Abstract
Double-stranded (ds) RNA interacts with host Toll-like receptor (TLR-3), leading to the induction of anti-viral host responses. The present study was designed to compare different routes of administration of a synthetic dsRNA (polyI:C) for induction of innate responses in chicken spleen and lungs. Chickens were treated with polyI:C via the aerosol, intra-air sac (i.a.s.), and intramuscular (IM) routes. Spleen and lungs were collected at 0, 2, 6, 12, and 24 h post-treatment and the expression of innate defence genes was quantified by real-time reverse transcriptase polymerase chain reaction (RT-PCR). There was an up-regulation of interferon (IFN)-β, TLR-3, and Toll/interleukin 1 receptor domain-containing adaptor protein inducing IFN-β (TRIF) at 6 h post-treatment in the spleen via IM administration of polyI:C. There was also an increase in the expression of TLR-3 and TRIF in the spleen at 2 h post-treatment via the i.a.s. route. The expression of IFN-α and TRIF was upregulated at 6 h post-treatment via the i.a.s. route in the lungs. Overall, our results indicate that administration of polyI:C can locally and systemically induce the expression of innate response genes depending on the route.
Résumé
L’ARN double brin (dsARN) interagit avec les récepteurs Toll-like de l’hôte (TLR-3), menant ainsi à l’induction de réponses anti-virales par l’hôte. La présente étude visait à comparer différentes voies d’administration de dsARN (polyI:C) pour l’induction d’une réponse innée dans la rate et les poumons de poulet. Des poulets ont été traités avec polyI:C par voie aérosol, intra-sac aérien (i.a.s.) et intramusculaire (IM). La rate et les poumons ont été prélevés au temps 0 ainsi que 2, 6, 12 et 24 h post-traitement et l’expression des gènes de défense innée quantifiée par réaction d’amplification en chaîne par la polymérase en temps réel utilisant la transcriptase réverse (RT-PCR). Il y avait une régulation à la hausse de l’interféron (IFN)-β, de TLR-3, et de la protéine adaptatrice contenant le domaine pour le récepteur Toll/interleukin-1 induisant l’IFN-β (TRIF) dans la rate 6 h suivant l’administration IM de polyI:C. Il y avait également une augmentation de l’expression de TLR-3 et TRIF dans la rate 2 h post-traitement via la voie i.a.s. L’expression d’IFN-α et de TRIF était régulée à la hausse dans les poumons 6 h post-traitement via la voie i.a.s. De manière générale, nos résultats indiquent que l’administration de polyI:C peut induire l’expression locale et systémique des gènes de la réponse innée en fonction de la voie d’administration.
(Traduit par Docteur Serge Messier)
It has been long known that nucleic acids play a role in induction of anti-viral responses leading to control of virus replication (1). The discovery of Toll-like receptors (TLRs) in vertebrates and the role of these receptors in the initiation of innate defence mechanisms has shed more light on the mechanisms by which synthetic double-stranded (ds) RNA molecules, such as polyinosinic-polycytidylic acid or polyI:C, generate their effects on host cells (2). The TLR repertoire of chickens is somewhat similar to that of mammals in which orthologues for mammalian TLR-1, 2, 3, 4, 5, and 7 have been identified in the chicken genome, although some of the mammalian TLRs do not seem to have an orthologue in the chicken (3,4). More recently, chicken TLR-21 has been identified, which has a function similar to that of mammalian TLR-9 in binding to unmethylated CpG DNA (5). For anti-viral responses, several TLRs, including TLR-3, TLR-7, TLR-8, and possibly TLR-9 may play a role for recognition of viruses in mammals. For example, dsRNA derived from viruses has been shown to bind to TLR-3 and activate the nuclear factor (NF)-κB pathway, which leads to the production of type I interferons (IFN), including IFN-a and IFN-b (6). In this process, TLR-3 activates Toll/IL-1R (TIR)-domain-containing adaptor protein inducing interferon-β (TRIF) that results in activation of NF-κB and interferon- regulatory factors (IRFs) (7). In turn, type I IFNs induce the expression a family of 2′5′ oligoadenylate synthetase (OAS) genes, which induce the activation of RNase L leading to inhibition of virus replication (8). It is noteworthy that in chickens, an orthologue of human OASL has been identified (8,9).
