Abstract
Hypericum perforatum extract (HPE) has been proved a drug effective to many viral diseases. The purpose of this paper was to investigate the therapeutic efficacy and immuno-enhancement of HPE for chickens which were already challenged with infectious bursal disease virus (IBDV BC-6/85). Chickens infected with IBDV were treated with HPE for 5 consecutive days, the observation of immune organ indexes and pathological changes index, determination of IFN-α and detection of IBDV with RT-PCR were employed to assess in vivo whether or not HPE had the certain therapeutic efficacy on infectious bursal disease (IBD), and if HPE was able to improve the immunologic function. The results showed that 1330 and 667.9 mg/kg body weight (BW) per day of HPE had significant therapeutic efficacy and improvement immunologic functions for chickens infected experimentally with IBDV.
Résumé
Il a été démontré qu’un extrait d’Hypericum perforatum (HPE) était un médicament efficace contre plusieurs maladies virales. L’objectif de cette étude était d’évaluer l’efficacité thérapeutique et immuno-stimulante de HPE chez des poulets déjà inoculés avec le virus de la maladie de Gumboro (IBDV BC-6/85). Les poulets infectés avec IBDV étaient traités avec HPE pendant 5 jours consécutifs, l’examen des indices des organes immunitaires et des changements pathologiques, la détermination d’IFN-α et la détection d’IBDV par RT-PCR ont été utilisés pour évaluer in vivo si HPE avait ou non une certaine efficacité thérapeutique sur la maladie de Gumboro (IBD), et si HPE était en mesure d’améliorer la fonction immunitaire. Les résultats ont démontré que des doses quotidiennes de 1330 et 667,9 mg/kg de poids vif (BW) d’HPE avaient une efficacité thérapeutique significative et amélioraient les fonctions immunitaires de poulets infectés expérimentalement par IBDV.
(Traduit par Docteur Serge Messier)
Introduction
Infectious bursal disease (IBD), induced by infectious bursal disease virus (IBDV), has developed into one of the most severe infectious diseases of chickens. The virus causes acute morbidity and death through invasion of the bursae in chickens, especially those 4 to 6 weeks old; it kills mature B lymphocytes and those which are still differentiating (1,2). For IBDV-infected chickens, it is easier to be infected with Newcastle disease, Marek’s disease, infectious bronchitis, or other diseases (3). In an early stage, IBDV caused lower mortality (≤ 10%) in chickens; however, new epidemics show characteristics of genetic variation, and some of variants can result in 100% morbidity and mortality (4,5). Because of the higher mutation rates of IBDV, it is difficult to use current vaccines to prevent disease caused by a new variant, especially a very virulent one. Researchers’ attention, therefore, has been drawn to the development of new drugs to prevent the outbreak and spread of IBD.
Hypericum perforatum, is one species of the genus Guttiferae that grows in Europe, West Asia, North Africa, and North America. It is used in Traditional Chinese Medicine for depression, tumors, detoxification, to stop bleeding, and for other functions (6). Recent studies show that there are many active components in Hypericum perforatum, such as hypericin, pesudohypericin, hyperforin, proanthocyanidin, and rutoside. Hypericin, in particular, has been proven to be highly effective in virus inhibition (7). Many studies, therefore, have focused on the antiviral activity of HPE on lipid-enveloped and non-enveloped DNA and RNA viruses, such as human immunodeficiency virus (HIV), hepatitis virus, labialis virus, and varicellavirus (8,9). Besides the research on antiviral activity, immunity improvement of HPE for piglets infected with porcine reproductive and respiratory syndrome virus (PRRSV) also has been done (10). There are, however, no reports on using HPE for inhibition of IBDV and immunity improvement for chickens infected with the virus. The aim of this study, therefore, was to determine whether or not HPE inhibits IBDV in vivo effectively and improves the immunologic function by recovering immune organs damaged by virus and inducing secretion of IFN-α.
