Abstract
Engineered zinc finger nucleases (ZFNs) induce DNA double-strand breaks at specific recognition sequences and can promote efficient introduction of desired insertions, deletions or substitutions at or near the cut site via homology-directed repair (HDR) with a double- and/or single-stranded donor DNA template. However, mutagenic events caused by error-prone non-homologous end-joining (NHEJ)-mediated repair are introduced with equal or higher frequency at the nuclease cleavage site. Furthermore, unintended mutations can also result from NHEJ-mediated repair of off-target nuclease cleavage sites. Here, we describe a simple and general method for converting engineered ZFNs into zinc finger nickases (ZFNickases) by inactivating the catalytic activity of one monomer in a ZFN dimer. ZFNickases show robust strand-specific nicking activity in vitro. In addition, we demonstrate that ZFNickases can stimulate HDR at their nicking site in human cells, albeit at a lower frequency than by the ZFNs from which they were derived. Finally, we find that ZFNickases appear to induce greatly reduced levels of mutagenic NHEJ at their target nicking site. ZFNickases thus provide a promising means for inducing HDR-mediated gene modifications while reducing unwanted mutagenesis caused by error-prone NHEJ.
INTRODUCTION
Zinc finger nucleases (ZFNs) are chimeras of engineered zinc finger domains fused to the non-specific nuclease domain of the restriction enzyme FokI (1). Dimers of ZFNs generate site-specific DNA double-strand breaks (DSBs) with each ZFN monomer cutting one DNA strand (2). Obligate heterodimeric versions of the FokI nuclease domain have been engineered that minimize homodimeric interactions between ZFN monomers within a pair (3–6).
ZFNs, as well as engineered homing endonucleases and transcription activator-like effector nucleases (TALENs), can be used to improve the efficiency of homology-directed repair (HDR) in a variety of different organisms and cell types (7–9). Repair of a nuclease-induced DSB mediated by an exogenous ‘donor template’ can be exploited to introduce sequence alterations or insertions at or near the site of the break. Although nuclease-induced HDR is highly efficient, repair of a DSB can also occur by the non-homologous end-joining (NHEJ) pathway. NHEJ-mediated repair of nuclease-induced DSBs has been shown to be error-prone, leading to insertion or deletion mutations (indels) at the site of the break (10) or the formation of chromosomal translocations (11). NHEJ and HDR are believed to be competing pathways (12). Thus, although HDR-mediated alterations can be efficiently introduced using engineered nucleases, alleles can also acquire NHEJ-mediated mutations [e.g. (13,14)]. Unwanted alterations at other off-target genomic sites can also be introduced by NHEJ-mediated repair; e.g. two recent reports have shown that ZFNs introduce a greater spectrum of off-target DSBs (and therefore NHEJ-mediated mutations) than previously appreciated (15,16).
Given the potential undesirable consequences of introducing DSBs in living cells, we hypothesized that it might be possible to induce DNA repair with single strand breaks (SSBs or nicks) as a less mutagenic alternative to DSBs. Thousands of SSBs naturally occur per day in human cells, generally without deleterious consequences (17). The concept of harnessing the benign nature of nicks for stimulation of homologous recombination has been previously suggested in the context of theoretical models and recombination induced by RAG proteins (18–21). In addition, homing endonucleases have been demonstrated to stimulate HDR when converted to nickases (22–27). However, conferring novel DNA binding specificities to this class of enzymes without disrupting catalytic activity has proven to be challenging because the domains for DNA recognition and cleavage are not structurally independent (8) as they are for ZFNs and TALENs.
Here, we describe a general method for creating site-specific zinc finger nickases (ZFNickases). To do this, we employed obligate heterodimeric ZFNs (4) and introduced a mutation that had previously been described to inactivate FokI cleavage activity (D450A) (28–30) into one monomer, thereby directing a break to only one strand, as recently shown with the native FokI enzyme (31). We demonstrate that ZFNickases can generate DNA single strand breaks efficiently in vitro and can also induce targeted HDR in cultured human cells with significantly lower rates of associated NHEJ-mediated mutation at the nicking site. ZFNickases provide an important additional tool for performing highly precise genome editing with reduced levels of NHEJ-mediated mutagenesis.
MATERIALS AND METHODS
Qualitative in vitro analysis of ZFN and ZFNickase activities
For in vitro protein expression experiments, we constructed vectors derived from pMLM290 and pMLM292 [Addgene plasmids 21872 and 21873, respectively (14)]. Zinc finger domains in the ZFNs HX735, VF2468 and VF2471 have been described previously (14) and were cloned as XbaI/BamHI fragments into pMLM290 and pMLM292 vectors modified to contain 3xFLAG instead of 1xFLAG epitopes. Previously reported zinc finger domains designed to bind the human CCR5 gene (32,33) were assembled from overlapping oligonucleotides generated by DNAWorks (34) and cloned into the 3xFLAG-pMLM290 and 3xFLAG-pMLM292 vectors. The nuclease inactivating D450A mutation (numbered relative to the native FokI enzyme) (28) was introduced by QuikChange Lightning Site-Directed Mutagenesis (Agilent). The protein lysates were prepared following manufacturer’s instructions for the T7 TnT Quick Coupled Transcription/Translation System (Promega) using 1 μg plasmid template per 50 μl lysate and incubating for 90 min at 30°C.
