Abstract
Background
Generalized anxiety disorder (GAD) is a prevalent health condition which seriously affects both patient quality of life and the National Health System. The aim of this research was to carry out a post hoc cost-effectiveness analysis of the effect of pregabalin versus selective serotonin reuptake inhibitors (SSRIs)/serotonin norepinephrine reuptake inhibitors (SNRIs) in treated benzodiazepine-refractory outpatients with GAD.
Methods
This post hoc cost-effectiveness analysis used secondary data extracted from the 6-month cohort, prospective, noninterventional ADAN study, which was conducted to ascertain the cost of illness in GAD subjects diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. Benzodiazepine-refractory subjects were those who claimed persistent symptoms of anxiety and showed a suboptimal response (Hamilton Anxiety Rating Scale ≥ 16) to benzodiazepines, alone or in combination, over 6 months. Patients could switch to pregabalin (as monotherapy or addon) or to an SSRI or SNRI, alone or in combination. Effectiveness was expressed as quality-adjusted life years gained, and the perspective was that of the National Health System in the year 2008. A sensitivity analysis was performed using bootstrapping techniques (10,000 resamples were obtained) in order to obtain a cost-effectiveness plane and a corresponding acceptability curve.
Results
A total of 282 subjects (mean Hamilton Anxiety Rating Scale score 25.8) were identified, comprising 157 in a pregabalin group and 125 in an SSRI/SNRI group. Compared with SSRI/SNRI, pregabalin (average dose 163 mg/day) was associated with higher quality-adjusted life years gained (0.1086 ± 0.0953 versus 0.0967 ± 0.1003, P = 0.334), but increased health care costs (€1014 ± 762 versus €846 ± 620, P = 0.166) and drug costs (€376 ± 252 versus 220 ± 140, P < 0.001), resulting in an incremental cost-effectiveness ratio of €25,304 (95% confidence interval dominant 149,430) per quality-adjusted life years gained for health care costs and €25,454 (dominant 124,562) when drug costs were considered alone. Eighty-six percent of resamples fell below the threshold of €30,000 per quality-adjusted life years.
Conclusion
This evaluation suggests that pregabalin may be cost-effective in comparison with SSRIs/SNRIs in benzodiazepine-refractory outpatients with GAD treated in mental health care settings under usual medical practice in Spain.
Keywords: cost-effectiveness, generalized anxiety disorder, benzodiazepine-refractory patients, pregabalin, quality-adjusted life years
Introduction
Generalized anxiety disorder (GAD) is characterized by excessive, uncontrolled, and often irrational and disproportionate concern about daily issues.1 In addition to agitation, irritability, difficulty with concentration, muscle tension, sleep disturbance, and fatigue, patients also suffer from somatic anxiety symptoms, which can be associated with both psychiatric and organic disease.2 Usually, subjects experience symptoms for 5–10 years before being diagnosed with GAD and receiving appropriate treatment.3 Anxiety disorders are considered to be the most prevalent psychiatric health conditions, with GAD being one of the most common in primary care and mental health care settings,4–6 and have been reported to account for represent 13% of the conditions seen in psychiatric outpatient clinics.7,8 Normal functioning in people with GAD is substantially impaired,9,10 and affected patients have lower perceived quality of life and a lower degree of social functioning than patients with major health conditions.11 Misdiagnosis or underdiagnosis, which translates into late or inappropriate treatment, can lead to a vicious circle of exacerbated existing illness and the development of new illnesses, fostering further anxiety, demoralization, and depression. The chronic nature of GAD and the vicious circle of medical and psychiatric conditions make GAD an anxiety disorder which causes considerable impairment, resulting in high use of health care resources.12–14 Thus, the burden of this disease is considerable for the individual and for the health care system.
Regardless of the GAD diagnosis, many patients consulting with anxiety symptoms remain symptomatic despite using treatments with an anxiolytic effect and, after a few months, may become refractory to therapy.15,16 Benzodiazepines have been shown to be useful for rapid, short-term relief of somatic symptoms of GAD,17 and are often used to help alleviate restlessness associated with initiation of antidepressant therapy. However, these agents are restricted to short-term use in many countries because of their potential for dependency. According to current treatment guidelines,1 effective pharmacotherapies that may be used on a long-term basis in patients with GAD include selective serotonin reuptake inhibitors (SSRIs), such as paroxetine, escitalopram, and sertraline, serotonergic noradrenergic reuptake inhibitors (SNRIs), such as venlafaxine and duloxetine, and a calcium channel modulators, pregabalin. Pregabalin is a third-generation anticonvulsant drug licensed for the treatment of GAD in Europe.18–21
Nowadays, health policy decision-makers should not only be aware of the clinical evidence of the effectiveness of a drug, but also of its financial implications, to be able to determine the efficiency of new treatments and thus make optimal use of existing limited economic resources. Economic evaluations are an appropriate method of estimating the economic consequences associated with management of anxiety disorders.22 Few formal economic evaluations of such agents have been reported in the literature published to date. The cost-effectiveness of pregabalin versus venlafaxine XR was recently examined from the point of view of the Spanish National Health System, but this evaluation used a simulation model and data from a short-term clinical trial,23 which health care decision-makers may consider as not being representative of real-world clinical practice. Thus, the objective of the present study is to explore the relative efficiency of pregabalin versus SSRIs and SNRIs in benzodiazepine-refractory outpatients with GAD treated according to current medical practice in mental health care settings in Spain.24
Methods
Data source
To perform this post hoc cost-effectiveness analysis, data were extracted from the 6-month, cohort ADAN (Amplification of Definition of ANxiety) study, which assessed the effect of broadening the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnostic criteria for GAD according to clinical evolution in the patients, resource utilization, and corresponding costs.24 The ADAN study was a multicenter, epidemiologic, noninterventional, prospective study conducted in Spanish psychiatric outpatient clinics between 2007 and 2008. Trained psychiatrists, with at least 5 years’ experience in the diagnosis of mental health diseases, who participated in the study were asked to select consecutive, newly diagnosed GAD patients, according to DSM-IV criteria25 and so-called broad criteria, until the predetermined sample size was obtained.
Men and women aged years 18 or over, who had provided their written informed consent to participate in the study, were refractory to previous benzodiazepine therapy, and had not been previously treated with pregabalin were included regardless of their previous treatment. For the cost-effectiveness analysis shown here, only data from patients with a standard diagnosis of GAD according to DSM-IV criteria were included. The reason for doing this is that, to date, broader criteria are still pending acceptance by the scientific community in a new version of the Diagnostic and Statistical Manual of Mental Disorders (ie, the DSM-V), so such broader criteria are tentative only. Benzodiazapine-refractory patients was defined as having persistent symptoms and/or suboptimal response, a Hamilton Anxiety Rating Scale (HAM-A) scale26,27 score ≥ 16, and a Clinical Global Impression-Severity (CGI-S) score 28 ≥ 3 at baseline after a course of any standard-dose benzodiazepine regimen, alone or in combination, given for at least 6 months prior to the baseline study visit. In addition to the main objective, the ADAN study also assessed self-perceived health-related quality of life using the EQ-5D questionnaire,29,30 use of health care resources, and related costs.