Expression of TLR-3 has been confirmed in several chicken tissues (3). However, there is little information available about the function of TLR-3 in chickens, although there is some evidence to suggest that TLR-3 is required for recognition of dsRNA and to elicit type I IFN production in chickens (10,11). In this regard, polyI:C has been used as a TLR-3 ligand to address questions related to anti-viral responses in different species, including chickens. Earlier reports indicated that chickens can respond to polyI:C, although the repertoire and underlying molecular mechanisms of these responses were not determined (12). More recently, in vitro studies showed that chicken cells including monocytes and heterophils can respond to polyI:C alone or in combination with CpG-ODN, eliciting antiviral and antibacterial responses (13,14). Furthermore, recent studies clearly showed that polyI:C induces the expression of type I IFN and TLR-3 in chicken splenocytes in vitro (11,15).
Given the anti-viral activity of polyI:C, it may be used as an adjuvant for vaccines against viral infections of chickens. However, there is paucity of information about the in vivo effects of this compound in chickens. Therefore, the main objective of the present study was to determine the effect of route of administration on induction of innate responses in systemic (spleen) and local (lung) tissues of treated chickens. We hypothesized that administration of polyI:C via different routes will lead to differential temporal and spatial induction of expression of innate response genes in chickens. To test this hypothesis, we examined the differential expression of type I IFN genes, as well as TLR-3, TRIF, and OAS in the spleen and lungs of chickens treated with polyI:C via 3 different routes.
Sixty 1-day-old chicks were randomly divided into 3 groups. Twenty birds in each group were treated with 200 μg/bird of polyI:C (P0913; Sigma Aldrich; Oakville, Ontario) via intra-muscular (IM) (injection was given in the upper third of the right pectoral muscle), intra-air sac (i.a.s.) (injection was given immediately posterior to the last rib), and aerosol routes on day 14 post-hatch. The dosage of polyI:C was chosen based on a pilot experiment (unpublished data). At 2, 6, 12, and 24 h post-treatment, equal numbers of birds from each group (5 birds per treatment at each time point) were euthanized by CO2 inhalation according to the University of Guelph Animal Care Committee guidelines. Another group of birds (n = 10) was kept in a separate room and used as a negative control. The results of our pilot experiments indicated that treatment of chickens with the polyI:C diluent (normal saline) (negative control) via the above routes did not result in a significant induction of host response (unpublished data). The spleen and lungs of each bird were kept in a solution (RNAlater solution; Invitrogen, Burlington, Ontario) immediately after euthanasia until further use. The RNA extraction and cDNA synthesis were done as described previously (16).
Five genes, IFN-α, IFN-β, TLR-3, TRIF, and OAS were selected as target genes for relative quantification. The B-actin, IFN-α, TLR-3, and TRIF primers were designed as previously described (16,17). In case of IFNβ (Accession number# X92479) and OAS (Accession number# NM_205041) primers were GCCTCCAGCTCCTTCAGAATACG (F), CTGGATCTGGTTGAGGAGGCTGT (R) and AGAACTGC AGAAGAACTTTGT (F), GCTTCAACATCTCCTTGTACC (R), respectively.
Each real-time PCR assay was carried out as previously described (16,18). The optimum thermal cycling parameters for IFN-β and OAS were: denaturation at 95°C for 10 min; 45 cycles of amplification at 95°C for 10 s, 64°C for 5 s (60°C/5 s for OAS), 72°C for 10 s; melting curve analysis at 95°C/1 s, 65°C/30 s, and 95°C/1 s; and cooling at 40°C/30 s. All real-time assays were conducted using a real-time PCR system (LightCycler 480 instrument; Roche Diagnostics GmbH, Mannheim, Germany).
The efficiency of real-time PCR and relative quantification of target genes was calculated based on the methods previously described. The efficiencies ranged from 1.8 to 2.00. The expression of target genes was calculated relative to the expression of β-actin based on the Pfaffl’s formula (19). Subsequently, relative expression of genes in polyI:C-treated chickens was compared to that of control chickens and fold changes in gene expression were calculated using computer software REST-MCS program (20). Fold changes were subjected to analysis of variance (ANOVA) using computer software (Minitab 12, Minitab Inc., State College, Pennsylvania, USA) to determine the effect of treatment with polyI:C, route of administration, and time on expression of genes. Comparisons were considered significant at P ≤ 0.05 and considered approaching significance when 0.05 < P < 0.1.
