Executive Summary
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective
The objective of this evidence-based analysis was to determine the effectiveness and cost-effectiveness of smoking cessation interventions in the management of chronic obstructive pulmonary disease (COPD).
Clinical Need: Condition and Target Population
Tobacco smoking is the main risk factor for COPD. It is estimated that 50% of older smokers develop COPD and more than 80% of COPD-associated morbidity is attributed to tobacco smoking. According to the Canadian Community Health Survey, 38.5% of Ontarians who smoke have COPD. In patients with a significant history of smoking, COPD is usually present with symptoms of progressive dyspnea (shortness of breath), cough, and sputum production. Patients with COPD who smoke have a particularly high level of nicotine dependence, and about 30.4% to 43% of patients with moderate to severe COPD continue to smoke. Despite the severe symptoms that COPD patients suffer, the majority of patients with COPD are unable to quit smoking on their own; each year only about 1% of smokers succeed in quitting on their own initiative.
Technology
Smoking cessation is the process of discontinuing the practice of inhaling a smoked substance. Smoking cessation can help to slow or halt the progression of COPD. Smoking cessation programs mainly target tobacco smoking, but may also encompass other substances that can be difficult to stop smoking due to the development of strong physical addictions or psychological dependencies resulting from their habitual use.
Smoking cessation strategies include both pharmacological and nonpharmacological (behavioural or psychosocial) approaches. The basic components of smoking cessation interventions include simple advice, written self-help materials, individual and group behavioural support, telephone quit lines, nicotine replacement therapy (NRT), and antidepressants. As nicotine addiction is a chronic, relapsing condition that usually requires several attempts to overcome, cessation support is often tailored to individual needs, while recognizing that in general, the more intensive the support, the greater the chance of success. Success at quitting smoking decreases in relation to:
a lack of motivation to quit,
a history of smoking more than a pack of cigarettes a day for more than 10 years,
a lack of social support, such as from family and friends, and
the presence of mental health disorders (such as depression).
Research Question
What are the effectiveness and cost-effectiveness of smoking cessation interventions compared with usual care for patients with COPD?
Research Methods
Literature Search
Search Strategy
A literature search was performed on June 24, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations (1950 to June Week 3 2010), EMBASE (1980 to 2010 Week 24), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Library, and the Centre for Reviews and Dissemination for studies published between 1950 and June 2010. A single reviewer reviewed the abstracts and obtained full-text articles for those studies meeting the eligibility criteria. Reference lists were also examined for any additional relevant studies not identified through the search. Data were extracted using a standardized data abstraction form.
Inclusion Criteria
English-language, full reports from 1950 to week 3 of June, 2010;
either randomized controlled trials (RCTs), systematic reviews and meta-analyses, or non-RCTs with controls;
a proven diagnosis of COPD;
adult patients (≥ 18 years);
a smoking cessation intervention that comprised at least one of the treatment arms;
≥ 6 months’ abstinence as an outcome; and
patients followed for ≥ 6 months.
Exclusion Criteria
case reports
case series
Outcomes of Interest
≥ 6 months’ abstinence
Quality of Evidence
The quality of each included study was assessed taking into consideration allocation concealment, randomization, blinding, power/sample size, withdrawals/dropouts, and intention-to-treat analyses.
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria. The following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Summary of Findings
Nine RCTs were identified from the literature search. The sample sizes ranged from 74 to 5,887 participants. A total of 8,291 participants were included in the nine studies. The mean age of the patients in the studies ranged from 54 to 64 years. The majority of studies used the Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD staging criteria to stage the disease in study subjects. Studies included patients with mild COPD (2 studies), mild-moderate COPD (3 studies), moderate–severe COPD (1 study) and severe–very severe COPD (1 study). One study included persons at risk of COPD in addition to those with mild, moderate, or severe COPD, and 1 study did not define the stages of COPD. The individual quality of the studies was high. Smoking cessation interventions varied across studies and included counselling or pharmacotherapy or a combination of both. Two studies were delivered in a hospital setting, whereas the remaining 7 studies were delivered in an outpatient setting. All studies reported a usual care group or a placebo-controlled group (for the drug-only trials). The follow-up periods ranged from 6 months to 5 years. Due to excessive clinical heterogeneity in the interventions, studies were first grouped into categories of similar interventions; statistical pooling was subsequently performed, where appropriate. When possible, pooled estimates using relative risks for abstinence rates with 95% confidence intervals were calculated. The remaining studies were reported separately.
Abstinence Rates
Table ES1 provides a summary of the pooled estimates for abstinence, at longest follow-up, from the trials included in this review. It also shows the respective GRADE qualities of evidence.
Table ES1: Summary of Results*.
| Intervention | Comparison | Number of Studies | Abstinence Rate Pooled Relative Risk (95% CI) | GRADE |
|---|---|---|---|---|
| Counselling | Usual Care | 2 | 5.85 (3.81−8.97)† | Moderate |
| Intensive Counselling ≥ 90 minutes | Usual Care | 1 | 7.70 (4.64−12.79)† | |
| Minimal Counselling < 90 minutes | Usual Care | 1 | 1.56 (0.65−3.72) | |
| Counselling + NRT | Usual Care | 3 | 4.28 (3.51−5.20)† | Moderate |
| Intensive Counselling ≥ 90 minutes + | Usual Care | |||
| NRT | Usual Care | 1 | 4.41 (3.60−5.39)† | |
| Minimal Counselling < 90 minutes + | 2 | 2.11 (0.90−4.91) | ||
| NRT | ||||
| Minimal Counselling < 90 minutes + Antidepressant | Usual Care | 1 | 1.91 (0.65−5.61) | Low |
| Minimal Counselling < 90 minutes + NRT + Antidepressant | Usual Care | 1 | 2.25 (0.87−5.85) | Low |
| NRT | Placebo | 1 | 3.01 (1.02−8.89)† | Moderate |
| Antidepressant | Placebo‡ | 2 | 2.09 (1.35−3.24)† | Moderate |
| Nortriptyline | Placebo | 1 | 2.54 (0.87−7.44) | Moderate |
| Bupropion | Placebo | 2 | 2.01 (1.24−3.24)† |
Abbreviations: CI, confidence interval; NRT, nicotine replacement therapy.
Statistically significant (P < 0.05).
One trial used in this comparison had 2 treatment arms each examining a different antidepressant.
Conclusions
Based on a moderate quality of evidence, compared with usual care, abstinence rates are significantly higher in COPD patients receiving intensive counselling or a combination of intensive counselling and NRT.
Based on limited and moderate quality of evidence, abstinence rates are significantly higher in COPD patients receiving NRT compared with placebo.
Based on a moderate quality of evidence, abstinence rates are significantly higher in COPD patients receiving the antidepressant bupropion compared to placebo.
Background
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective of Analysis
The objective of this evidence-based analysis was to examine the effectiveness and cost-effectiveness of smoking cessation interventions for patients with chronic obstructive pulmonary disease (COPD).
Clinical Need and Target Population
Tobacco smoking is the main risk factor for COPD. It is estimated that 50% of older smokers develop COPD and more than 80% of COPD-associated morbidity is attributed to tobacco smoking. (1) Patients with COPD who smoke have a particularly high level of nicotine dependence, and about 30% to 43% of patients with moderate to severe COPD continue to smoke. (2;3) Despite the severe symptoms that COPD patients suffer, the majority of patients with COPD are unable to quit smoking on their own; each year only about 1% of smokers succeed in quitting on their own initiative. (4)
Technology
Smoking cessation can help to slow or halt the progression of COPD. Smoking cessation programs mainly target tobacco smoking, but may also encompass other substances that can be difficult to stop smoking due to the development of strong physical addictions or psychological dependencies resulting from habitual use. Smoking cessation strategies include both pharmacological and nonpharmacological (behavioural or psychosocial) approaches. The basic components of smoking cessation interventions include simple advice, written self-help materials, individual and group behavioural support, telephone quit lines, nicotine replacement therapy (NRT), and antidepressants.
Since addiction to nicotine is a chronic relapsing condition that usually requires several attempts to overcome, cessation support is usually tailored to individual needs, but with the recognition that, in general, the more intensive the support, the greater the chance of success. Success at quitting smoking decreases in relation to:
a lack of motivation to quit,
a history of smoking more than a pack of cigarettes a day for more than 10 years,
a lack of social support, such as from family and friends, and
the presence of mental health disorders (such as depression).
Based on self-reported data from the 2003 Canadian Community Health Survey, 38.5% of Ontarians who smoke have been diagnosed with COPD. (5)
Regulatory Status
As shown in Table 1, several drugs to treat nicotine dependence are licensed by Health Canada. The only over-the-counter drug available is the nicotine transdermal patch.
Table 1: Summary of Drugs for Nicotine Dependence Licensed by Health Canada*.
| Drug Identification Number | Product | Active Ingredient | Strength | Route of Administration |
|---|---|---|---|---|
| 02057743 | Prostep TRD patch (30 mg per 7 sq cm) | Nicotine | 30 mg | Transdermal |
| 02291177 | Champix | Varenicline (Varenicline Titrate) | 0.5 mg | Oral |
| 02291185 | Champix | Varenicline (Varenicline Titrate) |
1.0 mg | Oral |
| 02298309 | Champix (kit) | Varenicline (Varenicline Titrate) |
0.5 mg and 1.0 mg | Oral |
| 0263399 | Ava-Bupropion | Bupropion hydrochloride | 100 mg | Oral |
| 02363402 | Ava-Bupropion | Bupropion hydrochloride | 150 mg | Oral |
| 02325357 | Bupropion SR | Bupropion hydrochloride | 150 mg | Oral |
| 02331616 | Bupropion SR | Bupropion hydrochloride | 100 mg | Oral |
| 02260239 | Novo-Bupropion | Bupropion hydrochloride | 150 mg | Oral |
| 02313421 | PMS-Bupropion SR | Bupropion hydrochloride | 150 mg | Oral |
| 02325373 | PMS-Bupropion | Bupropion hydrochloride | 100 mg | Oral |
| 02285657 | Ratio-Bupropion | Bupropion hydrochloride | 100 mg | Oral |
| 02285665 | Ratio-Bupropion SR | Bupropion hydrochloride | 150 mg | Oral |
| 02275074 | Sandoz Bupropion SR | Bupropion hydrochloride | 100 mg | Oral |
| 03375082 | Sandoz Bupropion SR | Bupropion hydrochloride | 100 mg | Oral |
| 02237825 | Wellbutrin SR | Bupropion hydrochloride | 150 mg | Oral |
| 02275090 | Wellbutrin XL | Bupropion hydrochloride | 150 mg | Oral |
| 02275104 | Wellbutrin XL | Bupropion hydrochloride | 150 mg | Oral |
| 02238441 | Zyban | Bupropion hydrochloride | 300 mg | Oral |
Abbreviations: SR, sustained release; XL, extended release.