Economic model design and patient data extraction procedure
A cost-effectiveness analysis was performed in this study. A cost-effectiveness analysis is a comparative analysis which has the purpose of estimating the ratio between the relative expenditure (cost) of a health-related intervention and the outcomes (effectiveness) it produces. Cost-effectiveness is typically expressed as an incremental cost-effectiveness ratio (ICER), ie, the ratio between the difference in costs and health benefits of two interventions. A threshold, or willingness to pay value, is often set by policy-makers, who may decide that only interventions within a given ICER threshold range are cost-effective, although decisions on funding may be more complex and subject to additional factors. In Spain, there is generally no accepted cost-effectiveness threshold value. However, an ICER ≤ €30,000 per quality-adjusted life year (QALY) gained is usually considered cost-effective.31
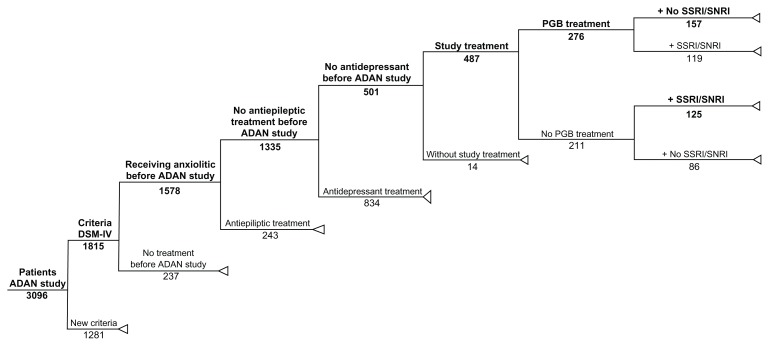
To conduct this post hoc cost-effectiveness analysis, two groups were identified for analysis from the ADAN trial,24 which included patients who met the DSM-IV criteria for the diagnosis of GAD. Subjects in both cohorts were benzodiazepine-refractory and consecutively selected from the blinded study database. Patients receiving pregabalin (as monotherapy or addon to the existing benzodiazepine treatment) from the baseline visit were used to form a pregabalin group, and subjects receiving SSRI/SNRI drugs were used to form an SSRI/SNRI group. The SSRI/SNRI drugs, included paroxetine, venlafaxine, escitalopram, duloxetine, mirtazapine, sertraline, fluoxetine, and citalopram, at the clinical discretion of the treating physicians. The patient disposition is shown in Figure 1.
Figure 1.
Tree decision model with extraction of data from the original ADAN cohort study.
Abbreviations: ADAN, Amplification of Definitiion of ANxiety; Criteria DSM-IV, GAD diagnosis according to DSM-IV criteria; DSM-N, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; New Criteria, GAD diagnosis according to new ADAN study criteria; GAD, generalized anxiety disorder; PGB, Pregabalin; SSRI, Selective Serotorin Reuptake Inhibitors; SNRI, Serotonin Norepinephine Reuptake Inhibitors.
Resource utilization and costs
Cost refers to the resources used for the intervention, usually measured in monetary terms, such as Euros. The cost of each treatment arm is equal to the sum of purchased medical and nonmedical resources used and unpurchased resources, such as the patient’s loss of productivity or unpaid family member/caregiver support (called “productivity costs” or “indirect costs”). For this financial evaluation, we selected a third party payer perspective, which is the one from the Spanish national health care system for the year 2008.32 Therefore, only health care resource utilization and corresponding costs were computed. The time frame used in the model was 6 months, as in the ADAN study. Subsequently, no time discounts were applied. Health care resource utilization was recorded at baseline and at the end-of-trial visit by means of a health care resource utilization questionnaire designed ad hoc for this study, representing only the noninterventional nature of both the original cohort study and the cost-effectiveness analysis. These resources, all related to GAD, included medical visits (primary care, specialists, and emergency room visits), days of hospitalizations, drug treatment for symptoms of GAD, and nonpharmacological treatment for the condition, such as physiotherapy, psychotherapy, and relaxation techniques (see Table 1).
Table 1.
Unit costs (€) of health care resources (Spain)
| Resources | Unit cost (€) |
|---|---|
| Non pharmacological treatment (per session) | |
| Psychosocial therapya | 45.0 |
| Supportive groupsb | 23.0 |
| Cognitive-behavioral therapyd | 50.0 |
| Psychoanalytical therapyb | 50.0 |
| Occupational therapya | 12.1 |
| Drug addict rehabilitation therapyc | 45.0 |
| Couple therapye | 110.0 |
| Psychotherapy dialecticb | 45.0 |
| Physiotherapyb | 31.0 |
| Rehabilitationb | 30.0 |
| Nursing home (per daytime session)a | 33.4 |
| Acupuncturea | 30.0 |
| Medical (per visit) | |
| Primary care (or general practitioner)a | 10.2 |
| Psychotherapista | 45.0 |
| Psychiatrista | 67.3 |
| Emergency rooma | 121.6 |
| Hospitalization (one day) | |
| Hospital stay in Psychiatric warda | 272.8 |
Notes:
Oblikue, 2008. eSALUD. SOIKOS;33
Fremap, Mutua de Accidentes de Trabajo y Enfermedades Profesionales de la Seguridad Social número 61; 2008;
Hospital de la Santa Creu i San Pau, Barcelona, Spain;
AEPC, Spanish Association of Behavioral Psychology;
General Council of Colleges of Psychology of Spain.
The cost of health care resource utilization was calculated by multiplying the number of resources used during the study by its unit price (Table 1), and was expressed during a 6-month period before the visit when recording took place. Thus, from a cost perspective, the study included two visits, ie, baseline and end-of-trial visits. The Spanish pharmaceutical drug catalog for 2008 was used to obtain the unit price of drugs, where cost was estimated as retail price plus value added tax of the cheapest generic medication available, or cheapest pharmaceutical medicinal product when a generic medication or reference price was unavailable. The cost of nonpharmacological treatments, medical visits, and hospitalizations was obtained from the eSALUD33 health care costs database for 2008 (Table 1) updated with the 2008 inflation health care rate.34 Finally, some nonpharmacological resources were priced according to expert opinion and/or directly from the vendor/provider.
Effectiveness measures
The measurements used to determine the efficiency of treatments for this cost-effectiveness analysis during the 6 months of the study were derived from the ADAN trial and were expressed as QALY gained, calculated by trapezoidal approximation using time trade-off values from the Spanish version of the EQ-5D questionnaire.30 The other effectiveness measurements were the change in the Spanish version of the HAM-A scale,27 such as response rate (percentage of patients with a reduction ≥ 50% at end-of-trial in comparison with the baseline intensity of anxiety symptoms assessed using the HAM-A scale) and percentage of subjects without anxiety symptoms at end-of-trial (HAM-A ≤ 9 points).