Materials and methods
Drugs
Hypericum perforatum extract was provided by Lanzhou Institute of Animal Husbandry & Veterinary Pharmaceutics Science, Chinese Academy of Agricultural Sciences. Because hypericin is considered the main active ingredients in the extract, the standards of HPE are usually evaluated by it. The reversed-phase high performance liquid chromatography (RP-HPLC) was used to detect the content of hypericin in HPE. Hypericin was separated by a Symmetry C18 column (4.6 mm × 250 mm, 5 μL) with methanol-acetonitile-1.0% sodium dihydrogen phosphate (170:10:10) as mobile phases. The wavelength used for detection was 588 nm, the flow rate was 1.0 mL/min, the column temperature was room temperature, and the injection volume was 20 μL. In this condition, the content of hypericin was 0.3% within HPE.
Virus
The IBDV (BC-6/85) whose median infective dose (ID50) for chickens was 105.0/0.05 mL (eye-drop) was provided by China Institute of Veterinary Drugs Control, Beijing. The virus was diluted to 100 ID50/0.1 mL and the suspension was added into 1000 U/mL penicillin and 100 μg/mL streptomycin, respectively, and stored at −70°C for later use.
Animals
Twenty day-old Hailanhe chickens, weighing 130 to 150 g, were purchased from a chicken farm in Gaobidian City, Hebei Province, China. To ensure that the chickens were BIDV-free, the agar gel precipitation (AGP) test was used to detect the virus at 10-days old and 18-days old, respectively. Chickens with virus or clinical signs of disease or abnormalities were not used in the study. All the chickens were tagged, allowed free access to water, and fed nonmedicated rations.
Treatments
Three hundred chickens were randomly divided into 6 groups. Chickens in the positive control group were only challenged with IBDV and no treatment was done in the negative control group. Chickens in the other groups were administered various dosages and drugs for 5 consecutive days and ensured that all drugs were taken. The treatment regimens are listed in Table I. All chickens had free access to water during the treatments. Chickens in each group were completely isolated from the other groups and reared in isolated pens. After infection with IBDV, the feed intake, diarrhea, death rate, and the other clinical manifestations of all chickens were recorded daily over the 17-day experiment. All procedures were performed strictly according to the legislation on the use and care of laboratory animals and the guidelines established by Beijing Laboratory Animal Research Centre.
Table I.
Animal grouping and treatment regimens
| IBDV challenge
|
Drugs treatments
|
||||||
|---|---|---|---|---|---|---|---|
| Groups | na | Times (d) | Dosage (mL) | Route | Times (d) | Dosageb | Route |
| HPE high-dose group | 50 | 20 | 0.1 | eye-drop | 22–26 | 1330 mg/kg BW q24h | drench |
| HPE middle-dose group | 50 | 20 | 0.1 | eye-drop | 22–26 | 667.9 mg/kg BW q24h | drench |
| HPE low-dose group | 50 | 20 | 0.1 | eye-drop | 22–26 | 333.9 mg/kg BW, q24h | drench |
| Egg-yolk antibody control group | 50 | 20 | 0.1 | eye-drop | 22–26 | 0.5 mL per chicken, q48h | IM |
| Positive control group | 50 | 20 | 0.1 | eye-drop | — | — | — |
| Negative control group | 50 | — | — | — | — | — | — |
BW — body weight; IM — intramuscular.
At 25 d (5 d after IBDV challenge), 10 chickens from each group were selected and killed for use; the remainder from each group were treated continuously with the drugs until 26 d.
1330, 667.9, and 333.9 mg of H. perforatum extract in 3 groups contained 4.0, 2.0, and 1.0 mg of hypericin, respectively.
On days 5, 7, 9, 12, and 16 after virus challenge, 10 chickens (including chickens which died during the experiment) were randomly selected from each group. Each chicken was weighed and recorded, followed by collecting blood samples without contamination for IFN-α analysis. Then all the selected chickens were euthanized. The bursae, spleens, and thymuses of each chicken were obtained aseptically for determining immune organ indices, pathological change indices, and viral isolation.
