Abstract
The detection and subspeciation of Campylobacter fetus subsp. venerealis (CFV) from veterinary samples is important for both clinical and economic reasons. Campylobacter fetus subsp. venerealis is the causative agent of bovine genital campylobacteriosis, a venereal disease that can lead to serious reproductive problems in cattle, and strict international regulations require animals and animal products to be CFV-free for trade. This study evaluated methods reported in the literature for CFV detection and reports the translation of an extensively tested CFV-specific polymerase chain reaction (PCR) primer set; including the VenSF/VenSR primers and a real-time, quantitative PCR (qPCR) platform using SYBR Green chemistry. Three methods of preputial sample preparation for direct qPCR were evaluated and a heat lysis DNA extraction method was shown to allow for CFV detection at the level of approximately one cell equivalent per reaction (or 1.0 × 103 CFU/mL) from prepuce. The optimized sample preparation and qPCR protocols were then used to evaluate 3 western Canadian bull cohorts, which included 377 bulls, for CFV. The qPCR assay detected 11 positive bulls for the CFV-specific parA gene target. DNA sequence data confirmed the identity of the amplified product and revealed that positive samples were comprised of 2 sequence types; one identical to previously reported CFV parA gene sequences and one with a 9% sequence divergence. These results add valuable information towards our understanding of an important CFV subspeciation target and offer a significantly improved format for an internationally recognized PCR test.
Résumé
La détection et l’identification précise de Campylobacter fetus ssp. venerealis (CFV) à partir d’échantillons vétérinaires sont importantes autant d’un point de vue clinique que pour des raisons économiques. Campylobacter fetus ssp. venerealis est l’agent étiologique de la campylobactériose génitale bovine, une maladie vénérienne qui peut entraîner de sérieux problèmes de reproduction chez les bovins, et une réglementation internationale stricte demande que les animaux et produits animaux soit exempt de CFV pour les échanges commerciaux. La présente étude visait à évaluer les méthodes publiées pour détecter CFV et fait rapport sur la traduction d’une paire d’amorces spécifiques à CFV largement utilisée dans une réaction d’amplification en chaîne par la polymérase (PCR); incluant les amorces VenSF/VenSR et un PCR quantitatif en temps réel (qPCR) utilisant la technologie SYBR Green. Trois méthodes de lavage pour la préparation de l’échantillon préputial en vue du qPCR ont été évaluées et une méthode d’extraction de l’ADN par lyse à la chaleur a permis de démontrer un seuil de détection de CFV à partir du prépuce équivalent à approximativement une cellule par réaction (ou 1,0 × 103 CFU/mL). La préparation d’échantillon optimale et les protocoles de qPCR ont par la suite été utilisés pour évaluer la présence de CFV dans trois cohortes de taureaux de l’ouest canadien (377 taureaux). L’épreuve qPCR a permis de détecter 11 taureaux positifs pour le gène parA, spécifique à CFV. Les résultats d’analyse des séquences d’ADN ont confirmé l’identité du produit amplifié et révélé que les échantillons positifs étaient constitués de 2 types de séquence; une identique aux séquences du gène parA rapportées précédemment et une avec 9 % de divergence dans la séquence. Ces résultats apportent de l’information supplémentaire valable pour la compréhension d’une cible importante dans l’identification de CFV et offre un format significativement amélioré pour une épreuve PCR reconnue internationalement.
Traduit par Docteur Serge Messier
Introduction
The importance of Campylobacter fetus has long been recognized in veterinary medicine. Campylobacter fetus is comprised of 2 recognized subspecies, C. fetus subsp. fetus (CFF) and C. fetus subsp. venerealis (CFV) (1). Campylobacter fetus subsp. fetus is known to colonize the intestinal tract of cattle and other animal species and is associated with sporadic abortion (2). Alternatively, CFV is highly adapted to the genital tract of cattle and is the causative agent of bovine genital campylobacteriosis (BGC) [also referred to as bovine venereal campylobacteriosis (BVC) or vibriosis], which results in infertility and abortion (2). Bovine genital campylobacteriosis is a notifiable disease of the Office international des épizootics (OIE) and animals or animal products must be certified CFV-free for international trade (3).
Detection of CFV in bulls is routinely done by collecting preputial scrapings into either saline or transport enrichment medium (TEM) for transport to the laboratory, followed by microaerophilic selective culture (4). Colonies with the correct macroscopic and microscopic characteristics are subspeciated primarily based on a single biochemical test where CFF can be grown in 1% glycine, while CFV cannot. Several limitations to this procedure are well-recognized and include many CFV strains being sensitive to polymyxin B (common in both TEM and selective media) (5), overgrowth of commensal bacteria (6), low sensitivity of culture (7), and C. fetus strains with an intermediate growth phenotype in glycine (termed C. fetus subsp. venerealis biovar “intermedius”) (8). Alternative detection and subspeciation approaches have included enzyme-linked immunoassays (9,10); direct immunofluorescence tests (11); and molecular tests, such as pulsed-field gel electrophoresis (12), amplified fragment length polymorphism (13,14), multilocus sequence typing (15), and PCR (7,14,16–18). Many of these tests have limited use as routine diagnostics because they are either not subspecies specific (9), require specialized reagents (10,11), or are too laborious and time-consuming for large sample numbers (13–15). In addition, most of these detection strategies require CFV to be isolated from the sample as a pure culture prior to identification. Presently, a PCR-based test is the most promising alternative to culture for the robust detection of CFV from field samples.