The EQ-5D is a standardized health-related quality of life scale and is a generic self-reported measure of health used frequently in clinical and economic evaluations, the details of which are published elsewhere.30 The HAM-A is a 14-item scale, with a score between 0 (absence) and 4 (severe) that explores the patients’ degree of anxiety.26,27 The possible score ranges from 0 to 56 points and allows a global score and two subscales, one for psychic symptoms and the other for somatic symptoms.26,27 HAM-A scores enable patients to be classified scores in the following categories: ≤9 “no or minimal anxiety”; 10–15 “mild anxiety”; 16–24 “moderate anxiety”; and >24 “severe anxiety”. While these classifications have not been clinically validated, to the best of our knowledge, they have been previously used by others. 35,36 The CGI-S scale is a seven-point scale that requires the clinician to rate the severity of the patient’s illness at the time of assessment relative to the clinician’s past experience with patients who have the same diagnosis. Considering total clinical experience, a patient is assessed on severity of illness at the time of rating as: 1, normal, not at all ill; 2, borderline mentally ill; 3, mildly ill; 4, moderately ill; 5, markedly ill; 6, severely ill; or 7, extremely ill.28
Cost-effectiveness analysis
The cost-effectiveness analysis was expressed as the ICER. It was calculated by dividing the difference in costs between pregabalin and SSRI/SNRI and the difference of their effectiveness: ICER = (Costpregabalin – CostSSRI/SNRI)/( Effectivenesspregabalin – EffectivenessSSRI/SNRI). To estimate the mean ICER with a nonparametric 95% percentile confidence interval (CI), bootstrapping (10,000 resamples) techniques were applied to draw the cost-effectiveness plane and the ICER acceptability curve for the base-case scenario, in accordance with Spanish guidelines for economic evaluation of health technologies.37 The base-case scenario was the one using the original data observed in the trial and was used as a source to feed this cost-effectiveness analysis. This approach enabled us to obtain the percentage of replications of ICER below €30,000 per QALY gained, and therefore could be considered as a cost-effective intervention.31
Sensitivity analysis
Two approaches were used to perform the sensitivity analysis. First, a probabilistic sensitivity analysis was performed using bootstrapping (10,000 resamples) techniques. The probabilistic sensitivity analysis used the data of the base-case scenario to be replicated 10,000 times to generate a probabilistic distribution of possible ICERs; then, allowing the estimate of 95% CI, the cost-effectiveness plane and the cost-effectiveness acceptability curve. In a second phase, we conducted a set of univariate sensitivity analyses using different sensitive variables each time. The sensitive variables considered for the univariate sensitivity analyses were health care costs, (medical visits, hospitalization, nonpharmacological treatment, and drugs), QALY gained, and trial duration (from 6 to 12 months). The values of such variables used in the base-case scenario were varied by ±50% each time, and the cost-effectiveness analysis was then repeated with the new value using resample techniques to calculate new ICERs with nonparametric percentile CIs. These allowed us to see how robust the findings observed in the base-case scenario were.
Statistical analysis
Descriptive statistics were calculated for the continuous variables in the study, including the assessment of central tendency and dispersion statistics, with a 95% CI when possible. The Kolmogorov-Smirnov test was used to check whether data demonstrated a Gaussian distribution. In the study, patients were classified according to the severity of symptoms, with HAM-A scores ≤ 9 indicating “no or minimal anxiety”, 10–15 “mild anxiety”, 16–24 “moderate anxiety”, and >24 “severe anxiety”. The percentage of patients without anxiety (HAM-A ≤ 9) and the percentage considered to be responders (HAM-A reduction ≥ 50% compared with baseline score) were also calculated.
For categorical variables, absolute and relative frequencies were calculated. For comparisons, the Student’s t-test and Chi-square test were used for continuous and categorical variables, respectively. Analysis of covariance or binary logistic regression models were carried out comparing pregabalin versus SSRI/SNRI groups, adjusting for baseline scores (CGI and EQ-5D scores) comorbidities (percentage of patients with a comorbid depressive disorder), and sociodemographic (marital status) data. All statistical tests were two-tailed, and an α-error of <0.05 was accepted as statistically significant. Data were analyzed using SPSS version 17.0 (SPSS Inc, Chicago, IL).
Results
In this cost-effectiveness analysis, two groups (Table 2) were formed. First was the SSRI/SNRI group, not including pregabalin, composed of 125 patients (mean baseline HAM-A score 25.5 ± 7.4 points) who received SSRI/SNRI treatment, or a combination of such therapies with existing therapies at the beginning of the study. The mean number of drugs used during the study was 2.3 (95% CI, 2.2–2.5), with 29.6% of patients using lorazepam concomitantly, 24.8% alprazolam, 10.4% clonazepam, 9.6% diazepam, and 8.0% other benzodiazepines. Second was the pregabalin group, composed of 157 patients (mean baseline HAM-A score 26.1 ± 7.4 points) who were treated with flexible doses of pregabalin (<7 5 mg/day, 30.4% of patients; 75–149 mg/day, 42.6%; 150–300 mg/day, 23.7%; >300 mg/day, 3.4%; average dose 163 mg/day), in monotherapy or as an addon to existing treatment at the beginning of the study. The mean number of drugs in this group was 2.0 (95% CI, 1.9–2.2). Added to pregabalin, 16.5% of patients were also treated concomitantly with alprazolam, 15.2% with lorazepam, 14.0% with mirtazapine, and 12.1% with diazepam.
Table 2.