Evidence-Based Analysis
Research Question
What are the effectiveness and cost-effectiveness of smoking cessation interventions compared with usual care for patients with COPD?
Research Methods
Literature Search
Search Strategy
A literature search was performed on June 24, 2010 using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations (1950 to June Week 3 2010), OVID EMBASE (1980 to 2010 Week 24), the EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from 1950 to June 2010. The detailed literature search strategy is shown in Appendix 1.
A single reviewer reviewed the abstracts and obtained full-text articles for those studies meeting the eligibility criteria. The reviewer also examined the reference lists for any additional relevant studies not identified through the search. Data were extracted using a standardized data abstraction form.
Inclusion Criteria
English-language, full reports from 1950 to week 3 of June, 2010;
either randomized controlled trials (RCTs), systematic reviews and meta-analyses, or non-RCTs with controls;
a proven diagnosis of COPD;
adult patients (≥ 18 years);
a smoking cessation intervention that comprised at least one of the treatment arms;
≥ 6 months’ abstinence as an outcome; and
patients followed for ≥ 6 months.
Exclusion Criteria
case reports
case series
Outcomes of Interest
≥ 6 months’ abstinence
Statistical Analysis
Due to substantial clinical heterogeneity across smoking cessation interventions, studies were first grouped into categories of interventions and then pooling was performed where appropriate. Pooled estimates (relative risks [RR] for abstinence with 95% confidence intervals [CI]) were calculated using a fixed-effects model. The remaining studies were reported descriptively. To further address heterogeneity, a priori subgroup analyses were performed based on intensity of smoking cessation counselling (SCC) and type of antidepressant.
Based on previous analyses, (6) the intensity of SCC was defined as
minimal or brief counselling: < 90 minutes in total; or
intensive counselling: ≥ 90 minutes in total.
Abstinence was defined as continuous abstinence (synonymous with prolonged or sustained abstinence) that was biochemically validated at all time points. It was anticipated a priori that there would be substantial differences among trials regarding components of smoking cessation strategies and methods of validation for abstinence.
Quality of Evidence
The quality of each included study was assessed taking into consideration the following 7 study design characteristics:
adequate allocation concealment;
randomization (study must include a description of the randomization procedure used, which must be a proper method);
power/sample size (adequate sample size based on a priori calculations; underpowered studies were identified, when possible, using post-hoc sample size power calculations);
blinding (if double blinding was not possible, a single blind study with unbiased assessment of outcome was considered adequate for this criterion);
< 20% withdrawals/dropouts;
intention-to-treat analysis conducted and done properly (i.e., withdrawals/dropouts considered in analysis); and
other criteria as appropriate for the particular research question and study design.
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (7) as presented below.
Quality refers to criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of the estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Results of Evidence-Based Analysis
The database search yielded 1,619 citations (with duplicates removed) published between 1950 and week 3 of June 2010. Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 summarizes the review of citations at title, abstract, and full text level.
Figure 1: Citation Flow Chart.
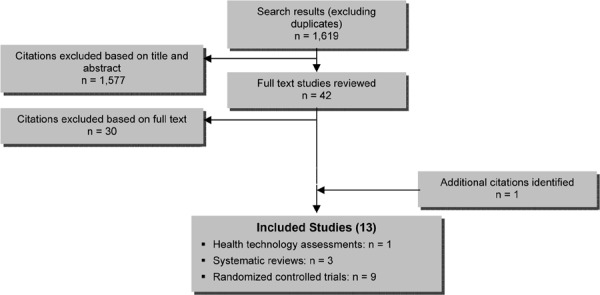
In total, 12 studies met the inclusion criteria: 3 systematic reviews and 9 RCTs. The references lists of the included studies were hand searched to identify any additional potentially relevant studies, and 1 additional citation, a health technology assessment, was added, bringing the total to 13 included citations. Detailed characteristics of the included studies are described in Appendix 2.
For each included study, the study design was identified and is summarized below in Table 2, which is a modified version of the hierarchy of study design by Goodman. (8)
Table 2: Body of Evidence Examined According to Study Design*.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studies | |
| Systematic review of RCTs | 4 |
| Large RCT† | 7 |
| Small RCT | 2 |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with contemporaneous controls | |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | |
| Database, registry, or cross-sectional study | |
| Case series | |
| Retrospective review, modelling | |
| Studies presented at an international conference or other sources of grey literature | |
| Expert opinion | |
| Total | 13 |
Abbreviation: RCT, randomized controlled trial.
Large RCT is defined as a sample size of at least 100.
Health Technology Assessments
One health technology assessment by Hoogendoorn et al (6) was identified. The objective was to assess the long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD based on a systematic review of RCTs on smoking cessation interventions in patients with COPD with 12-month biochemically validated abstinence rates.1
Interventions were grouped into usual care, minimal or brief counselling (< 90 minutes), intensive counselling (≥ 90 minutes) without pharmacotherapy, and intensive counselling (≥ 90 minutes) with pharmacotherapy. (6) Interventions offering pharmacotherapy on a noncompulsory basis were included in the pharmacotherapy category if it was used by more than 50% of the patients. Patients receiving the placebo drug also often received some form of counselling and were therefore grouped into the categories of minimal counselling or intensive counselling, depending on the duration of counselling. Absolute quit rates for each of the categories were used to populate the model. Two different abstinence rates were calculated:
continuous abstinence rate at 12 months postintervention
point-prevalence abstinence rate at 12 months postintervention.
Hoogendoorn et al (6) included 9 RCTs. The average 12-month continuous abstinence rates were: 1.4% for usual care, 2.6% for minimal counselling, 6% for intensive counselling, and 12.3% for intensive counselling with pharmacotherapy (Table 3). (6) Intensive counselling as well as intensive counselling plus pharmacotherapy were significantly more effective than usual care. The authors noted that the analysis did not take into account the duration and intensity of pharmacotherapy; thus, it is likely that longer duration and greater intensity of pharmacotherapy would lead to higher abstinence rates. (6)
Table 3: Abstinence Rates and Associated Intervention Costs for the Four Intervention Groups*.
| Intervention | 12-Month Continuous Abstinence Ratest† | |
|---|---|---|
| Average Rate, Percentage | Percent Difference With Usual Care (95% CI) | |
| Usual care | 1.4 | − |
| Minimal or brief counselling < 90 minutes | 2.6 | 1.2 (−1.3 to 3.7) |
| Intensive counselling ≥ 90 minutes | 6.0 | 4.6 (1.8−7.4) |
| Intensive counselling ≥ 90 minutes with pharmacotherapy | 12.3 | 10.9 (6.9−15.0) |
Abbreviation: CI, confidence interval.
Based on random effect meta-analysis performed on the absolute abstinence rates in trial arms.
Reproduced from Thorax, Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Molken MP, Vol. 65, p. 711–8, 2010 with permission from BMJ Publishing Group Ltd.
Systematic Reviews
Three systematic reviews were identified. (9;10) All 3 reviews included RCTs only.
In the 2009 systematic review by Strassmann et al (11), a network meta-analysis was performed to assess the effectiveness of smoking cessation interventions for patients with COPD. Behavioural interventions were classified as individual or group, self-help material, and telephone counselling; pharmacological interventions included nicotine replacement therapy (NRT), antidepressants, or other drugs. The primary outcome measure was prolonged biologically confirmed abstinence rates at 6 months or longer follow-up. If prolonged abstinence rates were not available, point-prevalence (defined as smoking cessation 7 days prior to follow-up) rates were considered. (11)
Strassmann et al (11) included 8 RCTs. Smoking cessation counselling (SCC) plus NRT was more effective than SCC alone, no intervention, or usual care (UC). Smoking cessation counselling plus antidepressant was the second most effective intervention. The intensity of SCC had an impact on prolonged abstinence rates. These results are summarized in Table 4.
Table 4: Relative Efficacy of Smoking Cessation Intervention on Prolonged Abstinence*.
| Intervention | Comparator | OR (95% CI) | P Value |
|---|---|---|---|
| Smoking cessation counselling + NRT | Nothing / usual care | 5.08 (4.32−5.97) | < 0.001 |
| Smoking cessation counselling + NRT | Smoking cessation counselling | 2.80 (1.49−5.26) | 0.001 |
| Smoking cessation counselling + NRT | Smoking cessation counselling + antidepressant | 1.53 (0.71−3.30) | 0.28 |
| Smoking cessation counselling + antidepressant | Nothing / usual care | 3.32 (1.53−7.21) | 0.002 |
| Smoking cessation counselling + antidepressant | Smoking cessation counselling | 1.83 (1.18−2.83) | 0.007 |
| Smoking cessation counselling | Nothing / usual care | 1.82 (0.96−3.44) | 0.07 |
Abbreviations: CI, confidence interval; NRT, nicotine replacement therapy; OR, odds ratio.
Source: Strassman et al, 2009 (11)
An in-depth analysis taking into consideration the intensity of SCC showed that the odds of prolonged abstinence were increased with high-intensity SCC versus low-intensity SCC. (11) However, only high-intensity SCC plus NRT was significantly more effective than low-intensity SCC plus NRT (odds ratio [OR], 1.81; 95% CI, 1.04–3.15; P = 0.04). When comparing high-intensity SCC alone with low-intensity SCC (OR, 1.46; 95% CI, 0.44–4.90; P = 0.54), and high-intensity SCC plus antidepressant with low-intensity SCC plus antidepressant (OR, 1.55; 95% CI, 0.35–6.91; P = 0.56), the ORs were not significant.
