Executive Summary
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-Term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective of Analysis
The objective of this analysis was to review empirical qualitative research on the experiences of patients with chronic obstructive pulmonary disease (COPD), informal caregivers (“carers”), and health care providers—from the point of diagnosis, through daily living and exacerbation episodes, to the end of life.
Clinical Need and Target Population
Qualitative empirical studies (from social sciences, clinical, and related fields) can offer important information about how patients experience their condition. This exploration of the qualitative literature offers insights into patients’ perspectives on COPD, their needs, and how interventions might affect their experiences. The experiences of caregivers are also explored.
Research Question
What do patients with COPD, their informal caregivers (“carers”), and health care providers experience over the course of COPD?
Research Methods
Literature Search
Search Strategy
Literature searches for studies published from January 1, 2000, to November 2010 were performed on November 29, 2010, using OVID MEDLINE; on November 26, 2010, using ISI Web of Science; and on November 28, 2010, using EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL). Titles and abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. One additional report, highly relevant to the synthesis, appeared in early 2011 during the drafting of this analysis and was included post hoc.
Inclusion Criteria
English-language full reports
studies published between January 1, 2000, and November 2010
primary qualitative empirical research (using any descriptive or interpretive qualitative methodology, including the qualitative component of mixed-methods studies) and secondary syntheses of primary qualitative empirical research
studies addressing any aspect of the experiences of living or dying with COPD from the perspective of persons at risk, patients, health care providers, or informal carers; studies addressing multiple conditions were included if COPD was addressed explicitly
Exclusion Criteria
studies addressing topics other than the experiences of living or dying with COPD from the perspective of persons at risk, patients, health care providers, or informal carers
studies labelled “qualitative” but not using a qualitative descriptive or interpretive methodology (e.g., case studies, experiments, or observational analysis using qualitative categorical variables)
quantitative research (i.e., using statistical hypothesis testing, using primarily quantitative data or analyses, or expressing results in quantitative or statistical terms)
studies that did not pose an empirical research objective or question, or involve the primary or secondary analysis of empirical data
Outcomes of Interest
qualitative descriptions and interpretations (narrative or theoretical) of personal and social experiences of COPD
Summary of Findings
Experiences at Diagnosis
Patients typically seek initial treatment for an acute episode rather than for chronic early symptoms of COPD.
Many patients initially misunderstand terms such as COPD, chronic obstructive pulmonary disease, or exacerbation.
Patients may not realize that COPD is incurable and fatal; some physicians themselves do not consider early COPD to be a fatal disease.
Smokers may not readily understand or agree with the idea that smoking caused or worsens their COPD. Those who believe there is a causal link may feel regret or shame.
Experiences of Living Day to Day
COPD patients experience alternating good days and bad days. A roller-coaster pattern of ups and downs becomes apparent, and COPD becomes a way of life.
Patients use many means (social, psychological, medical, organizational) to control what they can, and to cope with what they cannot. Economic hardship, comorbidities, language barriers, and low health literacy can make coping more difficult.
Increasing vulnerability and unpredictable setbacks make patients dependent on others for practical assistance, but functional limitations, institutional living or self-consciousness can isolate patients from the people they need.
For smokers, medical advice to quit can conflict with increased desire to smoke as a coping strategy.
Many of the factors that isolate COPD patients from social contact also isolate them from health care.
Experiences of Exacerbations
Patients may not always attribute repeated exacerbations to advancing disease, instead seeing them as temporary setbacks caused by activities, environmental factors, faltering self-management, or infection.
Lack of confidence in community-based services leads some patients to seek hospital admission, but patients also feel vulnerable when hospitalized. They may feel dependent on others for care or traumatized by hospital care routines.
Upon hospital discharge following an exacerbation, patients may face new levels of uncertainty about their illness, prognosis, care providers, and supports.
Experiences of the End of Life
Patients tend to be poorly informed about the long-term prognosis of COPD and what to expect toward the end of life; this lack of understanding impairs quality of life as the disease progresses.
As the end of life approaches, COPD patients face the usual challenges of daily living, but in a context of increasing exacerbations and deepening dependency. Activities and mobility decrease, and life may become confined.
Some clinicians have difficulty identifying the beginning of “the end of life,” given the unpredictable course of COPD. Long-term physician-patient relationships, familiarity and understanding, trust, good communication skills, sensitivity, and secure discussion settings can help facilitate end-of-life discussions.
Divergent meanings and goals of palliative care in COPD lead to confusion about whether such services are the responsibility of home care, primary care, specialty care, or even critical care. Palliative end-of-life care may not be anticipated prior to referral for such care. A palliative care referral can convey the demoralizing message that providers have “given up.”
Experiences of Carers
Carers’ challenges often echo patients’ challenges, and include anxiety, uncertainty about the future, helplessness, powerlessness, depression, difficulties maintaining employment, loss of mobility and freedoms, strained relationships, and growing social isolation.
Carers feel pressured by their many roles, struggling to maintain patience when they feel overwhelmed, and often feeling guilty about not doing enough.
Carers often face their own health problems and may have difficulty sustaining employment.
Synthesis: A Disease Trajectory Reflecting Patient Experiences
The flux of needs in COPD calls for service continuity and flexibility to allow both health care providers and patients to respond to the unpredictable yet increasing demands of the disease over time.
Background
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-Term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective of Analysis
The objective of this analysis was to review empirical qualitative research on the experiences of patients with chronic obstructive pulmonary disease (COPD), informal caregivers (“carers”), and health care providers—from the point of diagnosis, through daily living and exacerbation episodes, to the end of life.
Clinical Need and Target Population
Qualitative empirical studies (from social sciences, clinical, and related fields) can offer important information about how patients experience their condition. This exploration of the qualitative literature offers insights into patients’ perspectives on COPD, their needs, and how interventions might affect their experiences.
The findings of the qualitative research are summarized as they relate to 4 broad, episodic patient experiences over the course of COPD: diagnosis and prognosis; living day to day; exacerbations; and the end of life. A fifth category addresses carer experiences.
Systematic Review
Research Question
What do patients with COPD, their informal caregivers (“carers”), and health care providers experience over the course of COPD?
Research Methods
Literature Search
Search Strategy
Literature searches for studies published from January 1, 2000, through November 2010, were performed on November 29, 2010, using OVID MEDLINE; on November 26, 2010, using ISI Web of Science; and on November 28, 2010, using EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL). Titles and abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. One additional report, highly relevant to the synthesis, appeared in early 2011 during the drafting of this analysis and was included post hoc.
Inclusion Criteria
English-language full reports
studies published from January 1, 2000 through November 2010
primary qualitative empirical research (using any descriptive or interpretive qualitative methodology, including the qualitative component of mixed-methods studies) and secondary syntheses of primary qualitative empirical research
studies addressing any aspect of the experiences of living or dying with COPD from the perspective of persons at risk, patients, health care providers, or informal carers; studies addressing multiple conditions were included if COPD was addressed explicitly
Exclusion Criteria
studies addressing topics other than the experiences of living or dying with COPD, from the perspective of persons at risk, patients, health care providers, or informal carers
studies labelled “qualitative” but not using a qualitative descriptive or interpretive methodology (e.g., case studies, experiments, or observational analysis using qualitative categorical variables)
quantitative research (i.e., using statistical hypothesis testing, using primarily quantitative data or analyses, or expressing results in quantitative or statistical terms)
studies that did not pose an empirical research objective or question, or involve the primary or secondary analysis of empirical data
Outcomes of Interest
qualitative descriptions and interpretations (narrative or theoretical) of personal and social experiences of COPD
Qualitative Analysis
Full papers were retrieved and read by 2 investigators. Papers were grouped by broad topical focus and read closely by 1 investigator to generate a narrative summarizing the main findings under each topic. A second investigator reviewed the same papers, revised the narrative (by consensus with the first reviewer), and incorporated any relevant findings from papers in other topic groups (for example, some papers on smoking experiences also addressed day-to-day living issues). In all, each primary research paper was reviewed 2 to 3 times by at least 2 investigators.
A synthesis was developed to relate the findings to the clinical trajectory of COPD, highlighting key patient, caregiver, and health care provider experiences reported at specific phases of the disease course. Drafts of the full report were presented sequentially to the Ontario Health Technology Advisory Committee, the Medical Advisory Secretariat, and the COPD Expert Panel for multidisciplinary feedback.
Results of Systematic Review
The database search yielded 24,906 citations published between January 1, 2000, and November 2010 (including some duplicates). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded from the analysis.
Figure 1: Citation Flow Chart*.
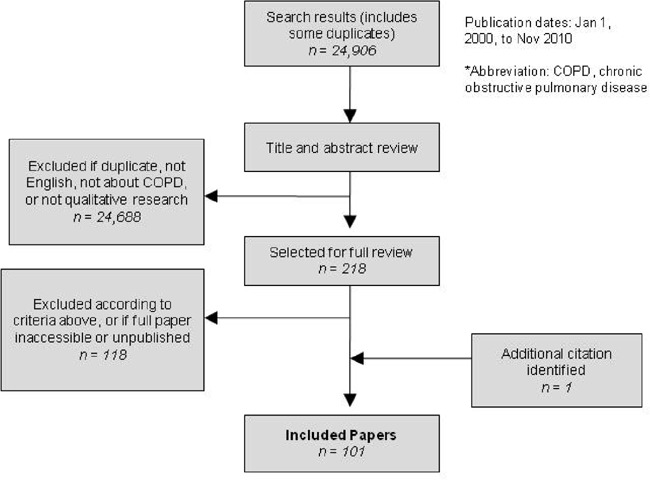
A total of 100 papers met the inclusion criteria. One additional report, highly relevant to the synthesis, appeared in early 2011 during the drafting of this analysis and was included post hoc.
Together, the papers included in the analysis represent an estimated 82 discrete studies and involve an estimated 1,404 people with COPD (“patients”), 397 health care providers in clinical or administrative professions, and 275 carers across many care and community settings. Studies from the United Kingdom and United States dominated the literature; Canadian research contributed 11 published papers from 7 studies (Table 1).
Table 1: Summary of Qualitative Studies Reviewed.
| Country | Patients, n* | Carers, n* | Health Care Providers, n* |
Studies, n* | Papers, n |
|---|---|---|---|---|---|
| United Kingdom | 450 | 90 | 158 | 30 | 36 |
| United States | 303 | 127 | 101 | 15 | 20 |
| Multinational | 148 | 0 | 0 | 2 | 2 |
| Canada | 119 | 15 | 27 | 7 | 11 |
| Australia | 109 | 28 | 16 | 5 | 8 |
| Netherlands | 102 | 0 | 20 | 4 | 5 |
| Sweden | 60 | 0 | 47 | 6 | 6 |
| Thailand | 31 | 0 | 0 | 1 | 1 |
| Taiwan | 25 | 0 | 0 | 2 | 2 |
| Norway | 21 | 0 | 8 | 2 | 2 |
| Iceland | 12 | 11 | 0 | 3 | 3 |
| Denmark | 10 | 0 | 0 | 1 | 1 |
| Hong Kong | 9 | 0 | 0 | 2 | 2 |
| Finland | 5 | 4 | 0 | 1 | 1 |
| New Zealand | 0 | 0 | 20 | 1 | 1 |
| Total | 1,404 | 275 | 397 | 82 | 101 |
Estimated.