Baseline socio-demographics and clinical characteristics of the study series according to treatment group (ADAN study, reference 24)
| Variable | Pregabalin N =157a | SSRI/SNRI N =125a | P |
|---|---|---|---|
| Gender (women), n (%) | 89 (61.4%) | 70 (60.9%) | 0.933 |
| Age (years), mean (SD) | 45.7 (13.8%) | 44.1 (13.7%) | 0.340 |
| Body mass index (kg/m2), mean (SD) | 25.0 (3.7%) | 24.5 (3.9%) | 0.295 |
| Marital status, n (%) | 0.044 | ||
| Married/cohabiting | 92 (59.0%) | 82 (65.6%) | |
| Single | 35 (22.4%) | 108 (27.2%) | |
| Widow(er) | 13 (8.3%) | 13 (1.6%) | |
| Divorced/separated | 16 (10.3%) | 34 (5.6%) | |
| Educational level, n (%) | 0.849 | ||
| No education | 6 (3.8%) | 2 (1.6%) | |
| Primary education | 49 (31.2%) | 37 (29.8%) | |
| Secondary education | 33 (21.0%) | 25 (20.2%) | |
| Intermediate educational level | 30 (19.1%) | 28 (22.6%) | |
| Higher education (university) | 36 (22.9%) | 31 (25.0%) | |
| Others | 3 (1.9%) | 1 (0.8%) | |
| Work status, n (%) | 0.176 | ||
| Active | 89 (56.7%) | 76 (61.3%) | |
| Housewife | 30 (19.1%) | 25 (20.2%) | |
| Sick leave | 5 (3.2%) | 5 (4.0%) | |
| Unemployed | 16 (10.2%) | 6 (4.8%) | |
| Retired | 16 (10.2%) | 7 (5.7%) | |
| Does not work (students) | 1 (0.6%) | 3 (2.4%) | |
| Others | 0 (0.0%) | 2 (1.6%) | |
| Health scales | |||
| CGI score, mean (SD) | 4.2 (0.7) | 3.9 (0.7) | 0.024 |
| HAM-A score, mean (SD) | 26.1 (7.4) | 25.5 (7.4) | 0.666 |
| Severe (>24), n (%) | 96 (63.6%) | 63 (54.8%) | 0.091 |
| Moderate (16 < HAM-A ≤ 24), n (%) | 43 (28.5%) | 41 (35.7%) | 0.392 |
| EQ-5D | |||
| Utility value, mean (SD) | 0.4760 (0.2970) | 0.5533 (0.2839) | 0.042 |
| Health status (VAS), mean (SD) | 47.5 (15.9) | 51.0 (14.6) | 0.139 |
| Previous benzodiazepine, n (%) | |||
| Alprazolam | 49 (31.2%) | 31 (24.8%) | 0.292 |
| Lorazepam | 29 (18.0%) | 37 (29.6%) | 0.040 |
| Diazepam | 26 (16.5%) | 12 (9.6%) | 0.127 |
| Clonazepam | 25 (15.9%) | 13 (10.4%) | 0.240 |
| Bromazepam | 24 (15.2%) | 19 (15.2%) | 0.883 |
| Others | 12 (7.6%) | 10 (8.0%) | 0.910 |
Notes:
Total number of patients analyzed (some patients failed to report all data).
Abbreviations: ADAN, Amplification of Definition of Anxiety; SSRI, Selective Serotonin Reuptake Inhibitors; SNRI, Serotonin-Norepinephrine Reuptake Inhibitors; p, statistical significance; SD, standard deviation; VAS, visual analogue scale; CGI, Clinical Global Impression; HAM-A scale, Hamilton-Anxiety scale; EQ-5D, EuroQol-5 Dimensions Quality of Life scale.
Both groups were similar, from a statistical standpoint, with regard to main sociodemographic characteristics (Table 2). However, clinical characteristics at baseline showed statistically significant differences in health scales, such as CGI-S scores, with scores being higher for the pregabalin group (4.2 ± 0.7 versus 3.9 ± 0.7, P = 0.024), and the mean utility value and health status assessed by the EQ-5D questionnaire being lower for the pregabalin group (0.4760 ± 0.2970 versus 0.5533 ± 0.2839, P = 0.042), meaning that patients included in the pregabalin group started with a more severe baseline status than those in the SSRI/SNRI group (Table 2). In addition, at baseline, the group of patients assigned to pregabalin had been treated with a significantly higher number of antidepressants than the control group (22.3% versus 6.4%, P < 0.001).
Total health care costs and effectiveness values are included in Tables 3 and 4. Compared with SSRI/SNRI, pregabalin showed higher numerical QALY gains after 6 months of treatment (0.1086 ± 0.0953 versus 0.0967 ± 0.1003, P = 0.334) after adjusting for gender, age, comorbidities, and baseline values. Moreover, the percentage of patients showing a response (HAM-A reduction ≥ 50%) at end-of-trial was also numerically higher in the pregabalin group (71.5% versus 64.3%, odds ratio = 1.5 [0.9–2.7], P = 0.271), but significant differences were not observed in the percentage of subjects without anxiety at the end of the study. Total health care costs were numerically higher in the pregabalin group compared with the SSRI/SNRI group (€1014 ± 762 versus €846 ± 620, P = 0.166). This difference was mainly due to the medical visit costs and, as expected, the cost of drugs (Tables 3 and 4).
Table 3.
Health care costs and effects according to treatment group
| Variable | Pregabalin N = 157a | SSRI/SNRIN =125a | Adjusted difference/RRb | Pc |
|---|---|---|---|---|
| Health care costs (€), mean (SD) | 1,014 (762) | 846 (620) | 111.9 (−46.6; 270.4) | 0.166 |
| Medical visitsd | 398 (518) | 394 (392) | −40 (−149.6; 69.5) | 0.473 |
| Hospitalizationd | 2 (22) | 0 (0) | 2.3 (−1.4; 6.1) | 0.223 |
| Non pharmacological treatmentd | 239 (422) | 233 (325) | 2.8 (−72.4; 78.0) | 0.942 |
| Drugse | 376 (252) | 220 (140) | 135.8 (94.7; 176.9) | <0.001 |
| Effectiveness (%) | ||||
| HAM-A score change with therapy | ||||
| Mean change (%) at end-of-trial | −64.8% | −63.7% | −1.0 (−7.8; 5.8) | 0.767 |
| Patients without anxiety (HAM-A ≤ 9), % | 55.0% | 55.7% | 1.2 (0.7; 2.1) | 0.552 |
| Responders (HAM-A reduction ≥50%), % | 71.3% | 64.0% | 1.1 (1.0; 1.3) | 0.189 |
| CGI mean variation (SD) at end-of-trial | −1.9 (1.0) | −1.8 (1.0) | 0.1 (−0.2; 0.3) | 0.470 |
| QALYs gained, mean (SD) | 0.1086 (0.0953) | 0.0967 (0.1003) | 0.008 (−0.015; 0.032) | 0.487 |
Notes:
Total number of patients analyzed; some patients failed to report all data;
adjusted by age, sex, co-morbidities and baseline values when applicable or adjusted relative risk (RR), in parenthesis, 95% confidence interval;
power was <80% in all comparison;
see table 1 for sources of unit cost sources;
Pharmaceutical price list from the Catálogo del Consejo General de Colegios Farmacéuticos de España, BOT base de datos del medicamento 2008.
Abbreviations: SSRI, Selective Serotonin Reuptake Inhibitors; SNRI, Serotonin-Norepinephrine Reuptake Inhibitors; p, statistical significance; SD, standard deviation; Health care costs, (medical visits + hospitalization + non pharmacological treatments + drugs) costs; HAM-A scale, Hamilton-Anxiety scale; CGI, Clinical Global Impression; QALY gained, quality-adjusted life year gained.
Table 4.
Cost-effectiveness analysis
| Variable | Pregabalin N = 157a | SSRI/SNRI N = 125a | Differenceb |
|---|---|---|---|
| Total health care costs (€), mean (95% CI) | 1,014 (894; 1,135) | 846 (736; 956) | 112 (−40; 270) |
| Total drug costs (€), mean (95% CI) | 376 (338; 415) | 220 (195; 244) | 136 (95; 177) |
| Effectiveness (QALY gained), mean (95% CI) | 0.1086 (0.0929; 0.1244) | 0.0967 (0.0781; 0.1154) | 0.008 (−0.015; 0.032) |
| Probabilistic ICERhealthcare cost (€)c | 25,304 (dominant; 149,430) | ||
| Probabilistic ICERdrugs (€)c | 25,454 (dominant; 124,562) |
Notes:
Total number of patients analyzed (some patients failed to report all data);
adjusted by age, sex, co morbidities and baseline values when applicable;
ICER calculated with bootstrapping techniques after 10,000 resamples with its 95% CI percentile.