Viral isolation and detection with RT-PCR
The bursae, which were obtained aseptically from 10 chickens on days 5, 7, 9, 12, and 16 after IBDV challenge, were frozen at −40°C and then thawed at room temperature 3 times to sufficiently destroy the cells. The bursae were ground and diluted into 10% to 20% suspensions with sterilized saline. Then suspensions were centrifugated at 3000 rpm for 10 min, and the supernatants were collected followed by addition of antibiotics and storage at −70°C for reverse-transcriptase polymerase chain reaction (RT-PCR).
Reverse-transcriptase polymerase chain reaction was used to detect IBDV in the supernatants. A primer pair was designed based on the sequences of a fragment of STC gene in the GenBank using the software package DNAStar (Version 5.0; DNAStar, Madison, Wisconsin, USA). The primers were synthesized by the Shanghai Sangon Biological Engineering Technology & Services Co., with the following sequences: IBDV-P1: 5′-GGACCGGCGTCCATTCCG-3′, IBDV-P2: 5′-TGTGCTTCACCTCACTGTG-3′. The extraction of viral RNA was performed using a Total RNA Kit (Sino-American Biotechnology Co.) according to the manufacturer’s instructions. The RT-PCR was performed by routine methods. And the amplified PCR products were examined with 2.0% agarose gel (containing 0.5 mg/L EB) electrophoresis.
Determination of immune organ indices and pathological change indices
The water on surface of bursae, spleens, and thymuses of each chicken were randomly selected on days 5, 7, 9, 12, and 16 after virus challenge was soaked up, followed by weighing with electronic balance. Then bursa index, spleen index, and thymus index were calculated using the formula:
Meanwhile more observations were made on hemorrhage of bursae, and muscles of thoraxes and legs. The pathological changes were assessed according to the degree of hemorrhage. The total pathological change index (TPCI) of bursae or muscles was the sum of all chicken pathological change indices (PCI) in one group. The PCI of bursae were marked according to degrees of hemorrhage: severe hemorrhage count as 4 points; moderate hemorrhage count as 3 points; slight hemorrhage count as 1 point; and focal hemorrhage is 0.2. Muscle PCIs were set as: severe hemorrhage count as 3 points per side, moderate hemorrhage count as 2 points per side, and slight hemorrhage count as 1 point per side. If 2 sides both had severe hemorrhage, it was counted as 6 points, and so on. Then on days 5 to 7, 9, 12, and 16 after IBDV challenge, the TPCI of muscle of the selected 10 chickens in each group were obtained and the integrative pathological change indices (IPCI) were established according to the formula:
Then the mean values of IPCI were calculated according to the data of IPCI obtained from days 5, 7, 9, 12, and 16.
IFN-α analysis
The samples which were collected on days 5, 7, 9, 12, and 16 after virus challenge were left at room temperature for 10 to 15 min followed by centrifugation at 1500 rpm for 15 min. The sera were collected carefully for IFN-α determination by using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (RapidBio Lab, USA).
Statistical analysis
The data obtained were analyzed together with statistical software package (SAS Version 9.0; SAS Institute, Cary, North Carolina, USA) to account for the effects of HPE for chickens’ PCI on day 16 and IFN-α on day 5, 7, 9, 12, and 16 after IBDV challenge. A parametric one-way analysis of variance (ANOVA) for repeated measures was used to examine intergroup differences. When a significant difference (P < 0.05) was found; Tukey’s test was used to compare the means.
Results
Clinical findings
After infection with IBDV, a small number of chickens in positive control group showed slight clinical signs such as decreased feed intake, fluffed feathers, and mess, somnolence, catalepsies. Only a few chickens discharged white or yellow watery feces, but there were almost no symptoms in other groups. Throughout the whole experiment, no chickens died in any group.
Viral detection by RT-PCR
The supernatants used to detect virus were extracted for RNA and submitted to PCR amplification. The RT-PCR products were visualized by the agarose gel electrophoresis. The gel image acquired by Image Acquisition and Analysis System (GDS8000PC, UVP, America) were used to determine the presence of IBDV. Supernatants containing the virus should give a fragment with size of about 267 base pairs (bp).
