Executive Summary
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective
The objective of this analysis was to determine the effectiveness of the influenza vaccination and the pneumococcal vaccination in patients with chronic obstructive pulmonary disease (COPD) in reducing the incidence of influenza-related illness or pneumococcal pneumonia.
Clinical Need: Condition and Target Population
Influenza Disease
Influenza is a global threat. It is believed that the risk of a pandemic of influenza still exists. Three pandemics occurred in the 20th century which resulted in millions of deaths worldwide. The fourth pandemic of H1N1 influenza occurred in 2009 and affected countries in all continents.
Rates of serious illness due to influenza viruses are high among older people and patients with chronic conditions such as COPD. The influenza viruses spread from person to person through sneezing and coughing. Infected persons can transfer the virus even a day before their symptoms start. The incubation period is 1 to 4 days with a mean of 2 days. Symptoms of influenza infection include fever, shivering, dry cough, headache, runny or stuffy nose, muscle ache, and sore throat. Other symptoms such as nausea, vomiting, and diarrhea can occur.
Complications of influenza infection include viral pneumonia, secondary bacterial pneumonia, and other secondary bacterial infections such as bronchitis, sinusitis, and otitis media. In viral pneumonia, patients develop acute fever and dyspnea, and may further show signs and symptoms of hypoxia. The organisms involved in bacterial pneumonia are commonly identified as Staphylococcus aureus and Hemophilus influenza. The incidence of secondary bacterial pneumonia is most common in the elderly and those with underlying conditions such as congestive heart disease and chronic bronchitis.
Healthy people usually recover within one week but in very young or very old people and those with underlying medical conditions such as COPD, heart disease, diabetes, and cancer, influenza is associated with higher risks and may lead to hospitalization and in some cases death. The cause of hospitalization or death in many cases is viral pneumonia or secondary bacterial pneumonia. Influenza infection can lead to the exacerbation of COPD or an underlying heart disease.
Streptococcal Pneumonia
Streptococcus pneumoniae, also known as pneumococcus, is an encapsulated Gram-positive bacterium that often colonizes in the nasopharynx of healthy children and adults. Pneumococcus can be transmitted from person to person during close contact. The bacteria can cause illnesses such as otitis media and sinusitis, and may become more aggressive and affect other areas of the body such as the lungs, brain, joints, and blood stream. More severe infections caused by pneumococcus are pneumonia, bacterial sepsis, meningitis, peritonitis, arthritis, osteomyelitis, and in rare cases, endocarditis and pericarditis.
People with impaired immune systems are susceptible to pneumococcal infection. Young children, elderly people, patients with underlying medical conditions including chronic lung or heart disease, human immunodeficiency virus (HIV) infection, sickle cell disease, and people who have undergone a splenectomy are at a higher risk for acquiring pneumococcal pneumonia.
Technology
Influenza and Pneumococcal Vaccines
Trivalent Influenza Vaccines in Canada
In Canada, 5 trivalent influenza vaccines are currently authorized for use by injection. Four of these are formulated for intramuscular use and the fifth product (Intanza®) is formulated for intradermal use.
The 4 vaccines for intramuscular use are:
Fluviral (GlaxoSmithKline), split virus, inactivated vaccine, for use in adults and children ≥ 6 months;
Vaxigrip (Sanofi Pasteur), split virus inactivated vaccine, for use in adults and children ≥ 6 months;
Agriflu (Novartis), surface antigen inactivated vaccine, for use in adults and children ≥ 6 months; and
Influvac (Abbott), surface antigen inactivated vaccine, for use in persons ≥ 18 years of age.
FluMist is a live attenuated virus in the form of an intranasal spray for persons aged 2 to 59 years. Immunization with current available influenza vaccines is not recommended for infants less than 6 months of age.
