells. The glycolytic effect was recapitulated through the overexpression of PFKFB3. We also demonstrate a shift from oxidative metabolism to glycolysis by the overexpression of PFKFB3, which is consistent with a highly glycolytic phenotype that could provide a survival advantage in a low-oxygen tension environment. However, we also demonstrated that low-pH adaptation itself leads to a decrease in intracellular ATP concentrations so it is possible that decreased energy levels can contribute to low-pH-induced activation of AMPK, and this indicates that low pH itself may propagate the glycolytic and aggressive phenotype of tumors.
Induction of AMPK resulted in an increase in glycolytic activity despite an oxygen-rich environment, which indicates that low-pH adaption can induce the Warburg effect. Therefore, it is possible that low pH induces an oxygen-sparing phenotype, which would be advantageous in a hypoxic tumor setting. Tumor cells that exhibit metabolic reprogramming have demonstrated survival advantages, increased metastasis, and chemoresistance [18]. Overexpression of PFKFB3 was sufficient to induce the shift in glycolytic rate and was induced downstream of AMPK after low pH adaptation; this is in addition to the two-fold reduction in ATP concentration in chronically acidified cells compared with normal-pH cells. This observation provides a reasonable mechanism suggesting that tumors sense environmental changes and have the ability to quickly adapt. Furthermore, it indicates that the mechanism of adaptation involves the activation of PFKFB3.
Another advantage of high glycolytic rates in tumors is the potential to increase pentose cycle activity. High glycolytic flux results in the reduction of NAD+ to NADH. The depletion of NAD+ is rectified through the oxidation of NADH to NAD+ in the conversion of pyruvate to lactate, allowing glycolysis to persist. Increases in both glycolysis and the pentose cycle are believed to play a role in circumventing toxicity from high levels of oxidative stress that result from low glucose levels [19]. We have shown here that we can reduce the glycolytic rate with compounds such as the AMPK inhibitor Compound C. Targeting AMPK could provide a strategy to counter the protective effects of glycolysis and sensitize tumors to therapy not only by decreasing the reducing power of cells but also because blocking glycolysis is believed to cause an accumulation of pernicious hydroperoxide radicals [20].
In addition to targeting AMPK, the glycolytic enzyme PFKFB3 may provide a more direct target. There is evidence that tumor cells are more resistant to glucose deprivation than nontransformed cells. This paradigm sets the stage for the development of 2-deoxy-d-glucose, which competes with glucose for uptake by chemically inducing a state of glucose deprivation [20]. Inhibition of PFKFB3 has also come to the forefront as an important potential therapeutic target because of its very high kinase activity. This markedly high kinase activity is hypothesized to have a sizeable role in driving the glycolytic phenotype seen in cancer cells [21]. This glycolytic targeting could be highly feasible given the frequency of tumor acidosis, independent of tumor oxygen tensions.
Many studies have focused on the use of AMPK as an antitumor therapy, and there has been promising chemoprevention results using the antidiabetic drug metformin to activate AMPK and mimic a cellular starvation state [22]. There is also a potential antitumor role of AMPK activation through p53 signaling or mTOR inhibition [23,24], and connections between AMPK and p53 have been characterized where activation of AMPK leads to activation of the tumor suppressor p53 and p53 target genes [25]. However, we have previously shown that, in DB-1, the induction of p53 may be protumorigenic because of the induction of downstream p53 antagonists by p53 itself [26]. Metformin is also used to stimulate glucose uptake [27], and the results presented here indicate that activation of AMPK could potentially be advantageous to already established tumors, possibly through enhancing the glycolytic flux.
There is also a potential role of mTOR in regulating autophagy. Autophagy is the cellular process of recycling cytoplasmic components when components are damaged or in excess, using lysosomal machinery. This process of recycling becomes important in starvation states, such as mediated by AMPK, to synthesize important proteins or break down cellular components to use as catabolic fuel. We also observed a decrease in mTOR phosphorylation, which could play a major role in tumor resistance to environmental and treatment stress by induction of autophagy. Low pH could also play a more direct role in mTOR regulation. Balgi et al. [28] demonstrated that mTOR is sensitive to pH changes and is inhibited in a low-pH environment. The group hypothesized that this down-regulation of mTOR signaling may aid in adaptation to an energetically unfavorable microenvironment. Both AMPK-mediated and direct down-regulation of mTOR by pH would support an autophagic process [29,30]. Future studies will explore the effect of pH on mTOR, which is involved in autophagy and possibly chemoresistance. However, in the interim, antitumor therapies focused on the use of AMPK should consider its potentially undesirable ability to induce a PFKFB3 and promote a glycolytic state.
Elucidation of mechanisms involved with low pH adaptation has important therapeutic implications. Tumors exhibiting an acidic extracellular pH are not suited to be treated with weak base chemotherapeutics, and pH must be taken into consideration in treatment protocols. Immediate tumor alkalinization with systemic administration of sodium bicarbonate has sensitized mammary tumors to doxorubicin and mitoxantrone in vivo [31,32]. The use of proton pump inhibitors to decrease cellular acid extrusion has also shown promise in vivo to sensitize tumors to weak base chemotherapy [33]. Conversely, exploiting the acidic extracellular pH by pairing a low-pH phenotype with a weak acid drug, such as melphalan can circumvent drug resistance conferred by low pH and has been successful in melanoma xenograft models [34]. Understanding tumor pH and the metabolic adaptations involved can help to adapt treatment protocols and improve therapeutic outcomes.