Findings covered 4 broad categories of patient experiences over the course of COPD: diagnosis, living day-to-day, exacerbations, and the end of life. A fifth category addressed carer experiences.
Experiences at Diagnosis
Receiving a COPD Diagnosis
Before a diagnosis of COPD, many people see early symptoms and limitations as their own “normal” way of life, and too mild or ordinary to warrant medical attention. (1;2;3;4) Patients may initially attribute their symptoms and limitations to smoking or aging; (1;5) they typically seek initial treatment for an acute episode rather than for the chronic, early symptoms of COPD. (6;7;8;9)
Physicians may not communicate a diagnosis of COPD clearly; (10;11;12) diagnostic and prognostic information is often given in stages, as medical events arise. (13) Patients may learn that they have COPD only after several visits, or from sources other than their physician. (8;12;14) In addition, terminology can confuse the message at diagnosis; for example, primary care clinicians may use other diagnostic terms (e.g., emphysema) or even euphemisms (e.g., chest problems). (8;15;16) Usually, the terms chronic obstructive pulmonary disease and COPD are unfamiliar to patients. (8;14;15;17)
Understanding the Implications of COPD
Patients sometimes worry that the C in COPD stands for cancer and are relieved to learn that it does not; smokers in particular mistakenly interpret COPD as good news in this context. (16;18) Patients may be unaware that COPD is incurable and fatal; (11;16) some physicians themselves do not consider early COPD to be a fatal disease. (13) The seriousness of COPD becomes clearer as the disease progresses and as patients realize that it will be a permanent feature of their lives. (19)
Some patients express a need for information at the time of diagnosis, including prognosis and management strategies, (9;10;11;14;20;21;22) but others prefer less explicit prognostic information and may avoid education or discussions for fear of learning things that will worry them. (15;20;21;22;23) The poor long-term prognosis of COPD is best communicated within a strong physician-patient relationship, and with compassion and empathy (10;13;20).
Role of Smoking
Smokers have divergent beliefs about whether smoking caused their COPD. Many know that smoking causes lung cancer, but few realize that it also causes COPD. (18;24;25) Smokers with COPD may favour other causal explanations, such as family history, hazard or pollution exposures, age, or chance. (3;4;17;26;27) Some may choose to believe that they have not smoked often enough, or recently enough, to bring on COPD. (19;28) Some are unaware that smoking will worsen their COPD, (14;28) and some find that quitting does not improve their health. (27;28) Clinicians’ advice to quit may be seen as routine, and not directly related to a diagnosis of COPD. (9)
Smokers who do attribute their COPD to smoking often feel profound regret, guilt, or shame. (1;3;19;27). Some clinicians reinforce this self-blame and stigma, (10;26;27) while others consciously avoid blaming. (13) Some smokers with COPD may feel that they are not entitled to health care or sympathy from others, (1;19;27) and some avoid health care visits to avoid “preaching” about smoking. (3)
Experiences of Living Day to Day
Daily Life
Patients with COPD experience an ongoing cycle of good and bad days. (29) Living with daily breathlessness is a perpetual, exhausting struggle, and living becomes hard work. (6;30) The experience of dyspnea involves not only breathlessness, but also fatigue and modified or limited activities (1;5;8;10;31;32) and negative mental states, including depression. (10;22;31;33) Basic activities of daily living may be affected, including sleeping, getting out of bed, bending over, bathing, dressing, eating, performing domestic chores or occupational duties, walking, driving, using public transportation, travelling, drinking, singing, dancing, having sex, shopping, playing instruments or sports, talking, carrying heavy objects, and even sitting in a doctor’s waiting room. (4;10;19;23;25;30;33;34;35;36;37;38; 39;40;41;42;43;44) Activities are delegated, limited, modified, or stopped due to breathlessness—sometimes temporarily, sometimes permanently (5;35;44;45;46;47)—and the loss of these activities affects enjoyment of life. (33;41) Pervasive (sometimes undertreated) pain often accompanies breathlessness. (32)
Emotions and Quality of Life
Patients may endure episodic fears, anxieties, panic, or dread (19;33)—of breathlessness, (5;22;34;37;45; 47;48;49) of being left alone, (50) of hospitalization, (19;37;38) or even of going home from the hospital. (19;37;38;51) Mortal fears often arise of suffocating, dying suddenly during an attack of dyspnea, or dying when asleep. (37;39;47;49;50;52)
Breathlessness and anxiety interact in complex ways. (6;32;53) Independence is threatened, as patients begin to need help for basic bodily care and functions due to severe breathlessness. (34;54) Self-confidence and self-esteem falter under feelings of powerlessness, helplessness, and hopelessness. (10;19;23;35;36;37;39;55;56) Sadness and vulnerability are common, (45;57) while anger, frustration, and irritability grow. (10;34;58)
The experience of overall quality of life with COPD is multidimensional, (59) and patients’ social and physical environments affect what they can achieve. (33) Objective quality-of-life instruments may be difficult to complete, and may fail to capture relevant domains. (60) Domains that patients use to evaluate their own quality of life include physical effects, medication dependency, disruption of relationships, emotional reactions, life disruptions, and self-esteem. (39)
Constant planning and balancing is required to incorporate the particular demands of COPD into daily life. (19;43;61;62) Coping becomes relentless, round-the-clock work. (63) Over time, patients become experts in their own condition and develop their own methods for coping. (49) Self-management strategies include energy conservation through pacing, planning, or modifying activities; (44;58;62;63;64;65) breathing techniques; (62;63;64;65;66) exercise; (62;63;64;65) exercise avoidance; (62;63) possessing medications and following medication regimens; (19;33;63;64;65) assistance equipment; (7;10) and controlling or avoiding threatening environments. (33;43;58;62;66) Many coping strategies require resources of time, money, or expertise. Economic hardship, comorbidities, language barriers, and low health literacy can all impair self-care, health care, and coping. (67)
With diminishing abilities and growing vulnerability come challenges to patients’ identity, and even to meaning of life. COPD affects patients’ ability to fulfill meaningful social roles, (6;19) and they reflect on and grieve the loss of identity, activity, or productivity. (4;6;19;33;39;48) Age and sex can affect role expectations and the challenges that come with them. (6;8) Patients may see themselves as being old or sick, (6;33) and they may greatly value the ability to even partially fulfill normal roles. (36;41) Some come to establish a relationship with their disease, build a different life around it, and seek new sources of meaning in their lives. (4;6;8;19;33) Some find comfort in spiritual beliefs, or in cultivating a broader perspective (such as appreciating what they have left, doing what they still can, and “making every day count”). (43;44;48) At later stages of disease, life may seem reduced to little more than existence, (45) or a continuous struggle for acceptance of profoundly diminished prospects. (30)
Despite the ongoing struggles that come with COPD, not all patients readily perceive a downward trajectory in their health over time. Some attribute worsening symptoms not to the progression of their disease, but rather, to temporary, immediate causes such as self-management failures or environmental changes. (2;31) The steady decline of COPD becomes most recognizable in hindsight, at later stages of disease. (9;16;42)
Relationships with Others
Increasing vulnerability and unpredictable setbacks make patients dependent on others for practical assistance, comfort, and moral support. (34;37;39;41) The involvement of others in caregiving can contribute richly to patients’ quality of life, ability to cope, and social relationships. (49;56;68) Conversely, COPD patients who live alone and are unable to hire extra help may become especially vulnerable. (10) Rehabilitation programs may replace some of the social contact and support that COPD patients lose as a result of their disease, and are valuable for this reason. (4;14;69) Incapacity due to advancing disease may require patients to relocate to other types of housing, assisted settings, or locations closer to health services, although not all can obtain optimal housing. (2;3) Both home-based and institution-based care presents patients with strong emotional and pragmatic tradeoffs. (30)
Sadly, patients’ isolation often grows together with their increasing need to “have someone there.” Functional limitations, institutional living, or self-consciousness can isolate COPD patients from the people they need. (8;9;10;30) Sometimes family and friends withdraw because of the disease. (23;70) Many aspects of COPD isolate patients from others, including the inability to walk, talk, leave home, or leave their chair or bed; fear of weather, second-hand smoke, or environmental pollution; inflexible planning and routines; strained or lost contact with family, friends, or coworkers; reluctance to ask for help or expectations that assistance will not help enough; embarrassment about the causes, symptoms, behaviour, or equipment associated with COPD; the strain of trying to appear normal; the invisibility of some distressing symptoms; and perceived lack of sympathy or compassion from others. (5;19;23;24;30;33;34;36;37;39;41;45;58;66;68;70)
Challenges of Smoking Cessation
COPD patients who smoke respond to advice to quit in complex ways. Some do not understand that smoking accelerates COPD, the onset of disability, and death. (8;17;28) Some become more motivated to quit upon realizing that they are experiencing the complications of smoking, (1;26;27;62) but others may feel incapable of doing do, or may see quitting as a never-ending struggle. (3;39) Paradoxically, the challenges of living with COPD can also increase the desire to smoke as a means of coping. (3;27;39) While COPD brings pain, difficulty, grievous loss, and a shrinking social world, smoking can provide pleasure, comfort, support, and sense of community with other smokers. (3;19;27;39) While COPD makes patients feel ill, tired, or old, smoking can bolster a stronger self-image. (3;19;39)
Interactions with the Health Care System
COPD patients often suffer poor relationships with health care providers, and experience hastiness, poor listening, or lack of compassion. (10) Patients sometimes feel that their subjective distress seems invisible to clinicians, who focus on objective health indicators. (10;24;42;53) Physicians infrequently investigate, address, or refer for the substantial nonmedical assistance needs of COPD patients. (5;9)
Access to care is a pervasive issue, for many reasons. Many of the factors that isolate COPD patients socially also isolate them from health care. (24;25;54) Some patients feel unwelcome when they visit health care providers because their condition seems hopeless, or because they continue to smoke. (3;10;24) Poor continuity of care is a common complaint, and can hamper both access to care and rapport between patient and health care provider. (25;71) Patients may feel compelled to be undemanding and agreeable to avoid losing their physician. (10;22;24;25) Medical visits become logistically difficult due to impaired mobility, time-limited oxygen supplies, etc., and for these reasons patients sometimes avoid visits during exacerbations, when they are most needed. (22;24;25;42;51) Some patients undermedicate to stockpile medications for future exacerbations, when they will self-medicate to avoid physician visits. (10)