Point-prevalence was examined in 2 studies. In 1 of these studies, the abstinence rate was not significantly different when comparing rewarding patients for smoking abstinence with lottery tickets and reimbursement for planned visits. (12) In the other study, a statistically significant improvement in abstinence rates was observed (OR, 2.71; 95% CI, 0.62–12.35) when comparing the use of the term “smoker’s lung” by nurses instead of chronic bronchitis when speaking to patients with COPD. (13)
Three of the included studies included mortality as an outcome. The mortality results were not pooled; 1 of the 3 studies found a statistically significant reduction in mortality when comparing the 14.5 year mortality in the smoking cessation intervention groups with the usual care group (OR, 0.74; 95% CI, 0.63–0.87). (14;15) No significant differences were observed at 1-year of follow-up in the other 2 studies (Tonnesen et al (16): OR, 0.74, 95% CI 0.22–2.41 comparing NRT with placebo; Brandt et al (13): OR, 1.88; 95% CI, 0.30–12.55 comparing diagnosis of “smoker’s lung” with chronic bronchitis).
Overall, the authors concluded that SCC plus NRT appears to be the most effective smoking cessation intervention followed by SCC plus antidepressant. Smoking cessation counselling without additional drug treatment is not much more effective than usual care. (11)
MAS Comments
Although the analysis was stratified by the intensity of counselling, the authors did not define the thresholds for low- and high-intensity counselling, nor how the thresholds were determined.
The authors reported that there was a lack of consistency across studies in reporting patient characteristics such as severity of COPD and the motivation to quit smoking; these are important characteristics which may influence the effectiveness of smoking cessation interventions.
The 2001 van der Meer et al (9) systematic review evaluated the effectiveness of smoking cessation interventions in patients with COPD; however, due to important heterogeneity across the identified RCTs, a meta-analysis was not performed and results were summarized descriptively.
Five RCTs, 2 of high quality, were included in this systematic review. (9) The results of the individual studies are summarized in Table 5.
Table 5: Abstinence Rates of Five Included Individual Studies*.
| Author, Year | Time of Assessment |
Intervention Sample Size (%) |
Control Sample Size (%) |
Abstinence Rates Risk Difference (95% CI) |
|---|---|---|---|---|
| Anthonisen et al, 1994 (17) | 1 year | 680† (34.7) | 177 (9.0) | 0.26 (0.23−0.28) |
| 674‡ (34.4) | 177 (9.0) | 0.25 (0.23−0.28) | ||
| 5 years | 408† (20.8) | 102 (5.2) | 0.16 (0.14−0.18) | |
| 427‡ (21.8) | 102 (5.2) | 0.17 (0.14−0.19) | ||
| Brandt et al, 1997 (13) | 1 year | 8 (40.0) | 5 (20.0) | 0.20 (-0.07 to 0.47) |
| Crowley et al, 1995 (12) | 6 months | 5 (13.9) | 5 (13.9) | 0 |
| Pederson et al, 1991 (18) | 6 months | 10 (33.3) | 6 (21.4) | 0.12 (-0.11 to 0.35) |
| Tashkin and Murray, 2001 (19) | 6 months | 32 (15.7) | 18 (9.0) | 0.07 (0.00−0.13) |
Abbreviation: CI, confidence interval.
Smoking cessation intervention + bronchodilator.
Smoking cessation intervention + placebo.
Source: van der Meer et al, 2001. (9)
Overall, the authors concluded that a combination of psychosocial intervention and pharmacological intervention is superior to no treatment or to psychological intervention alone. Due to the lack of sufficient high quality studies, no absolute or convincing evidence was found to support the effectiveness of psychosocial intervention for patients with COPD. (9) van der Meer et al (9) noted 2 important limitations with their review:
Heterogeneity existed across studies with regards to patient characteristics, outcome measurements, and the timing of measurements.
The description of counselling was often unclear, including the intensity of person-to-person clinical contact, types of counselling and behavioural therapies, and formats of psychosocial interventions.
Finally, the 2004 systematic review by Wagena et al (10) evaluated the effectiveness of smoking cessation interventions in patients with COPD. Behavioural interventions included:
self-help interventions (such as the use of pamphlets, audio tapes, videotapes, mailed information, or computer programs);
individual or group counselling; and/or
telephone counselling, with or without the use of pharmacotherapy (NRT, antidepressants, or both).
The primary outcome measure was biochemically confirmed abstinence rates at least 6 months following the intervention. (10)
Wagena et al (10) included 5 RCTs. Due to heterogeneity across studies with regards to the study populations and smoking cessation interventions, the results were not pooled. Although 3 trials found increased abstinence rates in the smoking cessation group compared with the control group, the differences were not statistically significant (Brandt et al (13): risk difference [RD], 0.16; 95% CI, -0.07 to 0.38 comparing using the term “smoker’s lung” instead of chronic bronchitis when speaking with patients; Pederson et al (18): RD, 0.11; 95% CI, -0.08 to 0.29 comparing individual counselling plus self-help smoking cessation manuals with physician’s advice to quit; and Crowley et al (12): RD, 0.10; 95% CI, -0.11 to 0.31 comparing contingent reinforcement using lottery tickets with reimbursing patients for their planned visits).
The Lung Health Study (17) evaluated the effect of an intensive smoking cessation intervention combined with either inhaled bronchodilator ipratropium bromide or placebo compared with the usual care group (no definitive smoking intervention) on lung function (forced expiratory volume in 1 second). The smoking cessation intervention consisted of free nicotine gum (NRT) and a 12-group session intervention for 10 weeks, starting with 4 meetings per week and gradually declining over the 10-week period. For those patients who quit smoking, a maintenance program aimed at preventing relapse by teaching coping skills for problems such as stress and weight gain was provided. (17)
After 12 months’ follow-up, lung function (defined by the forced expiratory volume in 1 second) was statistically improved in the smoking cessation intervention plus ipratropium bromide group compared with usual care (RD, 0.26; 95% CI, 0.23–0.28). The results remained significantly different at 5-year follow-up (RD, 0.16; 95% CI, 0.14–0.18). The combination of the smoking intervention plus the placebo bronchodilator was also significantly more effective than the usual care group at both 12 months and 5 years (RD, 0.25; 95% CI, 0.23–0.28 and RD, 0.17; 95% CI, 0.15–0.19, respectively). In a follow-up study at 11 years, Murray et al (20) demonstrated that the benefit in lung function remained.
Finally, 1 study reported the efficacy of bupropion sustained release for smoking cessation. (19) The results showed that the combination of bupropion sustained release 300 mg per day for 12 weeks in addition to 10 individual counselling sessions resulted in significantly higher prolonged abstinence rates after 26 weeks compared with placebo combined with the behavioural intervention (RD, 0.07; 95% CI, 0.00–0.13). However, at 12 months, the difference was no longer apparent (RD, 0.02; 95% CI, -0.04 to 0.07). (19)
Overall, the authors concluded that, with the exception of combined use of pharmacotherapy and counselling to reduce craving and withdrawal, the success rates of other smoking cessation interventions in patients with COPD is low. (10)
Randomized Controlled Trials
A total of 9 RCTs that met the inclusion criteria were identified and included in this review. The sample size of the studies ranged from 74 to 5,887 patients. A total of 8,291 participants were included in the 9 studies. The mean age of the patients ranged from 54 to 64 years. The majority of studies compared smoking cessation interventions to usual care; however, 3 studies (16;19;21) examined the use of pharmacotherapy compared to a placebo control group. Smoking cessation interventions included counselling alone and counselling plus pharmacotherapy. In trials where pharmacotherapy was compared to placebo, both the intervention and control arms received identical behavioural counselling.
The duration, intensity, and mode of behavioural counselling and the type of pharmacotherapy varied among studies. Antidepressants were used as pharmacotherapy in 2 of the studies. (19;21) Two studies were conducted in the hospital setting (18;22), while the remainder of studies were conducted in the outpatient setting. The severity of COPD in patients varied among studies; however, the majority of studies (6 of 9) were conducted in the mild-to-moderate COPD population based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria. One study (23) included patients with undiagnosed COPD. However, the patients were only eligible for the study if they had mild or moderate COPD according to the GOLD criteria as determined by spirometry. Studies had varying lengths of follow-up ranging from 6 months to 5 years. The majority of studies followed patients for 1 year. Table 6 provides an assessment of the quality of the individual studies. Appendix 3 provides a detailed summary of characteristics of included studies.
Table 6: Quality of Included Individual Studies*.
| Author, Year | Number of Patients |
Randomization | Allocation Concealment |
Blinding | Sample Size Calc. |
Withdrawals/ Dropouts |
ITT |
|---|---|---|---|---|---|---|---|
| Pederson et al, 1991 (18) | 74 | ✓ | ✓ | ✓ | ✓ | ||
| Sundblad et al, 2008 (22;24) | 247 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Anthonisen et al, 1994 (17) | 5887 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tashkin and Murray, 2001 (19) | 404 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hilberink et al, 2010 (25;26) | 667 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Wilson et al, 2008 (27) | 91 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tonnesen et al, 2006 (16;28) | 370 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Wagena et al, 2005 (21) | 255 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Kotz et al, 2009 (23;29) | 296 | ✓ | ✓ | ✓ | ✓ | ✓ |
Abbreviations: Calc., calculation; ITT, intention-to-treat.
Abstinence
Validation methods confirming abstinence varied among studies. In 2 studies, smoking cessation was validated by carboxyhemoglobin testing (18;30); in 5 studies by breath carbon monoxide testing; (12;13;16;19;22); in 2 studies by urinary cotinine testing (21;23); in 2 studies by salivary cotinine and carbon monoxide testing (17;27); in 1 study by salivary cotinine alone (31); and in 1 study by self-reported abstinence without a validation test. (26) When prolonged abstinence rates were unavailable, point-prevalence rates (defined as smoking cessation 7 days prior to follow-up) were considered. Time to assessment of abstinence varied among studies, and abstinence at the longest follow-up was used in the meta-analysis.