In conclusion, we show here for the first time that low pH is a principal factor in the paradigm of tumor metabolism and can be involved in the genesis of the glycolytic phenotype as well as contribute to the further deterioration of metabolic regulation. AMPK can potentially serve as a switch to induce PFKFB3, in which overexpression was sufficient to increase glucose consumption and induce an oxygen-sparing phenotype. The modulation of AMPK and the subsequent effect on tumor metabolism is an important approach to understanding low-pH-induced glycolytic signaling centering around PFKFB3.
Footnotes
This work was funded in part by grant 1R21CA139183-01A2 (R.B.).
References
- 1.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 2.Kaelin WG, Jr, Thompson CB. Q&A: Cancer: clues from cell metabolism. Nature. 2010;465(7298):562–564. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- 3.Bartrons R, Caro J. Hypoxia, glucose metabolism and the Warburg's effect. J Bioenerg Biomembr. 2007;39(3):223–229. doi: 10.1007/s10863-007-9080-3. [DOI] [PubMed] [Google Scholar]
- 4.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 5.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8(1):56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 6.Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97(5):646–653. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol (Oxf) 2009;196(1):55–63. doi: 10.1111/j.1748-1716.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 9.Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6(3):457–470. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23(5):537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86(3):174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Kim AS, Miller EJ, Wright TM, Li J, Qi D, Atsina K, Zaha V, Sakamoto K, Young LH. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51(1):24–32. doi: 10.1016/j.yjmcc.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10(20):1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 14.Obach M, Navarro-Sabaté A, Caro J, Kong X, Duran J, Gómez M, Perales JC, Ventura F, Rosa JL, Bartrons R. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem. 2004;279(51):53562–53570. doi: 10.1074/jbc.M406096200. [DOI] [PubMed] [Google Scholar]
- 15.Burd R, Wachsberger PR, Biaglow JE, Wahl ML, Lee I, Leeper DB. Absence of Crabtree effect in human melanoma cells adapted to growth at low pH: reversal by respiratory inhibitors. Cancer Res. 2001;61(14):5630–5635. [PubMed] [Google Scholar]
- 16.Paiva MA, Rutter-Locher Z, Goncalves LM, Providência LA, Davidson SM, Yellon DM, Mocanu MM. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300(6):H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, Yoshioka N, Bucala R, Koike T. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res. 2005;11(16):5784–5792. doi: 10.1158/1078-0432.CCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 18.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr. 2007;39(3):251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 19.Resendis-Antonio O, Checa A, Encarnacion S. Modeling core metabolism in cancer cells: surveying the topology underlying the Warburg effect. PLoS One. 2010;5(8):e12383. doi: 10.1371/journal.pone.0012383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons AL, Mattson DM, Dornfeld K, Spitz DR. Glucose deprivation-induced metabolic oxidative stress and cancer therapy. J Cancer Res Ther. 2009;5(suppl 1):S2–S6. doi: 10.4103/0973-1482.55133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo M, Kim JD, Neau D, Sehgal I, Lee YH. Structure-based development of small molecule PFKFB3 inhibitors: a framework for potential cancer therapeutic agents targeting the Warburg effect. PLoS One. 2011;6(9):e24179. doi: 10.1371/journal.pone.0024179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelman JA, Cantley LC. Chemoprevention meets glucose control. Cancer Prev Res (Phila) 2010;3(9):1049–1052. doi: 10.1158/1940-6207.CAPR-10-0178. [DOI] [PubMed] [Google Scholar]
- 23.Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Ther. 2012;133(1):108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? Eur J Cancer. 2010;46(13):2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Okoshi R, Ozaki T, Yamamoto H, Ando K, Koida N, Ono S, Koda T, Kamijo T, Nakagawara A, Kizaki H. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem. 2008;283(7):3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 26.Thangasamy T, Sittadjody S, Mitchell GC, Mendoza EE, Radhakrishnan VM, Limesand KH, Burd R. Quercetin abrogates chemoresistance in melanoma cells by modulating δNp73. BMC Cancer. 2010;10:282. doi: 10.1186/1471-2407-10-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol. 2009;27(20):3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 28.Balgi AD, Diering GH, Donohue E, Lam KK, Fonseca BD, Zimmerman C, Numata M, Roberge M. Regulation of mTORC1 signaling by pH. PLoS One. 2011;6(6):e21549. doi: 10.1371/journal.pone.0021549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardie DG. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer AJ, Codogno P. Autophagy: regulation by energy sensing. Curr Biol. 2011;21(6):R227–R229. doi: 10.1016/j.cub.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Raghunand N, Martinez-Zaguilan R, Wright SH, Gillies RJ. pH and drug resistance. II. Turnover of acidic vesicles and resistance to weakly basic chemotherapeutic drugs. Biochem Pharmacol. 1999;57(9):1047–1058. doi: 10.1016/s0006-2952(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 32.Raghunand N, Mahoney B, van Sluis R, Baggett B, Gillies RJ. Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone. Neoplasia. 2001;3(3):227–235. doi: 10.1038/sj.neo.7900151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1(6):779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 34.Kelley ST, Menon C, Buerk DG, Bauer TW, Fraker DL. Acidosis plus melphalan induces nitric oxide-mediated tumor regression in an isolated limb perfusion human melanoma xenograft model. Surgery. 2002;132(2):252–258. doi: 10.1067/msy.2002.125713. [DOI] [PubMed] [Google Scholar]