The results of virus detection by RT-PCR were as follows: on days 5, 7, and 9 after infection, a fragment was amplified in the supernatants in all groups, except the negative control group, indicating the presence of the IBDV in the supernatants. On day 12 after infection, positive results were obtained from the supernatants made from HPE low-dose group and the positive control group, while negative results were obtained for supernatants made from the other groups. On day 16 after infection, no virus was detected in all groups (Table II).
Table II.
Viral detection of bursae collected from the chickens after challenge
| Groups | Day 5 | Day 7 | Day 9 | Day 12 | Day 16 |
|---|---|---|---|---|---|
| HPE high-dose group | +a | + | + | − | − |
| HPE middle-dose group | + | + | + | − | − |
| HPE low-dose group | + | + | + | + | − |
| Egg-yolk antibody Igy control group | + | + | + | − | − |
| Positive control group | + | + | + | + | − |
| Negative control group | − | − | − | − | − |
Symbols: + — represents positive viral isolation; − — represents negative viral isolation.
Results of immune organ indexes and PCI
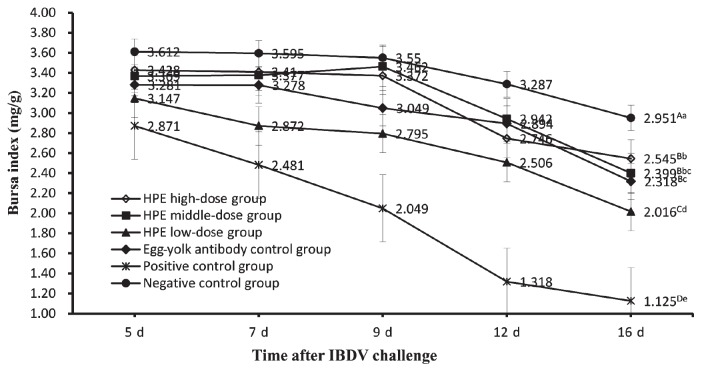
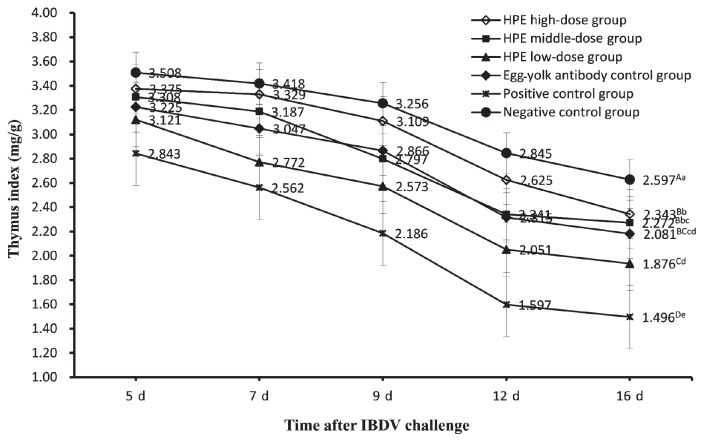
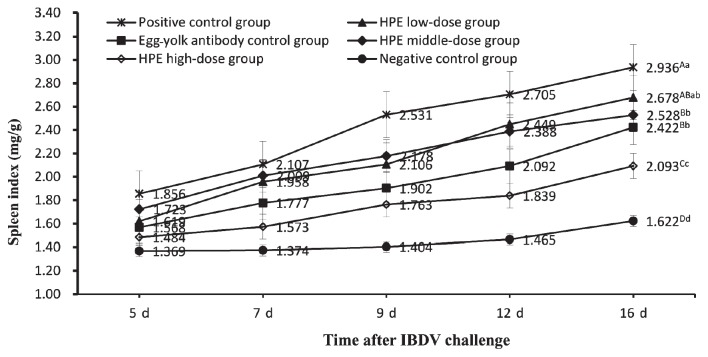
On days 5, 7, 9, 12, and 16 after IBDV challenge, the bursa, spleen, and thymus of each chicken were selected randomly from each group and weighed respectively by electronic balance. The weight (mg) of those organs was divided by body weight (g) according to the calculation methods of the organ index. Then the data of the organ indices and the significant difference of the organ indices between all groups on day 16 after virus challenge were obtained (Figures 1 to 3). Figure 1 is a graph of bursa index of chickens in all groups at different dates after challenge. The data showed that the bursa indices of chickens in all groups gradually declined in general. But the bursa indices of chickens in the positive control group declined more significantly than those in the other groups, the HPE low-dose group, and other groups except the negative control group. On day 16 after challenge, the bursa index of chickens in the HPE high-dose group, HPE middle-dose group, and egg-yolk antibody control group were not significantly different (P < 0.01) from the negative control group and HPE low-dose group. Figure 2 shows an increasing tendency of the spleen indices of the chickens in all groups, but the increasing degrees in 3 HPE dosage groups and egg-yolk antibody control group were remarkably higher than those in the negative control group and significantly lower than those in the positive control group. On day 16 after challenge, the spleen index of chickens in the HPE middle-dose group and egg-yolk antibody control group were significantly lower (P < 0.01) than indices in the positive control group and higher than those in HPE high-dose group, but no difference (P < 0.01) from those in the HPE low-dosage group. The spleen index of chickens in the negative control group