Interferon-α, IFN-β, TLR-3, TRIF, and OAS relative expression in the spleen
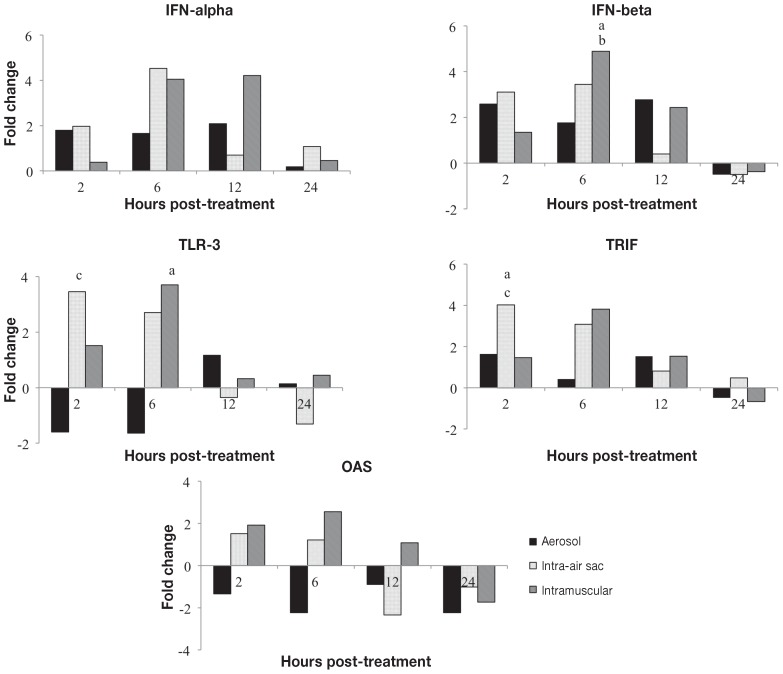
There was no significant up-regulation of IFN-α in the spleen after treatment with polyI:C. The expression of IFN-β was up-regulated in the spleen at 6 h post-treatment via the IM route, which approached significance (P = 0.08), compared with the other routes of administration at the same time point. The up-regulation of IFN-β at 6 h post-treatment in spleen through the IM route was significantly higher than the other time points (P ≤ 0.05) (Figure 1b). The expression of TLR-3 in the spleen was significantly higher at 2 h post-treatment through the i.a.s. route compared with the other 2 routes of administration and control (P ≤ 0.05) (Figure 1c). TLR-3 expression, however, was significantly higher at 6 h post-treatment via the IM route compared to other time points in the spleen (P ≤ 0.05) (Figure 1c). In the spleen, the expression of TRIF was significantly higher at 2 h post-treatment through the i.a.s. route compared with the other 2 routes of administration (P ≤ 0.05) (Figure 1d). The increase in TRIF expression at 2 h post-treatment through the i.a.s. route was also significantly higher when compared with the other time points (P ≤ 0.05) (Figure 1d). In addition, TRIF expression was higher at 6 h post-treatment via the IM route compared with the control, which approached statistical significance (P = 0.07) (Figure 1d). There was no significant effect of polyI:C treatment on induction of OAS gene expression in the spleen.
Figure 1.
Fold change of innate defence genes interferon (IFN)α, IFN-β, Toll-like receptor (TLR)-3, Toll/interleukin 1 receptor domain-containing adaptor protein inducing IFN-β (TRIF), and oligoadenylate synthetase (OAS) in spleen of chickens treated with polyI:C via the aerosol, intra-air sac, and intramuscular (IM) routes of administration. The data show the mean for the 5 biological replicates at each time point.
a,c Statistically significant (P ≤ 0.05) compared with the other time points and other treatments in the same time point, respectively.
b Statistical difference approaching significance at 0.05 < P < 0.1 compared with the other treatments at same time point.
Interferon-α, IFN-β, TLR-3, TRIF, and OAS relative expression in lungs
Treatment with polyI:C did not result in an increase in expression of TLR-3 or IFN-β in the lungs of treated chickens. The up-regulation of IFN-α expression at 6 h post-treatment in lungs by the i.a.s. route compared with the other routes of administration and control approached significance (P = 0.068) (Figure 2a). The expression of TRIF was up-regulated at 6 h post-treatment through the i.a.s. route compared with the other 2 routes of administration, which approached significance (P = 0.06) (Figure 2d). The up-regulation of TRIF at 6 h post-treatment was also significantly higher compared with the control birds (P ≤ 0.05) (Figure 2d). The increase in OAS expression at 2 h post-treatment through the aerosol route was significantly higher compared with the other time points (P ≤ 0.05) (Figure 2e). There was, however, an up-regulation in OAS expression at 2 h post-treatment through the i.a.s. route in lungs, which was significantly higher compared to 6 and 12 h post-treatment, as well as the control group (P ≤ 0.05) (Figure 2e).