Results of the pooled estimates for abstinence are summarized in Table 7. Quality of evidence according to GRADE, as shown in Appendix 5, was assessed as moderate for comparisons of counselling with usual care, counselling plus NRT with usual care, NRT with placebo, and antidepressant with placebo.
Table 7: Summary of Results − Abstinence at Longest Follow-Up*.
| Intervention | Comparison | Number of Studies | Abstinence Rate Pooled Relative Risk (95% CI) |
GRADE |
|---|---|---|---|---|
| Counselling | Usual Care | 2 | 5.85 (3.81−8.97)† | Moderate |
| Intensive Counselling ≥ 90 minutes | Usual Care | 1 | 7.70 (4.64−12.79)† | |
| Minimal Counselling < 90 minutes | Usual Care | 1 | 1.56 (0.65−3.72) | |
| Counselling + NRT | Usual Care | 3 | 4.28 (3.51−5.20)† | Moderate |
| Intensive Counselling ≥ 90 minutes + | Usual Care | |||
| NRT | Usual Care | 1 | 4.41 (3.60−5.39)† | |
| Minimal Counselling < 90 minutes + NRT | 2 | 2.11 (0.90−4.91) | ||
| Minimal Counselling < 90 minutes + Antidepressant | Usual Care | 1 | 1.91 (0.65−5.61) | Low |
| Minimal Counselling < 90 minutes + NRT + Antidepressant | Usual Care | 1 | 2.25 (0.87−5.85) | Low |
| NRT | Placebo | 1 | 3.01 (1.02−8.89)† | Moderate |
| Antidepressant | Placebo‡ | 2 | 2.09 (1.35−3.24)† | Moderate |
| Nortriptyline | Placebo | 1 | 2.54 (0.87−7.44) | Moderate |
| Bupropion | Placebo | 2 | 2.01 (1.24−3.24)† |
Abbreviations: CI, confidence interval; NRT, nicotine replacement therapy.
Statistically significant (P < 0.05).
One study had 2 treatment arms, each examining a different antidepressant.
Results
Counselling Versus Usual Care
Two studies examined the efficacy of SCC versus usual care in patients with COPD in an inpatient setting (Figure 2). One study (25) included an intervention of intensive counselling (defined as ≥ 90 minutes), while the other study (18) compared minimal counselling (defined as < 90 minutes of counselling) to that of usual care. As expected, high rates of statistical heterogeneity were observed when pooling these studies (I2 = 90%). Examining the studies separately showed that abstinence rates were statistically higher in those receiving intensive counselling compared to usual care (RR, 7.70; 95% CI, 4.64–12.79; P < 0.00001). No significant effect of minimal counselling on abstinence rates was found compared to usual care (RR, 1.56; 95% CI, 0.65–3.72; P = 0.32), but this may have been due to a lack of power as a result of a small study sample size.
Figure 2: Comparison of Abstinence for Counselling Versus Usual Care Groups at Longest Follow-Up*.
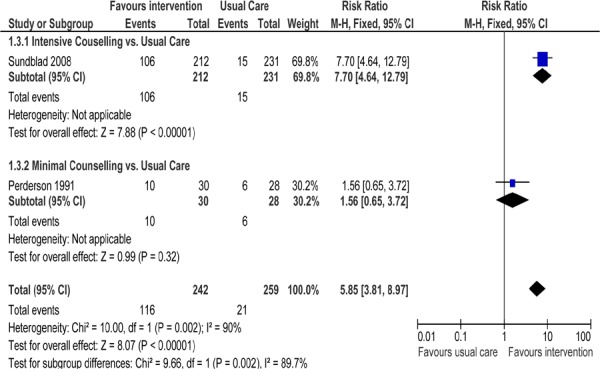
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Counselling Plus Nicotine Replacement Therapy Versus Usual Care
Three studies reported abstinence rates in patients with COPD receiving SCC plus NRT compared to those receiving usual care (Figure 3). One study (17) examined the effect of intensive counselling plus NRT while the other 2 studies (25;27) used minimal counselling plus NRT. As seen in Figure 3, there is a statistically significant difference in abstinence rates favouring the intervention groups compared with usual care (RR, 4.28; 95% CI, 3.51–5.20; P < 0.001). When subgrouped by intensity of counselling, only the study using intensive counselling plus NRT showed a significant difference in abstinence rates compared with usual care (P< 0.001).
Figure 3: Comparison of Abstinence for Counselling Plus NRT Versus Usual Care Groups at Longest Follow-Up*.
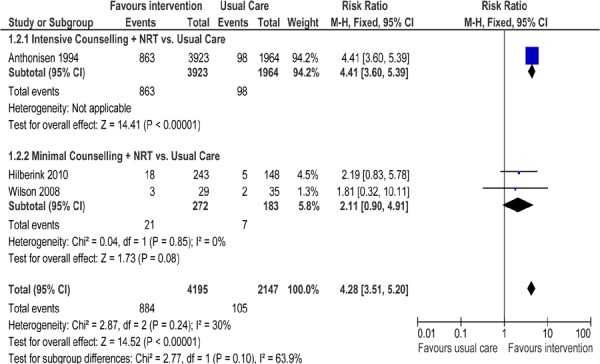
Abbreviations: CI, confidence interval; M–H, Mantel-Haenszel; NRT, nicotine replacement therapy.
Minimal Counselling Plus Antidepressant Versus Usual Care
One study reported (23) abstinence rates in patients with COPD receiving minimal counselling plus an antidepressant (nortriptyline) compared with those receiving usual care (Figure 4). No significant difference in abstinence rates was found between the intervention group and the usual care group (RR, 1.91; 95% CI, 0.65–5.61; P = 0.24). Although the study itself was of high quality, patients enrolled into this study had undiagnosed COPD and were only classified by the GOLD criteria upon entering the study. Since patients were unaware of their COPD diagnosis, they may not have been as motivated to quit smoking or to take their illness as seriously as patients who had been previously diagnosed with COPD.
Figure 4: Comparison of Abstinence for Minimal Counselling Plus Antidepressant Versus Usual Care Groups at Longest Follow-Up*.
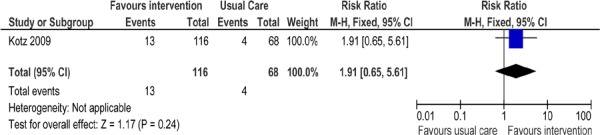
Abbreviations: CI, confidence interval; M–H, Mantel-Haenszel.
Minimal Counselling Plus NRT Plus Antidepressant Versus Usual Care
The efficacy of receiving minimal counselling, recommended NRT, and a prescribed antidepressant (bupropion) compared to usual care was examined in 1 study. (25) The study was conducted in the outpatient setting. As shown in Figure 5, there was no statistically significant difference in abstinence rates between the intervention and usual care arms (RR, 2.25; 95% CI, 0.87–5.85; P = 0.10). Although the study itself was of high quality, several factors may have contributed to the lack of success of the intervention including:
Figure 5: Comparison of Abstinence for Minimal Counselling Plus NRT Plus Antidepressant Versus Usual Care Groups at Longest Follow-Up*.
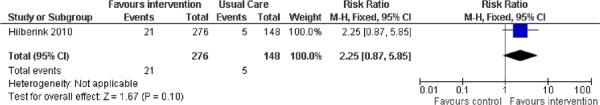
Abbreviations: CI, confidence interval; M–H, Mantel-Haenszel; NRT, nicotine replacement therapy.
the inclusion of some unmotivated COPD smokers,
less intensive counselling (the intervention was integrated into routine care), and
poor compliance with the use of bupropion and NRT noted at follow-up.
Nicotine Replacement Therapy Versus Placebo
One study (16) reported the efficacy of receiving NRT in a placebo-controlled trial. Both study arms received identical counselling. The study was conducted in an outpatient setting. As shown in Figure 6, there was a statistically significant difference in abstinence rates favouring the intervention group compared with the usual care group (RR, 3.01; 95% CI, 1.02–8.89; P = 0.05). The 1-year quit rate of 14% observed in the intervention group receiving the nicotine sublingual tablet is the same range as that reported in previous studies.
Figure 6: Comparison of Abstinence for NRT Versus Placebo Groups at Longest Follow-Up*.
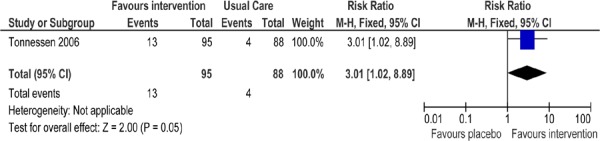
Abbreviations: CI, confidence interval; M–H, Mantel-Haenszel; NRT, nicotine replacement therapy.
Antidepressant Versus Placebo
Two studies (19;21) reported abstinence rates in patients with COPD receiving an antidepressant in a placebo-controlled trial (Figure 7). One study (21) included 2 arms, each examining a different antidepressant. As seen in Figure 7, there is a statistically significant difference in abstinence rates favouring the intervention groups as compared to usual care (RR, 2.09; 95% CI, 1.35–3.24; P < 0.001). When subgrouped by type of antidepressant, only those studies that prescribed bupropion showed a significant difference in abstinence rates as compared to usual care (P = 0.004).
Figure 7: Comparison of Abstinence for Antidepressant Versus Placebo Groups at Longest Follow-Up*.
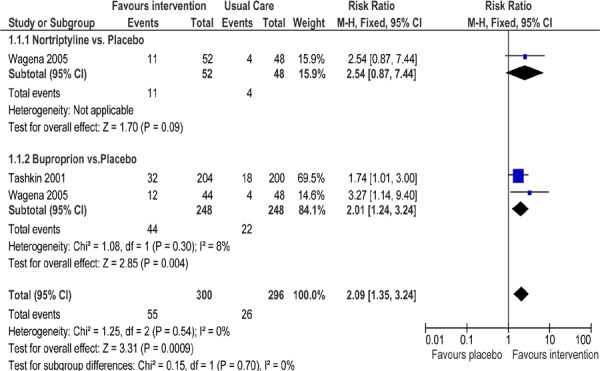
Abbreviations: CI, confidence interval; M–H, Mantel-Haenszel.