Abbreviations: SSRI, Selective Serotonin Reuptake Inhibitors; SNRI, Serotonin-Norepinephrine Reuptake Inhibitors; QALY gained, quality-adjusted life year gained; ICER, Incremental Cost-Effectiveness Ratio.
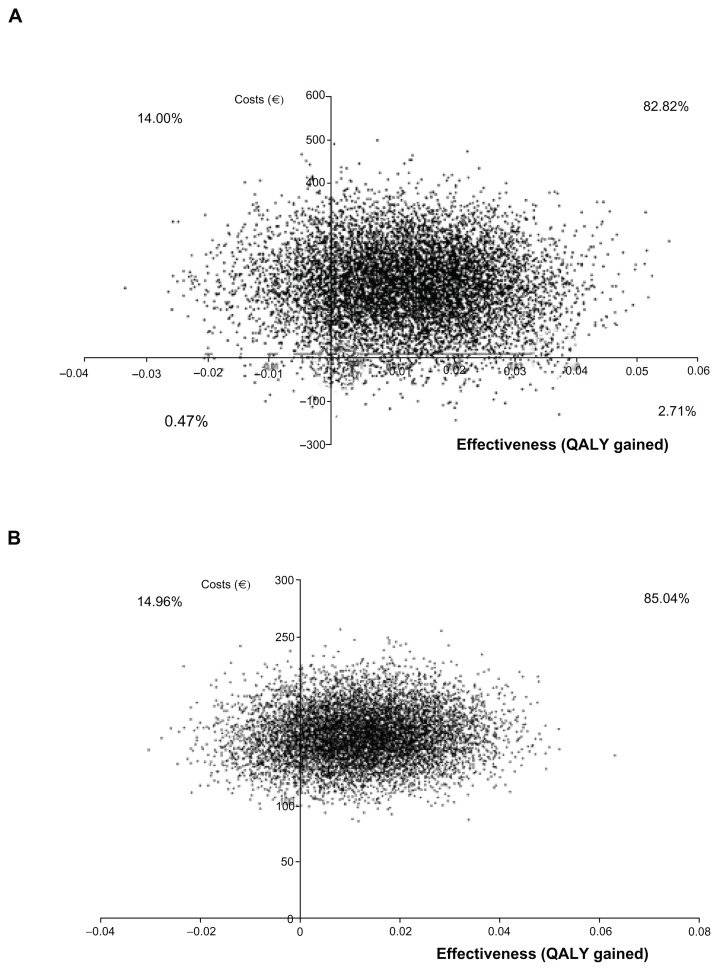
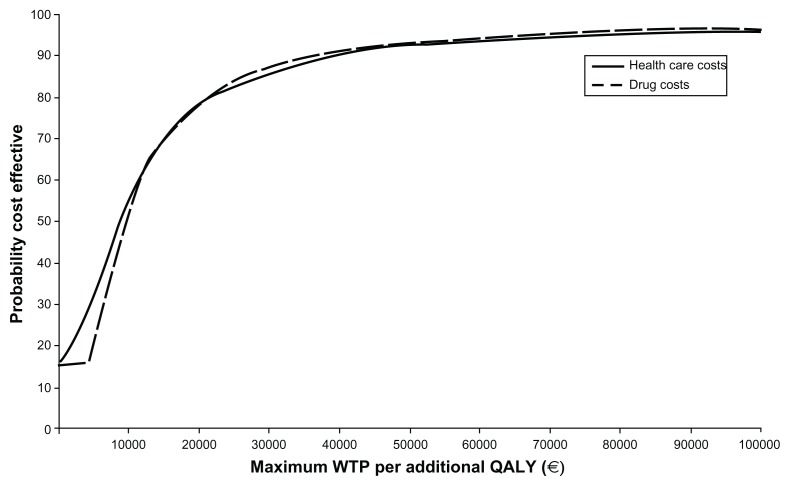
The probabilistic ICER of pregabalin over SSRI/SNRI drugs in total health care costs was €25,304 per QALY gained (95% CI dominant, 149,430, Table 4). The cost-effectiveness plane (Figure 2) showed that 68.82% of the resamples fell in the upper right quadrant (higher health care costs and more QALY gained). The cost-effectiveness acceptability curve constructed with those resamples showed that 86% fell under the willingness-to-pay threshold of €30,000 per QALY (Figure 3). In terms of drug costs only, the probability ICER after 10,000 resamples was €25,454 per QALY gained (95% CI dominant, 124,562), with 85.0% of samples in the upper right quadrant (Figure 2). In this case, 86% of the samples fell under the willingness-to-pay threshold of €30,000 per QALY gained (Figure 3).
Figure 2.
Cost-effectiveness plane for total health care cost (graph A) and total drug cost (graph B) of pregabalin over SSRI/SNRI.
Abbreviations: Total health care costs, (medical visits + hospitalization + non pharmacological treatments + drugs) costs; QALY gained; quality-adjusted life year gained. SSRI, Selective Serotonin Reuptake Inhibitors; SNRI, Serotonin-Norepinephrine Reuptake Inhibitors.
Figure 3.
Cost-effectiveness acceptability curve.
Abbreviations: WTP, Willingness to pay; QALY, Quality-Adjusted Life Year; Health care costs, (medical visits + hospitalization + non pharmacological treatments + drugs) costs.
Sensitivity analysis
The sensitivity analysis indicated the ICER to be robust as the main variable of the study range ±50% of the base-case value and after obtaining 10,000 bootstrap resamples in each case (Table 5). In most new scenarios, the probability of pregabalin being cost-effective was above 0.86 when the willingness-to-pay threshold was set up €30,000 per QALY, except when drug costs were multiplied by 1.5 (probability decreased to 0.78), or when the study time horizon increased up to 12 months (probability 0.80), which could still be considered cost-effective. When QALY gained was multiplied by 0.5 (0.50 reduction of the base-case scenario), the probability of pregabalin being cost-effective for a willingness-to-pay threshold of €30,000/QALY dropped to 70%.
Table 5.
Results of sensitivity analyses on key model assumptions and parameter estimates
| Variable | Difference in health care cost/QALY gain (2008 €) [mean (95% CI)] | % re-samples below a WTP of €30,000 |
|---|---|---|
| Medical visit costs (€) | ||
| ×0.5 basecase | 21,754 (dominant; 130,557) | 86% |
| ×1.5 basecase | −40,149 (dominant; 133,765) | 86% |
| Non pharmacological treatment costs (€) | ||
| ×0.5 basecase | 16,019 (dominant; 125,344) | 86% |
| ×1.5 basecase | −15,276 (dominant; 133,462) | 86% |
| Drug costs (€) | ||
| ×0.5 basecase | −12,383 (dominant; 80,128) | 92% |
| ×1.5 basecase | 36,619 (dominant; 213,692) | 78% |
| QALY gained | ||
| ×0.5 basecase | −17,605 (dominant; 229,569) | 70% |
| ×1.5 basecase | 9,550 (dominant; 89,653) | 90% |
| Extension of the trial to 12 months | 25,864 (dominant; 172,280) | 80% |
Abbreviations: QALY gained, quality-adjusted life year gained; WTP, Willingness to pay.