Experiences of Exacerbations
Recognition
Acute breathlessness is the most terrifying aspect of living with COPD. Patients do not always expect to recover from an exacerbation, and it raises fears of sudden death. (6;10;72;73) It can be frightening to be alone; breathlessness creates feelings of urgent need for help from others, and the presence of others may help alleviate terror. (14;23;34;70;73)
Patients vary in their recognition of clinically defined exacerbations, and often describe the experience with nonclinical language (e.g., attack, bad day, chest infection, crisis). (29;74) Indeed, the term exacerbation lacks meaning for patients across many countries and languages. (74) As well, patients’ own descriptions of events may not correspond with clinical measures; for example, patients may report that they have no problem breathing because they have ceased all activity due to severe breathlessness. (75) Restricting activity is a typical way to maintain a feeling of normalcy and avoiding asking for help. (5) Self-management strategies include decreased activity, medication, relaxation, and altered breathing patterns. (76) Because patients seem “normal” at rest, observers may underestimate how ill they really are. (24)
Many patients are first diagnosed with COPD during a severe exacerbation. (8) Although an exacerbation is considered a discrete event from a clinical point of view, patients experience exacerbations within a continuous flux of good days and bad days. Exacerbations are the low points in a familiar but unpredictable cycle, and are not always recognized as emerging medical crises. (29;72;75) Patients may feel reluctant or unable to seek medical help during an exacerbation. (22;25;29;42;51;75) A sudden inability to cope with life’s demands, in addition to frightening changes in symptoms, typically drives patients to seek care. (29) Some who defer care end up hospitalized and demoralized. (22)
Treatment
Patients may distrust clinicians’ competence when they don’t receive expected treatments, or when interventions are disappointing: for example, when they are not given antibiotics for what seems to be a chest infection; (10;39) when pulmonary rehabilitation doesn’t improve symptoms; or when hospital staff respond casually to symptoms that the patient believes are an emergency. (6;69;72;73) Lack of confidence in community-based services leads some patients to seek hospital admission. (77) Some exacerbations require hospital care, but patients may feel especially vulnerable while hospitalized; they may feel dependent on others for care, or traumatized by frightening, exhausting hospital care routines. (51;54;73) While in hospital, many patients wish for better communication about their treatments, progress, and post-discharge care. (51;78)
Recovery
Upon hospital discharge following an exacerbation, patients may face new levels of uncertainty about their illness, prognosis, care providers, and supports. (38;51;71) Patients may find security in self-treatments such as oxygen therapy and “standby” medications. (51) They typically hope that after recovering from an exacerbation, they will return to their normal daily life. (23;70) Exacerbations may not be recalled as considerable medical events; instead, they may be remembered for their impact on activities, plans, and daily life. (29) Patients may interpret recovery from an exacerbation as a sign of improvement in their COPD; temporary improvement obscures the overall downward trend in their health. (2)
Experiences of the End of Life
Understanding the Prognosis of COPD
Although it is fairly certain that COPD will eventually be fatal, the timing of decline and death is highly uncertain. (79) Such uncertainty may make physicians unsure about whether and when to discuss the prognosis of COPD with patients. (10) Patients often learn about their prognosis from a source other than their physician, and typically well after their initial diagnosis. (80) Patients tend to be poorly informed about the long-term prognosis of their disease and what to expect toward the end of life (especially compared to those with diseases such as cancer or acquired immune deficiency syndrome); this lack of understanding impairs their quality of life as the disease progresses. (11;15;80) Some may envision their death from COPD occurring at the end of their natural life (rather than prematurely), (2;10;11) and some may deliberately avoid contemplating death. (47) Nevertheless, although the long-term picture may be fuzzy, patients may fear and think about death, particularly during acute exacerbations, not knowing which one may be their last. (2;19;22;47)
Experiences of Dying
Patients may realize that death is imminent when COPD is very advanced and seems to take over every aspect of life, or entirely exhausts them. (19;81) They worry about how they might die—in particular, about suffocating during a final attack of breathlessness. (5;11;47) They may want health care providers to address their fears of dying breathless or in pain, (20;47) or to provide a clear new care plan. (20) The perception that clinicians are too busy inhibits some patients from sharing their psychosocial needs at the end of life. (7) Nurses may offer more time for conversation, and often play a translating role between physicians and patients. (13;82) Some patients prefer a hospital death, as hospitalization ensures that they will not be alone and alleviates family care burdens. (47)
As the end of life approaches, COPD patients face the usual challenges of daily living, but with increasing of exacerbations and deepening dependency. Activities and mobility decrease, and life may become confined to the home, or even to a single chair. (5;50) Declining health often deepens social isolation and loneliness, (45;46;57) but being involved with and appreciated by others provides end-stage COPD patients with a significant source of comfort, meaning in life, and reason to “go on.” (30;45) Patients become more dependent on their families, (25;34;36;37;39;41) and they may worry about the impact of their sudden death on surviving family members. (57) Peace of mind before dying may become an important goal. (47) Anxieties and fears about the future, and about dying, persist or grow. (50;57) Maintaining hope—for improvement, remission, or cure—becomes a central feature of psychological coping. (49;56;83)
Communication with Health Care Providers
Patients may look to their physicians for information, behaviour, or cues (e.g., language, or new medications) that can be interpreted as optimistic signs. (10;83;84) Patients may feel compelled to “take a chance” on dramatic interventions (e.g., lung volume reduction surgery) when they perceive that they have few options and “nothing to lose.” (85) A lack of hopeful messages from clinicians can be devastating, and referral to palliative care may be interpreted as hopelessness. (7;10;50) In contrast, however, the absence of prognostic information can also cause some patients make overly pessimistic assumptions. (5) When no more treatment options are available, patients may realize that they are approaching the end of life. (83) Upon hearing that their disease is terminal, patients typically compartmentalize, balance, integrate, or redirect their hopes elsewhere. (20)
There are many reasons why clinicians may find it difficult to communicate the terminal prognosis of COPD or initiate advance care planning. Some have difficulty identifying the beginning of “the end of life,” given the unpredictable course of COPD. (16;47;86) During appointments, there may be multiple health issues to address and inadequate time for conversations about long-term prospects. (16) During exacerbations, when patients’ thoughts of death are most acute and physicians are most engaged with their care, clinicians tend to focus on crisis management and often have insufficient time, opportunity, or privacy for difficult conversations, reflection on the bigger picture, and end-of-life planning. (7;16;79;87;88). Specialists care for patients only sporadically, and may not know them well. (79) Clinicians are aware that poorly timed end-of-life discussions may traumatize patients or families, and may hesitate for fear of harming them, or dashing their hopes; (8;20;79) some fear that patients might forgo life-enhancing interventions such as smoking cessation or exercise. (16) Not all clinicians are adequately informed or prepared to pursue palliative care for COPD. (13;87) As well, clinicians grapple with difficult emotions of their own, such as sadness or anxiety; some prefer to let patients initiate end-of-life discussions. (79) Many COPD patients are unwilling to discuss the prospect of dying, (11;20;22;46;79;81;82) but some want to know when death is imminent, so that they can prepare. (47) Long-term physician-patient relationships, familiarity and understanding, trust, good communication skills, sensitivity to patients’ receptiveness about end-of-life topics, and secure discussion settings can help facilitate end-of-life discussions. (20;34;82;86;89;90)
Patients may selectively forgo interventions such as intubation when they realize they are dying, but individual patients vary in their tolerance for burden-benefit ratios. (81;84) Patients’ preferences may change over the course of the disease. In particular, they may become willing to tolerate greater burdens for smaller incremental benefits, (84;86) and this calls into question the value of advance care plans. (84) Some patients prefer to make decisions only when they are needed. (84)
Palliative Care
The term palliative care lacks a stable definition among COPD health care providers. (7) Some consider the defining feature to be palliation itself (i.e., symptom management at any stage of life or disease, possibly in concert with therapeutic intervention), while others restrict the term to end-of-life care (i.e., comfort care in lieu of therapeutic care). (7) End-of-life palliative services have traditionally targeted patients with cancer, acquired immune deficiency syndrome, or neurodegenerative disease, but COPD patients face more uncertain prognoses, as well as chronic symptoms, limitations, and psychosocial challenges. (15;80;90) The transition point to “end of life” is difficult to pinpoint in the long, cyclic trajectory of COPD, so a chronic model of palliative care (i.e., one not focused on an “end stage”) may best suit patients’ needs. (15)
Divergent meanings and goals of palliative care in COPD lead to confusion about whether such services are the responsibility of home care, primary care, specialty care, or even critical care. (7;34;87) For COPD patients, a meaningful evaluation of palliative services would focus on care processes in addition to palliation-relevant outcomes. (91) For example, preserving continuity of care and established, compassionate provider relationships are important to COPD patients, and the loss of such relationships can cause suffering. (20;89;90;91) Other valued features may include supportiveness, communication, accessibility, clinical skill, teamwork, family involvement, patient education, personalized care, attention to patient values, and respect for patients’ lifestyle, culture, decisions, and wishes (80;90;91).