To generate target sites for in vitro analysis, annealed oligonucleotides with compatible overhangs were cloned into the BsaI restriction sites of pBAC-lacZ (using oligonucleotides OC152/OC153 for CCR5; Supplementary Table S1) as described previously (35) or the BglII/SpeI sites of pCP5 (a gift from Daniel Voytas; using oligonucleotides OC665/OC666 for VF2468, OC667/OC668 for VF2471 and OC671/OC672 for HX735; Supplementary Table S1) as described previously (36). Primers labeled with 6-carboxyfluorescein (6-FAM) were used to amplify DNA fragments for cleavage assays with the Expand High Fidelity PCR System (Roche Applied Science). The primers OC213/OC215 were used for pBAC-lacZ-derived targets and OC417/OC418 were used for pCP5-derived targets (see Supplementary Table S1 for sequences of primers).
Cleavage reactions were performed under light-protected conditions using opaque black tubes in 100 μl volumes with 10 μl protein lysate and 80 ng 6-FAM-labeled cleavage substrate in 1x NEBuffer 4 (New England Biolabs). Reactions were incubated at 37°C for 1 h, purified using a Minelute PCR Purification kit (Qiagen) according to the manufacturer’s instructions with final elution into 20 μl 0.1X buffer EB, and submitted to the DNA Core Facility at Massachusetts General Hospital (Cambridge, MA) for denaturing capillary electrophoresis with fluorescent detection. Analysis of the resulting data was performed using Peak Scanner Software v1.0 (Applied Biosystems).
Chromosomal EGFP repair assay in U2OS cells
For the generation of the reporter cell lines used in the chromosomal enhanced green fluorescent protein (EGFP) repair assay, the cleavage site for the HX735 ZFNs (14) was cloned between the lacZ open reading frame (ORF) and the 5′-truncated EGFP (∂GFP) gene in plasmid pLV.LacZ∂GFP (37). The donor plasmid used for these experiments harbors a 5′-truncated lacZ (∂lacZ) gene followed by the corrected EGFP ORF and also lacks a promoter (pUC. ∂LacZ-GFP) (38).
To generate ZFNs and ZFNickases for mammalian expression, zinc finger domains were cloned into a previously described dual expression plasmid in which the cytomegalovirus (CMV) promoter drives expression of two ZFN monomers separated by a self-cleaving T2A peptide (6). The D450A mutation was introduced into one or both of the FokI subunits in this vector as needed by subcloning or using the QuikChange Lightning Site-Directed Mutagenesis kit (Agilent).
For the chromosomal EGFP repair assay, U2OS-based reporter cell lines containing the LacZ-HX735-∂GFP target locus were generated by lentiviral transduction (LV.CMV.LacZ-HX735-∂GFP) with a viral dose that rendered <1% of cells resistant to geneticin-sulfate (0.4 mg/ml), thus preferentially generating reporter cells with a single copy target locus (37). Reporter cells, cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen), were seeded at a density of 50 000 cells/well in 24-well plates. After 24 h, the transfection was performed using X-tremeGENE HP DNA Transfection Reagent (Roche Applied Science) following the manufacturer’s instructions. Transfection cocktail included 150 ng of ZFN or ZFNickase expression plasmids, 600 ng of donor plasmid (pUC.∂LacZ-GFP) and 100 ng of a plasmid encoding for mCherry (39) to identify transfected cells. The extent of gene targeting was assessed after 8 days using flow cytometry (FACSCalibur; BD Biosciences) to determine the percentage of EGFP-positive cells in the fraction of mCherry-positive cells. Experiments were performed at least twice independently in duplicate.
Traffic Light Reporter assay in HEK293 cells
Traffic Light Reporter (TLR) experiments were performed as described by Certo et al. (22). Briefly, oligonucleotides each harboring one or two ZFN or ZFNickase target sites (oligonucleotides ams1228/ams1229 for the VF2468 and VF2471 sites and oligonucleotides ams1230/ams1231 for the CCR5 site) were cloned into the SbfI and SpeI restriction sites of the TLR2.1 plasmid. Note that the VF2468 and VF2471 targets overlap significantly, so only one TLR reporter encompassing both sites was created. Cell lines were generated by transduction of HEK293T cells with limiting amounts of a lentivirus containing a target site of interest cloned into the TLR, followed by selection in 1 μg/ml puromycin. The puromycin-resistant population was then bulk sorted by fluorescence-activated cell sorting (FACS) to isolate a polyclonal population of EGFP-negative, mCherry-negative cells. These cells were cultured in glutamine-free Dulbecco’s modified Eagle’s medium supplemented with 2 mM L-glutamine, 10% FBS and 1% penicillin/streptomycin (Invitrogen). For transfections, 1 × 105 reporter cells were plated per 24-well plate and transfected 24 h later with 0.5 μg of ZFN- or ZFNickase-encoding plasmids and 0.5 μg Donor-T2A-BFP plasmid (Addgene plasmid 31485) using Fugene6 reagent according to the manufacturer’s protocol (Roche Applied Science). Cells were split into a six-well plate 24 h post-transfection, and analyzed using a flow cytometer (LSRII or FACSAria; BD Biosciences) 72 h post-transfection. Transfection efficiency was controlled for by gating on 103 to 104 BFP-positive cells prior to HDR and NHEJ analysis. Experiments were performed independently three times.