Several CFV-specific PCR protocols have been proposed (Table I). The most cited of these is the multiplex PCR proposed by Hum et al (16) and has since become the benchmark to which novel PCR protocols and molecular tests are compared (7,12–15). Hum’s PCR is based on 2 PCR primer sets, where one primer set (MG3F/MG4R) amplifies a 960 base pair (bp) (12,16) or 750 bp (13,19) fragment of the C. fetus carbon starvation protein gene (found in both subspecies), while a second primer set (VenSF/VenSR) amplifies a 142 bp fragment of the parA gene found only in CFV. Recent genomic evaluation of CFV strains has located the parA gene within a genomic island specific to CFV (20,21) and to date, this sequence has only been reported from CFV strains. The Hum CFV-specific primers VenSF/VenSR are the most extensively tested to date for CFV detection and have demonstrated impressive consistency with alternative methodologies (Table I). The current drawback with this primer set is that they have only been used as a conventional PCR and they have been used primarily with isolated strains, not clinical samples.
Table I.
Use of Campylobacter fetus subsp. venerealis polymerase chain reaction (PCR) primer sets in the literature
| Primer set (amplicon in base pairs [bp]) | Study | Number of C. fetus isolates tested | Countries of isolate origin | Agreement of with biochemical identification | Agreement of with alternative typing method |
|---|---|---|---|---|---|
| VenSF/VenSR (142 bp) | 16 | 99 | Australia | 97 (98%) | ND |
| 13 | 69 | Netherlands, South Africa, Turkey, United Kingdom | 61 (88%) | 68 (99%); AFLP typing | |
| 12 | 31 | Argentina, Australia, Canada, England, France, Northern Ireland, Scotland, Sweden, Uruguay | 30 (97%) | 31 (100%); PFGE typing | |
| 14 | 65 | Belgium, France, Germany, Hungary, Netherlands, Spain, South Africa, Turkey, United Kingdom, United States | ND (Gold standard was AFLP typing from [13]- 57 [88%]) | 57 (88%); alternative PCR protocol, Cf C05 | |
| 15 | 140 | Argentina, Belgium, France, Germany, Hungary, Japan, Netherlands, Spain, South Africa, Turkey, United Kingdom, United States | 112 (80%) | 101 (71%); MLST typing | |
| 19 | 76 | Sweden, United Kingdom | 58 (76%) | ND | |
| 29 | 53 | Argentina, Australia, Belgium, Canada, Czech Republic, France, Sweden, Switzerland, Uruguay, Yugoslavia | 52 (98%) | 52 (98%); alternative PCR target, ISCfeI | |
| Cf C05 forward/reverse (54 bp) | 14 | 65 | Belgium, France, Germany, Hungary, Netherlands, Spain, South Africa, Turkey, United Kingdom, United States | ND (Gold standard was AFLP typing from [13]- 65 [100%]) | 57 (88%); alternative PCR protocol, VenSF/VenSR |
| 15 | 140 | Argentina, Belgium, France, Germany, Hungary, Japan, Netherlands, Spain, South Africa, Turkey, United Kingdom, United States | 112 (80%) | 107 (76%); MLST typing | |
| CFVF/CFVR (86 bp) | 7 | 6 | Australia, United States | ND (known isolates from culture collections- 6 [100%]) | ND |
| 4 sets primers (virB4 — 521 bp, virB6 — 186 bp, virB11 — 233 bp, virD4 — 101 bp) | 21 | 7 | Argentina, United States | ND (known isolates from culture collections- 7 [100%]) | ND |
ND — not determined; AFLP — amplified fragment length polymorphism; PFGE —pulsed-field gel electrophoresis; MLST — multilocus sequence typing.
The only real time, quantitative PCR (qPCR) assay that has been proposed for CFV detection has been a 5′ Taq nuclease assay based on an internal subsequence of the known parA fragment (7) (Table I). Quantitative PCR offers advantages in sample processing time, sensitivity, reduced risk of cross-contamination, as well as offering a quantification component (22). Attractive qualities in this assay include its targeting of a well-recognized CFV-specific gene (parA), its format to take advantage of qPCR chemistry and its validation for CFV detection directly from preputial samples. However, the assay was designed with a novel primer/probe set based on only 3 CFV parA sequences. No data are currently available on the genetic diversity of the parA gene within CFV and, given that the qPCR was only directly tested with 6 strains, it would be erroneous to ascribe the success of the VenSF/VenSR primer set (which has its annealing sites just outside the novel target region) to this newer qPCR. Thus, there are several promising methodologies for CFV detection, but at the moment, no completely ideal test.
Canada is known to be a CFV-positive country (23). However, our knowledge of the prevalence of CFV in Canada is limited by current culture-based diagnostics. The practical limitations of sampling cattle herds by attending veterinarians in the field, usually at great distance from laboratory facilities, followed by extended transport of those samples for processing, has hampered research and epidemiological studies. The ideal situation for CFV testing under these conditions would be a qPCR assay using PCR primers that have been extensively tested against C. fetus isolates, which generate an amplicon long enough for DNA sequencing, and that have been validated for CFV detection directly from preputial samples. This kind of test could be applied to address prevalence, determine the load of CFV in individual animals (quantification), and examine sequence variation within the CFV-specific PCR target. We decided that the VenSF/VenSR primer set, which has been tested by multiple, independent research groups on over 450 strains of CFV from countries around the world, offered the best platform from which to build and assess a qPCR assay.