Limitations
Due to the limited amount of studies available for this analysis, it was not possible to examine the characteristics of patients, such as severity of COPD and motivation to quit smoking, which could likely impact the effect of the smoking cessation intervention on abstinence rates.
Economic Analysis
The results of the economic analysis are summarized in issue 12 of the COPD series entitled Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model. This report can be accessed at
www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Economic_March.pdf.
Conclusions
Based on a moderate quality of evidence, compared with usual care, abstinence rates are significantly higher in COPD patients receiving intensive counselling or a combination of intensive counselling and NRT.
Based on limited moderate quality evidence, abstinence rates are significantly higher in COPD patients receiving NRT compared with placebo.
Based on moderate quality of evidence, abstinence rates are significantly higher in COPD patients receiving the antidepressant bupropion compared to placebo.
Glossary
- 6 Minute Walking Test (6MWT)
A measure of exercise capacity which measures the distance that a patient can quickly walk on a flat, hard surface in a period of 6 minutes. A widely used outcome measure in respiratory rehabilitation of patients with COPD.
- Acute exacerbations of chronic obstructive pulmonary disease (AECOPD)
A change in baseline symptoms that is beyond day-to-day variation, particularly increased breathlessness, cough, and/or sputum, which has an abrupt onset.
- Admission avoidance hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and avoid admission to hospital. After patients are assessed in the emergency department for an acute exacerbation, they are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home where they receive regular visits from a medical professional until the exacerbation has resolved.
- Ambulatory oxygen therapy
Provision of oxygen therapy during exercise and activities of daily living for individuals who demonstrate exertional desaturation.
- Bilevel positive airway pressure (BiPAP)
A continuous positive airway pressure mode used during noninvasive positive pressure ventilation (see definition below) that delivers preset levels of inspiratory and expiratory positive airway pressure. The pressure is higher when inhaling and falls when exhaling, making it easier to breathe.
- Cost-effectiveness acceptability curve (CEAC)
A method for summarizing uncertainty in estimates of cost-effectiveness.
- Cor pulmonale
Right heart failure, as a result of the effects of respiratory failure on the heart.
- Dyspnea
Difficulty breathing or breathlessness.
- Early discharge hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and decrease their length of stay in hospital. After being assessed in the emergency department for acute exacerbations, patients are admitted to the hospital where they receive the initial phase of their treatment. These patients are discharged early into a hospital-at-home program where they receive regular visits from a medical professional until the exacerbation has resolved.
- Forced expiratory volume in 1 second (FEV1)
A measure of lung function used for COPD severity staging; the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
- Forced vital capacity(FVC)
The amount of air that can be forcibly exhaled from the lungs after taking the deepest breath possible.
- Fraction of inspired
The percentage of oxygen participating in gas exchange.
- oxygen (FiO2)
- Hypercapnia
Occurs when there is too much carbon dioxide in the blood (arterial blood carbon dioxide > 45 to 60 mm Hg).
- Hypopnea
Slow or shallow breathing.
- Hypoxemia
Low arterial blood oxygen levels while breathing air at rest. May be severe (PaO2 ≤ 55 mm Hg), moderate (56 mm Hg ≤ PaO2 < 65 mm Hg), or mild-to-moderate (66 mm Hg < PaO2 ≤ 74 mm Hg).2
- Incremental cost-effectiveness ratio (ICER)
Ratio of the change in costs of a therapeutic intervention to the change in effects of the intervention compared to the alternative (often usual care).
- Intention-to-treat analysis (ITT)
An analysis based on the initial treatment the participant was assigned to, not on the treatment eventually administered.
- Invasive mechanical ventilation (IMV)
Mechanical ventilation via an artificial airway (endotracheal tube or tracheostomy tube).
- Long-term oxygen therapy (LTOT)
Continuous oxygen use for about 15 hours per day. Use is typically restricted to patients fulfilling specific criteria.
- Multidisciplinary care
Defined as care provided by a team (compared to a single provider). Typically involves professionals from a range of disciplines working together to deliver comprehensive care that addresses as many of the patient’s health care and psychosocial needs as possible.
- Nicotine replacement therapy (NRT)
The administration of nicotine to the body by means other than tobacco, usually as part of smoking cessation.
- Noninvasive positive pressure ventilation (NPPV)
Noninvasive method of delivering ventilator support (without the use of an endotracheal tube) using positive pressure. Provides ventilatory support through a facial or nasal mask and reduces inspiratory work.
- Partial pressure of carbon dioxide (PaCO2)
The pressure of carbon dioxide dissolved in arterial blood. This measures how well carbon dioxide is able to move out of the body.
- Partial pressure of oxygen (PaO2)
The pressure of oxygen dissolved in arterial blood. This measures how well oxygen is able to move from the airspace of the lungs into the blood.
- Palliative oxygen therapy
Use of oxygen for mildly hypoxemic or nonhypoxemic individuals to relieve symptoms of breathlessness. Used short term. This therapy is “palliative” in that treatment is not curative of the underlying disease.
- Pulmonary rehabilitation
Multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary rehabilitation programs.
- Pulse oximetry
A noninvasive sensor, which is attached to the finger, toe, or ear to detect oxygen saturation of arterial blood.
- Quality-adjusted life-years (QALYs)
A measure of disease burden that includes both the quantity and the quality of the life lived that is used to help assess the value for money of a medical intervention.
- Respiratory failure
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. It can be either acute (acute respiratory failure, ARF) or chronic, and is classified as either hypoxemic (type I) or hypercapnic (type II) respiratory failure. Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD.
- Short-burst oxygen therapy
Short-duration, intermittent, supplemental oxygen administered either before or after exercise to relieve breathlessness with exercise.
- Sleep apnea
Interruption of breathing during sleep due to obstruction of the airway or alterations in the brain. Associated with excessive daytime sleepiness.
- Smoking cessation
The process of discontinuing the practice of inhaling a smoked substance.
- Spirometry
The gold standard test for diagnosing COPD. Patients breathe into a mouthpiece attached to a spirometer which measures airflow limitation.
- SpO2
Oxygen saturation of arterial blood as measured by a pulse oximeter.
- Stable COPD
The profile of COPD patients which predominates when patients are not experiencing an acute exacerbation.
- Supplemental oxygen therapy
Oxygen use during periods of exercise or exertion to relieve hypoxemia.
- Telemedicine (or telehealth)
Refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services.
- Telemonitoring (or remote monitoring)
Refers to the use of medical devices to remotely collect a patient’s vital signs and/or other biologic health data and the transmission of those data to a monitoring station for interpretation by a health care provider.
- Telephone only support
Refers to disease/disorder management support provided by a health care provider to a patient who is at home via telephone or videoconferencing technology in the absence of transmission of patient biologic data.
- Ventilator-associated pneumonia (VAP)
Pneumonia that occurs in patients undergoing mechanical ventilation while in a hospital.
Acknowledgements
Medical Information Officer
Kellee Kaulback
Editorial Staff
Linda Senzilet
Irina Alecu
COPD Expert Advisory Panel
The role of the expert panel was to provide direction on the scope of the project and the relevant outcomes measures of effectiveness, to review the evidence-based analyses and to identify any societal or systemic issues that are relevant to intervention effectiveness. However, the statements, conclusions and views expressed in this report do not necessarily represent the views of the expert panel members.
Jeremy Grimshaw, MD, MBChB, PhD (Chair)
Senior Scientist, Ottawa Hospital Research Institute Professor, Department of Medicine, University of Ottawa
Dina Brooks, PhD
Professor, Department of Physical Therapy, University of Toronto
Debbie Coutts, RRT, CRE
Andrea Gershon, MD, MSc, FRCP(C)
Scientist, Institute for Clinical Evaluative Sciences Respirologist, Sunnybrook Health Sciences Centre
Assistant Professor, Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto
Mita Giacomini, BSc, MPH, MA, PhD
Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Ron Goeree, BA, MA
Director, PATH Research Institute, St. Joseph’s Hospital (Hamilton)
Associate Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Roger Goldstein, MBCHB, FRCP(C), FRCP(UK)
NSA Chair in Respiratory Rehabilitation Research
Director, Respiratory Services, and Senior Scientist, West Park Healthcare Centre Professor, Medicine and Physical Therapy, University of Toronto
Alan G Kaplan, MD, CCFP(EM), FCFP
Chairperson, Family Physician Airways Group of Canada
Chairperson, Special Interest Focused Care Group in Respiratory Medicine, College of Family Physicians of Canada
Clinical Lecturer, Department of Family and Community Medicine, University of Toronto
DE O’Donnell, MD, FRCP(C)
Director, COPD Centre, Kingston General Hospital
Professor, Department of Medicine, Queen’s University
Asad Razzaque, MD
Family Physician
Holger Schünemann, MD, PhD, MSc, FRCP(C)
Michael Gent Chair in Healthcare Research
Chair, Department of Clinical Epidemiology & Biostatistics, McMaster University
Professor, Department of Clinical Epidemiology & Biostatistics and Medicine, McMaster University
Tasnim Sinuff, MD, PhD, FRCP(C)
Clinician Scientist, Sunnybrook Health Sciences Centre
Assistant Professor, Department of Medicine, University of Toronto
Laura Watling, RRT, BSc(HK)
Clinical Practice Leader/Clinical Coordinator, Respiratory Therapy, West Park Healthcare Centre
Appendices
Appendix 1: Literature Search Strategy
Search date: June 24, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Wiley Cochrane Library, Centre for Reviews and Dissemination
Database: Ovid MEDLINE(R) <1950 to June Week 3 2010> Search Strategy:
--------------------------------------------------------------------------------
exp Pulmonary Disease, Chronic Obstructive/ (13760)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti, ab. (20719)
(copd or coad).ti,ab. (15726)
chronic airflow obstruction.ti,ab. (484)
exp Emphysema/ (6878)
((chronic adj2 bronchitis) or emphysema).ti,ab. (22456)
or/1–6 (52513)
exp Smoking Cessation/ (14779)
(smok* adj2 (cessation or quit* or stop* or ceas*)).ti,ab. (15464)
8 or 9 (21492)
7 and 10 (1161)
limit 11 to (english language and humans) (890)
limit 12 to (case reports or comment or editorial or letter) (77)
12 not 13 (813)
Database: EMBASE <1980 to 2010 Week 24> Search Strategy:
--------------------------------------------------------------------------------
exp chronic obstructive lung disease/ (35741)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (19329)
(copd or coad).ti,ab. (15705)
chronic airflow obstruction.ti,ab. (453)
exp emphysema/ (14502)
exp chronic bronchitis/ (6190)
((chronic adj2 bronchitis) or emphysema).ti,ab. (14538)
or/1–7 (58318)
exp smoking cessation/(20183)
(smok* adj2 (cessation or quit* or stop* or ceas*)).ti,ab. (13485)
9 or 10 (23418)
8 and 11 (1736)
limit 12 to (human and english language) (1392)
limit 13 to (editorial or letter or note) (218)
case report/ (1108743)
14 or 15 (1108959)
Database: CINAHL
Search Strategy:
--------------------------------------------------------------------------------
S9 (S5 or S6 or S7) and (S4 and S8) (353)
S8 S5 or S6 or S7 (9655)
S7 smok* and (cessation or quit* or stop* or ceas*) (9655)
S6 (MH “Smoking Cessation Programs”) (1047)
S5 (MH “Smoking Cessation”) (6902)
S4 S1 or S2 or S3 (7064)
S3 chronic bronchitis or Emphysema or chronic obstructive lung (1701)
S2 chronic obstructive pulmonary or copd or chronic airway obstruction (5631)
S1 (MH “Pulmonary Disease, Chronic Obstructive+”) (4177)
Appendix 2: Characteristics of Included Studies
The characteristics of the included randomized control trials are described in Table A1.