RESULTS
In vitro enzymatic activities of ZFNs and ZFNickases
Based on recent work demonstrating that mutational inactivation of one monomer in a FokI dimer can convert this nuclease into a nickase (31), we reasoned that a similar strategy might be used to convert a ZFN dimer into a nickase. To test this possibility, we used four previously described ZFN pairs targeted to sites in three endogenous human genes; one to the HOXB13 gene (HX735), two to the VEGF-A gene (VF2468 and VF2471) and one to the CCR5 gene (CCR5) (14,32,33). For each ZFN pair, we arbitrarily designated one of the monomers as the ‘Left monomer’ and the other as the ‘Right monomer’ (Supplementary Table S2) and generated variants of each monomer harboring a previously described mutation (D450A) that inactivates the catalytic activity of the FokI nuclease domain (28).
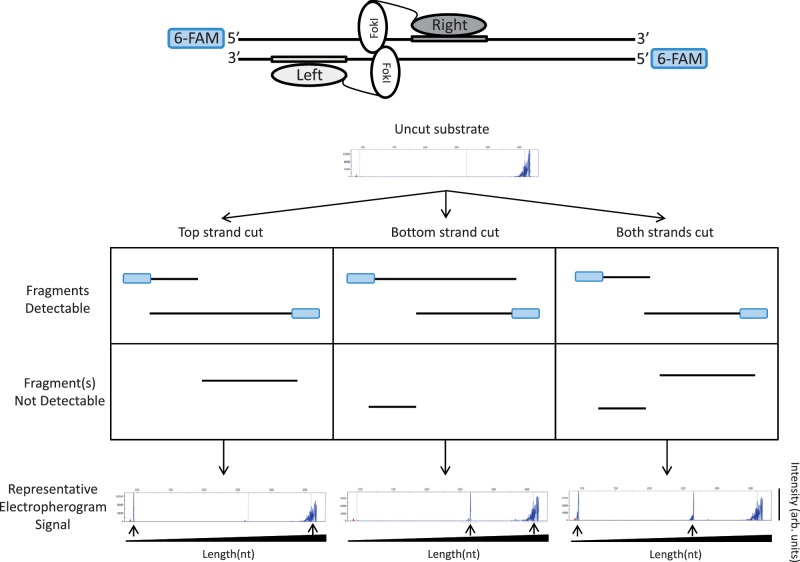
To test whether inactivation of one monomer in a ZFN pair might result in generation of a zinc finger nickase (ZFNickase), we developed a qualitative version of an in vitro assay similar to one recently described by Sanders et al. (31) that allowed us to assess the introduction of breaks into either strand of a double-stranded DNA fragment. In this system, a target site for a ZFN is positioned asymmetrically within a DNA fragment that is fluorescently labeled on the 5′-ends of both DNA strands (Figure 1). This DNA is then incubated with different combinations of active and inactive Left/Right monomers that have been co-expressed in vitro using a coupled transcription/translation system (40). Following this incubation, fluorescently labeled DNA strands of various sizes are generated depending upon whether the top or bottom strands are cut or not cut (Figure 1). These fluorescently labeled products can be analyzed under denaturing conditions using capillary electrophoresis, which separates DNA molecules based on size and enables visualization of 6-FAM-labeled DNA strands.
Figure 1.
A qualitative in vitro assay to detect cleavage and nicking by ZFNs and ZFNickases. An asymmetrically positioned full ZFN target site is placed within a DNA fragment that has been labeled on both its 5′-ends with 6-FAM fluorescent dye (depicted in blue). Only 6-FAM labeled strands will be detected in the denaturing capillary electrophoresis assay. In the example shown, the ZFN target site is positioned toward the left end of the DNA fragment. In this configuration, if nicking of the top strand occurs, this results in the generation of one short and one full-length 6-FAM labeled product. If nicking of the bottom strand occurs, this results in the generation of one medium-length and one full-length 6-FAM labeled product. Cleavage of both strands results in the generation of short and medium-length products. Sample electropherograms are shown with arbitrary intensity units on the y-axis and DNA strand length on the x-axis. DNA strands expected from nicking or cleavage reactions are designated by black arrows. Note that full-length DNA strands due to incomplete enzyme reactions may be present in addition to the expected products.
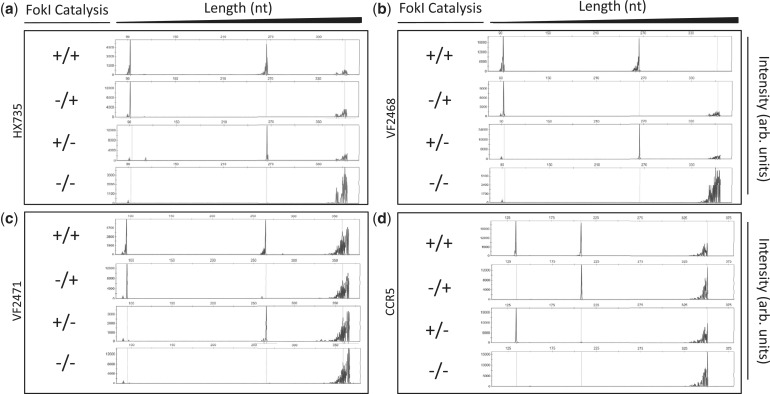
Using this qualitative in vitro assay, we tested the effects of various combinations of active/inactive monomers for the HX735, VF2468, VF2471 and CCR5 ZFNs on their target DNA sites. As expected, with all four active Left/active Right pairs, both strands in the target sites were cleaved and with all inactive Left/inactive Right pairs, no cleavage of either strand was observed (Figure 2a–d, top and bottom panels). However, when pairs of active/inactive monomers were tested, we observed preferential cleavage of only one DNA strand, the strand with which the active ZFN monomer is expected to make its primary DNA base contacts (Figures 2a–d, middle panels). Analysis of the electropherograms in Figure 2 reveals that the cleavage positions for all of our ZFNickases are either identical or within 1 nt of the cleavage positions of their matched parental ZFNs (data not shown). These results demonstrate that introduction of an inactivating FokI mutation into one monomer in a ZFN obligate heterodimer pair provides a general method for converting ZFNs into ZFNickases and that nicking activity can be preferentially directed to one particular strand of the DNA.