was significantly lower (P < 0.01) than that in HPE high-dose group. Figure 3 shows a decreasing tendency in the thymus indices of chickens in all groups, but the data in the positive control group were always lower than those in the HPE low-dose group and remarkably lower than that in the other groups. On day 16 after challenge, there was no significant difference (P < 0.01) among HPE high-dose group, HPE middle-dose group, and egg-yolk antibody control group, except for the difference (P < 0.05) between the HPE high-dose group and the egg-yolk antibody control group. The thymus index of chickens in the HPE high-dose group was significantly lower (P < 0.01) than that in negative control group. The HPE low-dose group was significantly higher (P < 0.01) than that in positive control group, but was not significantly lower (P < 0.01) than that in egg-yolk antibody control group.
Figure 1.
The bursa index of chickens in all groups on days 5, 7, 9, 12, and 16 after IBDV challenge. Each point and vertical bar represents the mean and standard error (n = 10). Different capital letters indicated significant difference (P < 0.01) and different small letters indicated difference (P < 0.05) at day 16 after challenge, but the same letter indicated not.
Figure 3.
The thymus index of chickens in all groups on days 5, 7, 9, 12, and 16 after IBDV challenge. Each point and vertical bar represents the mean and standard error (n = 10). Different capital letters indicated significant difference (P < 0.01) and different small letters indicated difference (P < 0.05) at day 16 after challenge, but the same letter indicated not.
Figure 2.
The spleen index of chickens in all groups on days 5, 7, 9, 12, and 16 after IBDV challenge. Each point and vertical bar represents the mean and standard error (n = 10). Different capital letters indicated significant difference (P < 0.01) and different small letters indicated difference (p < 0.05) at day 16 after challenge, but the same letter indicated not.
The PCI of bursa and muscle were marked respectively according to the degrees of hemorrhage on days 5, 7, 9, 12, and 16 after IBDV challenge. The TPCI of different days and IPCI mean values were obtained and all the groups were arranged according to the descending order of IPCI mean values (Table III). Data showed that the TPCI of bursa and muscle in each group rose slightly at the beginning then began to slide on day 7 in general except the negative control group. Of course, there are some exceptions. The TPCI and IPCI mean values in positive control group were largest. And there were significant difference (P < 0.01) in IPCI mean values between positive control group and other groups. Between the HPE low-dose group and the egg-yolk antibody control group, IPCI mean values were no significant difference (P < 0.01) but difference (P < 0.05). The IPCI mean values in the egg-yolk antibody control group were significant higher (P < 0.01) than those in the HPE middle-dose group and the HPE high-dose group.
Table III.