Our goals in this study were to translate the well-established VenSF/VenSR primer set to a SYBR Green qPCR platform, optimize the detection of CFV directly in preputial samples, compare the detection limits of this qPCR assay to a traditional culture method, and apply the newly formatted assay to screen several cohorts of western Canadian bulls for CFV. Amplicons generated from positive clinical samples were sequenced to gain a greater understanding of the CFV parA target and revealed 2 parA sequence types; one identical to published sequences and one novel sequence type.
Materials and methods
Bacterial culture
The type stain for Campylobacter fetus subsp. venerealis (ATCC 19438T) was obtained from the American Type Culture Collection. Pure cultures were grown under microaerophilic conditions (GasPak, EZ CampyPouch System; BD diagnostics, Mississauga, Ontario) at 37°C for 48 h on either TSA II 5% sheep blood agar (BD diagnostics) or in Bacto brain heart infusion broth (BD diagnostics) supplemented with 0.2% yeast extract, 0.07% agar and 220 rpm shaking.
Animal sampling
Preputial scrapings were collected by attending veterinarians using standard sampling techniques reported for Tritrichomonas foetus. Briefly, the prepuce of each bull was scraped with an artificial insemination pipette attached to a 20 mL syringe with suction applied. Collected preputial fluid and tissue was transferred into screw-capped tubes containing 2 mL of phosphate buffered (saline) solution (PBS; 20 mM phosphate, 150 mM NaCl). Samples to be cultured were packed in warm containers and immediately transported to the laboratory for processing. Field samples were refrigerated until transport to the laboratory with ice packs and stored at −80°C until analysis.
Samples were obtained from 3 cohorts of cattle. The first cohort (termed the yearling bull cohort) was comprised of 30 yearling bulls from a Saskatchewan community pasture. These bulls were tested for venereal diseases before the breeding season and had not been exposed to any females or older bulls since being placed in the community pasture in the early winter of 2010. At arrival, samples were categorized by visual inspection as clear (no visible red color), bloody-clear (moderate or light red color), or bloody (dark red color). Samples were initially screened with the VenSF/VenSR primers (16) (as follows) in a pre-optimized SYBR Green assay [PCR conditions as specified by Hum et al (16)] using a heat lysis prepuce preparation method (7) and yearling bull cohort samples confirmed negative were used to test prepuce DNA extraction methods (kit, chelex, or heat lysis) and as background to determine the assay’s analytical sensitivity.
The second cohort was a collection of 12 bulls housed at the Western College of Veterinary Medicine (termed in-house bull cohort). These animals were purchased by the researchers after failing screening for commercial breeding programs. Three successive preputial samples were taken from the in-house bulls, 1 to 2 wk apart and were evaluated by both culture and qPCR for CFV.
The third cohort was a collection of samples submitted by private veterinarians from western Canada collected from bulls tested in herds with a history of reproductive failure (termed survey bull cohort). Samples were collected from May 2009 through June 2010 and were submitted to the researchers for CFV testing.
Culturing of preputial samples
Culture for CFV was done on the in-house bull cohort samples using a filter-based, non-selective media methodology optimized for Campylobacter isolation (24). Fresh preputial samples (transported to the laboratory and processed within 3 h of collection) were thoroughly mixed and 100 μL or 300 μL volumes were spread onto 5% blood agar plates containing a 0.65 μm mixed cellulose ester membrane filter (Millipore; Billerica, Massachusetts, USA). Plates were incubated, filter-side up, at 37°C for 30 min to allow motile cells to cross the membrane, after which time the filters were removed and plates were returned to 37°C incubated for 48 h in microaerophilic conditions. Suspect colonies were identified macroscopically as small, smooth, translucent colonies and microscopically examined. Gram-negative cells with Campylobacter-like morphologies (rods or spirals) were subcultured and identified by cpn60 sequencing (25).
Cloning of the parA gene region
A segment from the parA gene of CFV ATCC 19438T, corresponding to nucleotides 30 566 to 30 706 of the CFV-specific genomic island (GenBank Accession: EU443150), was amplified from genomic DNA using the previously described primers (VenSF: 5′-CTT AGC AGT TTG CGA TAT TGC CAT T-3′ and VenSR: 5′-GCT TTT GAG ATA ACA ATA AGA GCT T-3′) and PCR program (16). The 142 bp product was ligated into the pGEM-T Easy plasmid and used to transform competent E. coli JM109 cells (Invitrogen, Burlington, Ontario). Plasmids with the target insert (termed parA-containing plasmids) were confirmed by sequencing, quantified, and used as qPCR assay standards.
Prepuce sample processing
Prepuce samples were prepared for qPCR analysis by 1 of 3 methods. The first method was a commercial stool DNA extraction kit (QIAamp DNA Stool Mini Kit; Qiagen, Mississauga, Ontario), as per manufacturer’s instructions. The second processing method was a slight modification of a resin-based DNA extraction protocol designed for Tritrichomonas foetus detection in prepuce described by Chen and Li (26). Briefly, 500 μL of preputial sample was centrifuged at 12 000 × g for 3 min and the pellet resuspended in chelex solution [5% chelex 100 resin in ultrapure water (BioRad, Mississauga, Ontario)]. Samples were vortexed for 20 s, followed by a 10 min heat treatment at 95°C to 100°C and then 10 min cooling on ice. Samples were again vortexed for 15 s and centrifuged at 12 000 × g for 10 min. Supernatant was used as the PCR template. The third method was the heat lysis protocol described by McMillen et al (7). Briefly, 200 μL of preputial sample was centrifuged for 5 min at 12 000 × g and the pellet resuspended in 100 μL ultrapure water. Samples were thoroughly vortexed and heated for 10 min at 95°C, followed by a centrifugation at 2000 × g for 30 s. Supernatant was used as the PCR template.