Critical care clinicians caring for end-stage COPD in an intensive care setting identify special challenges, such as meeting high emotional needs, effectively managing dyspnea and anxiety, and negotiating life support and rescue-oriented critical care as these become more futile. (87) Important quality domains for hospital-based palliative care include (92) teams with appropriate and well-trained members; formalized care pathways; communication with patients regarding care options and preferences; available specialist and generalist services; and communication and collaboration with other acute and community care providers. (92)
Experiences of Carers
Psychosocial Effects
Carers’ challenges often echo those of COPD patients, including anxiety, uncertainty about the future, helplessness, powerlessness, depression, difficulties maintaining employment, loss of mobility and freedoms, strained relationships, and growing social isolation. (48;83;93;94;95) Like patients, carers may also be poorly informed about the nature of COPD, its management, and long-term prospects; this uncertainty contributes to carer stress. (93) They ride an “emotional roller coaster” over the course of the disease, with its evolving demands. (94) Carers monitor patients to anticipate their needs, and many actively manage patients’ activities to control breathing. (94)
Carers may face overwhelming anxiety during (and in anticipation of) patients’ episodes of breathlessness; they feel compelled to help, but there is little they can do. (53;94;96;97) The unpredictability of exacerbations can make it frightening for carers to leave patients; fears tend to increase at night and often impair carers’ sleep. (93;94;97;98) Acute episodes of breathlessness may heighten carers’ uncertainty and pessimism. (83;93;95) However, carers may also come to accept the unpredictability of COPD and develop new resilience and strategies for confronting problems as they arise. (93;99;100)
The patient’s declining health increases carer fatigue and depression. (98) COPD often puts stresses on relationships between carers and their loved ones; carers may grieve the loss of certain patient character traits, medications may create frightening personality changes, breathing problems may impair communication and intimacy, and compulsory togetherness may bring challenges. (69;94;98)
Multiple Roles
Carers can feel pressured by their many roles (“nurse, doctor, psychologist, and carer” in addition to family member), struggle to maintain patience when they feel overwhelmed, and feel guilty about not doing enough. (12;93) Toward the end of life, carers often serve as the “backbone” of the care team, with additional responsibilities and burdens. (7) Care duties can bring resentment, satisfaction, or both. (93) Burnout results when caregivers have no breaks or escape from caregiving roles, or no one to share the burden or boredom. (48;98)
Strong social support, including reassuring and frequent contact with health care providers, helps carers cope with and sustain their role, but caregiving also limits carers’ capacity for needed interactions with others. (12;28;48;94;95;98) The reasons for social isolation are many, including reluctance to leave the patient, and the need for privacy. (94;95) Many carers find it difficult to ask for help; they may feel duty-bound to provide the care themselves, or they may feel that they alone fully understand the patient’s needs. (12;53;94;95;96;98) During acute episodes, patients may call on family members first, leaving them responsible to call for professional help. (96) During crises, carers’ desire to control caregiving may be cast aside. (95) Hospitalization may appeal when carers feel burnt out, or when patients feel inadequately cared for at home. (77) Carers may interpret palliative end-of-life care as an unwelcome sign that patients and physicians have “given up.” (50) Professional support that is responsive to urgent, unpredictable needs may be the most important kind of support for carers. (94)
Increased Burden
Carers often face their own health problems, or struggle to care adequately for themselves as they place the patient’s needs before their own. (95;98) Carers with serious health problems may worry about becoming unable to care for the patient. (95) Patients also fear burdening their families, as their disabilities and dependence grow, and may feel shame or try to be stronger because of this. (22;33;45;46;57;101). Family caregivers may also face difficulties sustaining employment and supporting their family. (69) With added disease-related expenses and the patient’s loss of income, carers’ paid employment becomes both more important and more challenging. (69) Some carers continue to work out of financial necessity, while others choose to work for the outlet it offers. (69;98) At work, however, carers may be preoccupied, and contact with the patient is important; they appreciate understanding and flexible employers. (69)
Synthesis: A Disease Trajectory Reflecting Patient Experiences
After the qualitative research findings were gathered they were synthesized, and evidence-grounded insights were related to prevailing clinical theories of the COPD disease trajectory. A more patient-centred model of the COPD trajectory was then proposed for clinical, health services, and policy applications.
Clinicians widely recognize the COPD trajectory as one of steady decline in health status and function, punctuated by increasing exacerbations, and ending in death from COPD. The shape of the theorized trajectory has evolved over decades, to relate key COPD stages to interventions, services, and patient needs. In 1977, Fletcher and Peto (102) modelled lung function against years of life to illustrate how smoking cessation at different stages could affect longevity (Figure 2). This model is still used clinically to explain the value of quitting smoking to patients, for example. (13)
Figure 2: COPD Disease Trajectory: Traditional View*.
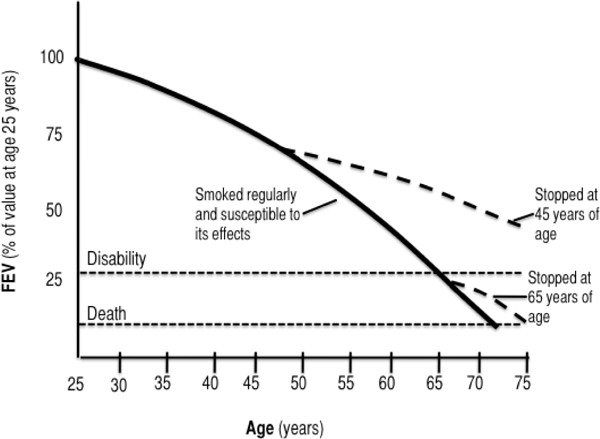
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV, forced expiratory volume.
Reprinted from BMJ, Vol. 6077; Fletcher C, Peto R: The Natural History of Chronic Airflow Obstruction, p. 1645-1648 with permission from BMJ Publishing Group Ltd.
In 2001, Lynn (103) presented a cyclic trajectory for end-stage organ failure (including lung failure), suggesting that the downward trajectory of COPD is punctuated with health crises and recoveries along its course, but lacks a distinctive end-of-life turning point at which to initiate hospice services. This differs crucially from the trajectory of cancer, with its characteristic sharp downward inflection in the terminal stage. Lynn’s theorized trajectory has been supported empirically by a prospective cohort analysis, modelling activities of daily living over time for end-stage organ failure. (104)
More recently, in an editorial commentary on 1 of the qualitative studies included in this review, (13) Lehman (105) proposed a more patient-centred trajectory for understanding COPD in the context of physician-patient discussions about prognosis and end of life (Figure 3).
Figure 3: COPD Disease Trajectory: Updated Clinical View*.
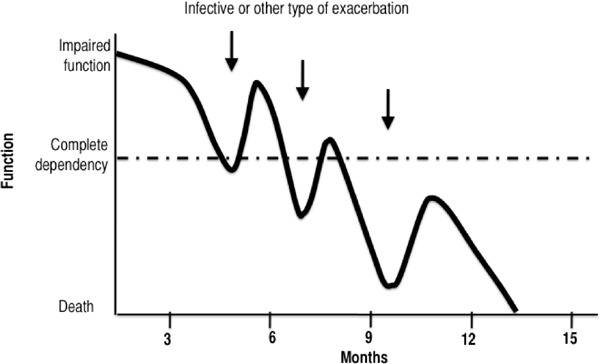
Abbreviation: COPD, chronic obstructive pulmonary disease.
Reprinted from Br J Gen Pract, Vol. 54; Lehman R. How Long Can I Go on Like This? Dying from Cardiorespiratory Disease, p. 892–893, with permission from the Royal College of General Practitioners.
Lehman’s model preserves the roller coaster–like cycles of Lynn’s curve and the lack of a discrete transition to an end stage, but it replaces Fletcher and Peto’s lung function (y-axis) with patients’ overall functioning. The model highlights patients’ inevitable yet rocky transition to a state of complete dependency, when they need social services, support, technologies, and interventions to do what they can no longer do for themselves. Support services also often aim to control the level of care, deferring hospitalization or long-term care as long as is reasonable. Both physicians and patients expect death from an exacerbation, but “the doctor may be little better than anyone else at predicting which dip is going to be the final one.” (105)
Patients with other chronic, fatal diseases, such as cancer, normally reach each of 2 crucial turning points—total dependency and end of life—only once. These are usually regarded of as points of no return, and allow for predictable service transitions between major institutions of health care: home, home care, hospital, and hospice. On the COPD roller coaster, however, patients’ needs vacillate above and below the line of total dependency, and no one specific event may demarcate the “end of life” stage, except perhaps the final acute exacerbation, which is difficult to predict and may be relatively brief.
Over a period of years, COPD patients may move repeatedly between levels, institutions, and providers of care. Demands on informal caregivers also wax and wane as needs change over time. Such a disease trajectory creates recurring challenges and the need for timely decisions, smooth transitions, appropriateness, and continuity of care. Many aspects of palliative care are needed throughout the disease course, with a gradual shift in emphasis from controlling symptoms to coping with dying: “The patient’s physiology almost never dictates an abrupt change from ‘cure’ to ‘care.’ Instead, aggressive and palliative treatments will be mixed, with advance care planning occurring alongside emergency medical services, dyspnea relief alongside pacemaker placement, and family support alongside resuscitation.” (103) COPD requires a different model and philosophy of palliative care than the 2-stage hospice model that has evolved for diseases such as cancer. (2;83;103)
The review of qualitative research studies described above suggests 2 further refinements to our understanding of debilitation and dying with COPD, as well as related service needs over time. Figure 4 represents a slight modification to Lehman’s trajectory, representing the prevailing clinical view of the COPD course, one of “…inexorable decline: a prolonged period of disabling dyspnea and increasingly frequent hospital admission reflecting deteriorating lung function and usually presaging a premature death.” (15) Accordingly, Figure 4 depicts increasing frequency and severity of crises over time. In addition, a major source of clinical uncertainty (as well as failed communication between physicians and patients) comes from the difficulty of predicting time to disease progression or death for a given patient. Figure 3 depicts this uncertainty with a broken x-axis, representing uncertain units of time despite a relatively certain decline.
Figure 4: COPD Disease Trajectory: Current Clinical View*.
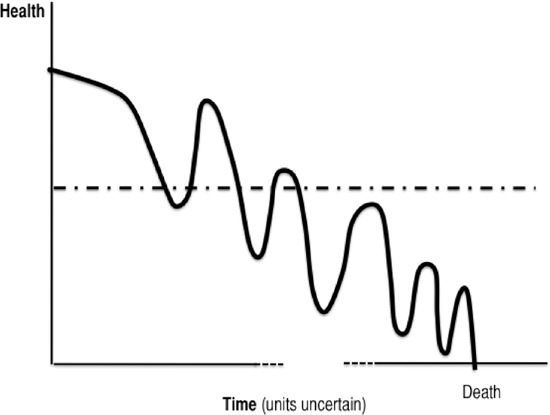
Abbreviation: COPD, chronic obstructive pulmonary disease.
Adapted from Br J Gen Pract, Vol. 54; Lehman R: How Long Can I Go on Like This? Dying from Cardiorespiratory Disease, p. 892–893 and verbal characterization in Thorax, Vol. 55; Gore JM, Brophy CJ, Greenstone MA. How Well Do We Care for Patients with End Stage Chronic Obstructive Pulmonary Disease (COPD)? A Comparison of Palliative Care and Quality of Life in COPD and Lung Cancer, p. 1000–1006.
Figure 5 proposes an even more patient-centred trajectory, based on relevant findings from this review. This proposed trajectory focuses on the problem of staging the disease course in ways that are meaningful to clinicians, patients, and health policy makers, with a view to supporting better planning, targeting, and continuity of services and interventions.
Figure 5: COPD Disease Trajectory: Common Patient Experiences and Expectations*.

Abbreviation: COPD, chronic obstructive pulmonary disease.
Note: The clinical trajectory of Figure 4 appears in grey here, for comparison.
This model is proposed with 3 caveats. First, this trajectory does not apply to all patients at all stages of the disease; rather, it characterizes what many patients seem to experience and expect, especially at early and middle stages. Second, most patients would not depict their experiences using a graph, as we do here; the illustration is simply meant to help translate patient experiences for clinical, health services, and policy audiences. Finally, this model does not capture the entirety of the patient experience covered in the extensive body of qualitative research on COPD.
Patient experiences may deviate from clinical expectations in a number of ways. Before diagnosis, patients experience the suboptimal health that clinicians might call “early COPD,” but that patients know as their own “normal,” and not necessarily as illness. On the occasion of their diagnosis, many patients are seeking care not for their “normal” symptoms, but because they are feeling unusually unwell (what they might interpret as an “infection,” but what a clinician might recognize as an “exacerbation”). It may be difficult for patients to pinpoint a moment of diagnosis; the nature of their condition and the exact diagnosis come into focus over time, using personal experience and piecemeal information from various sources.