Data of TPCI and IPCI
| TPCI of bursae
|
TPCI of muscles
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | n | Day 5 | Day 7 | Day 9 | Day 12 | Day 16 | Day 5 | Day 7 | Day 9 | Day 12 | Day 16 | IPCI mean valuesa |
| Positive control group | 10 | 26.2 | 30.0 | 30.0 | 28.0 | 29.0 | 45 | 48 | 46 | 47 | 45 | 74.64Aa |
| HPE low-dose group | 10 | 25.0 | 28.0 | 26.2 | 24.4 | 22.0 | 38 | 37 | 36 | 39 | 35 | 62.12Bb |
| Egg-yolk antibody control group | 10 | 21.2 | 25.0 | 24.0 | 23.6 | 20.4 | 39 | 36 | 38 | 36 | 33 | 59.24Bc |
| HPE middle-dose group | 10 | 15.6 | 15.4 | 17.4 | 17.0 | 16.6 | 35 | 33 | 36 | 36 | 33 | 51.00Cd |
| HPE high-dose group | 10 | 15.4 | 18.0 | 18.2 | 15.4 | 16.0 | 32 | 30 | 34 | 37 | 32 | 49.60Cd |
| Negative control group | 10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0.00De |
IPCI mean values were marked with different capital letters were significantly different (P < 0.01); different small letters indicated difference (P < 0.05), but the same letter did not.
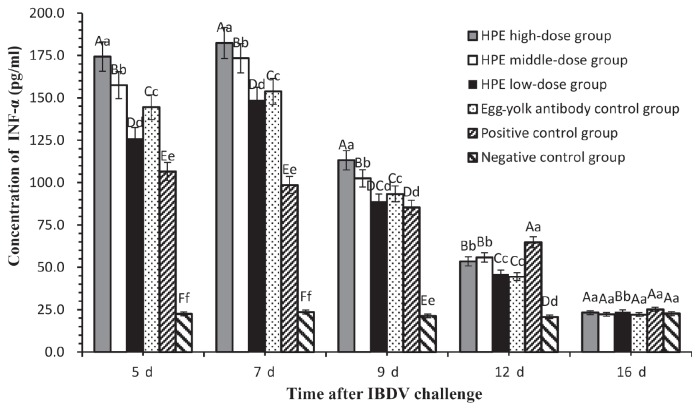
Results of IFN-α analysis
On days 5, 7, 9, 12, and 16 after IBDV challenge, sera of 10 chickens in each group were collected and IFN-α were determined with an ELISA. On day 7, the IFN-α in 3 HPE dosage groups and the egg-yolk antibody control group were the highest compared with that of the other days. The IFN-α in the positive control group, however, decreased over time and was significantly lower (P < 0.01) than that in the other groups on days 5 and 7, except the negative control group which had no significant change in IFN-α throughout the experiment. On day 9, IFN-α in the positive control group was not significantly lower (P < 0.01) than that in HPE low-dose group, and on day 12 it was significantly higher than that in other groups. The IFN-α in the HPE high-dose group were significant higher (P < 0.01) than that in the other groups on days 5, 7, and 9. On day 16 there was no significant difference among all groups (Figure 4).
Figure 4.
Concentration of IFN-α at days 5, 7, 9, 12, and 16 after IBDV challenge. Values are expressed as means and 5% error bars. Different capital letters indicated significant difference (P < 0.01) and different small letters indicated difference (P < 0.05), but the same letter indicated not.
Discussion
Hypericin is considered the most active moiety in HPE and exhibits many pharmacological effects, especially in antiretroviral activity (11). In this experiment, although the content of hypericin in HPE was no more than 0.3%, it improved the anti-IBDV effect and immuno-enhancement. It is still unknown if those effects are achieved by the hypericin only or by synergistic action of hypericin with other compounds in HPE.
Throughout the experiment, no chicken died in all groups after infection with BC-6/85 IBDV, which indicated that the virus was unable to result in chickens’ death. But BC-6/85 IBDV caused damage or hemorrhage to bursae, muscles of thoraxes and legs, spleens, and thymuses. Therefore CPI and the detection of virus were deployed to assess the therapeutic or preventive effect produced by drugs or vaccines against BC-6/85 IBDV.
Sensitivity and accuracy are the main benefits of RT-PCR over conventional methods (12). There remain a number of problems associated with its use, however, especially the inherent variability of RNA (13). Based on the conserved motifs of IBDV reported by Mundt and Muller (14), a valid pair of primers was designed and ideal PCR products were obtained. The results of viral detection showed that inhibitions of IBDV in chickens with higher dosage of HPE and egg-yolk antibody were provided to be effective.