Table A1: Characteristics of Included Randomized Control Trials*.
| Author, Year | Patients | Comparison | Outcomes Definitions | Follow-up Period | Authors’ Conclusions | Notes |
|---|---|---|---|---|---|---|
| Pederson et al, 1991 (18) | Mean age: 53.4 years (SD 13.7) - Smokers admitted to hospital with exacerbation of COPD. COPD defined according to the American College of Chest Physicians and the American Thoracic Society (ACCP-ATS) Mean FEV1FCV = 0.52 (SD = 0.17) Severity of COPD: Probably severe or very severe according to GOLD Motivation to quit: Advised to quit Total sample: N = 74 |
Self-help manual + counselling session by trained smoking cessation counselor (3 to eight 15–20 minute counselling sessions on alternate days while in hospital (min 45 minutes, max 160 minutes) [minimal counselling to intensive counselling]. vs. Usual care |
Abstinence: Self-reported and verified by COHB analysis from blood drawn at 6 months. Mortality: All-cause, cardiovascular death, lung cancer death. | 3 months, 6 months | No significant difference between the 2 groups. | Because of acute exacerbation, it was felt that inpatients were more likely to be amenable to quitting smoking. - Patients in the treatment group received smoking cessation on alternate days for the duration of their hospital stay. - Follow-up visits were used to offer support and encouragement and to answer patient’s questions. - Physicians were blinded to group membership. |
| Sundblad et al, 2008 (22) | Age range: 40–60 years. - Smokers with COPD according to Siafakas et al (32) and according to European Respiratory Society Guidelines. - Smoked at least 8 cigarettes per day) - Average pack-years: 34.9 (SD 12.8) Severity of COPD:- 71% had mild COPD, FEV1 > 70% of predicted. |
Comprehensive smoking cessation program Included 11-day hospitalizations, use of NRT and recommended physical exercise, 1 hour daily with trained cessation nurse (660 minutes), structured educational program (physician, physiotherapist, dietician, psychologist, occupational therapist, group discussion with spouse [intensive |
Abstinence: At 3-year follow-up, confirmed by carbon monoxide testing using piCO Smokerlyzer. Abstinence from smoking for at least 6 months. Questionnaires were used to gather information about the smoking habits. |
1 year, 3 years | This comprehensive smoking cessation program, including several components, resulted in a high rate of smoke-free patients after 1 year (52%) and long-lasting effect, with 38% of the smokers with COPD remaining free from smoking after 3 years. | Smokers had to be smoking more than 8 cigarettes/day. - Smoking cessation program lasted for 1 year, and included a 2-day stay at the pulmonary rehabilitation clinic. - Patients were hospitalized for 11 days to build up motivation to stop smoking through information and personal support. - Carbon monoxide testing |
| - 23% had moderate COPD, FEV1 = 69-50% of predicted. - 6% had severe COPD, FEV1 ≤ 49% of predicted. Motivation to quit: Not stated Total sample: N = 247 |
counselling+ NRT]. vs. Usual care |
Nicotine dependence was classified according to Fagerstrom test for nicotine independence. | performed in a random sample of patients. - NRT and distractive activities such as physical exercise were recommended. - Each participant met 1 hour daily with a trained smoking cessation nurse. - Structured educational program including a physician, physiotherapist, dietician, lab technician, psychologist, and occupational therapist, each with a specific role in the education program. - Spouses of smokers were invited to stay in the hospital if they accepted. |
|||
| Lung Health Study Anthonisen et al, 1994; (17) Anthonisen et al, 2005 (14) |
Age range: 35–60 years. - Presence of mild airway obstruction. - Smokers smoking 10 cigarettes/day during 30 days preceding the screening test. Severity of COPD: Mild to moderate, FEV1/FCV < 70% and 55% < FEV1 < 90% Motivation to quit: Not stated Total sample: N = 5887 |
Smoking intervention: ipratropium bromide [intensive counselling + bronchodilator]. vs. Smoking intervention: placebo [intensive counselling + NRT]. vs. Usual care Smoking intervention: physician message, individual session with interventionist for behavioral interview, group orientation meeting, 12 intensive group sessions, clinic visits every 4 months |
Abstinence: Smoking cessation confirmed by salivary cotinine/carbon monoxide testing. Participants with cotinine levels > 20 ng/mL were considered to be smokers. Mortality: All-cause, cardiovascular mortality and lung cancer mortality. Lung function: FEV1 |
1 year, 5 years, 14.5 years | An aggressive smoking intervention program significantly reduces age-related decline in middle-aged smokers with mild airway obstruction. - Use of anticholinergic bronchodilators results in a relatively small improvement in FEV1 that appears to be reversed after the drug is discontinued. Use of bronchodilator did not influence the long-term decline of FEV1. - All-cause mortality was significantly lower in the smoking intervention groups than in the usual |
Spouses and significant others of both intervention groups were included in the cessation program if they wished and were treated the same way as participants. - Women tended to have greater improvement in lung function in response to smoking cessation than did men. - In addition to change in smoking status, determinants of the degree of improvement in, or stabilization of, FEV1 included baseline function, baseline bronchodilator responsiveness, race, methacholine reactivity, intervention group, and age. - Smoking cessation reduced the frequency of lower respiratory illness physician visits. |
| for 5 years, maintenance program for quitters, extended intervention program for patients still smoking or relapsing, and NRT gum. | care group. Smoking cessation intervention programs have a substantial effect on subsequent mortality even when successful in the minority of participants. - No linear relationship was found between smoking reduction and FEV1. |
- Quitting smoking for an interval followed by relapse to smoking appeared to provide a measurable and lasting benefit in comparison to continuous smoking. Attempts to quit smoking can prevent some loss of lung function. - Changes in AR were primarily related to changes in FEV1 The greater the decline in FEV1, the greater the increase in airway reactivity. Smoking cessation had a small additional benefit in AR beyond its favorable effects on FEV1 changes. |
||||
| Tashkin et al, 2001 (19) | Aged > 35 years. - Smoked 15 or more cigarettes/day for the previous year and had not stopped smoking for more than 3 months during that year. Severity of COPD: Mild-Moderate. Patients with Stage I and II COPD (FEV1/FVC ≤ 0.70) according to ATS guidelines and presence of clinically defined COPD (emphysema, chronic bronchitis, and smoking-related small airways disease). Motivation to quit: Motivated to quit Total sample: N = 404 |
Counselling: All patients received brief, face-to-face counselling at each of the 9 visits to the clinic + 1 telephone session 3 days after the quit date. Bupropion SR 150mg—days 1–3: once per day; days 4–84: 150 mg twice per day + personalized counselling by trained counsellor. [intensive counselling + antidepressant]. vs. Placebo + personalized counselling for 12 weeks [intensive counselling]. |
Continuous abstinence: Zero cigarettes per day confirmed by exhaled carbon monoxide values of ≤ 10 ppm. Point-prevalence abstinence: Defined as abstinence during the previous 7 days. |
6 months | Bupropion SR is a well-tolerated and effective aid to smoking cessation in people with mild to moderate COPD. | 12-week treatment phase with follow-up at 6 months. - Block randomization stratified by centre. - Target cessation date was selected. - Predictors of abstinence were tested using multivariable logistic regression, controlling for smoking history, age, sex, centre, and treatment group assignment. - Proportions of patients with either Stage I or Stage II COPD were evenly distributed between treatment groups, as were participants with sub-diagnoses of emphysema, bronchitis, or small airways disease. |
| Hilberink et al, 2010 (25) | Aged > 35 years - Current smokers. - Diagnosis of COPD confirmed by GP. - Recorded medication with ICPC code R95/96. - At least 3 prescriptions for bronchodilators in the past year. - At least 3 prescriptions for inhaled anti-inflammatory medication in the past year. Severity of COPD: Not defined, but probably mild to moderate according to GOLD. Motivation to quit: Not stated Total sample: N = 667 |
Counselling + NRT [minimal counselling + NRT]. vs. Counselling + NRT + bupropion [minimal counselling + NRT + antidepressant]. vs. Usual Care SMOCC intervention: A multi-faceted strategy containing:
|
Point-prevalence abstinence: Self-reported abstinence, biochemically verified by urinary cotinine levels of < 50 ng/mL. | 6 months, 12 months | The program doubled the cessation rates (statistically nonsignificant). Too few participants used additional bupropion SR to prove its effectiveness. | - Professionally directed intervention consisted of 4-hour group training on COPD, smoking, and smoking cessation. More individual support was provided by an outreach visitor by means of counselling and feedback about performance at the practice location. Support materials included information on smoking and smoking cessation, educational tools for patients, and questionnaires assessing smoking habits. - Data collection included motivation to quit smoking and Fagerstrom test. Severity of COPD was determined according to Medical Research Council Questionnaire and self-efficacy. - Patients were divided into 3 categories: preparers, contemplators, and pre-contemplators. |