Figure 2.
Site-specific nicking of DNA in vitro by ZFNickases. Substrates labeled with 6-FAM fluorescent dye harboring (a) HX735, (b) VF2468, (c) VF2471 or (d) CCR5 binding sites were incubated with active Left/active Right (+/+), inactive Left/active Right (−/+), active Left/inactive Right (+/−) and inactive Left/inactive Right (−/−) ZFN monomers. Cleavage products were subjected to denaturing capillary electrophoresis. Axes are arbitrary intensity units (y-axis) and DNA strand length (x-axis). The y-axis is differentially scaled for each plot, whereas the x-axis is scaled uniformly for all plots. Representative electropherograms are shown, but all experiments were performed in triplicate (data not shown). Note that the HX735, VF2468 and VF2471 targets were cloned into the pCP5 vector that results in asymmetric placement left of center within the substrate similar to the configuration depicted in Figure 1. However, the CCR5 target is cloned into pBAC-lacZ, which results in binding site placement right of center relative to the substrate; when the top strand is cleaved in this configuration, the fragment generated is longer than when the bottom strand is cleaved.
Testing ZFNickase activities using a human cell-based chromosomal EGFP reporter assay
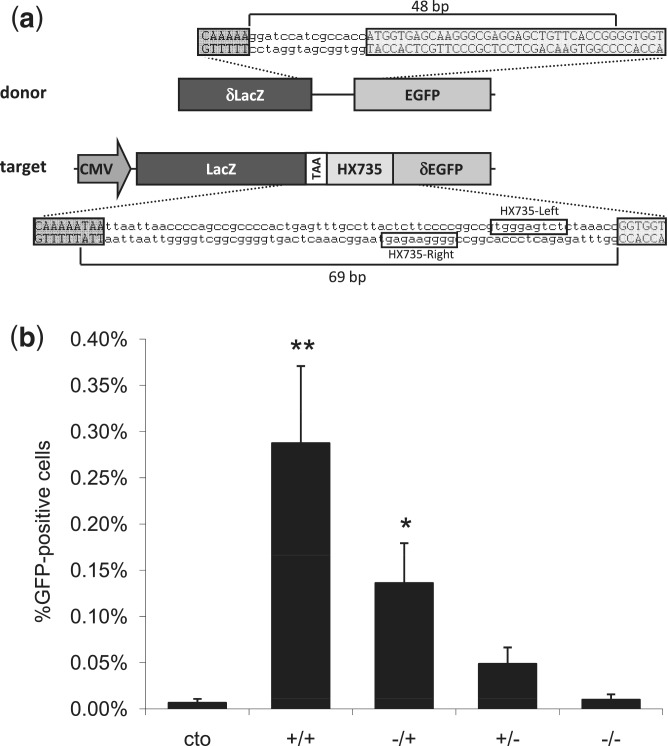
Having established the activities of ZFNickases in vitro, we next wished to test whether these nickases, like their nuclease counterparts, could induce HDR at their target sites in human cells. To do this, we used a previously described, human cell-based reporter gene assay for monitoring HDR (37). In this assay, a 5′ truncated EGFP reporter gene bearing a ZFN target site of interest is chromosomally integrated using a viral-based vector (Figure 3a). HDR with an appropriate donor construct leads to restoration of an intact EGFP reporter gene. Thus, the percentages of EGFP-positive cells arising after the co-delivery of ZFNs or ZFNickases and the donor plasmid reflect the abilities of ZFNs to stimulate HDR at the target locus.
Figure 3.
Assessment of ZFNickase-mediated HDR using a human cell-based chromosomal EGFP reporter assay. (a) A schematic of the U2OS.LacZ-HX735-∂GFP reporter construct integrated in a U2OS cell line. Note that the orientation of the binding site in the reporter is inverted relative to the configuration at the HX735 endogenous locus, for which the Left and Right designations were originally (but arbitrarily) assigned. (b) ZFN and ZFNickase-mediated HDR in a U2OS EGFP reporter line. Cells were co-transfected with the donor plasmid and plasmids encoding HX735 ZFN pairs composed of active Left/active Right (+/+), inactive Left/active Right (−/+), active Left/inactive Right (+/−) and inactive Left/inactive Right (−/−) FokI domains. The graph shows the percentage of EGFP-positive cells 8 days following transfection. Statistically significant differences in HDR-based gene correction relative to donor-only control (cto) are indicated by * (P < 0.05) or ** (P < 0.01).
We tested ZFNs and corresponding ZFNickases targeted to the HX735 site for their abilities to induce HDR in the EGFP reporter assay. ZFNs targeted to the HX735 locus were able to stimulate gene repair, inducing EGFP expression in 0.29% of transfected cells, a statistically significant increase relative to the 0.01% of cells that expressed EGFP upon transfection with a catalytically inactive ZFN (Figure 3b). Interestingly, expression of corresponding HX735 ZFNickases designed to nick one strand or the other restored EGFP expression in 0.14 and 0.05% of cells, although only the former increase in HDR was statistically significant relative to the level observed with the catalytically inactive ZFN. Thus, these results suggest that a ZFNickase can induce HDR in this EGFP reporter gene assay, albeit at a lower level than that observed with its parental ZFN.