Pneumococcal Vaccine
Pneumococcal polysaccharide vaccines were developed more than 50 years ago and have progressed from 2-valent vaccines to the current 23-valent vaccines to prevent diseases caused by 23 of the most common serotypes of S pneumoniae. Canada-wide estimates suggest that approximately 90% of cases of pneumococcal bacteremia and meningitis are caused by these 23 serotypes. Health Canada has issued licenses for 2 types of 23-valent vaccines to be injected intramuscularly or subcutaneously:
Pneumovax 23® (Merck & Co Inc. Whitehouse Station, NJ, USA), and
Pneumo 23® (Sanofi Pasteur SA, Lion, France) for persons 2 years of age and older.
Other types of pneumococcal vaccines licensed in Canada are for pediatric use. Pneumococcal polysaccharide vaccine is injected only once. A second dose is applied only in some conditions.
Research Questions
What is the effectiveness of the influenza vaccination and the pneumococcal vaccination compared with no vaccination in COPD patients?
What is the safety of these 2 vaccines in COPD patients?
What is the budget impact and cost-effectiveness of these 2 vaccines in COPD patients?
Research Methods
Literature search
Search Strategy
A literature search was performed on July 5, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2000 to July 5, 2010. The search was updated monthly through the AutoAlert function of the search up to January 31, 2011. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Articles with an unknown eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established. Data extraction was carried out by the author.
Inclusion Criteria
studies comparing clinical efficacy of the influenza vaccine or the pneumococcal vaccine with no vaccine or placebo;
randomized controlled trials published between January 1, 2000 and January 31, 2011;
studies including patients with COPD only;
studies investigating the efficacy of types of vaccines approved by Health Canada;
English language studies.
Exclusion Criteria
non-randomized controlled trials;
studies investigating vaccines for other diseases;
studies comparing different variations of vaccines;
studies in which patients received 2 or more types of vaccines;
studies comparing different routes of administering vaccines;
studies not reporting clinical efficacy of the vaccine or reporting immune response only;
studies investigating the efficacy of vaccines not approved by Health Canada.
Outcomes of Interest
Primary Outcomes
Influenza vaccination: Episodes of acute respiratory illness due to the influenza virus.
Pneumococcal vaccination: Time to the first episode of community-acquired pneumonia either due to pneumococcus or of unknown etiology.
Secondary Outcomes
rate of hospitalization and mechanical ventilation
mortality rate
adverse events
Quality of Evidence
The quality of each included study was assessed taking into consideration allocation concealment, randomization, blinding, power/sample size, withdrawals/dropouts, and intention-to-treat analyses. The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria. The following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate |
| Very Low | Any estimate of effect is very uncertain. |
Summary of Efficacy of the Influenza Vaccination in Immunocompetent Patients With COPD
Clinical Effectiveness
The influenza vaccination was associated with significantly fewer episodes of influenza-related acute respiratory illness (ARI). The incidence density of influenza-related ARI was:
All patients: vaccine group: (total of 4 cases) = 6.8 episodes per 100 person-years; placebo group: (total of 17 cases) = 28.1 episodes per 100 person-years, (relative risk [RR], 0.2; 95% confidence interval [CI], 0.06−0.70; P = 0.005).
Patients with severe airflow obstruction (forced expiratory volume in 1 second [FEV1] < 50% predicted): vaccine group: (total of 1 case) = 4.6 episodes per 100 person-years; placebo group: (total of 7 cases) = 31.2 episodes per 100 person-years, (RR, 0.1; 95% CI, 0.003−1.1; P = 0.04).
Patients with moderate airflow obstruction (FEV1 50%−69% predicted): vaccine group: (total of 2 cases) = 13.2 episodes per 100 person-years; placebo group: (total of 4 cases) = 23.8 episodes per 100 person-years, (RR, 0.5; 95% CI, 0.05−3.8; P = 0.5).
Patients with mild airflow obstruction (FEV1 ≥ 70% predicted): vaccine group: (total of 1 case) = 4.5 episodes per 100 person-years; placebo group: (total of 6 cases) = 28.2 episodes per 100 person-years, (RR, 0.2; 95% CI, 0.003−1.3; P = 0.06).