Campylobacter fetus subsp. venerealis qPCR
All qPCR reactions were run on a plate containing a no template control (NTC), an extraction negative control (ultrapure water extracted with the appropriate method at the same time as the samples) and a standard curve composed of parA-containing plasmids added at concentrations of 100 to 107 plasmid copies/reaction. All reactions were done in duplicate. Each reaction consisted of 1 × iQ SYBR Green supermix (BioRad), 400 nM each of VenSF and VenSR and 2 μL of template DNA, in a final volume of 25 μL. A thermocycler (MyiQ; BioRad) was used for all reactions with the following program: 95°C for 3 min, followed by 37 cycles of 95°C for 15 s, 60°C for 15 s, 72°C for 15 s, and a final extension at 72°C for 5 min. A melt curve was subsequently run for 81 cycles at 0.5°C increments from 55°C to 95°C for 30 s at each time point. Fluorescent signals were measured every cycle at the end of the annealing step and continuously during the melt curve data collection. The resulting data was analyzed using computer software (iQ5 Optical System Software; BioRad). A sample was classified as positive for CFV if both technical replicates within the assay were positive (generated the correct melt peak signal above background; 78.5°C ± 0.5°C) and the sample remained positive after 2 independent sample extractions and assays. Amplified products were sequenced to confirm parA target identity. An alternative qPCR assay for general C. fetus detection (targeting a cpn60 gene region identical in both subspecies) was also used to evaluate positive CFV samples, as previously described (27).
Results
Optimization of VenSF/VenSR qPCR
Two parameters were tested to optimize the performance of the VenSF/VenSR primers with SYBR Green chemistry, namely annealing temperature and amplification cycle number, by doing test assays and evaluating both the melt curve peaks (generated within the data analysis software) and by visualizing PCR products on a 1.75% agarose gel. The optimal annealing temperature of 60°C was determined by running the assay with a temperature gradient from 50°C (annealing temperature used in original Hum assay) to 70°C, using CFV genomic DNA as template. An annealing temperature of 60°C was determined to be the highest temperature that generated a saturated PCR signal (data not shown). The optimal qPCR cycle number was determined by running the assay with 36, 37, and 40 cycles with a set of clear, bloody-clear, and bloody heat-lysed and commercial stool kit prepared samples from the yearling bull cohort. Thirty-seven cycles of amplification was found to amplify the entire range of the standard curve (4.02 × 100 to 4.02 × 107 target copies/reaction) while generating the least amount of background fluorescence due to non-specific amplicons (data not shown).
Optimization of sample processing method
Preputial samples were processed with a commercial stool DNA extraction kit, a chelex-resin extraction method or a heat lysis extraction method, and the relative inhibitory properties of the resulting extracts were determined. Given the sample to sample variability of preputial samples, pools of 3 appearance types were generated before use. Equal volumes of 3 preputial samples of similar appearance from the yearling bull cohort were pooled, creating pools of clear (non-bloody samples that contained scraped tissue), bloody-clear (intermediate samples that contained traces of blood but were still translucent) and bloody (dark red samples that were not translucent) samples. Total DNA was extracted from sample pools using each of the 3 methods and tested as neat (undiluted), 1:10 and 1:100 dilutions (with ultrapure water). The DNA extracts were spiked with parA-containing plasmids at concentrations of 100 to 107 copies/μL. The detection of the parA-containing plasmid by each extraction method at each dilution was compared to the standard curve (plasmid in ultrapure water). The samples processed by the commercial stool DNA extraction kit showed no appreciable qPCR inhibition for any dilution of the extract compared to the standard in ultrapure water (detected plasmid copies were within 0.1 log values of the known spiked copy number). Conversely, both the chelex-resin and heat lysis extraction methods behaved comparably, with a notable decrease in detection of target DNA from bloody samples in the neat (undiluted) DNA extracts (detected on average 0.5 log values less of the known spiked plasmid; data not shown). Near optimal detection (within 0.1 log values) was restored with a 1:10 dilution of the DNA extracts. The chelex-resin extraction method offered no apparent advantage over the heat lysis method and was not tested further. The neat extract from the commercial stool kit and the 1:10 extract from the heat lysis method were carried on for further evaluation.
To evaluate the ability of the DNA extraction methods to recover CFV DNA from whole bacterial cells in a preputial sample, a dilution series of a CFV cell suspension (collected in PBS from 48 h CFV culture plates and adjusted to OD600 of 0.8) was spiked into PBS, clear, bloody-clear, and bloody sample pools. Dilution series were then generated using PBS or uninoculated prepuce sample as diluent where appropriate. Each sample in the dilution series was divided equally and processed by either the commercial stool DNA extraction kit or the heat lysis method.