| Wilson et al, 2008 (27) | Mean age: 61 years (SD84) - Smokers with diagnosis of COPD attending the regional respiratory centre. |
Individual support by nurse (5 weekly hour-long sessions) + 12-week course of NRT for those wishing to stop (300 minutes) [intensive counselling + NRT]. | Abstinence: Self-report of complete cessation confirmed by biochemical validation (carbon monoxide ≤ 10 ppm and salivary cotinine ≤ 10 ng/mL). | 12 months | Patients with COPD were unable to stop smoking, regardless of the type of support they received. | Study objective was to gain an insight into the nurse’s role in changing the smoking behavior of adults with COPD requiring secondary care. - Hypothesis was that intensive |
|
Mean (SD) pack-years: 41.4 (20). Severity of COPD: Not defined Motivation to quit: Not stated Total sample: N = 91 |
vs. Group support (5 weekly, hour-long sessions) by nurse + 12-week course of NRT for those wishing to stop [intensive counselling + NRT]. vs. Control (minimal counselling 5-10 min by GP + leaflet about smoking cessation). |
Stages of change—
Nicotine dependence: Measured by heaviness of smoking index. Dyspnea: Assessed by Medical Research Council dyspnea scale. |
nursing sessions (individual or group) would increase cessation rates. - Respiratory nurses and a physician providing individual and group support received standardized training. - Stage of Change criteria were used to categorize motivation and assist nurses to stage-match interventions. |
|||
| Tonnesen et al, 2006 (16) | Aged >18 years - Patients with COPD. Severity of COPD: Mild: 9% had FEV1 > 80% Moderate: 53% had < 50% < FEV1 < 80% Severe: 30% had < 30% <FEV1 < 50% Very Severe: 8% had FEV1 < 50% Motivation to quit: Not stated Total sample: N = 370 |
2 mg nicotine sublingual tablet + low behavioral support [intensive counselling + NRT]. vs. 2 mg nicotine sublingual tablet + high behavioral support [intensive counselling + NRT]. vs. Placebo + low behavioral support [intensive counselling]. vs. Placebo + low behavioral support [intensive counselling]. Low behavioral support: (individual + telephone sessions (total of 150 minutes by a respiratory nurse) + take-home |
Point-prevalence abstinence at 6 and 12 months: Self-reported smoking during previous week; cessation verified by exhaled carbon monoxide level < 10 ppm. Point-prevalence smoking reduction: Degree of smoking reduction after 12 months. Those still smoking but who reduced their smoking to less than 7 cigarettes daily or who reduced their daily smoking to less than 50%. Sustained abstinence: Self-reported smoking cessation at all visits from week 2 to 12 months verified by exhaled carbon monoxide level < 10 ppm. |
6 months, 12 months | The results demonstrate that the use of sublingual nicotine tablets in addition to a nurse-run smoking program results in higher rates of smoking cessation compared to placebo. |
Low support: (4 individual visits + 6 telephone calls: total hours = 2.5). High support: (7 individual visits + 5 telephone calls; total hours =4.5). NRT or placebo tablets were taken for 12 weeks with possibility of continued use for up to 12 months. Recommended study dose of medication was dependent on baseline cigarette consumption (≥16 cigarettes = 1-2 tablets per hour [min 10 and max 40]; 10–15 cigarettes = 1–2 tablets per hour [min 6 and max 30]; 6–9 cigarettes = 1 tablet per hour [min 3 and max 10]). - The term “smoker’s lung” was used to explain COPD to patients. - Nurses received standardized training on counselling and counselling guidelines. |
| material). High behavioral support: (individual + telephone sessions (total of 270 minutes by a respiratory nurse) + take-home material). |
HRQOL: Changes in QOL measures according to the SGRQ. A reduction in a total SGRQ score of ≥ 4 is considered to represent a clinically significant improvement. | - Nicotine dependence assessed by Fagerstrom test. - Motivation to quit smoking assessed on 10-cm visual analog scale. |
||||
| Kotz et al, 2009 (23) | Mean age: 53.8 years, SD 7.0. - Patients with smoking history of ≥ 10 pack-years and had airflow limitation defined as FEV1/FCV < 70%, FEV1 ≥ 50% according to GOLD guidelines. Severity of COPD: Mild-Moderate GOLD I (n): 160 GOLD II (n): 136 Motivation to quit: Motivated to quit Total sample: N = 296 |
Confrontation with spirometry results during face-to-face session + 1 telephone session by respiratory nurse (165 minutes) + nortriptyline for 7 weeks [intensive counselling + antidepressant]. vs. Face-to-face session + 1 telephone session by a respiratory nurse (165 min) + nortriptyline for 7 weeks without confrontation [intensive counselling + antidepressant]. vs. Usual care (care as usual for smoking cessation provided by patient’s own GP [minimal counselling]. |
Prolonged abstinence: defined as urine cotinine–validated (< 50 ng/mL) abstinence from smoking at all 3 follow-up visits. | 5 weeks, 26 weeks, 52 weeks | Study did not provide evidence that confrontational approach to smoking cessation increases the rate of long-term abstinence from smoking compared with equally intensive treatment without confrontation with spirometry or usual care. | Nortriptyline dose: Days 1–3: 25 mg OD Days 4–7: 50 mg OD Days 8–49: 75 mg OD |
| Wagena et al, 2005 (21) | Age range: 30–70 years. - Patients with COPD or at risk of COPD according to GOLD. - Had a smoking history of at least 5 years, with an average of 10 cigarettes per day in the last year. Severity of COPD: Stages 0— III Motivation to quit: Had motivation to stop smoking Total sample: N = 255 |
All patients received individual face-to-face (60–minute) and telephone counselling (30–minute) sessions by a respiratory nurse. Bupropion hydrochloride SR [intensive counselling + antidepressant]. vs. Nortriptyline hydrochloride [intensive counselling + antidepressant]. vs. Placebo [intensive counselling]. |
Prolonged abstinence: Zero cigarettes per day from weeks 4 to week 26— confirmed by urinary cotinine values of 60 ng/mL or less. Point-prevalence abstinence: No smoking for previous 7 days assessed at weeks 4, 12, and 26— confirmed by urinary cotinine values of < 60 ng/mL. |
4 weeks, 12 weeks, 26 weeks | A small but nonsignificant difference in prolonged abstinence rates was observed between the 2 groups. Smaller differences in prolonged abstinence were observed in patients at risk of COPD (Stage 0). - Authors concluded that bupropion SR treatment is an efficacious aid to smoking cessation in patients with COPD. Nortriptyline treatment seems to be a useful alternative to bupropion. |
Includes patients at risk for COPD (Stage 0 according to GOLD). - Patients were stratified based on disease severity according to European Respiratory Society and GOLD. |
Abbreviations: AR, airway reactivity; ATS, American Thoracic Society; COHB, carboxyhemoglobin; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GP, general practitioner; ICPC, International Classification for Primary Care; max, maximum; min, minimum; NRT, nicotine replacement therapy; OD, once daily; ppm, parts per million; SD, standard deviation; SGRQ, St. George’s Respiratory Questionnaire; SMOCC, Smoking Cessation in Patients With COPD; SR, sustained release.
Appendix 3: GRADE Quality of Evidence
The GRADE quality of evidence of the randomized controlled trials is shown below in Table A2.
Table A2: GRADE Quality of Evidence (Outcome = Abstinence Rate)*.
| No. of Studies | Design | Study Quality | Inconsistency | Indirectness | Imprecision | Other Modifying Factors | Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Comparison: Counselling vs. Usual Care | |||||||
| 2 | RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
| Comparison: Counselling + NRT vs. Usual Care | |||||||
| 3 | RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
| Comparison: Minimal Counselling (< 90 minutes) + Antidepressant vs. Usual Care | |||||||
| 1 | RCT | No serious limitations | n/a | Some serious limitations† | No serious limitations | n/a | Low |
| Comparison: Minimal Counselling (< 90 minutes) + NRT + Antidepressant vs. Usual Care | |||||||
| 1 | RCT | Serious limitations‡ | n/a | No serious limitations | No serious limitations | n/a | Low |
| Comparison: NRT vs. Placebo | |||||||
| 1 | RCT | No serious limitations | n/a | No serious limitations | No serious limitations | n/a | Moderate |
| Comparison: Antidepressant vs. Placebo | |||||||
| 1 | RCT | No serious limitations | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; n/a, not applicable; No., number; NRT, nicotine replacement therapy; RCT, randomized controlled trial.
Study quality was downgraded for the outcome of abstinence for the comparison of minimal counselling plus antidepressant versus usual care due to some limitations in the directness. Patients enrolled in the study had undiagnosed COPD; however, they were eligible for enrollment in the study once they had been classified by the GOLD stage criteria. Because patients entering into the study were originally undiagnosed, they may not have been as motivated or may not have taken their illness as seriously as those who had been diagnosed with COPD.
Study quality was downgraded due to limitations in the study design. The study was underpowered to detect a difference; also, at follow-up, there was poor compliance with the prescribed and recommended NRT and bupropion, affecting the overall success of the intervention.