Patients experience alternating good days and bad days, and a “roller coaster” pattern of ups and downs becomes apparent. COPD becomes as much as a way of life as an illness; patients use many means (social, psychological, medical, organizational) to control what they can, and to cope with what they cannot. The sense of what is normal, and the tolerability of health problems and interventions, both evolve with the disease.
Bad days, or exacerbations, may not be experienced as a net decline in health, but rather as temporary setbacks. Sporadic exacerbations may not be attributed to advancing disease, but rather to specific activities, changes in the weather, environmental factors, faltering self-management, or infection.
Although patients pass back and forth over Lehman’s line of total dependency during some exacerbations, they may not expect permanent total dependency. Sporadic dependency is challenging and disruptive, and patients may yearn for others who will “be there” for them during crises, but informal social support and formal social services are difficult to establish around intermittent and emergent needs. The patient’s social life also follows its own steady downward trajectory.
Late in the disease, the severity, duration, or frequency of bad days reveals a permanent decline in health. Even so, patients may still envision death (from COPD) to be in the distant, unpredictable future. The term “chronic” conveys a lifelong disease, rather than a terminal illness. Patients hope to recover from exacerbations, but also fear dying from suffocation or breathlessness during these crises. Palliative end-of-life care may not be envisioned until patients are actually referred for such care, and the referral may convey the demoralizing message that providers have “given up.”
COPD interventions and services tend to be organized around key points in the disease trajectory, such as diagnosis, daily living with disability, exacerbations requiring hospitalization, complete dependency, and dying, yet the contours of the COPD disease trajectory do not offer discrete turning points around which to plan. Rather, the flux of needs in COPD calls for service continuity and flexibility to allow health care providers and patients to respond to the unpredictable yet increasing demands of the disease over time.
Conclusions
Experiences at Diagnosis
Patients typically seek initial treatment for an acute episode rather than for chronic early symptoms of COPD.
Many patients initially misunderstand terms such as COPD, chronic obstructive pulmonary disease, or exacerbation.
Patients may not realize that COPD is incurable and fatal; some physicians themselves to not consider early COPD to be a fatal disease.
Smokers may not readily understand or agree with the idea that smoking causes or worsens COPD. Those who accept the causal link may feel regret or shame.
Experiences of Living Day to Day
COPD patients experience alternating good days and bad days. A roller-coaster pattern of ups and downs becomes apparent, and COPD becomes a way of life.
Patients use many means (social, psychological, medical, organizational) to control what they can, and to cope with what they cannot. Economic hardship, comorbidities, language barriers, and low health literacy can make coping more difficult.
Increasing vulnerability and unpredictable setbacks make patients dependent on others for practical assistance, but functional limitations, institutional living or self-consciousness can isolate patients from the people they need.
For smokers, medical advice to quit can conflict with increased desire to smoke as a coping strategy.
Many of the factors that isolate COPD patients from social contact also isolate them from health care.
Experiences of Exacerbations
Patients may not always attribute repeated exacerbations to advancing disease, instead seeing them as temporary setbacks caused by activities, environmental factors, faltering self-management, or infection.
Lack of confidence in community-based services leads some patients to seek hospital admission, but patients also feel vulnerable when hospitalized. They may feel dependent on others for care or traumatized by hospital care routines.
Upon hospital discharge following an exacerbation, patients may face new levels of uncertainty about their illness, prognosis, care providers, and supports.
Experiences of the End of Life
Patients tend to be poorly informed about the long-term prognosis of COPD and what to expect toward the end of life; this lack of understanding impairs quality of life as the disease progresses.
As the end of life approaches, COPD patients face the usual challenges of daily living, but in a context of increasing exacerbations and deepening dependency. Activities and mobility decrease, and life may become confined.
Some clinicians have difficulty identifying the beginning of “the end of life,” given the unpredictable course of COPD. Long-term physician-patient relationships, familiarity and understanding, trust, good communication skills, sensitivity, and secure discussion settings can help facilitate end-of-life discussions.
Divergent definitions and goals of palliative care in COPD lead to confusion about whether such services are the responsibility of home care, primary care, specialty care, or even critical care. Palliative end-of-life care may not be anticipated prior to referral for such care. A palliative care referral can convey the demoralizing message that providers have “given up.”
Experiences of Carers
Carers’ challenges often echo patients’ challenges, and include anxiety, uncertainty about the future, helplessness, powerlessness, depression, difficulties maintaining employment, loss of mobility and freedoms, strained relationships, and growing social isolation.
Carers feel pressured by their many roles, struggling to maintain patience when they feel overwhelmed, and often feeling guilty about not doing enough.
Carers often face their own health problems and may have difficulty sustaining employment.
Synthesis: A Disease Trajectory Reflecting Patient Experience
The flux of needs in COPD calls for service continuity and flexibility to allow both health care providers and patients to respond to the unpredictable yet increasing demands of the disease over time.
Glossary
- 6 Minute Walking Test (6MWT)
A measure of exercise capacity which measures the distance that a patient can quickly walk on a flat, hard surface in a period of 6 minutes. A widely used outcome measure in respiratory rehabilitation of patients with COPD.
- Acute exacerbations of chronic obstructive pulmonary disease (AECOPD)
A change in baseline symptoms that is beyond day-to-day variation, particularly increased breathlessness, cough, and/or sputum, which has an abrupt onset.
- Admission avoidance hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and avoid admission to hospital. After patients are assessed in the emergency department for an acute exacerbation, they are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home where they receive regular visits from a medical professional until the exacerbation has resolved.
- Ambulatory oxygen therapy
Provision of oxygen therapy during exercise and activities of daily living for individuals who demonstrate exertional desaturation.
- Bilevel positive airway pressure (BiPAP)
A continuous positive airway pressure mode used during noninvasive positive pressure ventilation (see definition below) that delivers preset levels of inspiratory and expiratory positive airway pressure. The pressure is higher when inhaling and falls when exhaling, making it easier to breathe.
- Cost-effectiveness acceptability curve (CEAC)
A method for summarizing uncertainty in estimates of cost-effectiveness.
- Cor pulmonale
Right heart failure, as a result of the effects of respiratory failure on the heart.
- Dyspnea
Difficulty breathing or breathlessness.
- Early discharge hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and decrease their length of stay in hospital. After being assessed in the emergency department for acute exacerbations, patients are admitted to the hospital where they receive the initial phase of their treatment. These patients are discharged early into a hospital-at-home program where they receive regular visits from a medical professional until the exacerbation has resolved.
- Forced expiratory volume in 1 second (FEV1)
A measure of lung function used for COPD severity staging; the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
- Forced vital capacity (FVC)
The amount of air that can be forcibly exhaled from the lungs after taking the deepest breath possible.
- Fraction of inspired oxygen (FiO2)
The percentage of oxygen participating in gas exchange.
- Hypercapnia
Occurs when there is too much carbon dioxide in the blood (arterial blood carbon dioxide > 45 to 60 mm Hg).
- Hypopnea
Slow or shallow breathing.
- Hypoxemia
Low arterial blood oxygen levels while breathing air at rest. May be severe (PaO2 ≤ 55 mm Hg), moderate (56 mm Hg ≤ PaO2 < 65 mm Hg), or mild-to-moderate (66 mm Hg < PaO2 ≤ 74 mm Hg).1
- Incremental cost-effectiveness ratio (ICER)
Ratio of the change in costs of a therapeutic intervention to the change in effects of the intervention compared to the alternative (often usual care).
- Intention-to-treat analysis (ITT)
An analysis based on the initial treatment the participant was assigned to, not on the treatment eventually administered.
- Invasive mechanical ventilation (IMV)
Mechanical ventilation via an artificial airway (endotracheal tube or tracheostomy tube).
- Long-term oxygen therapy (LTOT)
Continuous oxygen use for about 15 hours per day. Use is typically restricted to patients fulfilling specific criteria.
- Multidisciplinary care
Defined as care provided by a team (compared to a single provider). Typically involves professionals from a range of disciplines working together to deliver comprehensive care that addresses as many of the patient’s health care and psychosocial needs as possible.
- Nicotine replacement therapy (NRT)
The administration of nicotine to the body by means other than tobacco, usually as part of smoking cessation.
- Noninvasive positive pressure ventilation (NPPV)
Noninvasive method of delivering ventilator support (without the use of an endotracheal tube) using positive pressure. Provides ventilatory support through a facial or nasal mask and reduces inspiratory work.
- Partial pressure of carbon dioxide (PaCO2
The pressure of carbon dioxide dissolved in arterial blood. This measures how well carbon dioxide is able to move out of the body.
- Partial pressure of oxygen (PaO2)
The pressure of oxygen dissolved in arterial blood. This measures how well oxygen is able to move from the airspace of the lungs into the blood.
- Palliative oxygen therapy
Use of oxygen for mildly hypoxemic or nonhypoxemic individuals to relieve symptoms of breathlessness. Used short term. This therapy is “palliative” in that treatment is not curative of the underlying disease.
- Pulmonary rehabilitation
Multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary rehabilitation programs.
- Pulse oximetry
A noninvasive sensor, which is attached to the finger, toe, or ear to detect oxygen saturation of arterial blood.
- Quality-adjusted life- years (QALYs)
A measure of disease burden that includes both the quantity and the quality of the life lived that is used to help assess the value for money of a medical intervention.
- Respiratory failure
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. It can be either acute (acute respiratory failure, ARF) or chronic, and is classified as either hypoxemic (type I) or hypercapnic (type II) respiratory failure. Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD.
- Short-burst oxygen therapy
Short-duration, intermittent, supplemental oxygen administered either before or after exercise to relieve breathlessness with exercise.
- Sleep apnea
Interruption of breathing during sleep due to obstruction of the airway or alterations in the brain. Associated with excessive daytime sleepiness.
- Smoking cessation
The process of discontinuing the practice of inhaling a smoked substance.
- Spirometry
The gold standard test for diagnosing COPD. Patients breathe into a mouthpiece attached to a spirometer which measures airflow limitation.
- SpO2
Oxygen saturation of arterial blood as measured by a pulse oximeter.
- Stable COPD
The profile of COPD patients which predominates when patients are not experiencing an acute exacerbation.
- Supplemental oxygen therapy
Oxygen use during periods of exercise or exertion to relieve hypoxemia.
- Telemedicine (or telehealth)
Refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services.
- Telemonitoring (or remote monitoring)
Refers to the use of medical devices to remotely collect a patient’s vital signs and/or other biologic health data and the transmission of those data to a monitoring station for interpretation by a health care provider.
- Telephone only support
Refers to disease/disorder management support provided by a health care provider to a patient who is at home via telephone or videoconferencing technology in the absence of transmission of patient biologic data.