Bursae, spleens, and thymuses are damaged to different extents when IBDV infects chickens; bursae and thymuses shrink and spleens swell. This was especially noticeable in the bursae in which B lymphocytes are destroyed and the bursae shrank with virus proliferation (15,16). So it is necessary to assess the immune organs damage made by IBDV with those organs indices (17). The data on bursa and thymus indices showed that the damage of bursae and thymuses of the positive group was much greater than that in the HPE dose groups and egg-yolk antibody control group on day 16 after virus challenge. The bursae and thymuses in the HPE dose groups and egg-yolk antibody control group were damaged to different extents by IBDV according to the results. The bursa and thymus indices in those groups were significantly lower (P < 0.01) on day 16 after virus challenge than those in the negative group. Although the bursa and thymus indices in the negative group slightly decreased, it was normal for the rate of body weight gain which was higher than that of bursa or thymus over time. Spleen, served as one of peripheral immune organs, will be swollen with the activation and proliferation of T- and B-cells induced strongly by the infection with virus. According to the spleen index in the positive and negative control groups, HPE and egg-yolk antibodies could protect the spleen to a certain extent, but not completely, from damage by IBDV.
The results of PCI showed that, except for the negative control group, the TPCI of bursa and muscle in each group rose slightly at the beginning then began to slide on day 7, but the downward trend of the positive control group was less obvious than that in the HPE treatment groups and egg-yolk antibody control group. A possible explanation for this was that HPE and egg-yolk antibody had suppressed IBDV and those tissues damaged by the virus began to recover. In addition, there was a linear relationship between the dosage of HPE and the TPCI of the bursa and muscle. The results showed no significant difference between IPCI mean values in the HPE middle-dose group compared with the HPE high-dose group, but there was a significant difference between the HPE middle-dose group and the egg-yolk antibody control group, which suggests that the HPE middle dosage [667.9 mg/kg · body weight (BW) per day] would be a candidate to treat diseases or repair the tissues damaged by IBDV.
Interferon is secreted by immune cells when stimulated by virus or other inducer and serves as an antivirus, enhancing immune response (18,19). Determination of INF-α showed that the INF-α in the positive control group was significantly higher than that in negative control group on days 5, 7, 9, 12, and 16 after virus challenge, which indicated that the immune cells were stimulated by IBDV and had secreted a large amount of INF-α. However, the INF-α was significantly lower (P < 0.01) than the HPE dose groups and the egg-yolk antibody control group on days 5 and 7, which indicated that at 24 h after the last administration, INF-α was induced not only by IBDV but by HPE or egg-yolk antibody. Furthermore, the ability of the HPE middle-dose to induce INF-α was significantly higher (P < 0.01) than that of the egg-yolk antibody. On day 9 INF-α in HPE dose groups and egg-yolk antibody control group declined sharply, but in the positive control group it declined slightly. On day 12 INF-α in positive control group was significantly higher (P < 0.01) than that in other groups. The sharp decline of INF-α in HPE dose groups was likely associated with HPE concentration and the amount of IBDV in chickens.
The results of this study demonstrate that HPE has a certain therapeutic efficacy on IBD infected artificially with the virus and immuno-enhancement, especially at the dosage of 1330 or 667.9 mg/kg BW per day.
Acknowledgments
This work was supported by National Science & Technology Pillar Program during the Eleventh Five-year Plan Period: The development and application of safety and environmental-protection veterinary drugs (2006BAD31B05). The authors thank the College of Veterinary Medicine, China Agricultural University which offered isolated pens and other experimental facilities.