Assessment of HDR and NHEJ induced by ZFNs and ZFNickases in human cells
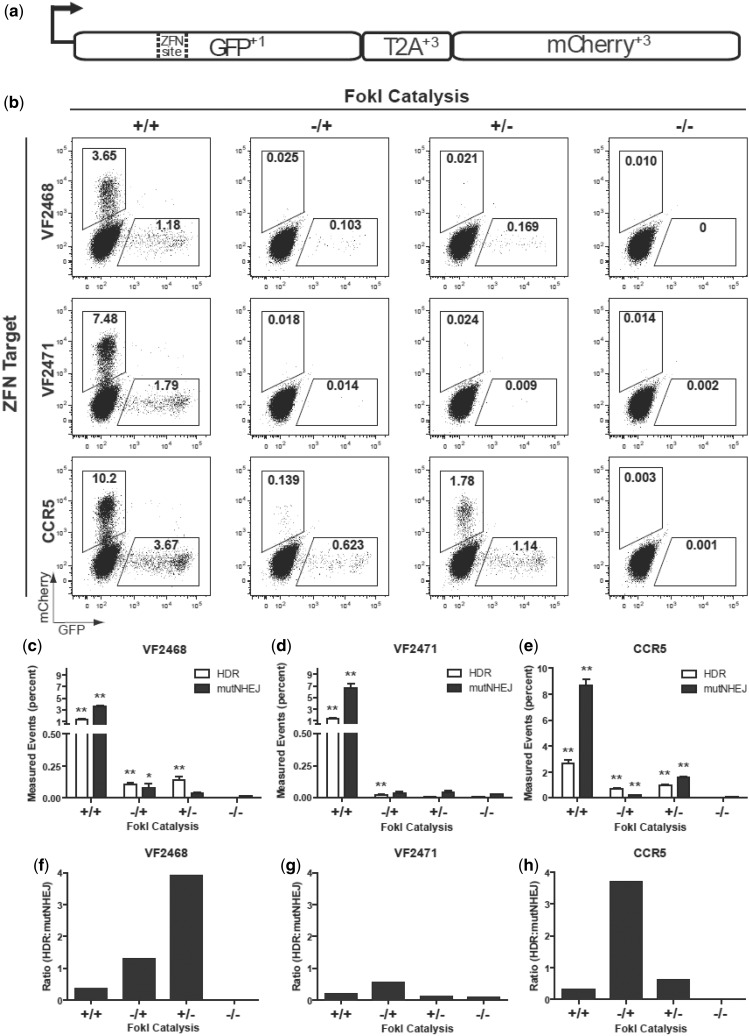
To further test the ability of ZFNickases to induce HDR and to simultaneously assess the rate of NHEJ-mediated mutagenesis at the same target site, we used the recently described ‘TLR’ (22). In this assay, the reporter harbors a nuclease target site (or sites) of interest positioned within a defective EGFP coding sequence that is followed by an mCherry coding sequence joined out of frame to the EGFP gene via a T2A peptide sequence. HDR with an exogenously provided donor template reconstitutes a functional EGFP coding sequence, turning cells green, whereas NHEJ-induced indels can create frameshifts that place the downstream mCherry protein in-frame, turning cells red (Figure 4a). Thus, with these reporter cells, the extent of nuclease-induced HDR and NHEJ can be monitored simultaneously for a given target site using flow cytometry.
Figure 4.
Assessment of ZFNickase-mediated HDR and NHEJ using a human cell-based TLR assay. (a) Schematic of the ‘TLR’ HDR-mediated correction of the EGFP gene with a co-transfected donor template results in EGFP-positive cells. Mutagenic NHEJ events at the nuclease target site result in mCherry-positive cells. (b) Representative flow cytometry plots showing percentages of EGFP-positive and mCherry-positive cells following transfection of TLR cell lines with plasmid encoding the indicated ZFNs and ZFNickases and the donor template. In the experiments shown, cells have been gated for BFP expression (encoded by the plasmid harboring the donor template) to normalize for transfection efficiencies. (c–e) Bar graphs showing mean percentages of EGFP-positive and mCherry-positive cells for experiments performed with the VF2468, VF2471 and CCR5 ZFNs and ZFNickases. Results were derived from three independent experiments with SEM shown. Statistically significant differences in HDR and mutagenic NHEJ rates relative to donor-only control (−/−) are indicated by * (P < 0.05) or ** (P < 0.01). (f–h) Ratios of percentage of EGFP-positive cells to percentage of mCherry-positive cells for the VF2468, VF2471 and CCR5 ZFNs and ZFNickases using the data from (c–e). Data used to create Figure 4c–h is available in Supplementary Table S3.