- Ventilator-associated pneumonia (VAP)
Pneumonia that occurs in patients undergoing mechanical ventilation while in a hospital.
Acknowledgements
Editorial Staff
Jeanne McKane
COPD Expert Advisory Panel
The role of the expert panel was to provide direction on the scope of the project and the relevant outcomes measures of effectiveness, to review the evidence-based analyses and to identify any societal or systemic issues that are relevant to intervention effectiveness. However, the statements, conclusions and views expressed in this report do not necessarily represent the views of the expert panel members.
Jeremy Grimshaw, MD, MBChB, PhD (Chair)
Senior Scientist, Ottawa Hospital Research Institute
Professor, Department of Medicine, University of Ottawa
Dina Brooks, PhD
Professor, Department of Physical Therapy, University of Toronto
Debbie Coutts, RRT, CRE
Andrea Gershon, MD, MSc, FRCP(C)
Scientist, Institute for Clinical Evaluative Sciences
Respirologist, Sunnybrook Health Sciences Centre
Assistant Professor, Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto
Mita Giacomini, BSc, MPH, MA, PhD
Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Ron Goeree, BA, MA
Director, PATH Research Institute, St. Joseph’s Hospital (Hamilton)
Associate Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Roger Goldstein, MBCHB, FRCP(C), FRCP(UK)
NSA Chair in Respiratory Rehabilitation Research
Director, Respiratory Services, and Senior Scientist, West Park Healthcare Centre
Professor, Medicine and Physical Therapy, University of Toronto
Alan G Kaplan, MD, CCFP(EM), FCFP
Chairperson, Family Physician Airways Group of Canada
Chairperson, Special Interest Focused Care Group in Respiratory Medicine, College of Family Physicians of Canada
Clinical Lecturer, Department of Family and Community Medicine, University of Toronto
DE O’Donnell, MD, FRCP(C)
Director, COPD Centre, Kingston General Hospital
Professor, Department of Medicine, Queen’s University
Asad Razzaque, MD
Family Physician
Holger Schünemann, MD, PhD, MSc, FRCP(C)
Michael Gent Chair in Healthcare Research
Chair, Department of Clinical Epidemiology & Biostatistics, McMaster University
Professor, Department of Clinical Epidemiology & Biostatistics and Medicine, McMaster University
Tasnim Sinuff, MD, PhD, FRCP(C)
Clinician Scientist, Sunnybrook Health Sciences Centre
Assistant Professor, Department of Medicine, University of Toronto
Laura Watling, RRT, BSc(HK)
Clinical Practice Leader/Clinical Coordinator, Respiratory Therapy, West Park Healthcare Centre
This study was funded by the Canadian Institutes of Health Research, Grant # TOO-105430. Deirdre DeJean and Andrea Smith were supported by CIHR Frederick Banting and Charles Best Canada Graduate Scholarships (CGS) Doctoral Awards. We thank the Ontario Ministry of Health and Long-Term Care for its support for the Centre for Health Economics and Policy Analysis (CHEPA) at McMaster University, whose collegial environment made this project possible.
Appendices
Appendix 1: Literature Search Strategies
OVID MEDLINE
exp Pulmonary Disease, Chronic Obstructive
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti, ab.
(copd or coad).ti, ab.
chronic airflow obstruction.ti, ab.
exp Emphysema/
((chronic adj2 bronchitis) or emphysema).ti, ab.
or/1-6
limit 7 to (english language and humans and yr=“2000 -Current”)
ISI Web of Science
Chronic obstructive lung* disease* (in title)
chronic obstructive pulmonary disease* (in title)
chronic obstructive pulmonary disorder* (in title)
chronic obstructive airway* disease* (in title)
chronic obstructive airway* disorder* (in title)
chronic obstructive airflow* disease* (in title)
chronic obstructive airflow* disorder* (in title)
chronic obstructive respiratory disease*
chronic obstructive respiratory disorder*
(copd or coad) (in title)
chronic airflow obstruction (in title)
chronic bronchitis (in topic)
emphysema (in title)
or/1-13
limit to English, human, January 1, 2000 to Current
EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL)
(MH “Pulmonary Disease, Chronic Obstructive+”)
(chronic obstructive and (lung* or pulmonary or airway* or airflow or respiratory) and (disease* or disorder*))
copd or coad
(MH “Emphysema+”)
chronic bronchitis or emphysema
S1 or S2 or S3 or S4 or S5
Limiters - Published Date from: 20000101-20101231 and English and Human
Suggested Citation
This report should be cited as follows:
Giacomini M, DeJean D, Simeonov D, Smith A. Experiences of living and dying with COPD: a systematic review and synthesis of the qualitative empirical literature. Ont Health Technol Assess Ser [Internet]. 2012 March;12(13):1-47. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Qualitative_March.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in Excerpta Medica/EMBASE and the Center for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: MASinfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All analyses in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: MASinfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-9157-7 (PDF)
© Queen’s Printer for Ontario, 2012
About the Medical Advisory Secretariat
Effective April 5, 2011, the Medical Advisory Secretariat (MAS) became a part of Health Quality Ontario (HQO), an independent body funded by the Ministry of Health and Long-Term Care. The mandate of MAS is to provide evidence-based recommendations on the coordinated uptake of health services and health technologies in Ontario to the Ministry of Health and Long-Term Care and to the health care system. This mandate helps to ensure that residents of Ontario have access to the best available and most appropriate health services and technologies to improve patient outcomes.
To fulfill its mandate, MAS conducts systematic reviews of evidence and consults with experts in the health care services community. The resulting evidence-based analyses are reviewed by the Ontario Health Technology Advisory Committee—to which MAS also provides a secretariat function—and published in the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, MAS systematically reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, the Secretariat collects and analyzes information about how a new technology fits within current practice and existing treatment alternatives. Details about the technology’s diffusion into current health care practices add an important dimension to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist decision-makers in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals wishing to comment on an analysis prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by MAS for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data and information provided by experts and applicants to MAS to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the MAS website for a list of all evidence-based analyses: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
List of Tables
| Table 1: Summary of Qualitative Studies Reviewed |
List of Figures
List of Abbreviations
- COPD
Chronic obstructive pulmonary disease
- FEV
Forced expiratory volume
Footnotes
The mild-to-moderate classification was created for the purposes of the report.
References
- 1.Arne M, Emtner M, Janson S, Wilde-Larsson B. COPD patients perspectives at the time of diagnosis: a qualitative study. Prim Care Respir J. 2007;16(14):215–21. doi: 10.3132/pcrj.2007.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinnock H, Kendall M, Murray SA, Worth A, Levack P, Porter M, et al. Living and dying with severe chronic obstructive pulmonary disease: multi-perspective longitudinal qualitative study. BMJ. 2011:342–d142. doi: 10.1136/bmj.d142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsdottir R, Jonsdottir H. The experience of women with advanced chronic obstructive pulmonary disease of repeatedly relapsing to smoking. Scand J Caring Sci. 2007;21(3):297–304. doi: 10.1111/j.1471-6712.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan SCC. Chronic obstructive pulmonary disease and engagement in occupation. Am J Occup Ther. 2004;58(4):408–15. doi: 10.5014/ajot.58.4.408. [DOI] [PubMed] [Google Scholar]
- 5.Habraken JM, Pols J, Bindels PJE, Willems DL. The silence of patients with end-stage COPD: a qualitative study. Br J Gen Pract. 2008;58(557):844–9. doi: 10.3399/bjgp08X376186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gysels M, Bausewein C, Higginson IJ. Experiences of breathlessness: a systematic review of the qualitative literature. Palliat Support Care. 2007;5(3):281–302. doi: 10.1017/s1478951507000454. [DOI] [PubMed] [Google Scholar]
- 7.Spence A, Hasson F, Waldron M, Kernohan WG, McLaughlin D, Watson B, et al. Professionals delivering palliative care to people with COPD: qualitative study. Palliat Med. 2009;23(2):126–31. doi: 10.1177/0269216308098804. [DOI] [PubMed] [Google Scholar]
- 8.Walters JA, Hansen EC, Walters EH, Wood-Baker R. Under-diagnosis of chronic obstructive pulmonary disease: a qualitative study in primary care. Respir Med. 2008;102(5):738–43. doi: 10.1016/j.rmed.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Gysels M, Higginson IJ. The experience of breathlessness: the social course of chronic obstructive pulmonary disease. J Pain Symptom Manage. 2010;39(3):555–63. doi: 10.1016/j.jpainsymman.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Oliver SM. Living with failing lungs: the doctor-patient relationship. Fam Pract. 2001;18(4):430–9. doi: 10.1093/fampra/18.4.430. [DOI] [PubMed] [Google Scholar]
- 11.Gardiner C, Gott M, Small N, Payne S, Seamark D, Barnes S, et al. Living with advanced chronic obstructive pulmonary disease: patients concerns regarding death and dying. Palliat Med. 2009;23(8):691–7. doi: 10.1177/0269216309107003. [DOI] [PubMed] [Google Scholar]
- 12.Hasson F, Spence A, Waldron M, Kernohan G, McLaughlin D, Watson B, et al. Experiences and needs of bereaved carers during palliative and end-of-life care for people with chronic obstructive pulmonary disease. J Palliat Care. 2009;25(3):157–63. [PubMed] [Google Scholar]
- 13.Halliwell J, Mulcahy P, Buetow S, Bray Y, Coster G, Osman LM. GP discussion of prognosis with patients with severe chronic obstructive pulmonary disease: a qualitative study. Br J Gen Pract. 2004;54(509):904–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers S, Dyas J, Molyneux AWP, Ward MJ, Revill SM. Evaluation of the information needs of patients with chronic obstructive pulmonary disease following pulmonary rehabilitation: a focus group study. Chron Respir Dis. 2007;4(4):195–203. doi: 10.1177/1479972307080698. [DOI] [PubMed] [Google Scholar]