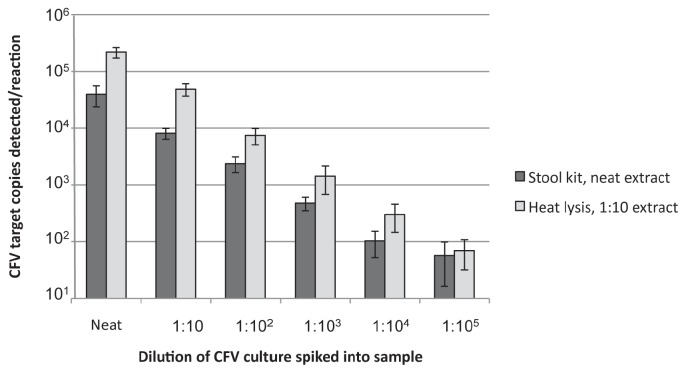
The neat stool kit DNA extracts and the 1:10 heat lysis DNA extracts from each dilution were assayed (Figure 1). For each method, the numbers of CFV targets detected for each dilution were consistent regardless of the sample background. For example, an average of 2.7 × 104 target copies/reaction were detected in PBS samples spiked with neat CFV culture and processed with the commercial stool kit, 5.0 × 104 target copies/reaction were detected in clear prepuce, 5.7 × 104 target copies/reaction were detected in bloody-clear prepuce, and 2.5 × 104 target copies/reaction were detected in bloody prepuce (visualized in Figure 1 by the small range of the error bars). Conversely, the number of CFV target copies detected per reaction between the sample preparation methods differed appreciably, with the 1:10 diluted heat lysis extraction consistently giving higher counts. For example, 4.0 × 104 +/− 1.6 × 104 copies/reaction were detected in neat spiked samples processed with the commercial stool kit while 2.2 × 105 +/− 4.7 × 104 target copies/reaction were detected in the same samples processed as the 1:10 diluted heat lysis extraction (Figure 1). It was only at the level of 101 to 102 copies/reaction where the 2 processing methods detected comparable levels of CFV target. The heat lysis method, with a 1:10 dilution of the processed extract, gave the least qPCR inhibition along with the best target detection and was, therefore, used as the prepuce processing method for the remainder of the study.
Figure 1.
Detection of C. fetus subsp. venerealis (CFV) from preputial samples spiked with CFV cell suspensions. The starting optical density (OD600) CFV cell suspension was 0.8. For each extraction method [commercial stool kit (dark grey bars), heat lysis (light grey bars)], CFV cells were spiked into phosphate buffered (saline) solution (PBS). Clear, bloody-clear, and bloody preputial samples were processed as indicated and assayed by quantitative polymerase chain reaction (qPCR). Results were averaged across the 4 sample types at each spiking level and are reported as average parA copy number per reaction. Error bars indicate standard deviation.
Comparison of culture and qPCR sensitivity using a quantified spiked sample
To evaluate the detection limit of the VenSF/VenSR qPCR using the 1:10 diluted heat lysis prepuce extraction method, CFV culture was spiked into a mixed prepuce pool (containing equal parts clear, bloody-clear, and bloody samples). A dilution series from 1.4 × 108 to 1.4 × 100 CFU/mL was made using prepuce sample as diluent and samples were processed for culture detection and qPCR. For culture, when 300 μL of preputial sample was plated (the largest liquid volume that could be accommodated on the filters used), the sample had to contain 1.4 × 104 CFU/mL of CFV before CFV colonies were visible. If the volume of preputial sample plated was reduced to 100 μL, 1.4 × 106 CFU/mL were necessary before CFV colonies could be visually identified on culture plates. These culture detection limits are consistent with previous reports, where preputial samples needed to be spiked with an average of 2.5 × 104 CFU/mL or greater of CFV cells to isolate CFV colonies from selective medium (Skirrow’s) (7). Alternatively, preputial samples containing 1.4 × 103 CFU/mL of CFV were reliably positive in qPCR assay reactions. This equates to an assay detection limit of ~1.4 cells/reaction.
Survey of Canadian cattle cohorts
The VenSF/VenSR qPCR assay was utilized to test preputial samples from 3 western Canadian bull cohorts, which represent a range of animals and conditions, including yearling bulls housed together (yearling cohort), mature bulls housed together, and sampled repeatedly over time (in-house bulls), and mature bulls from different geographic locations (survey bulls). Of the 377 animals tested, a positive signal for the parA target was detected from 11 animals (Table II). One cohort (the in-house bulls) was also tested by culture, which yielded negative results in all cases (Table II).
Table II.
Summary of C. fetus subsp. venerealis (CFV) detection in Canadian bulls
| qPCR
|
||||
|---|---|---|---|---|
| Cohort | Total animals | Culture | Positive | Negative |
| Yearling bulls | 30 | ND | 1 | 29 |
| In-house bullsa | 12 | All negative | 2 | 34 |
| Survey bulls | 335 | ND | 8b | 327 |
| Totals | 377 | 0 | 11 | 390 |
Cohort included 12 bulls that were sampled 3 times, approximately a week apart, generating 36 samples to be processed.
Five of these samples were also positive by an alternative C. fetus qPCR test (27).
qPCR — quantitative polymerase chain reaction; ND — Not done.
Positive samples included 1 yearling bull (SPB10-3A), 2 of the in-house bulls [Bull 3 upon first sampling (V1-10-03) and Bull 10 upon third sampling (V4-10-10)] and 8 bulls from the geographic survey (WCVM_331, WCVM_338, WCVM_340, LVBF2007, LVBF2028, LVBF86, LVBF89, and CFB_714). A quantifiable value for target copies/reaction was determined for 6 of the positives and ranged from 8.4 × 100 to 4.1 × 102 target copies/reaction. The remaining 5 positives were detected beyond the limit of the standard curve (fewer than 4.5 × 100 copies/reaction) and, as such, can only be reported as detectable, but not quantifiable. DNA sequence data was obtained from all 11 samples confirming the amplified product was the parA gene from CFV and 9 sequences were full length and of sufficient quality to allow for sequence comparison (GenBank Accession HQ644122-HQ644130) (Figure 2). The 3 published parA sequences from strains ATCC 19438T, 98-118432, and 84-112 were included as references. Three sequences (LVBF2028, WCVM_331, and WCVM_340) were identical to the 3 reference stains over the 91 bp of sequence between the PCR primers while the remaining 6 sequences (LVBF2086, LVBF2007, LVBF2089, SPB10-3A, V1-10-03, and V4-10-10) were identical to each other but differed by 8 bp from the reference strains (Figure 2).