Figure 2.
Fold change of innate defence genes interferon (IFN)α, IFN-β, Toll-like receptor (TLR)-3, Toll/interleukin 1 receptor domain-containing adaptor protein inducing IFN-β (TRIF), and oligoadenylate synthetase (OAS) in lungs of chickens treated with polyI:C via the aerosol, intra-air sac, and intramuscular (IM) routes of administration. The data show the mean for the 5 biological replicates at each time point.
a,c Statistically significant (P ≤ 0.05) compared with the other time points and other treatments in the same time point, respectively.
b Statistical difference approaching significance at 0.05 < P < 0.1 compared with the other treatments at same time point.
In this study, we investigated the effects of different routes of administration of polyI:C on the induction of innate defence genes in the spleen and lungs of chickens. Spleen was selected because it serves as an important systemic secondary lymphoid tissue in chickens, while the respiratory system is the primary site of infection or port of entry for several important poultry viral pathogens, including influenza virus, infectious bronchitis virus, Newcastle disease virus, and Marek’s disease virus.
Among the interferons, we found that IFN-β expression was up- regulated in spleens of chickens that received polyI:C via the IM route. This finding is consistent with the previous reports in other species. For example, intra-nasal administration of a vaccine plus polyI:C to mice was shown to induce the up-regulation of IFNs in the nasal-associated lymphoid tissue (21). In addition to interferons, we examined the expression of other genes, which may be involved in the process of polyI:C-mediated activation of the immune system. We noted an up-regulation in the expression of TLR-3 and TRIF in the spleen, which approached significance (P = 0.07), at 6 h post-treatment when polyI:C was given via the IM route. There was also an increase in TLR-3 and TRIF expression at 2 h post-treatment in the spleen in the the i.a.s. group. Karpala and colleagues (11) have highlighted the activation of TLR-3 by polyI:C administration in vitro and concluded that this activation leads to production of IFN-β in chicken cell lines. Importantly, in our experiments, the up-regulation of TLR-3 and TRIF coincided with an increase in the expression of IFN-β, which confirms and extends the report by Karpala and colleagues (11). On the other hand, the up-regulation of TRIF expression in lungs of chickens treated with polyI:C via the i.a.s. route coincided with the up-regulation of IFN-α in lungs (P = 0.068). Others have shown that dsRNA activates the TLR-3 pathway in lung epithelial cells in vitro (22). The IFN-inducible gene OAS and its up-regulation may indicate activation of the IFN pathway. Indeed, we discovered that OAS was up-regulated at 2 h post-treatment, especially in the lungs of chickens that were treated through the aerosol or i.a.s. routes. In a recent study, we have shown that polyI:C-treated chicken splenocytes express OAS at 2 and 4 h post-treatment (15).
Among the routes of administration, the aerosol route appeared to be least capable of inducing innate defence genes, especially type I IFN and TLR-3. Failure to induce the expression of these genes might be due to the size of the droplets that were used in the present study. The entrapment of polyI:C molecules in the upper respiratory tract abrogates the ability of this TLR agonist to activate its receptors in the lungs. The direction of airflow in chicken lungs that passes through air sacs, however, might have decreased the amount of available polyI:C. The IM route appeared to be better at eliciting the activation of innate defence genes in the spleen compared to aerosol and i.a.s. routes. In contrast, administration of polyI:C via the i.a.s. route was associated with a significant up-regulation of TLR-3 and TRIF genes in the spleen, while in the lungs it was associated with up-regulation of and IFN-α and TRIF genes, which only approached significance. The latter finding is, to some extent, in agreement with the report that administration of polyI:C with a vaccine containing the hemagglutinin (HA) antigen via the intranasal route up- regulated TLR-3 and IFN-α genes in nasal-associated lymphoid tissues (20). In addition, the differences observed between responses in lung and spleen could be due to composition of cells that reside in these 2 tissues. Different responses in lung and spleen also highlight the effects of routes of administration. Therefore, based on our observations, local and systemic administration of polyI:C in chickens can induce the expression of innate defence genes that have potential anti-viral activities.