The Kaplan-Meier survival analysis showed a significant difference between the vaccinated group and the placebo group regarding the probability of not acquiring influenza-related ARI (log-rank test P value = 0.003). Overall, the vaccine effectiveness was 76%. For categories of mild, moderate, or severe COPD the vaccine effectiveness was 84%, 45%, and 85% respectively.
With respect to hospitalization, fewer patients in the vaccine group compared with the placebo group were hospitalized due to influenza-related ARIs, although these differences were not statistically significant. The incidence density of influenza-related ARIs that required hospitalization was 3.4 episodes per 100 person-years in the vaccine group and 8.3 episodes per 100 person-years in the placebo group (RR, 0.4; 95% CI, 0.04−2.5; P = 0.3; log-rank test P value = 0.2). Also, no statistically significant differences between the 2 groups were observed for the 3 categories of severity of COPD.
Fewer patients in the vaccine group compared with the placebo group required mechanical ventilation due to influenza-related ARIs. However, these differences were not statistically significant. The incidence density of influenza-related ARIs that required mechanical ventilation was 0 episodes per 100 person-years in the vaccine group and 5 episodes per 100 person-years in the placebo group (RR, 0.0; 95% CI, 0−2.5; P = 0.1; log-rank test P value = 0.4). In addition, no statistically significant differences between the 2 groups were observed for the 3 categories of severity of COPD. The effectiveness of the influenza vaccine in preventing influenza-related ARIs and influenza-related hospitalization was not related to age, sex, severity of COPD, smoking status, or comorbid diseases.
safety
Overall, significantly more patients in the vaccine group than the placebo group experienced local adverse reactions (vaccine: 17 [27%], placebo: 4 [6%]; P = 0.002). Significantly more patients in the vaccine group than the placebo group experienced swelling (vaccine 4, placebo 0; P = 0.04) and itching (vaccine 4, placebo 0; P = 0.04). Systemic reactions included headache, myalgia, fever, and skin rash and there were no significant differences between the 2 groups for these reactions (vaccine: 47 [76%], placebo: 51 [81%], P = 0.5).
With respect to lung function, dyspneic symptoms, and exercise capacity, there were no significant differences between the 2 groups at 1 week and at 4 weeks in: FEV1, maximum inspiratory pressure at residual volume, oxygen saturation level of arterial blood, visual analogue scale for dyspneic symptoms, and the 6 Minute Walking Test for exercise capacity.
There was no significant difference between the 2 groups with regard to the probability of not acquiring total ARIs (influenza-related and/or non-influenza-related); (log-rank test P value = 0.6).
Summary of Efficacy of the Pneumococcal Vaccination in Immunocompetent Patients With COPD
Clinical Effectiveness
The Kaplan-Meier survival analysis showed no significant differences between the group receiving the penumoccocal vaccination and the control group for time to the first episode of community-acquired pneumonia due to pneumococcus or of unknown etiology (log-rank test 1.15; P = 0.28). Overall, vaccine efficacy was 24% (95% CI, −24 to 54; P = 0.33).
With respect to the incidence of pneumococcal pneumonia, the Kaplan-Meier survival analysis showed a significant difference between the 2 groups (vaccine: 0/298; control: 5/298; log-rank test 5.03; P = 0.03).
Hospital admission rates and median length of hospital stays were lower in the vaccine group, but the difference was not statistically significant. The mortality rate was not different between the 2 groups.
Subgroup Analysis
The Kaplan-Meier survival analysis showed significant differences between the vaccine and control groups for pneumonia due to pneumococcus and pneumonia of unknown etiology, and when data were analyzed according to subgroups of patients (age < 65 years, and severe airflow obstruction FEV1 < 40% predicted). The accumulated percentage of patients without pneumonia (due to pneumococcus and of unknown etiology) across time was significantly lower in the vaccine group than in the control group in patients younger than 65 years of age (log-rank test 6.68; P = 0.0097) and patients with a FEV1 less than 40% predicted (log-rank test 3.85; P = 0.0498).