Figure 2.
Sequence alignment of the parA gene target obtained from positive samples. Published sequences from strains ATCC 19438T (EU443150), 98-118432 (AY903214), and 84-112 (AY750964) have been included as references. The amplification primer sequences (VenSF and VenSR) have been removed for analysis and only bases differing from the ATCC strain are indicated. N — ambiguous nucleotide in the sequence data.
An alternate qPCR assay targeting C. fetus in general was used in an attempt to independently confirm the presence of C. fetus in the positive samples. Only 5 of the 11 samples (all from the survey cohort) were positive for C. fetus by this species-level test (Table II); however, the published detection limit for the C. fetus general assay is 102 copies/reaction (27) and all our positive samples were quantified at or below this concentration.
Discussion
This study reports the translation of the well-established CFV-specific PCR primers VenSF/VenSR from Hum et al (16) to a qPCR assay platform using SYBR Green chemistry, evaluation and optimization of preputial sample processing for this platform, determination of the assays sensitivity limits, and application of this assay to western Canadian bull cohorts for the detection of CFV. The published VenSF/VenSR primers have become the PCR standard for subspeciation of C. fetus isolates. This primer set has been tested on strains of C. fetus from around the world (Table I) (13,15,16). The limitation of the original assay has been recognized in the poor analytical sensitivity of the PCR, leading to its use primarily on cultured pure isolates. Recent efforts to understand the genetic differences between CFV and CFF have expanded our understanding of the VenSF/VenSR gene target, parA, and support its use as a CFV-specific genomic marker (20,21). The 142 bp PCR product generated by the VenSF/VenSR primer set is an ideal size for SYBR Green assays and optimization of this PCR for the qPCR platform required only minor adjustments to the annealing temperature (increase from 50°C to 60°C) and amplification cycle number (increase from 30 to 37 cycles) from the original PCR protocol (16).
Preputial samples are complex to analyze, as sample to sample variability is high and any sample could contain a mixture of tissue, blood, feces, or other contaminants. Many of these contaminating compounds are inhibitory to PCR (28). We evaluated 3 preputial processing methods, representing 3 levels of processing. The DNA extracts from a commercial stool DNA extraction kit method were found to be the “cleanest” extracts (carried through the least amount of qPCR inhibitors), but the extensive processing resulted in less overall DNA being extracted from whole cells (Figure 1). The commercial kit was also the most expensive and time-consuming processing method tested. The second processing method was a resin-based partial purification. The theory behind this process is that the chelex resin binds and removes some qPCR inhibitors from the sample without losing DNA in extraction steps. Resin-processed samples were not any better than heat lysed samples, which required fewer reagents and less processing time since the samples were simply washed, boiled, and tested. The heat lysis method was not expected to remove qPCR inhibitors and it was not surprising that reduced detection of target DNA was seen with the most contaminated samples (bloody). A 1:10 dilution of the neat heat lysed extract removed the qPCR inhibition and allowed better detection of CFV DNA from whole cells compared to neat commercial stool kit extracts (Figure 1). The combination of the simplest sample preparation and most target DNA recovered within the assay made the 1:10 dilution of the heat lysis DNA extract the sample preparation method of choice for this study.
We determined the assay’s analytical sensitivity by spiking known numbers of CFV cells into samples and assaying for target DNA. The qPCR assay could reliably detect 103 CFU/mL of sample, which equates to detecting ~1 copy/reaction. This exceeds the conventional PCR application of the VenSF/VenSR primers, reported to require 103 copies/reaction (7) and is equivalent to the alternative CFV qPCR protocol currently published (which also detects ~1 copy/reaction) (7).
We did not directly test the analytical specificity of this qPCR assay in this study. A primary reason for choosing the VenSF/VenSR primer set for this assay is that these primers have already been tested for target specificity by several international research groups on over 450 isolates (Table I). The parA gene target has also previously been determined to be absent from other organisms known to inhabit the prepuce (7). In addition, to ensure that our qPCR did not generate false positive results, all PCR amplicons generated in this study were sequenced and confirmed to be derived from the parA gene.
We cannot draw any conclusions at this time regarding diagnostic sensitivity and specificity because the direct comparison between culture (the gold standard) and our qPCR was too small. A major limitation in investigating CFV prevalence and epidemiology currently is that culture is a poor test for CFV. In the surveys we undertook, culture of most prepuce samples was simply not possible. We are currently undertaking further studies to address questions about the detection of CFV in known positive bulls by culture and qPCR and acknowledge that the qPCR assay presented herein will be a research tool until more work can be done to further validate it as a diagnostic test.
The qPCR assay was then used to evaluate preputial samples from a range of western Canadian bull cohorts. Canada is recognized as a country with confirmed BGC cases (23). The in-house bull cohort was evaluated by culture for CFV and all animals were found to be negative. However, the CFV-specific parA gene could be detected at low levels from all 3 cohorts (Table II). It is difficult to translate detected quantities of CFV back to a consistent measure of prepuce scraping, as each sampling of an animal collects differing amounts of preputial fluid, tissue, blood, and contaminants and the sample is often mixed with PBS or transport media after collection. What these results do indicate is that the parA target gene could be reproducibly detected from 11 preputial samples tested. We cannot comment on whether this detection is clinically significant, as background carriage rates of CFV in bulls are unknown at this level.