References
- 1.Müller H, Schnitzler D, Bernstein F, Becht H, Cornelissen D, Lütticken DH. Infectious bursal disease of poultry: Antigenic structure of the virus and control. Vet Microbiol. 1992;33:175–183. doi: 10.1016/0378-1135(92)90045-u. [DOI] [PubMed] [Google Scholar]
- 2.Reddy SK, Silim A. Comparison of neutralizing antigens of recent isolates of infectious bursal disease virus. Arch Virol. 1991;117:287–2101. doi: 10.1007/BF01310772. [DOI] [PubMed] [Google Scholar]
- 3.Jackwood DJ, Saif YM, Hughes JH. Nucleic acid and structural proteins of infectious bursal disease virus isolates belonging to serotype I and II. Avian Dis. 1984;28:990–1016. [PubMed] [Google Scholar]
- 4.Rosenberger JK, Cloud SS. Isolation and characterization of variant infectious bursal disease viruses. Proc 123rd Ann Meet Amer Vet Med Assoc Abst; 1986. p. 81. [Google Scholar]
- 5.Jackwood DH, Saif YM. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987;31:766–770. [PubMed] [Google Scholar]
- 6.Lavagna SM, Secci D, Chimenti P, Bonsignore L, Ottaviani A, Bizzarri B. Efficacy of Hypericum and Calendula oils in the epithelial reconstruction of surgical wounds in childbirth with caesarean section. Farmaco. 2001;56:451–453. doi: 10.1016/s0014-827x(01)01060-6. [DOI] [PubMed] [Google Scholar]
- 7.Di Matteo V, Di Giovanni G. Effect of acute administration of hypericum perforatum CO2 extract on dopamine and serotonin release in the rat central nervous system. Pharmacopsychiatry. 2000;1:14–18. doi: 10.1055/s-2000-8449. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Colacino JM, Larson SH, Spitzer W. Virucidal activity of hypericin against enveloped and nonenveloped DNA and RNA viruses. Antiviral Res. 1990;3:313–325. doi: 10.1016/0166-3542(90)90015-y. [DOI] [PubMed] [Google Scholar]
- 9.Serkedjieva J, Manolova N, Zgorniak-Nowosielska I, Zawilińska Barbara, Grzybek Jan. Antiviral activity of the infusion (SHS-174) from flowers of Sambucus nigra L., aerial parts of Hypericum perforatum L., and roots of Saponaria officinalis L. against influenza and herpes simplex viruses. Phytotherapy Research. 1990;4:97–100. [Google Scholar]
- 10.Xiuying Pu, Jianping Liang, Ruofeng Shang, et al. Influence of Hypericum perforatum extract on piglet infected with porcine respiratory and reproductive syndrome virus. Agricultural Science in China. 2009;8:34–38. [Google Scholar]
- 11.Miskovsky P. Hypericin-A new antiviral and antitumor photosensitizer: Mechanism of action and interaction with biological macromolecules. Current Drug Targets. 2002;3:55–84. doi: 10.2174/1389450023348091. [DOI] [PubMed] [Google Scholar]
- 12.Huggett J, Dhedal K, Bustin S, Zumla A. Real-time RT-PCR normalization; Strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 13.Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–166. [PMC free article] [PubMed] [Google Scholar]
- 14.Mundt E, Muller H. Complete nucleotide sequences of 5′- and 3′-noncoding regions of both genome segments of different strains of infectious bursal disease virus. Virology. 1995;209:10–18. doi: 10.1006/viro.1995.1226. [DOI] [PubMed] [Google Scholar]
- 15.Rodenberg J, Sharma JM, Balzer S, Nordgren RM, Naqi S. Flow cytometric analysis of B-cell and T-cell subpopulation in specific pathogen-free chickens infected with infectious bursal disease virus. Avian Dis. 1994:3816–21. [PubMed] [Google Scholar]
- 16.Tankuura N, Sharma JM. Appearance of T-cells in the bursa of Fabrieius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens. Avian Dis. 1997;41:638–645. [PubMed] [Google Scholar]
- 17.Rivas AL, Fabricant J. Indications of immunodepression in chickens infected with various strain of Marek’s disease virus. Avian Dis. 1988;32:1–8. [PubMed] [Google Scholar]
- 18.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type I interferon-producing cells in human blood. Science. 1999;84:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 19.Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of novel interferon regulatory factor IRF-5, results in the induction of distinct interferon alpha genes. Biol Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]