- 15.Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55(12):1000–6. doi: 10.1136/thorax.55.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gott M, Gardiner C, Payne S, Small N, Seamark D, Halpin D. Barriers to advance care planning for older people with chronic obstructive pulmonary disease: patient and professional perspectives. Australasian Ageing. 2009;28(32):A100. [Google Scholar]
- 17.Hansen EC, Walters J, Baker RW. Explaining chronic obstructive pulmonary disease (COPD): perceptions of the role played by smoking. Sociol Health Illn. 2007;29(5):730–49. doi: 10.1111/j.1467-9566.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- 18.Parker DR, Goldman RE, Eaton CB. A qualitative study of individuals at risk for or who have chronic obstructive pulmonary disease: what do they understand about their disease? Lung. 2008;186(5):313–6. doi: 10.1007/s00408-008-9091-9. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist G, Hallberg LRM. “Feelings of guilt due to self-inflicted disease”: a grounded theory of suffering from chronic obstructive pulmonary disease (COPD) J Health Psychol. 2010;15(3):456–66. doi: 10.1177/1359105309353646. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JR, Engelberg R, Young JP, Vig LK, Reinke LF, Wenrich MD, et al. An approach to understanding the interaction of hope and desire for explicit prognostic information among individuals with severe chronic obstructive pulmonary disease or advanced cancer. J Palliat Med. 2008;11(4):610–20. doi: 10.1089/jpm.2007.0209. [DOI] [PubMed] [Google Scholar]
- 21.Harris M, Wildgoose D, Veale AJ, Smith BJ. Providing reviews of evidence to COPD patients: qualitative study of barriers and facilitating factors to patient-mediated practice change. Chron Respir Dis. 2010;7(1):19–28. doi: 10.1177/1479972309358634. [DOI] [PubMed] [Google Scholar]
- 22.Hasson F, Spence A, Waldron M, Kernohan G, McLaughlin D, Watson B. I can not get a breath: experiences of living with advanced chronic obstructive pulmonary disease. Int J Palliat Nurs. 2008;14(11):526–31. doi: 10.12968/ijpn.2008.14.11.31756. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill ES. Illness representations and coping of women with chronic obstructive pulmonary disease: a pilot study. Heart Lung. 2002;31(4):295–302. doi: 10.1067/mhl.2002.123712. [DOI] [PubMed] [Google Scholar]
- 24.Gysels M, Higginson IJ. Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage. 2008;36(5):451–60. doi: 10.1016/j.jpainsymman.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Shipman C, White S, Gysels M, White P. Access to care in advanced COPD: factors that influence contact with general practice services. Prim Care Respir J. 2009;18(4):273–8. doi: 10.4104/pcrj.2009.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey PH, Montgomery P, Boyles CM. COPD stories of complex causal “truths” “Sure I’ve smoked all my life/but I also put in 37 years at the mine”. J Clin Nurs. 2009;18(14):1994–2002. doi: 10.1111/j.1365-2702.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 27.Schofield I, Kerr S, Tolson D. An exploration of the smoking-related health beliefs of older people with chronic obstructive pulmonary disease. J Clin Nurs. 2007;16(9):1726–35. doi: 10.1111/j.1365-2702.2007.01701.x. [DOI] [PubMed] [Google Scholar]
- 28.Caress A, Luker K, Chalmers K. Promoting the health of people with chronic obstructive pulmonary disease: patients’ and carers’ views. J Clin Nurs. 2010;19(3-4):564–73. doi: 10.1111/j.1365-2702.2009.02982.x. [DOI] [PubMed] [Google Scholar]
- 29.Adams R, Chavannes N, Jones K, Ostergaard MS, Price D. Exacerbations of chronic obstructive pulmonary disease—a patients’ perspective. Prim Care Respir J. 2006;15(2):102–9. doi: 10.1016/j.pcrj.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elofsson LC, Öhlén J. Meanings of being old and living with chronic obstructive pulmonary disease. Palliat Med. 2004;18(7):611–8. doi: 10.1191/0269216304pm922oa. [DOI] [PubMed] [Google Scholar]
- 31.Victorson DE, Anton S, Hamilton A, Yount S, Cella D. A conceptual model of the experience of dyspnea and functional limitations in chronic obstructive pulmonary disease. Value Health. 2009;12(6):1018–25. doi: 10.1111/j.1524-4733.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 32.Lohne V, Heer HCD, Andersen M, Miaskowski C, Kongerud J, Rustoen T. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung. 2010;39(3):226–34. doi: 10.1016/j.hrtlng.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Nicolson P, Anderson P. Quality of life, distress and self-esteem: a focus group study of people with chronic bronchitis. Br J Health Psychol. 2003;8(Pt3):251–70. doi: 10.1348/135910703322370842. [DOI] [PubMed] [Google Scholar]
- 34.Barnett M. Caring for a patient with COPD: a reflective account. Nurs Stand. 2005 May-24;19(36):41–6. doi: 10.7748/ns.19.36.41.s58. [DOI] [PubMed] [Google Scholar]
- 35.Clancy K, Hallet C, Caress A. The meaning of living with chronic obstructive pulmonary disease. J Nurs Healthc Chronic Illn. 2009;1(1):78–86. [Google Scholar]
- 36.Fraser DD, Kee CC, Minick P. Living with chronic obstructive pulmonary disease: insiders’ perspectives. J Adv Nurs. 2006;55(5):550–8. doi: 10.1111/j.1365-2648.2006.03946.x. [DOI] [PubMed] [Google Scholar]
- 37.Guthrie SJ, Hill KM, Muers ME. Living with severe COPD. A qualitative exploration of the experience of patients in Leeds. Respir Med. 2001;95(3):196–204. doi: 10.1053/rmed.2000.1021. [DOI] [PubMed] [Google Scholar]
- 38.Jeng C, Tsao L-I, Ho C-H, Chang P-C. Experiences of daily activities within two weeks after hospital discharge among Taiwanese elderly patients with chronic obstructive pulmonary disease. J Nurs Res. 2002;10(3):168–76. doi: 10.1097/01.jnr.0000347596.74262.8a. [DOI] [PubMed] [Google Scholar]
- 39.Nicolson P, Anderson P. The patient’s burden: physical and psychological effects of acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 2000;45(suppl2):25–32. doi: 10.1093/jac/45.suppl_2.25. [DOI] [PubMed] [Google Scholar]
- 40.Odencrants S, Ehnfors M, Grobe SJ. Living with chronic obstructive pulmonary disease: part I. [Struggling with meal-related situations: experiences among persons with COPD]. Scand J Caring Sci. 2005;19(3):230–9. doi: 10.1111/j.1471-6712.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 41.Williams V, Bruton A, Ellis-Hill C, McPherson K. What really matters to patients living with chronic obstructive pulmonary disease? An exploratory study. Chron Respir Dis. 2007;4(2):77–85. doi: 10.1177/1479972307078482. [DOI] [PubMed] [Google Scholar]
- 42.Wilson DM, Ross C, Goodridge D, Davis P, Landreville A, Roebuck K. The care needs of community-dwelling seniors suffering from advanced chronic obstructive pulmonary disease. Can J Aging. 2008;27(4):347–57. doi: 10.3138/cja.27.4.347. [DOI] [PubMed] [Google Scholar]
- 43.Bentkover JD, Beresford J, Rogers B, Schachtner AE, Swensen A, Dastani HB. Patient, caregiver, and physician perceptions of management of chronic obstructive pulmonary disease: findings from qualitative research. Value Health. 2008;11(3) [Google Scholar]
- 44.Kerr A, Ballinger C. Living with chronic lung disease: an occupational perspective. J Occup Sci. 2010;17(1):34–9. [Google Scholar]
- 45.Ek K, Ternestedt B-M. Living with chronic obstructive pulmonary disease at the end of life: a phenomenological study. J Adv Nurs. 2008;62(4):470–8. doi: 10.1111/j.1365-2648.2008.04611.x. [DOI] [PubMed] [Google Scholar]
- 46.Jones I, Kirby A, Ormiston P, Loomba Y, Chan K-K, Rout J, et al. The needs of patients dying of chronic obstructive pulmonary disease in the community. Fam Pract. 2004;21(3):310–3. doi: 10.1093/fampra/cmh317. [DOI] [PubMed] [Google Scholar]
- 47.Hall S, Legault A, Côté J. Dying means suffocating: perceptions of people living with severe COPD facing the end of life. Int J Palliat Nurs. 2010;16(9):451–7. doi: 10.12968/ijpn.2010.16.9.78640. [DOI] [PubMed] [Google Scholar]
- 48.Seamark DA, Blake SD, Seamark CJ, Halpin DM. Living with severe chronic obstructive pulmonary disease (COPD): perceptions of patients and their carers. An interpretative phenomenological analysis. Palliat Med. 2004;18(7):619–25. doi: 10.1191/0269216304pm928oa. [DOI] [PubMed] [Google Scholar]
- 49.Duangpaeng S, Eusawas P, Laungamornlert S, Gasemgitvatana S, Sritanyarat W. Chronic dyspnea self-management of Thai adults with COPD. Thai J Nurs Res. 2002;6(4):200–15. [Google Scholar]
- 50.Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med. 2004;98(5):439–45. doi: 10.1016/j.rmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Gruffydd-Jones K, Langley-Johnson C, Dyer C, Badlan K, Ward S. What are the needs of patients following discharge from hospital after an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Prim Care Respir J. 2007;16(6):363–8. doi: 10.3132/pcrj.2007.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shackell BS, Jones RCM, Harding G, Pearse S, Campbell J. “Am I going to see the next morning?” A qualitative study of patients’ perspectives of sleep in COPD. Prim Care Respir J. 2007;16(6):378–83. doi: 10.3132/pcrj.2007.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey PH. The dyspnea-anxiety-dyspnea cycle—COPD patients’ stories of breathlessness: “It’s scary/when you can’t breathe”. Qual Health Res. 2004;14(6):760–78. doi: 10.1177/1049732304265973. [DOI] [PubMed] [Google Scholar]
- 54.Lomborg K, Bjorn A, Dahl R, Kirkevold M. Body care experienced by people hospitalized with severe respiratory disease. J Adv Nurs. 2005;50(3):262–71. doi: 10.1111/j.1365-2648.2005.03389.x. [DOI] [PubMed] [Google Scholar]
- 55.Torheim H, Gjengedal E. How to cope with the mask? Experiences of mask treatment in patients with acute chronic obstructive pulmonary disease-exacerbations. Scand J Caring Sci. 2010;24(3):499–506. doi: 10.1111/j.1471-6712.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- 56.Milne L, Moyle W, Cooke M. Hope: a construct central to living with chronic obstructive pulmonary disease. Int J Older People Nurs. 2009;4(4):299–306. doi: 10.1111/j.1748-3743.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 57.Fitzsimons D, Mullan D, Wilson JS, Conway B, Corcoran B, Dempster M, et al. The challenge of patients’ unmet palliative care needs in the final stages of chronic illness. Palliat Med. 2007;21(4):313–22. doi: 10.1177/0269216307077711. [DOI] [PubMed] [Google Scholar]
- 58.Gullick J, Stainton MC. Living with chronic obstructive pulmonary disease: developing conscious body management in a shrinking life-world. J Adv Nurs. 2008;64(6):605–14. doi: 10.1111/j.1365-2648.2008.04823.x. [DOI] [PubMed] [Google Scholar]