Although the parA gene target for this assay has only been reported from CFV isolates, we attempted to verify independently that C. fetus was present in the samples using a species-specific qPCR assay targeting a different gene target (27). We were able to confirm that 5/11 (45%) samples were positive by the species-level test. This low confirmation rate was most likely a limitation of the species-level test, which has a detection limit above almost all of our samples (102 copies/reaction) (27), and not a CFV-assay specificity problem.
To gain a better understanding of the parA target detected in this study, we sequenced the qPCR products from positive clinical samples. Nine full-length, high quality sequences were obtained and compared to the 3 reference parA sequences available in the public domain (Figure 2). Three of the sequences (obtained from survey bulls from across western Canada) are identical to reference isolates. However, the remaining 6 sequences were identical to each other, but differed from the reference strains at 8 nucleotide positions. This translates into a 2 amino acid difference between parA proteins. The biological significance of this sequence variation is unknown but it does have implications for diagnostics. For example, the 5′ Taq nuclease CFV assay previously described (7) was based solely on the parA reference sequences and it is questionable whether it would have detected the 6 alternative sequences types seen in this study, as the reverse PCR primer for this assay would have had 4 mismatches to the template sequence. Willoughby et al (19) reported poor agreement of phenotypic and PCR results for C. fetus isolates from the UK. Since identification of CFV is determined by the generation of a PCR product, if CFV isolates from the UK have a sequence variation within the VenSF/VenSR landing sites, PCR would fail and CFV strains would be falsely identified as CFF by the PCR test. Further investigation of the parA gene target sequence from a range of CFV isolates is needed to ascertain the extent of the conservation within this CFV-specific target.
The qPCR assay and sample processing method described in this study offer laboratories a sensitive, inexpensive test that can be applied to a range of preputial samples. The VenSF/VenSR primer set is grounded in 13 y of testing by the international scientific community and continues to be reliable in the face of new discoveries regarding CFV genetics. The finding in this study that the CFV parA gene target can have sequence variation suggests new avenues for investigation and new questions about this important veterinary pathogen. Research into the parA gene from CFV will continue to make CFV detection more robust and informative in the future.
Acknowledgments
The authors gratefully thank Alvaro Garcia Guerra for access to the in-house bull cohort and assistance with sample collection, as well as cattle producers and veterinarians from across western Canada for participation in the CFV survey. Funding was provided by the WCVM Vitamin Class Action Settlement Fund, the Canadian Advancing Agriculture Program (CAAP) from Agriculture and Agri-Food Canada, and the Alberta Beef Producers. Dr. Chanban was supported by a Saskatchewan Health Research Foundation Post doctoral fellowship and Shirley Chu was supported by the WCVM Interprovincial Undergraduate Student Summer Research Program.
References
- 1.Véron M, Chatelain R. Taxonomic study of the genus Campylobacter Sebald and Véron and designation of the neotype strain for the type species, Campylobacter fetus (Smith and Taylor) Seblad and Véron. Int J Syst Bacteriol. 1973;23:122–134. [Google Scholar]
- 2.Skirrow MB. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113–149. doi: 10.1016/s0021-9975(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 3.OIE. Bovine Genital Campylobacteriosis. Paris: Office international des épizooties; 2010. [Google Scholar]
- 4.OIE. Bovine Genital Campylobacteriosis. OIE Terrestrial Manual. 2008;Chapter 2.4.5:661–670. [Google Scholar]
- 5.Hum S, Brunner J, McInnes A, Mendoza G, Stephens J. Evaluation of cultural methods and selective media for the isolation of Campylobacter fetus subsp venerealis from cattle. Aust Vet J. 1994;71:184–186. doi: 10.1111/j.1751-0813.1994.tb03385.x. [DOI] [PubMed] [Google Scholar]
- 6.Lander KP. The application of a transport and enrichment medium to the diagnosis of Campylobacter fetus infections in bulls. Br Vet J. 1990;146:334–340. doi: 10.1016/s0007-1935(11)80026-6. [DOI] [PubMed] [Google Scholar]
- 7.McMillen L, Fordyce G, Doogan VJ, Lew AE. Comparison of culture and a novel 5′ Taq nuclease assay for direct detection of Campylobacter fetus subsp. venerealis in clinical specimens from cattle. J Clin Microbiol. 2006;44:938–945. doi: 10.1128/JCM.44.3.938-945.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama SM, Garcia MM, Taylor DE. Differentiation of the subspecies of Campylobacter fetus by genomic sizing. Int J Syst Bacteriol. 1992;42:446–450. doi: 10.1099/00207713-42-3-446. [DOI] [PubMed] [Google Scholar]