We derived polyclonal HEK293T cell lines harboring the TLR with targets for either the VF2468 and VF2471 ZFNs [a single cell line with both overlapping targets present, just as they occur in the endogenous locus (14)] or the CCR5 ZFNs. For each ZFN pair, we transfected combinations of plasmids encoding active and/or inactive ZFN monomers together with a donor template for correcting the EGFP gene. Flow cytometry was then used to determine HDR and NHEJ rates by quantifying the percentages of EGFP-positive and mCherry-positive cells, respectively (Figure 4b–e). For all three target sites, ZFNs tested showed robust activities, inducing high percentages of EGFP-positive cells (indicative of HDR events) and even higher percentages of mCherry-positive cells (indicative of NHEJ events) in transfected cells (Figure 4b, left column). All but one of the six ZFNickases tested for the three target sites induced significantly higher levels of EGFP-positive cells compared with negative controls (Figure 4b, c, d and e). The percentage of EGFP-positive cells for the VF2468 and CCR5 ZFNickases are 3- to 10-fold lower than what was observed with their corresponding ZFNs (Figure 4c and e, compare second and third white-colored columns with the first white-colored column), suggesting that HDR is induced by ZFNickases but again at a lower rate than is observed with the parental ZFNs. The activities of the VF2471 ZFNickases were detectable but quite low despite the high activity of the VF2471 ZFNs (Figure 4d). However, for all three target sites, the ZFNickases consistently induce lower percentages of mCherry-positive cells relative to their matched ZFNs, suggesting that fewer mutagenic NHEJ-mediated events are occurring with the nickases compared with the nucleases (Figure 4c, d and e). In addition, the ratio of the percentage of EGFP-positive cells to the percentage of mCherry-positive cells is higher for five of the six ZFNickases compared with the parental ZFN (Figure 4f, g and h). We also found, in accordance with previous studies conducted with homing endonucleases and nickases, that increased donor template concentrations were associated with increased nuclease- and nickase-induced HDR frequencies (Supplementary Figure S1 and data not shown) (22,23,27). Interestingly, the effect of donor template concentration appears to be more dramatic on nickase-induced HDR than on nuclease-induced HDR (Supplementary Figure S1 and data not shown). These results suggest that ZFNickases induce higher levels of HDR events relative to NHEJ events compared with ZFNs. Our results indicate that ZFNickases may offer the benefit of significantly reduced NHEJ rates albeit with a reduction in HDR activity in cells.
DISCUSSION
In this report, we describe a general and simple method for converting ZFNs into ZFNickases. Introduction of a previously described D450A mutation into one monomer of a ZFN pair can generate a ZFNickase. This result parallels recent work from Halford and colleagues in which they used a similar approach to convert the wild-type FokI restriction enzyme into a nickase. Our qualitative in vitro data demonstrate that each ZFNickase preferentially cuts one DNA strand at a position either identical or within 1 nt of the cut positions of its matched ZFN. Furthermore, the data show that each ZFN monomer cuts the DNA strand to which it makes most of its DNA base contacts, providing direct experimental support for the model of binding and cleavage illustrated in Figure 1. Testing in two different human cell-based reporter systems revealed that ZFNickases can induce HDR-mediated repair, albeit at lower levels than matched ZFNs from which they were derived. Of the eight ZFNickases we tested (two pairs each derived from ZFNs targeted to four different target sites; data presented in Figures 3 and 4), six induced statistically significant levels of HDR. The levels of HDR we observed with the ZFNickases ranged widely, from between 2-fold and >100-fold lower than those observed with the corresponding ZFNs from which they were derived. However, for at least some of the ZFNickases we tested (e.g. HX735 −/+, VF2468 −/+ and +/− and CCR5 −/+ and +/−), the levels of HDR induced were of sufficiently high frequency (≥0.1%) to be useful for research applications and some potential therapeutic strategies. Our observations that ZFNickases can induce HDR events and that HDR efficiency is positively correlated with the concentration of donor present in cells are consistent with the findings of others using homing endonucleases engineered to induce nicks (22–27). However, to our knowledge, our findings are the first to report that nickases derived from ZFNs can be used to induce HDR events.
Although absolute rates of HDR were lower for ZFNickases than ZFNs in our human cell-based reporter assays, we also observed a consistent reduction in mutagenic NHEJ rates in the TLR assay. This reduction is not entirely surprising given that nicks are typically repaired without causing mutations (17). However, we do not know the origin of the residual NHEJ-mediated events we observed with some of the ZFNickases we tested. Possible explanations include conversion of a nick into a DSB that may occur with replication fork collapse (see below) or weak residual homodimerization of the active ZFNickase monomer that may lead to cleavage at the intended target site. Use of improved second-generation FokI heterodimer variants (3) may reduce activity due to the latter mechanism [we used first-generation FokI heterodimer variants (4) for this study].
Importantly, for five of the six ZFNickases we tested in the TLR assay, the ratio of HDR to NHEJ events was increased compared with the three matched ZFNs from which they were derived. These results demonstrate that ZFNickases can induce HDR events with relatively lower rates of NHEJ-mediated mutations created at the nick site. We do not currently know the mechanism of the ZFNickase-mediated HDR or the improved HDR:NHEJ ratios we observe. One possibility for the improved HDR:NHEJ ratios is that a nick in the path of a DNA replication fork may be converted to a DSB leading to fork collapse, the repair of which would be expected to lead to repair by either NHEJ or HDR. A potential hypothesis for why we observe a preferential shift from NHEJ to HDR with ZFNickases may be the more frequent repair of nick-induced replication fork collapse by HDR (41), in part due to the availability of repair factors for homologous recombination during DNA replication in S-phase (42). Interestingly, for every target site we tested in our human cell-based assays, one ZFNickase combination consistently outperformed the other with respect to absolute HDR rates and, for those assayed using the TLR assay, improved HDR:NHEJ ratios. This reproducible difference does not appear to be correlated with whether the nicked strand is transcribed or not, and there were no strand cleavage preferences discernible from the in vitro data. It is possible that strand-dependent differences in HDR activity arise due to different DNA-binding affinities of zinc finger domains in each monomer and how this may affect asymmetric accessibility to the break by cellular repair machinery. Regardless of the precise mechanism, our results suggest that testing both potential ZFNickases for a given target site is worthwhile to identify the most active nickase possible.