In this evaluation, we also analyzed the group of subjects treated with pregabalin in a subanalysis comparing pregabalin in monotherapy versus pregabalin as an addon therapy, with the aim of determining whether a difference in cost or health benefit exists between these two treatment populations at baseline and at end-of-trial. This subanalysis (data not shown) found that both populations, although unequal, were similar and comparable at baseline and at end-of-trial in terms of improved quality of life or QALY gain. The only difference, as expected, was drug costs, which were higher for patients treated with pregabalin plus other additional antianxiety treatments, compared with those treated with pregabalin alone.
Discussion
This paper estimates the cost-effectiveness of treatment for GAD with pregabalin or SSRI/SNRI drugs in benzodiazepine- refractory patients in order to provide health decision-makers with insights on the efficiency of these therapies. The design used in this evaluation allowed us to run the analysis in GAD patient cohorts with similar sociodemographic and clinical characteristics and, interestingly, to collect real world data from such patients. This is of particular interest for health decision-makers who have the opportunity to support their estimations and decisions in realistic data, instead of findings calculated from simulations, modeling, or clinical trials only.
Although SSRIs, SNRIs, and pregabalin are recommended as the primary approaches for treating GAD, benzodiazepines are still widely used.16 Benzodiazepines have a rapid onset of action, but it is recognized by regulatory authorities and clinical guidelines that their potential for abuse and dependence limits their use.17,38 Long-term benzodiazepine use needs to be monitored for signs of both dependence and tolerance. In addition, many patients still remain symptomatic, or fail to respond at all. The existing data and clinical experience suggest that alternative or adjunctive use of benzodiazepines added to antidepressants or anticonvulsants, such as pregabalin, are reasonable strategies to consider after weighing their associated risk profile.16 In this population, adding pregabalin to the treatment of such benzodiazepine-refractory patients compared with adding SSRI/SNRI drugs showed that treating subjects with pregabalin would cost about €112 more per patient in health care costs over a 6-month period. However, this incremental cost was accompanied by better results in terms of both increased clinical response (better reduction of anxiety symptoms and percentage of responders), and much better quality of life as assessed by QALY gain (ie, lower trade-off of years of perfect health), resulting in an affordable increased cost of €25,304 per QALY gained, which in our health care context used is considered to be a costeffective intervention.31 When this operation was repeated to obtain 10,000 samples for management of the level of uncertainty, the robustness of the results was confirmed in most scenarios included in the sensitivity analysis carried out; most of the new incremental cost-effectiveness ratios fell below the willingness-to-pay threshold of €30,000 per QALY. This indicates that treatment with pregabalin seems to be cost-effective compared with SSRI/SNRI treatment in benzodiazepine-refractory outpatients with GAD in most clinical situations. As expected, the only exception was when the QALY gain was reduced by 50% or when the drug costs are increased again by 50%, even though those scenarios were considered highly unlikely.
In this evaluation, we also analyzed the group of subjects treated with pregabalin in a subanalysis comparing pregabalin in monotherapy versus pregabalin as an addon therapy, with the aim of determining whether a difference in cost or health benefit exists between these two treatment populations. Because this subanalysis found that both subgroups obtained a similar and comparable QALY gain, with the only expected difference being in drug costs (higher for patients treated with pregabalin plus other additional antianxiety treatments compared with pregabalin alone), the question arises regarding whether the effectiveness of “therapy additional to pregabalin” in these patients was similar to that of the other group during the 6 months of the study. If the cost of this additional medication, which apparently does not provide higher effectiveness, was avoided, the result of the cost-effectiveness analysis and therefore treatment with pregabalin alone, rather than with an SSRI/SNRI drug, would still be more cost-effective. However, due to the fact that the sample size was small (n = 56) and patients were not randomized to the two groups, further studies with larger sample should be carried out to confirm this hypothesis.
Reviewing the literature on cost-effectiveness studies in anxiety disorders to date, we found eight cost-effectiveness analyses published on panic disorder, five on GAD, one on social phobia, one on post-traumatic stress disorder, and another involving several anxiety disorders.22 Of the five cost-effectiveness analyses focusing on GAD,23,39–42 three used DSM-IV diagnostic criteria for GAD (the others used ICD-10 codification), and only one of them presented QALY gain as a measure of effectiveness, then incorporating both quantity and quality of life in a combined summary effectiveness measurement. Guest et al examined the cost-effectiveness of venlafaxine XL versus diazepam over 6 months from the perspective of the UK National Health Service. They used a deterministic decision analytic model and reported an estimate of the incremental cost for each additional patient successfully treated with venlafaxine XL (versus diazepam), and an incremental cost for each additional patient in whom a relapse would be avoided at 2000/2001 price levels. They concluded that starting treatment with venlafaxine rather than diazepam was more effective clinically and cost-effective for managing nondepressed patients with GAD in the UK. The investigators’ CGI improvement score was used as the key clinical measure in the model, but the authors recognized that CGI measurements might be less robust than HAM-A scores.41 Jörgensson et al used a similar model and reported higher rates of first-line treatment success and lower discontinuation rates due to adverse events over 9 months in patients treated with escitalopram (versus paroxetine), as well as cost savings (at 2004 price levels) from a societal perspective. Treatment success and relapse were defined in the model using the CGI alone or in combination with HAM-A threshold values and evidence of treatment discontinuation due to lack of efficacy. The authors did not report estimates of the cost-effectiveness of escitalopram versus paroxetine.42 Therefore, there is only one published cost-effectiveness analysis using DSM-IV diagnostic criteria for GAD, QALY gain as a measure of effectiveness, and a third party payer’s perspective comparing pregabalin with an SSRI/SNRI drug.23 A particular strength was its stochastic (as opposed to deterministic) nature, which takes into consideration the uncertainty inherent in estimates of the average change in HAM-A scores with treatment. However, the data used were obtained from the short-term (8-week) PEACE (Pregabalin Efficacy in Anxiety Clinical Evaluation) trial.43
The strengths of our study are that it is the first analysis involving all the perspectives mentioned above, uses a representative sample of 282 patients and, importantly, captures real-world outcomes over a period of 6 months. In terms of cost, comparing the total health care costs shown by our analysis with those in the studies cited above, our analysis seems more complete because the costs of all medical visits and hospitalization were included, in addition to nonpharmacological treatment. As pointed out in a systematic review published in 2009,44 it would be useful to agree on measures of effectiveness used in cost-effectiveness analysis, eg, standard measures of QALY gained, or perhaps disability-adjusted life years avoided, which is recommended in most guidelines for financial evaluation and assessment of health technologies.39,45,46 Assuming that any analysis of the real situation carries some degree of uncertainty, a sensitivity analysis of 10,000 samples using bootstrapping techniques was carried out to minimize errors and increase the certainty of cost estimates and QALY gained over the total duration of the study, thereby determining the robustness of the analysis and its conclusions.