Vaccine effectiveness was 76% (95% CI, 20−93; P = 0.01) for patients who were less than 65 years of age and −14% (95% CI, −107 to 38; P = 0.8) for those who were 65 years of age or older. Vaccine effectiveness for patients with a FEV1 less than 40% predicted and FEV1 greater than or equal to 40% predicted was 48% (95% CI, −7 to 80; P = 0.08) and −11% (95% CI, −132 to 47; P = 0.95), respectively. For patients who were less than 65 years of age (FEV1 < 40% predicted), vaccine effectiveness was 91% (95% CI, 35−99; P = 0.002).
Cox modelling showed that the effectiveness of the vaccine was dependent on the age of the patient. The vaccine was not effective in patients 65 years of age or older (hazard ratio, 1.53; 95% CI, 0.61−a2.17; P = 0.66) but it reduced the risk of acquiring pneumonia by 80% in patients less than 65 years of age (hazard ratio, 0.19; 95% CI, 0.06−0.66; P = 0.01).
safety
No patients reported any local or systemic adverse reactions to the vaccine.
Background
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective of Analysis
The objective of this analysis was to determine the effectiveness of the influenza vaccination and the pneumococcal vaccination in patients with COPD in reducing the incidence of influenza-related illness or pneumococcal pneumonia.
Clinical Need and Target Population
Influenza Disease
Influenza is a global threat. It is believed that the risk of a pandemic of influenza still exists. Three pandemics occurred in the 20th century which resulted in millions of deaths worldwide. (1) The fourth pandemic of H1N1 influenza occurred in 2009 and affected countries on all continents.
Rates of serious illness due to influenza viruses are high among older people and patients with chronic conditions such as COPD. (2) The influenza viruses spread from person to person through sneezing and coughing. Infected persons can transfer the virus even a day before their symptoms start. (3) The incubation period is 1 to 4 days with a mean length of 2 days. (1) Symptoms of influenza infection include fever, shivering, dry cough, headache, runny or stuffy nose, muscle ache, and sore throat. Other symptoms such as nausea, vomiting, and diarrhea can occur.
Complications of influenza infection include viral pneumonia, secondary bacterial pneumonia, and other secondary bacterial infections such as bronchitis, sinusitis, and otitis media. In viral pneumonia, patients develop an acute fever and dyspnea, and may further show signs and symptoms of hypoxia. The organisms involved in bacterial pneumonia are commonly Staphylococcus aureus and Hemophilus influenza. The incidence of secondary bacterial pneumonia is most common in the elderly and those with underlying conditions such as congestive heart disease and chronic bronchitis. (4)
Healthy people usually recover within one week, but in very young or very old people and those with underlying medical conditions such as COPD, heart disease, diabetes, and cancer, influenza is associated with higher risks and may lead to hospitalization and in some cases death. The cause of hospitalization or death in many cases is viral pneumonia or secondary bacterial pneumonia. (3) Influenza infection can lead to the exacerbation of COPD or an underlying heart disease. (2)
Strains of Influenza Virus
Influenza viruses exist in 3 forms: A, B, and C. Influenza A is generally responsible for epidemics and pandemics while influenza B generally causes milder and less severe outbreaks in smaller communities such as schools or camps. (1) Virus strains are characterized by different hemagglutinin (H) and neuraminidase (N) subclasses. Sixteen H subtypes (H1 to H16) and 9 N subtypes (N1 to N9) have been identified for influenza A viruses. Major shifts in the antigenic profiles of the viruses can cause epidemics. However, minor antigenic shifts can cause less severe outbreaks.
Influenza Ecology
Influenza A viruses are primarily viruses of water-based birds (5) which are natural reservoirs for a variety of H and N combinations. Influenza A viruses infect a wide range of species such as humans, pigs, wild birds, domestic poultry, domestic cats, civets, tigers, seals, aquatic mammals, and horses. (4) Influenza B and C are viruses that affect humans, with only a few reports of sporadic infections in mammalian hosts such as seals, pigs, and dogs. (4;5)
Avian influenza in birds is usually mild or asymptomatic and the virus can be secreted in high titres through the cloacae for a period of up to 30 days. The practice of free-ranging poultry close to the family dwelling facilitates the transfer of the virus to a human.