Finally, based on our observations, the most critical time points in terms of the induction of innate defence genes either locally or systemically, are 2 and 6 h post-treatment. This observation agrees with the report that polyI:C induces the up-regulation of innate defence genes such as IFN-α and IFN-β at 3 and 6 h post-treatment in chicken splenocytes in vitro (15).
In conclusion, we confirmed and extended the previous findings that polyI:C is immunostimulatory for chickens and induces the expression of several innate defence genes in chicken tissues. In addition, we concluded that both the IM and i.a.s. routes of administration can induce innate defence genes in the spleen and lung of chickens. Despite the fact that administration of polyI:C via various routes in chickens warrants further experimentation, the use of TLR-3 agonist through IM or i.a.s. routes may be exploited to boost the immune response against viral infections.
Acknowledgments
This study was funded by Natural Sciences and Engineering Research Council of Canada (NSERC)-CRD Partnerships Program, and Poultry Industry Council. The authors thank the staff of the animal isolation facility of University of Guelph for their assistance and handling of the animals.
References
- 1.Rotem Z, Cox RA, Isaacs A. Inhibition of virus multiplication by foreign nucleic acid. Nature. 1963;197:564–566. doi: 10.1038/197564a0. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal M, Philbin VJ, Smith AL. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet Immunol Immunopathol. 2005;104:117–127. doi: 10.1016/j.vetimm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz A, Shen S, Adelson DL, Xavier S, Zhu JJ. Identification and sequence analysis of chicken Toll-like receptors. Immunogenetics. 2005;56:743–753. doi: 10.1007/s00251-004-0740-8. [DOI] [PubMed] [Google Scholar]
- 5.Brownlie R, Zhu J, Allan B, et al. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol Immunol. 2009;46:3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. J Interferon Cytokine Res. 2011;31:49–57. doi: 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsumi R, Hamada K, Sekiya S, et al. 2′,5′ Oligoadenylate synthetase gene in chicken: Gene structure, distribution of alleles and their expression. Biochimica Biophysica Acta. 2000;1494:263–268. doi: 10.1016/s0167-4781(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz H, Schneider K, Ohnemus A, et al. Chicken toll-like receptor 3 recognizes its cognate ligand when ectopically expressed in human cells. J Interferon Cytokine Res. 2007;27:97–101. doi: 10.1089/jir.2006.0098. [DOI] [PubMed] [Google Scholar]
- 11.Karpala AJ, Lowenthal JW, Bean AG. Activation of the TLR3 pathway regulates IFNbeta production in chickens. Dev Comp Immunol. 2008;32:435–444. doi: 10.1016/j.dci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein MS. Development of tissue viral resistance in chickens following administration of interferon inducers. J Immunol. 1972;108:1506–1516. [PubMed] [Google Scholar]
- 13.Kogut MH, Iqbal M, He H, Philbin V, Kaiser P, Smith A. Expression and function of Toll-like receptors in chicken heterophils. Dev Comp Immunol. 2005;29:791–807. doi: 10.1016/j.dci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.He H, Genovese KJ, Nisbet DJ, Kogut MH. Synergy of CpG oligodeoxynucleotide and double-stranded RNA (polyI:C) on nitric oxide induction in chicken peripheral blood monocytes. Mol Immunol. 2007;44:3234–3242. doi: 10.1016/j.molimm.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva AI, Kulkarni RR, Sharif S. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev Comp Immunol. 2011;35:28–34. doi: 10.1016/j.dci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parvizi P, Read L, Abdul-Careem MF, Lusty C, Sharif S. Cytokine gene expression in splenic CD4(+) and CD8(+) T-cell subsets of chickens infected with Marek’s disease virus. Viral Immunol. 2009;22:31–38. doi: 10.1089/vim.2008.0062. [DOI] [PubMed] [Google Scholar]
- 17.Wheaton S, Lambourne MD, Sarson AJ, Brisbin JT, Mayameei A, Sharif S. Molecular cloning and expression analysis of chicken MyD88 and TRIF genes. DNA Seq. 2007;18:480–486. doi: 10.1080/10425170701295856. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Careem MF, Haq K, Shanmuganathan S, et al. Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek’s disease virus. Virology. 2009;393:250–257. doi: 10.1016/j.virol.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichinohe T, Watanabe I, Ito S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005;79:2910–2919. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillot L, Le Goffic R, Bloch S, et al. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]