However, some limitations of our cost-effectiveness analysis approach should be noted. First is the observational design of the original cohort data source, ie, the ADAN study, with its inherent limitations, in particular, the fact that it was not a clinical trial. However, rather than just being considered a methodological weakness of our analysis, it could also be accepted as an advantage for payers or for the National Healthcare Service because the study was based on real-world data enabling health decision-makers to estimate actual costs and thereby improve resource utilization. Second, the study sample size could be considered small, with power below 80% in effectiveness comparisons, meaning that the study may be limited in terms of guaranteeing than differences in effectiveness could exist between the two groups of GAD therapy analyzed in this study. However, the outcomes used in the cost-effectiveness analysis were similar (and not different from a statistical perspective) at baseline. Despite the pregabalin group being more severely affected at baseline, after 6 months of treatment they had a better quality of life gain than the group treated with SSRI/SNRI drugs, indicating that the randomized controlled trial did demonstrate a real-world clinical benefit for pregabalin, and appears to provide benefit additional to that seen in patient treated with SSRI/SNRI drugs. Third, the patients included in this analysis were benzodiazepine-refractory and met specific criteria in the ADAN study protocol. Although there is no consensus in the scientific community on how to define refractory criteria, the criteria used here seem to fall within the scope of that used in other reports in the literature.3,46,48 Moreover, our cost-effectiveness analysis could be criticized for focusing on benzodiazepine-refractory outpatients without including other types of patients with GAD. While this is true, most patients with GAD seen on an outpatient basis in psychiatry clinics in Spain fall in the refractory subtype, so the results of this cost-effectiveness analysis would therefore be applicable to a considerable number of subjects in seen in standard psychiatry clinics. Another possible limitation is the fact this evaluation was only from the perspective of the Spanish national health system, and did not include indirect costs resulting from loss of productivity, which could be significant for patients with GAD, nor did it include out-of-pocket costs or resources paid for by the patients themselves. The main reason for this is that, in the Spanish health care context, the national health system is more concerned about the costs of the resources it funds rather than the costs of components which do not fall within its scope of coverage. Moreover, due to the absence of specific questions in the patient diary during the study regarding source of funding, some of the used or prescribed nonpharmacological treatments in this study could have been paid for by the patient, such as some types of massage, acupuncture sessions, yoga/tai chi sessions, and naturopathy.
In conclusion, despite the limitations of the analysis, pregabalin appears to be cost-effective in comparison with SSRI/SNRI drugs in the treatment of benzodiazepine- refractory outpatients with a DSM-IV diagnosis of GAD treated in mental health centers in usual medical practice in Spain.
Footnotes
Disclosure
Data collection and analysis were funded by Pfizer Inc. AB and JR are employees of Pfizer SLU. MdS-C was an employee of Trial Form Support, a consultancy agency engaged by Pfizer Inc, at the time of conducting this research. MdS-C is an employee of Pfizer SLU at present. The other authors report no conflicts of interests in this work.
References
- 1.Bandelow B, Zohar J, Hollander E, Kasper S, Möller HJ WFSBP task force on treatment guidelines for anxiety obsessive-compulsive post-traumatic stress disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders – first revision. World J Biol Psychiatry. 2009;4:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- 2.Judd LL, Kessler RC, Paulus MP, Zeller PV, Wittchen HU, Kunovac JL. Comorbidity as a fundamental feature of generalized anxiety disorders: results from the National Comorbidity Study (NCS) Acta Psychiatr Scand Suppl. 1998;393:6–11. doi: 10.1111/j.1600-0447.1998.tb05960.x. [DOI] [PubMed] [Google Scholar]
- 3.Ballenger JC, Davidson JR, Lecrubier Y, et al. Consensus statement of generalized anxiety disorder from the international consensus group on depression and anxiety. J Clin Psychiatry. 2001;62( Suppl 11):53–58. [PubMed] [Google Scholar]
- 4.Wittchen HU, Kessler RC, Beesdo K, Krause P, Hofler M, Hoyer J. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63( Suppl 8):24–34. [PubMed] [Google Scholar]
- 5.Alonso J, Angermeyer MC, Bernert S, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(Suppl 420):21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Wittchen HU. Patterns and correlates of generalized anxiety disorder in community samples. J Clin Psychiatry. 2002;63( Suppl 8):4–10. [PubMed] [Google Scholar]
- 7.Chocron Bentata L, Vilalta Franch J, Legazpi Rodriguez I, Auquer K, Franch L. Prevalence of psychopathology at a primary care center. Aten Primaria. 1995;16:586–590. Spanish. [PubMed] [Google Scholar]
- 8.Caballero L, Bobes J, Vilardaga I, Rejas J. Clinical prevalence and reason for visit of patients with generalized anxiety disorder seen in the psychiatry out-patient clinics in Spain. Results of the LIGANDO study. Actas Esp Psiquiatr. 2009;37:17–20. Spanish. [PubMed] [Google Scholar]
- 9.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 10.Lieb R, Becker E, Altamura C. The epidemiology of generalized anxiety disorder in Europe. Eur Neuropsychopharmacol. 2005;15:445–452. doi: 10.1016/j.euroneuro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Katzman MA. Current considerations in the treatment of generalized anxiety disorder. CNS Drugs. 2009;23:103–120. doi: 10.2165/00023210-200923020-00002. [DOI] [PubMed] [Google Scholar]
- 12.Andlin-Sobocki P, Wittchen HU. Cost of anxiety disorders in Europe. Eur J Neurol. 2005;12( Suppl 1):39–44. doi: 10.1111/j.1468-1331.2005.01196.x. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin TP, Khandker RK, Kruzikas DT, Tummala R. Overlap of anxiety and depression in a managed care population: prevalence and association with resource utilization. J Clin Psychiatry. 2006;67:1187–1193. doi: 10.4088/jcp.v67n0803. [DOI] [PubMed] [Google Scholar]
- 14.Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety. 2002;16:162–171. doi: 10.1002/da.10065. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MH. Refractory generalized anxiety disorder. J Clin Psychiatry. 2009;70( Suppl 2):32–38. doi: 10.4088/jcp.s.7002.06. [DOI] [PubMed] [Google Scholar]