Influenza Diagnosis
Infection by the influenza virus results in a rise in the serum antibody titre. Demonstration of fourfold or greater rise in the hemagglutination inhibition (HI) antibody titre in the convalescent serum as compared with the acute serum is considered as diagnostic of the infection. (4)
Influenza Vaccination
Influenza vaccination is the primary method of influenza prevention and has been available for about 70 years. (3) It has been shown that during 10 seasons, influenza vaccination significantly reduced the risk of hospitalization for pneumonia or influenza, and the risk of death among community-dwelling elderly persons. Nichol et al (6) have shown that in people with chronic lung diseases, vaccination resulted in a 52% reduction in hospitalizations and a 70% reduction in death rates during influenza seasons. In this study, hospitalization rates for pneumonia and influenza among unvaccinated people were twice as high in the influenza seasons as they were in the interim (non-influenza) periods. During the influenza seasons, those who received the vaccine had fewer hospitalizations for pneumonia and influenza compared with those who were not vaccinated (adjusted risk ratio [RR], 0.48; 95% confidence interval [CI], 0.28−0.82), and they had lower risk for death (adjusted odds ratio [OR], 0.30; 95% CI, 0.21−0.43).
Several studies have shown that antibody response decreases in the elderly compared with young people following subsequent vaccinations. Gardner et al (7) studied antibody responses to annual influenza vaccination over 4 years in a healthy elderly population. In the first year following vaccination, 32% of the persons produced a fourfold rise in antibody titre to any vaccine component included in the vaccine. However, this percentage decreased after subsequent vaccinations with the same component (10% following the second and the third vaccination, 12% following the fourth vaccination, and 6% following the fifth vaccination). However, in any given year, the percentage of people with post vaccination titres greater than or equal to 40 to A/Texas was not less than 50% (first year 84%, second year 50%, third year 84%, fourth year 82%, and fifth year 76%).
Global Prevalence and Incidence of Influenza
During the 20th century there were 3 pandemics of influenza: year 1918, year 1957, and year 1968. The fourth influenza outbreak occurred in 2009. The 1918 pandemic had the highest mortality rate causing approximately 40 million deaths worldwide. In 1957 the appearance of influenza A2 type H2N2 caused over 2 million deaths worldwide. The pandemic that occurred in 1968 was the result of the influenza type H3N2 that emerged in Hong Kong. The avian influenza caused by H5N1 emerged in 1997 and re-emerged in 2004 to 2005. (1)
From April 2009 to January 2010, more than 211 countries and overseas territories reported laboratory confirmed cases of influenza A (H1N1) 2009. Pandemic influenza A (H1N1) 2009 remained predominant while seasonal influenza types A (H1N1), A (H3N2), and B viruses circulated at very low levels in many countries during this period. (8)
A highly pathogenic avian influenza A (H5N1) is present in poultry. Since December 2003, a total of 478 confirmed human cases and 286 deaths due to influenza A (H5N1) have been reported by 15 countries. (8)
In Canada, the national influenza surveillance is coordinated through the Centre for Immunization and Respiratory Infectious Diseases (CIRID) and the Public Health Agency of Canada (PHAC). The Flu Watch program provides a national picture of influenza activities through collecting information from different sources.
Streptococcal Pneumonia
Pathogenic Bacteria
Streptococcus pneumoniae, also known as pneumococcus, is an encapsulated Gram-positive bacterium that often colonizes in the nasopharynx of healthy children and adults. Pneumococcus can be transmitted from person to person during close contact. The bacteria can cause illnesses such as otitis media and sinusitis, and may even become more aggressive and affect other areas of the body such as the lungs, brain, joints, and blood stream. More severe infections caused by pneumococcus are pneumonia, bacterial sepsis, meningitis, peritonitis, arthritis, osteomyelitis, and in rare cases endocarditis and pericarditis.