Suggested Citation
This report should be cited as follows:
Thabane M; COPD Working Group. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2012 March; 12(4) 1–50. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Smoking_Cessation_March.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in Excerpta Medica/EMBASE and the Center for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: MASinfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All analyses in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: MASinfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-7010-7 (PDF)
© Queen’s Printer for Ontario, 2012
About the Medical Advisory Secretariat
Effective April 5, 2011, the Medical Advisory Secretariat (MAS) became a part of Health Quality Ontario (HQO), an independent body funded by the Ministry of Health and Long-Term Care. The mandate of MAS is to provide evidence-based recommendations on the coordinated uptake of health services and health technologies in Ontario to the Ministry of Health and Long-Term Care and to the health care system. This mandate helps to ensure that residents of Ontario have access to the best available and most appropriate health services and technologies to improve patient outcomes.
To fulfill its mandate, MAS conducts systematic reviews of evidence and consults with experts in the health care services community. The resulting evidence-based analyses are reviewed by the Ontario Health Technology Advisory Committee—to which MAS also provides a secretariat function—and published in the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, MAS systematically reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, the Secretariat collects and analyzes information about how a new technology fits within current practice and existing treatment alternatives. Details about the technology’s diffusion into current health care practices add an important dimension to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist decision-makers in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals wishing to comment on an analysis prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by MAS for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data and information provided by experts and applicants to MAS to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the MAS website for a list of all evidence-based analyses: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
List of Tables
| Table ES1: Summary of Results* |
| Table 1: Summary of Drugs for Nicotine Dependence Licensed by Health Canada* |
| Table 2: Body of Evidence Examined According to Study Design* |
| Table 3: Abstinence Rates and Associated Intervention Costs for the Four Intervention Groups* |
| Table 4: Relative Efficacy of Smoking Cessation Intervention on Prolonged Abstinence* |
| Table 5: Abstinence Rates of Five Included Individual Studies* |
| Table 6: Quality of Included Individual Studies* |
| Table 7: Summary of Results – Abstinence at Longest Follow-Up* |
| Table A1: Characteristics of Included Randomized Control Trials* |
| Table A2: GRADE Quality of Evidence (Outcome = Abstinence Rate)* |
List of Figures
| Figure 1: Citation Flow Chart |
| Figure 2: Comparison of Abstinence for Counselling Versus Usual Care Groups at Longest Follow-Up* |
| Figure 3: Comparison of Abstinence for Counselling Plus NRT Versus Usual Care Groups at Longest Follow-Up* |
| Figure 4: Comparison of Abstinence for Minimal Counselling Plus Antidepressant Versus Usual Care Groups at Longest Follow-Up* |
| Figure 5: Comparison of Abstinence for Minimal Counselling Plus NRT Plus Antidepressant Versus Usual Care Groups at Longest Follow-Up* |
| Figure 6: Comparison of Abstinence for NRT Versus Placebo Groups at Longest Follow-Up* |
| Figure 7: Comparison of Abstinence for Antidepressant Versus Placebo Groups at Longest Follow-Up* |
List of Abbreviations
- COPD
Chronic obstructive pulmonary disease
- CI
Confidence interval
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- MAS
Medical Advisory Secretariat
- NRT
Nicotine replacement therapy
- OR
Odds ratio
- RCT
Randomized controlled trial
- RD
Risk difference
- RR
Relative risk
- SCC
Smoking cessation counselling
Footnotes
In addition to evaluating the effectiveness of the smoking cessation interventions, a dynamic Dutch COPD population model was developed to estimate the impact of increased implementation of smoking cessation interventions and usual care and the cost-effectiveness of the interventions. These are out of scope of this systematic review, and so the results are not summarized here.
The mild-to-moderate classification was created for the purposes of the report.
References
- 1.Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006 Nov;61(11):935–9. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007 Feb 22;356(8):775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z. Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008 Jan 1;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 4.Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003 Dec 18;12:1053–7. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vozoris NT, Stanbrook MB. Smoking prevalence, behaviours, and cessation among individuals with COPD or asthma. Respir Med. 2010 Sep 15; doi: 10.1016/j.rmed.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Molken MP. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010 Aug 65;(8):711–8. doi: 10.1136/thx.2009.131631. [DOI] [PubMed] [Google Scholar]
- 7.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun;19(328):7454–1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman C. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care. 1996. Literature searching and evidence interpretation for assessing health care practices; 81 pp. SBU Report No. 119E. [DOI] [PubMed] [Google Scholar]
- 9.van der Meer RM WEORJAvSOP. Cochrane Database of Systematic Reviews. Issue 1. 2001. Smoking cessation for chronic obstructive pulmonary disease. Art. No.: CD002999. DOI: 10.1002/14651858.CD002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagena EJ, van der Meer RM, Ostelo RJWG, Jacobs JE. Van Schayck CP. The efficacy of smoking cessation strategies in people with chronic obstructive pulmonary disease: Results from a systematic review. Respir Med. 2004;98(9):805–15. doi: 10.1016/j.rmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Strassmann R, Bausch B, Spaar A, Kleijnen J, Braendli O, Puhan MA. Smoking cessation interventions in COPD: a network meta-analysis of randomised trials. Eur Respir J. 2009 Sep;34(3):634–40. doi: 10.1183/09031936.00167708. [DOI] [PubMed] [Google Scholar]
- 12.Crowley TJ, Macdonald MJ, Walter MI. Behavioral anti-smoking trial in chronic obstructive pulmonary disease patients. Psychopharmacology (Berl) 1995 May;119(2):193–204. doi: 10.1007/BF02246161. [DOI] [PubMed] [Google Scholar]
- 13.Brandt CJ, Ellegaard H, Joensen M, Kallan FV, Sorknaes AD, Tougaard L. Effect of diagnosis of ‘smoker’s lung’. Lancet. 1997;349(9047):253. doi: 10.1016/s0140-6736(05)64863-5. [DOI] [PubMed] [Google Scholar]
- 14.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5–year mortality: a randomized clinical trial. Ann Intern Med. 2005(142):233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Tashkin DP, Murray RP. Smoking cessation in chronic obstructive pulmonary disease. Respir Med. 2009 Jul;103(7):963–74. doi: 10.1016/j.rmed.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Tonnesen P, Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest. 2006 Aug;130(2):334–42. doi: 10.1378/chest.130.2.334. [DOI] [PubMed] [Google Scholar]
- 17.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994 Nov 16;272(19):1497–505. [PubMed] [Google Scholar]
- 18.Pederson LL, Wanklin JM, Lefcoe NM. The effects of counseling on smoking cessation among patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. Int J Addict. 1991 Jan;26(1):107–19. doi: 10.3109/10826089109056242. [DOI] [PubMed] [Google Scholar]
- 19.Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001 May 19;357(9268):1571–5. doi: 10.1016/s0140-6736(00)04724-3. [DOI] [PubMed] [Google Scholar]
- 20.Murray RP, Anthonisen NR, Connett JE, Wise RA, Lindgren PG, Greene PG. Effects of multiple attempts to quit smoking and relapses to smoking on pulmonary function. J Clin Epidemiol. 1998;51(12):1317–26. doi: 10.1016/s0895-4356(98)00120-6. [DOI] [PubMed] [Google Scholar]
- 21.Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, Van Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med. 2005 Oct 24;165(19):2286–92. doi: 10.1001/archinte.165.19.2286. [DOI] [PubMed] [Google Scholar]
- 22.Sundblad BM, Larsson K, Nathell L. High rate of smoking abstinence in COPD patients: Smoking cessation by hospitalization. Nicotine Tob Res. 2008 May;10(5):883–90. doi: 10.1080/14622200802023890. [DOI] [PubMed] [Google Scholar]
- 23.Kotz D, Wesseling G, Huibers MJ, van Schayck OC. Efficacy of confronting smokers with airflow limitation for smoking cessation. Eur Respir J. 2009 Apr;33(4):754–62. doi: 10.1183/09031936.00116308. [DOI] [PubMed] [Google Scholar]
- 24.Sundblad BM, Larsson K, Nathell L. Low awareness of COPD among physicians. Clin Respir J. 2008 Jan;2(1):11–6. doi: 10.1111/j.1752-699X.2007.00020.x. [DOI] [PubMed] [Google Scholar]
- 25.Hilberink SR, Jacobs JE, Breteler M, de Vries H, Grol R. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: Impact after 1 year of two complex interventions [Internet] [[updated 2010; cited 2010 Apr 1]]. Available from: www.elsevier.com/locate/pateducou . [DOI] [PubMed]
- 26.Hilberink SR, Jacobs JE, Bottema BJ, de VH, Grol RP. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months’ results. Prev Med. 2005 Nov;41(5–6):822–7. doi: 10.1016/j.ypmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JS, Fitzsimons D, Bradbury I, Stuart EJ. Does additional support by nurses enhance the effect of a brief smoking cessation intervention in people with moderate to severe chronic obstructive pulmonary disease? A randomised controlled trial. Int J Nurs Stud. 2008 Apr;45(4):508–17. doi: 10.1016/j.ijnurstu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Sundblad BM, Larsson K, Nathell L. Lung function testing influences the attitude toward smoking cessation. Nicotine Tob Res. 2010 Jan;12(1):37–42. doi: 10.1093/ntr/ntp170. [DOI] [PubMed] [Google Scholar]
- 29.Kotz D, van de KK, Jobsis Q, Van Schayck CP. Effects of tobacco exposure on lung health and pulmonary biomarkers in young healthy smokers aged 12–25 years: a systematic review. Expert Rev Respir Med. 2007 Dec;1(3):403–18. doi: 10.1586/17476348.1.3.403. [DOI] [PubMed] [Google Scholar]
- 30.Borglykke A, Pisinger C, Jorgensen T, Ibsen H. The effectiveness of smoking cessation groups offered to hospitalised patients with symptoms of exacerbations of chronic obstructive pulmonary disease (COPD) Clin Respir J. 2008 Jul;2(3):158–65. doi: 10.1111/j.1752-699X.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- 31.Christenhusz L, Seydel E, Pieterse M, van d V, van-der PJ. The SMOKE study: 1 year evaluation of an intensive multi-component smoking cessation programme for COPD outpatients [Abstract] Eur Respir J. 2005(26) Abstract. [Google Scholar]
- 32.Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J. 1995 Aug;8(8):1398–420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]