- 9.Hum S, Stephens LR, Quinn C. Diagnosis by ELISA of bovine abortion due to Campylobacter fetus. Aust Vet J. 1991;68:272–275. doi: 10.1111/j.1751-0813.1991.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 10.Devenish J, Brooks B, Perry K, et al. Validation of a monoclonal antibody-based capture enzyme-linked immunosorbent assay for detection of Campylobacter fetus. Clin Diagn Lab Immunol. 2005;12:1261–1268. doi: 10.1128/CDLI.12.11.1261-1268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez AH, Bardon JC, Nosoda BP, Cordeviola JM, Sarmiento F, Gau JA. Herd diagnosis on Trichomoniasis and Campylobacteriosis in bovine utilizing the empty cow as indicator. Vet Arg. 1986;111:962–966. [Google Scholar]
- 12.On SL, Harrington CS. Evaluation of numerical analysis of PFGE-DNA profiles for differentiating Campylobacter fetus subspecies by comparison with phenotypic, PCR and 16S rDNA sequencing methods. J Appl Microbiol. 2001;90:285–293. doi: 10.1046/j.1365-2672.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagenaar JA, van Bergen MA, Newell DG, Grogono-Thomas R, Duim B. Comparative study using amplified fragment length polymorphism fingerprinting, PCR genotyping, and phenotyping to differentiate Campylobacter fetus strains isolated from animals. J Clin Microbiol. 2001;39:2283–2286. doi: 10.1128/JCM.39.6.2283-2286.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Bergen MA, Simons G, van der Graaf-van Bloois L, et al. Amplified fragment length polymorphism based identification of genetic markers and novel PCR assay for differentiation of Campylobacter fetus subspecies. J Med Microbiol. 2005;54:1217–1224. doi: 10.1099/jmm.0.46186-0. [DOI] [PubMed] [Google Scholar]
- 15.van Bergen MA, Dingle KE, Maiden MC, et al. Clonal nature of Campylobacter fetus as defined by multilocus sequence typing. J Clin Microbiol. 2005;43:5888–5898. doi: 10.1128/JCM.43.12.5888-5898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hum S, Quinn K, Brunner J, On SL. Evaluation of a PCR assay for identification and differentiation of Campylobacter fetus subspecies. Aust Vet J. 1997;75:827–831. doi: 10.1111/j.1751-0813.1997.tb15665.x. [DOI] [PubMed] [Google Scholar]
- 17.Tu ZC, Eisner W, Kreiswirth BN, Blaser MJ. Genetic divergence of Campylobacter fetus strains of mammal and reptile origins. J Clin Microbiol. 2005;43:3334–3340. doi: 10.1128/JCM.43.7.3334-3340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Clark CG, Taylor TM, et al. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J Clin Microbiol. 2002;40:4744–4747. doi: 10.1128/JCM.40.12.4744-4747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willoughby K, Nettleton PF, Quirie M, et al. A multiplex polymerase chain reaction to detect and differentiate Campylobacter fetus subspecies fetus and Campylobacter fetus — species venerealis: Use on UK isolates of C. fetus and other Campylobacter spp. J Appl Microbiol. 2005;99:758–766. doi: 10.1111/j.1365-2672.2005.02680.x. [DOI] [PubMed] [Google Scholar]
- 20.Gorkiewicz G, Kienesberger S, Schober C, et al. A genomic island defines subspecies-specific virulence features of the host-adapted pathogen Campylobacter fetus subsp. venerealis. J Bacteriol. 2010;192:502–517. doi: 10.1128/JB.00803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moolhuijzen PM, Lew-Tabor AE, Wlodek BM, et al. Genomic analysis of Campylobacter fetus subspecies: Identification of candidate virulence determinants and diagnostic assay targets. BMC Microbiol. 2009;9:86. doi: 10.1186/1471-2180-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay IM. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect. 2004;10:190–212. doi: 10.1111/j.1198-743x.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 23.Mshelia GD, Amin JD, Woldehiwet Z, Murray RD, Egwu GO. Epidemiology of bovine venereal campylobacteriosis: Geographic distribution and recent advances in molecular diagnostic techniques. Reprod Domest Anim. 2010;45:e221–e230. doi: 10.1111/j.1439-0531.2009.01546.x. [DOI] [PubMed] [Google Scholar]
- 24.Le Roux E, Lastovica AJ. The Cape Town Protocol: How to isolate the most campylobacters for your dollar, pound, franc, yen, etc. In: Lastovica AJ, Newell DG, Lastovica EE, editors. Proceedings of the 9th International Workshop on Campylobacter, Helicobacter and Related Organisms. Cape Town: Institute of Child Health; 1998. pp. 30–33. [Google Scholar]
- 25.Hill JE, Paccagnella A, Law K, et al. Identification of Campylobacter spp. and discrimination from Helicobacter and Arcobacter spp. by direct sequencing of PCR-amplified cpn60 sequences and comparison to cpnDB, a chaperonin reference sequence database. J Med Microbiol. 2006;55:393–399. doi: 10.1099/jmm.0.46282-0. [DOI] [PubMed] [Google Scholar]
- 26.Chen XG, Li J. Increasing the sensitivity of PCR detection in bovine preputial smegma spiked with Tritrichomonas foetus by the addition of agar and resin. Parasitol Res. 2001;87:556–558. doi: 10.1007/s004360100401. [DOI] [PubMed] [Google Scholar]
- 27.Chaban B, Musil KM, Himsworth CG, Hill JE. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl Environ Microbiol. 2009;75:3055–3061. doi: 10.1128/AEM.00101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rådström P, Knutsson R, Wolffs P, Lövenklev M, Löfström C. Pre-PCR processing: Strategies to generate PCR-compatible samples. Mol Biotechnol. 2004;26:133–146. doi: 10.1385/MB:26:2:133. [DOI] [PubMed] [Google Scholar]
- 29.Abril C, Vilei EM, Brodard I, Burnens A, Frey J, Miserez R. Discovery of insertion element ISCfe1: A new tool for Campylobacter fetus subspecies differentiation. Clin Microbiol Infect. 2007;13:993–1000. doi: 10.1111/j.1469-0691.2007.01787.x. [DOI] [PubMed] [Google Scholar]