- 59.Bendtsen P, Leijon M, Sofie Sommer A, Kristenson M. Measuring health-related quality of life in patients with chronic obstructive pulmonary disease in a routine hospital setting: feasibility and perceived value. Health Qual Life Outcomes. 2003;1 doi: 10.1186/1477-7525-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farquhar M, Ewing G, Higginson IJ, Booth S. The experience of using the SEIQoL-DW with patients with advanced chronic obstructive pulmonary disease (COPD): issues of process and outcome. Qual Life Res. 2010;19(5):619–29. doi: 10.1007/s11136-010-9631-7. [DOI] [PubMed] [Google Scholar]
- 61.Jeon YH, Jowsey T, Yen L, Glasgow NJ, Essue B, Kljakovic M, et al. Achieving a balanced life in the face of chronic illness. Aust J Prim Health. 2010;16(1):66–74. doi: 10.1071/py09039. [DOI] [PubMed] [Google Scholar]
- 62.Chen K-H, Chen M-L, Lee S, Cho H-Y, Weng L-C. Self-management behaviours for patients with chronic obstructive pulmonary disease: a qualitative study. J Adv Nurs. 2008;64(6):595–604. doi: 10.1111/j.1365-2648.2008.04821.x. [DOI] [PubMed] [Google Scholar]
- 63.Cicutto L, Brooks D, Henderson K. Self-care issues from the perspective of individuals with chronic obstructive pulmonary disease. Patient Educ Couns. 2004;55(2):168–76. doi: 10.1016/j.pec.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271–7. doi: 10.1016/S0738-3991(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 65.Monninkhof E, van der Aa M, van der Valk P, van der Palen J, Zielhuis G, Koning K, et al. A qualitative evaluation of a comprehensive self-management programme for COPD patients: effectiveness from the patients’ perspective. Patient Educ Couns. 2004;55(2):177–84. doi: 10.1016/j.pec.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Nield M. Dyspnea self-management in African Americans with chronic lung disease. Heart Lung. 2000;29(1):50–5. doi: 10.1016/s0147-9563(00)90037-2. [DOI] [PubMed] [Google Scholar]
- 67.Jeon YH, Essue B, Jan S, Wells R, Whitworth JA. Economic hardship associated with managing chronic illness: a qualitative inquiry. BMC Health Serv Res. 2009;9(1):182. doi: 10.1186/1472-6963-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mars GMJ, Kempen GIJM, Mesters I, Proot IM, Van Eijk JTM. Characteristics of social participation as defined by older adults with a chronic physical illness. Disabil Rehabil. 2008;30(17):1298–308. doi: 10.1080/09638280701623554. [DOI] [PubMed] [Google Scholar]
- 69.Gysels M, Higginson IJ. Reconciling employment with caring for a husband with an advanced illness. BMC Health Serv Res. 2009;9(1):216. doi: 10.1186/1472-6963-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomsen D, Jensen BØ. Patients’ experiences of everyday life after lung transplantation. J Clin Nurs. 2009;18(24):3472–9. doi: 10.1111/j.1365-2702.2009.02828.x. [DOI] [PubMed] [Google Scholar]
- 71.Cowie L, Morgan M, White P, Gulliford M. Experience of continuity of care of patients with multiple long-term conditions in England. J Health Serv Res Policy. 2009;14(2):82–7. doi: 10.1258/jhsrp.2009.008111. [DOI] [PubMed] [Google Scholar]
- 72.Bailey PH, Tilley S. Storytelling and the interpretation of meaning in qualitative research. J Adv Nurs. 2002;38(6):574–83. doi: 10.1046/j.1365-2648.2000.02224.x. [DOI] [PubMed] [Google Scholar]
- 73.Heinzer MMV, Bish C, Detwiler R. Acute dyspnea as perceived by patients with chronic obstructive pulmonary disease. Clin Nurs Res. 2003;12(1):85–101. doi: 10.1177/1054773803238742. [DOI] [PubMed] [Google Scholar]
- 74.Kessler R, Ståhl E, Vogelmeier C, Haughney J, Trudeau E, Löfdahl C-G, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview based study. Chest. 2006;130(1):133–42. doi: 10.1378/chest.130.1.133. [DOI] [PubMed] [Google Scholar]
- 75.Michaels C, Meek PM. The language of breathing among individuals with chronic obstructive pulmonary disease. Heart Lung. 2004;33(6):390–400. doi: 10.1016/j.hrtlng.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Thomas LA. Effective dyspnea management strategies identified by elders with end-stage chronic obstructive pulmonary disease. Appl Nurs Res. 2009;22(2):79–85. doi: 10.1016/j.apnr.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 77.Yu DSF, Lee DTF, Wu J. The revolving door syndrome: the Chinese COPD patients’ perspectives. J Clin Nurs. 2007;16(9):1758–60. doi: 10.1111/j.1365-2702.2007.02089.x. [DOI] [PubMed] [Google Scholar]
- 78.Forbes MOS. Prolonged ventilator dependence: perspective of the chronic obstructive pulmonary disease patient. Clin Nurs Res. 2007;16(3):231–50. doi: 10.1177/1054773807302781. [DOI] [PubMed] [Google Scholar]
- 79.Crawford A. Respiratory practitioners’ experience of end-of-life discussions in COPD. Br J Nurs. 2010;19(18):1164–9. doi: 10.12968/bjon.2010.19.18.79049. [DOI] [PubMed] [Google Scholar]
- 80.Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Patients’ perspectives on physician skill in end-of-life care: differences between patients with COPD, cancer, and AIDS. Chest. 2002;122(1):356–62. doi: 10.1378/chest.122.1.356. [DOI] [PubMed] [Google Scholar]
- 81.Fried TR, O’Leary JR. Using the experiences of bereaved caregivers to inform patient- and caregiver-centered advance care planning. J Gen Intern Med. 2008;23(1):1602–7. doi: 10.1007/s11606-008-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reinke LF, Shannon SE, Engelberg RA, Young JP, Curtis JR. Supporting hope and prognostic information: nurses’ perspectives on their role when patients have life-limiting prognoses. J Pain Symptom Manage. 2010;39(6):982–92. doi: 10.1016/j.jpainsymman.2009.11.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reinke LF, Engelberg RA, Shannon SE, Wenrich MD, Vig EK, Back AL. Transitions regarding palliative and end-of-life care in severe chronic obstructive pulmonary disease or advanced cancer: themes identified by patients, families, and clinicians. J Palliat Med. 2008;11(4):601–9. doi: 10.1089/jpm.2007.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fried TR, Bradley EH. What matters to seriously ill older persons making end-of-life treatment decisions? A qualitative study. J Palliat Med. 2003;6(2):237–44. doi: 10.1089/109662103764978489. [DOI] [PubMed] [Google Scholar]
- 85.Gullick JG, Colleen Stainton M. Taking a chance: the experience of lung volume reduction procedures for chronic obstructive pulmonary disease. Chronic Illn. 2009;5(4):293–304. doi: 10.1177/1742395309349547. [DOI] [PubMed] [Google Scholar]
- 86.Borgsteede SD, Deliens L, Graafland-Riedstra C, Francke AL, van der Wal G, Willems DL. Communication about euthanasia in general practice: opinions and experiences of patients and their general practitioners. Patient Educ Couns. 2007;66(2):156–61. doi: 10.1016/j.pec.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Goodridge D, Duggleby W, Gjevre J, Rennie D. Caring for critically ill patients with advanced COPD at the end of life: a qualitative study. Intensive Crit Care Nurs. 2008;24(3):162–70. doi: 10.1016/j.iccn.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Goodridge D, Duggleby W, Gjevre J, Rennie D. Exploring the quality of dying of patients with chronic obstructive pulmonary disease in the intensive care unit: a mixed methods study. Nurs Crit Care. 2009;14(2):51–60. doi: 10.1111/j.1478-5153.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 89.Back AL, Young JP, McCown E, Engelberg RA, Vig EK, Reinke LF, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009 Mar 9;169(5):474–9. doi: 10.1001/archinternmed.2008.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41–9. doi: 10.1111/j.1525-1497.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borgsteede SD, Graafland-Riedstra C, Deliens L, Francke AL, van Eijk JT, Willems DL. Good end-of-life care according to patients and their GPs. Br J Gen Pract. 2006;56(552):20–6. [PMC free article] [PubMed] [Google Scholar]
- 92.Roberts CM, Seiger A, Buckingham R, Stone RA. Clinician perceived good practice in end-of-life care for patients with COPD. Palliat Med. 2008;22(7):855–8. doi: 10.1177/0269216308096722. [DOI] [PubMed] [Google Scholar]
- 93.Spence A, Hasson F, Waldron M, Kernohan G, McLaughlin D, Cochrane B, et al. Active carers: living with chronic obstructive pulmonary disease. Int J Palliat Nurs. 2008;14(8):368–72. doi: 10.12968/ijpn.2008.14.8.30771. [DOI] [PubMed] [Google Scholar]
- 94.Boyle AH. Living with a spouse with chronic obstructive pulmonary disease: the meaning of wives’ experiences. J Nurs Healthc Chronic Illn. 2009;1(4):273–82. [Google Scholar]
- 95.Gysels MH, Higginson IJ. Caring for a person in advanced illness and suffering from breathlessness at home: threats and resources. Palliat Support Care. 2009;7(2):153–62. doi: 10.1017/S1478951509000200. [DOI] [PubMed] [Google Scholar]
- 96.Bailey PH. Death stories: acute exacerbations of chronic obstructive pulmonary disease. Qual Health Res. 2001;11(3):322–38. doi: 10.1177/104973201129119136. [DOI] [PubMed] [Google Scholar]
- 97.Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care. 2003;1(4):337–44. doi: 10.1017/s1478951503030499. [DOI] [PubMed] [Google Scholar]
- 98.Bergs D. “The hidden client”-women caring for husbands with COPD: their experience of quality of life. J Clin Nurs. 2002;11(5):613–21. doi: 10.1046/j.1365-2702.2002.00651.x. [DOI] [PubMed] [Google Scholar]
- 99.Jonsdottir H. Research-as-if-practice: a study of family nursing partnership with couples experiencing severe breathing difficulties. J Fam Nurs. 2007;13(4):443–60. doi: 10.1177/1074840707309210. [DOI] [PubMed] [Google Scholar]
- 100.Kanervisto M, Kaistila T, Paavilainen E. Severe chronic obstructive pulmonary disease in a family’s everyday life in Finland: perceptions of people with chronic obstructive pulmonary disease and their spouses. Nurs Health Sci. 2007;9(1):40–7. doi: 10.1111/j.1442-2018.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 101.Cotterell P. Striving for independence: experiences and needs of service users with life limiting conditions. J Adv Nurs. 2008;62(6):665–73. doi: 10.1111/j.1365-2648.2008.04638.x. [DOI] [PubMed] [Google Scholar]
- 102.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;6077(1):1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lynn J. Serving patients who may die soon and their families: the role of hospice and other services. JAMA. 2001;285(7):925–32. doi: 10.1001/jama.285.7.925. [DOI] [PubMed] [Google Scholar]
- 104.Lunney JR, Lynn J, Folley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289(18):2387–92. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 105.Lehman R. How long can I go on like this? Dying from cardiorespiratory disease. Br J Gen Pract. 2004;54(509):892–3. [PMC free article] [PubMed] [Google Scholar]