Our work demonstrates that ZFNickases with predictable strand nicking activities can be easily derived from ZFNs and that these enzymes can be used in cells to induce HDR with improved HDR:NHEJ ratios. It will be of interest in future experiments to test whether ZFNickase-induced HDR rates can be further increased by using improved FokI heterodimer frameworks and hyperactive FokI variants (3,43). Our observation of reduced mutagenic NHEJ events at the target nicking site suggest that ZFNickases will also likely induce fewer mutations at potential off-target sites elsewhere in the genome, a prediction that can easily be tested for ZFNs with known off-target sites (15,16). In addition, site-specific nickases may generally be of interest for the study of biological phenomena such as replication fork dynamics. Our results suggest ZFNickases may provide a means to induce HDR with reduced mutagenesis caused by NHEJ and that additional optimization of this platform should be an important goal for future investigation.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1–3 and Supplementary Figure 1.
FUNDING
The National Institutes of Health (NIH) Director's Pioneer Award DP1 OD006862 (to J.K.J.); NIH R01 GM088040 (to J.K.J.), P50 HG005550 (to J.K.J.), RL1 CA133832 (to A.M.S.), UL1 DE019582 (to A.M.S.), R01 AI068885 (to A.P.M.), and T32 GM07270 (to M.T.C.); the Jim and Ann Orr Massachusetts General Hospital Research Scholar award (to J.K.J.); the European Commission (PERSIST–222878 to T.C.); National Science Foundation Graduate Research Fellowship (to C.L.R.); Ford Foundation Predoctoral Fellowship (to C.L.R.). Funding for open access charge: NIH Director’s Pioneer Award (DP1 OD006862 to J.K.J).
Conflict of interest statement. A.M.S. serves as Chief Scientific Officer for Cellectis therapeutics, for which he receives a combination of stock and salary as compensation. A.M.S. is a founder and member of the board of directors of Pregenen Inc., for which he receives stock compensation. J.K.J. is a member of the scientific advisory board of Transposagen Biopharmaceuticals, Inc.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Fabienne Lütge (Hannover Medical School) for technical assistance, Daniel Collette (Massachusetts General Hospital DNA Core) for help with capillary electrophoresis data analysis, Steve Halford and Eva Vanamee for helpful suggestions in the early stages of this project and Ralph Scully and Lee Zou for helpful discussions regarding DNA repair.
REFERENCES
- 1.Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc. Natl Acad. Sci. USA. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mani M, Smith J, Kandavelou K, Berg JM, Chandrasegaran S. Binding of two zinc finger nuclease monomers to two specific sites is required for effective double-strand DNA cleavage. Biochem. Biophys. Res. Commun. 2005;334:1191–1197. doi: 10.1016/j.bbrc.2005.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyon Y, Vo TD, Mendel MC, Greenberg SG, Wang J, Xia DF, Miller JC, Urnov FD, Gregory PD, Holmes MC. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods. 2011;8:74–79. doi: 10.1038/nmeth.1539. [DOI] [PubMed] [Google Scholar]
- 4.Miller JC, Holmes MC, Wang J, Guschin DY, Lee YL, Rupniewski I, Beausejour CM, Waite AJ, Wang NS, Kim KA, et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 5.Szczepek M, Brondani V, Buchel J, Serrano L, Segal DJ, Cathomen T. Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat. Biotechnol. 2007;25:786–793. doi: 10.1038/nbt1317. [DOI] [PubMed] [Google Scholar]
- 6.Sollu C, Pars K, Cornu TI, Thibodeau-Beganny S, Maeder ML, Joung JK, Heilbronn R, Cathomen T. Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res. 2010;38:8269–8276. doi: 10.1093/nar/gkq720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handel EM, Cathomen T. Zinc-finger nuclease based genome surgery: it's all about specificity. Curr. Gene Ther. 2011;11:28–37. doi: 10.2174/156652311794520120. [DOI] [PubMed] [Google Scholar]
- 8.Arnould S, Delenda C, Grizot S, Desseaux C, Paques F, Silva GH, Smith J. The I-CreI meganuclease and its engineered derivatives: applications from cell modification to gene therapy. Protein Eng. Des. Sel. 2011;24:27–31. doi: 10.1093/protein/gzq083. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanove AJ, Voytas DF. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333:1843–1846. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- 10.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet E, Simsek D, Tomishima M, DeKelver R, Choi VM, Gregory P, Urnov F, Weinstock DM, Jasin M. Chromosomal translocations induced at specified loci in human stem cells. Proc. Natl Acad. Sci. USA. 2009;106:10620–10625. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartlerode AJ, Scully R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem. J. 2009;423:157–168. doi: 10.1042/BJ20090942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou J, Sweeney CL, Chou BK, Choi U, Pan J, Wang H, Dowey SN, Cheng L, Malech HL. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117:5561–5572. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol. Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattanayak V, Ramirez CL, Joung JK, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat. Methods. 2011;8:765–770. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman G, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 2011;29:816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- 17.Holmquist GP. Endogenous lesions, S-phase-independent spontaneous mutations, and evolutionary strategies for base excision repair. Mutat. Res. 1998;400:59–68. doi: 10.1016/s0027-5107(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 18.Weinstock DM, Jasin M. Alternative pathways for the repair of RAG-induced DNA breaks. Mol. Cell Biol. 2006;26:131–139. doi: 10.1128/MCB.26.1.131-139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee GS, Neiditch MB, Salus SS, Roth DB. RAG proteins shepherd double-strand breaks to a specific pathway, suppressing error-prone repair, but RAG nicking initiates homologous recombination. Cell. 2004;117:171–184. doi: 10.1016/s0092-8674(04)00301-0. [DOI] [PubMed] [Google Scholar]