- 16.Davidson JR. First-line pharmacotherapy approaches for generalized anxiety disorder. J Clin Psychiatry. 2009;70( Suppl 2):25–31. doi: 10.4088/jcp.s.7002.05. [DOI] [PubMed] [Google Scholar]
- 17.Rickels K, Downing R, Schweizer E, Hassman H. Antidepressants for the treatment of generalized anxiety disorder: a placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50:884–895. doi: 10.1001/archpsyc.1993.01820230054005. [DOI] [PubMed] [Google Scholar]
- 18.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 19.Dooley DJ, Donovan CM, Pugsley TA. Stimulus-dependent modulation of [(3)H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther. 2000;295:1086–1093. [PubMed] [Google Scholar]
- 20.Maneuf YP, Hughes J, McKnight AT. Gabapentin inhibits the substance P-facilitated K(+)-evoked release of [(3)H]glutamate from rat caudal trigeminal nucleus slices. Pain. 2001;93:91–96. doi: 10.1016/S0304-3959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 21.Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 22.Konnopka A, Leichsenring F, Leibing E, König HH. Cost-of-illness studies and cost-effectiveness analyses in anxiety disorders: a systematic review. J Affect Disord. 2009;114:14–31. doi: 10.1016/j.jad.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Vera-Llonch M, Dukes E, Rejas J, Sofrygin O, Mychaskiw M, Oster G. Cost-effectiveness of pregabalin versus venlafaxine in the treatment of generalized anxiety disorder: findings from a Spanish perspective. Eur J Health Econ. 2010;11:35–44. doi: 10.1007/s10198-009-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Álvarez E, Carrasco JL, Olivares JM, et al. Broadening of generalized anxiety disorders definition does not affect the response to therapy: findings from the ADAN study. Eur Psychiatry. 2010;25(Suppl 1) doi: 10.2174/1745017901208010158. Abstract PW01-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Lobo A, Chamorro L, Luque A, Dal-Ré R, Badía X, Baró E. Validación de las versiones en espanol de la Montgomery-Asberg Depression Scale y la Hamilton Anxiety Rating Scale para la evaluación de la depresión y de la ansiedad. Med Clin (Barc) 2002;118:493–499. doi: 10.1016/s0025-7753(02)72429-9. Spanish. [DOI] [PubMed] [Google Scholar]
- 28.Guy W. ECDEU Assessment Manual for Psychopharmacology Revised. Rockville, MD: US Department of Health Education, and Welfare, Psychopharmacology Research Branch; 1976. [Google Scholar]
- 29.EuroQoL Group. EuroQoL – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 30.Badía X, Roset M, Montserrat S, Herdman M, Segura A. The Spanish version of EuroQoL: a description and its applications. European Quality of Life scale. Med Clin (Barc) 1999;112(Suppl 1):79–85. Spanish. [PubMed] [Google Scholar]
- 31.Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. Qué es una tecnología sanitaria eficiente en Espana? Gac Sanit. 2002;4:334–343. doi: 10.1016/s0213-9111(02)71933-x. Spanish. [DOI] [PubMed] [Google Scholar]
- 32.Catálogo del Consejo General de Colegios Farmacéuticos de Espana, BOT base de datos del medicamento 2008.
- 33.Social SCdEeEdlSyP., editor. Oblikue. eSALUD. Base de datos de costes sanitarios. Soikos; Barcelona: 2008. [Google Scholar]
- 34.INE. Spain 2008. [Accessed Aug 2008]. [ http://www.ine.es]
- 35.Revicki D, Brandenburg M, Matza LS, et al. Relationship between anxiety severity and health utility index scores in generalized anxiety disorder. Presented at the Collegium of Internationale Neuro-Psycharmacologicum; Chicago, IL. July 9–13, 2006. [Google Scholar]
- 36.Morlock R, Shah H, Feltner D, Brandenburg N, Matza LS, Revicki D. Estimation of health-state utilities for patients with generalized anxiety disorder. Presented at the Collegium of Internationale Neuro-Psycharmacologicum; Chicago, IL. July 9–13; 2006. [Google Scholar]
- 37.Bastida JL, Oliva J, Antonanzas F, et al. A proposed guideline for economic evaluation of health technologies. Gac Sanit. 2010;24:154–170. doi: 10.1016/j.gaceta.2009.07.011. Spanish. [DOI] [PubMed] [Google Scholar]
- 38.Gao K, Sheehan DV, Calabrese JR. Atypical antipsychotics in primary generalized anxiety disorder or comorbid with mood disorders. Expert Rev Neurother. 2009;9:1147–1158. doi: 10.1586/ern.09.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heuzenröeder L, Donnelly M, Haby MM, et al. Cost-effectiveness of psychological and pharmacological interventions for generalized anxiety disorder and panic disorder. Psychiatry. 2004;38:602–612. doi: 10.1080/j.1440-1614.2004.01423.x. [DOI] [PubMed] [Google Scholar]
- 40.Issakidis C, Sanderson K, Corry J, Andrews G, Lapsley H. Modelling the population cost-effectiveness of current and evidence-based optimal treatment for anxiety disorders. Psychol. Med. 2004;34:19–35. doi: 10.1017/s003329170300881x. [DOI] [PubMed] [Google Scholar]
- 41.Guest JF, Russ J, Lenox-Smith A. Cost-effectiveness of venlafaxine XL compared with diazepam in the treatment of generalized anxiety disorder in the United Kingdom. Eur J Health Econ. 2005;6:136–145. doi: 10.1007/s10198-004-0272-z. [DOI] [PubMed] [Google Scholar]
- 42.Jørgensen TR, Stein DJ, Despiegel N, Drost PB, Hemels ME, Baldwin DS. Cost-effectiveness analysis of escitalopram compared with paroxetine in treatment of generalized anxiety disorder in the United Kingdom. Ann Pharmacother. 2006;40:1752–1758. doi: 10.1345/aph.1H156. [DOI] [PubMed] [Google Scholar]
- 43.Baldinetti F, Bandelow B, Kasper S, et al. Efficacy of pregabalin and venlafaxine-XR in generalized anxiety disorder: results of a double-blind, placebo-controlled 8-week trial. Int Clin Psychopharmacol. 2009;24:87–96. doi: 10.1097/yic.0b013e32831d7980. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein MC, O’Brien B, Hornberger J, et al. ISPOR Task Force on Good Research Practices. Principles of good practice for decision analytic modelling in health care evaluation: report of the ISPOR Task Force on good research practices – modeling studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 45.National Institute for Clinical Excellence. Guide to the Methods of Technology Appraisal. London, UK: National Institute for Clinical Excellence; 2004. [PubMed] [Google Scholar]
- 46.Pollack MH, Otto MW, Roy-Byrne PP, et al. Novel treatment approaches for refractory anxiety disorders. Depress Anxiety. 2008;25:467–476. doi: 10.1002/da.20329. [DOI] [PubMed] [Google Scholar]
- 47.Simon NM, Connor KM, LeBeau RT, et al. Quetiapine augmentation of paroxetine CR for the treatment of refractory generalized anxiety disorder: preliminary findings. Psychopharmacology (Berl) 2008;197:675–681. doi: 10.1007/s00213-008-1087-x. [DOI] [PubMed] [Google Scholar]
- 48.Pollack MH, Simon NM, Zalta AK, et al. Olanzapine augmentation of fluoxetine for refractory generalized anxiety disorder: a placebo controlled study. Biol Psychiatry. 2006;59:211–215. doi: 10.1016/j.biopsych.2005.07.005. [DOI] [PubMed] [Google Scholar]