- 20.Holliday R. A mechanism for gene conversion in fungi. Genet. Res. 2007;89:285–307. doi: 10.1017/S0016672308009476. [DOI] [PubMed] [Google Scholar]
- 21.Meselson MS, Radding CM. A general model for genetic recombination. Proc. Natl Acad. Sci. USA. 1975;72:358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Certo MT, Ryu BY, Annis JE, Garibov M, Jarjour J, Rawlings DJ, Scharenberg AM. Tracking genome engineering outcome at individual DNA breakpoints. Nat. Methods. 2011;8:671–676. doi: 10.1038/nmeth.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger MJ, McConnell-Smith A, Stoddard BL, Miller AD. Single-strand nicks induce homologous recombination with less toxicity than double-strand breaks using an AAV vector template. Nucleic Acids Res. 2011;39:926–935. doi: 10.1093/nar/gkq826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SH, Stoddard BL, Xu SY. Natural and engineered nicking endonucleases–from cleavage mechanism to engineering of strand-specificity. Nucleic Acids Res. 2011;39:1–18. doi: 10.1093/nar/gkq742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Nierop GP, de Vries AA, Holkers M, Vrijsen KR, Goncalves MA. Stimulation of homology-directed gene targeting at an endogenous human locus by a nicking endonuclease. Nucleic Acids Res. 2009;37:5725–5736. doi: 10.1093/nar/gkp643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McConnell Smith A, Takeuchi R, Pellenz S, Davis L, Maizels N, Monnat RJ, Jr, Stoddard BL. Generation of a nicking enzyme that stimulates site-specific gene conversion from the I-AniI LAGLIDADG homing endonuclease. Proc. Natl Acad. Sci. USA. 2009;106:5099–5104. doi: 10.1073/pnas.0810588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis L, Maizels N. DNA nicks promote efficient and safe targeted gene correction. PLoS One. 2011;6:e23981. doi: 10.1371/journal.pone.0023981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I. FokI dimerization is required for DNA cleavage. Proc. Natl Acad. Sci. USA. 1998;95:10570–10575. doi: 10.1073/pnas.95.18.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waugh DS, Sauer RT. Single amino acid substitutions uncouple the DNA binding and strand scission activities of Fok I endonuclease. Proc. Natl Acad. Sci. USA. 1993;90:9596–9600. doi: 10.1073/pnas.90.20.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beumer K, Bhattacharyya G, Bibikova M, Trautman JK, Carroll D. Efficient gene targeting in Drosophila with zinc-finger nucleases. Genetics. 2006;172:2391–2403. doi: 10.1534/genetics.105.052829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders KL, Catto LE, Bellamy SR, Halford SE. Targeting individual subunits of the FokI restriction endonuclease to specific DNA strands. Nucleic Acids Res. 2009;37:2105–2115. doi: 10.1093/nar/gkp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, Ando D, Urnov FD, Galli C, Gregory PD, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 33.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008;26: 808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoover DM, Lubkowski J. DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res. 2002;30:e43. doi: 10.1093/nar/30.10.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeder ML, Thibodeau-Beganny S, Sander JD, Voytas DF, Joung JK. Oligomerized pool engineering (OPEN): an 'open-source' protocol for making customized zinc-finger arrays. Nat. Protoc. 2009;4:1471–1501. doi: 10.1038/nprot.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature. 2009;459:442–445. doi: 10.1038/nature07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alwin S, Gere MB, Guhl E, Effertz K, Barbas CF, 3rd, Segal DJ, Weitzman MD, Cathomen T. Custom zinc-finger nucleases for use in human cells. Mol. Ther. 2005;12:610–617. doi: 10.1016/j.ymthe.2005.06.094. [DOI] [PubMed] [Google Scholar]
- 38.Cornu TI, Thibodeau-Beganny S, Guhl E, Alwin S, Eichtinger M, Joung JK, Cathomen T. DNA-binding specificity is a major determinant of the activity and toxicity of zinc-finger nucleases. Mol. Ther. 2008;16:352–358. doi: 10.1038/sj.mt.6300357. [DOI] [PubMed] [Google Scholar]
- 39.Mussolino C, Morbitzer R, Lutge F, Dannemann N, Lahaye T, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–9293. doi: 10.1093/nar/gkr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cradick TJ, Keck K, Bradshaw S, Jamieson AC, McCaffrey AP. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol. Ther. 2010;18:947–954. doi: 10.1038/mt.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleh-Gohari N, Bryant HE, Schultz N, Parker KM, Cassel TN, Helleday T. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Mol. Cell Biol. 2005;25:7158–7169. doi: 10.1128/MCB.25.16.7158-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartlerode A, Odate S, Shim I, Brown J, Scully R. Cell cycle-dependent induction of homologous recombination by a tightly regulated I-SceI fusion protein. PLoS One. 2011;6: e16501. doi: 10.1371/journal.pone.0016501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo J, Gaj T, Barbas CF., 3rd Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J. Mol. Biol. 2010;400:96–107. doi: 10.1016/j.jmb.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.