High Risk Groups
People with impaired immune systems are susceptible to pneumococcal infection. Young children, elderly people, and patients with underlying medical conditions including chronic lung or heart disease, human immunodeficiency virus (HIV) infection, sickle cell disease, and people who have undergone splenectomy are at a higher risk for acquiring pneumococcal pneumonia.
Limitations of Trials of Pneumococcal Vaccine Efficacy
Although randomized controlled trials (RCTs) would provide the most definitive data about vaccine efficacy in the COPD population, identification of an organism-specific effect such as pneumococcal pneumonia, which is difficult to isolate in clinical samples (9), limits the implementation of these trials.
It has been estimated that an RCT would require between 120,000 and 482,000 patients to demonstrate a benefit in a clinically relevant outcome such as pneumococcal pneumonia. (10) These trials are prohibitive in terms of costs and logistics. In addition, conducting a placebo-controlled trial in patients with COPD may raise ethical concerns, as the pneumococcal vaccination of this at risk group is considered to be the standard of care in many countries.
Studies of Pneumococcal Vaccine Efficacy in the General Population
Recommendations for pneumococcal vaccinations target people who are at high risk for invasive pneumococcal disease. However, the use of a pneumococcal vaccine in the elderly or in high risk populations is still controversial and has been the subject of many meta-analyses and systematic reviews. It is not clear whether effectiveness wanes over time and/or with age. Presence of significant heterogeneity between the results of the trials makes it difficult to estimate the true effect of the pneumococcal vaccine in adults. Albeit, it seems that the strongest evidence is for the end point of pneumococcal bacteremia.
Some studies have found that the pneumococcal vaccine was not protective against pneumococcal pneumonia without bacteremia. For example, a large retrospective cohort study of 47,365 participants 65 years of age and older (11) did not find an association between the pneumococcal vaccination and a reduced risk of community-acquired pneumonia (CAP), regardless of the need for hospitalization (hazard ratio [HR], 1.07; 95% CI, 0.99−1.14). However, this study found a significant reduction in the risk of pneumococcal bacteremia (HR, 0.56; 95% CI, 0.33−0.93; P = 0.03). Another finding of this study was a higher risk of hospitalization due to pneumonia among those vaccinated (HR, 1.14; 95% CI, 1.02−1.28; P = 0.02). The patient population for the above study consisted of members of the Group Health Cooperative, a health maintenance organization in Washington State, and there were significant differences in baseline characteristics of those who received the vaccine and those who did not. For example, significantly more patients in the vaccine group had coronary artery disease, diabetes mellitus, chronic lung failure, and were immunocompromised.
Cornu et al (12) conducted a meta-analysis of the properly conducted RCTs published from 1996 to 2000, comparing pneumococcal polysaccharide vaccine (PPSV) with a placebo in immunocompetent adults. Fourteen trials were identified, which included 48,837 participants. Their findings included a significant reduction in the incidence of definite pneumococcal pneumonia1 (OR 0.29; 95% CI, 0.2−0.42) without significant heterogeneity, a significant reduction in presumptive pneumococcal pneumonia2 (OR 0.6; 95% CI, 0.37−0.96) with significant heterogeneity, and no significant effect on all-cause pneumonia (OR 0.78; 95% CI, 0.58−1.07) with significant heterogeneity. A subgroup analysis of trials conducted in gold miners in South Africa showed a significant reduction in all-cause pneumonia (OR 0.52; 95% CI, 0.43−0.63) without significant heterogeneity. The meta-analysis also found a significant reduction in mortality due to pneumonia (OR 0.68; 95% CI, 0.51−0.92) without significant heterogeneity.
Cornu et al (12) also performed an analysis of a subgroup of patients over 55 years of age. The study could not follow the prior plan for analyzing patients over 65 years of age because the age of the patients was dichotomized differently. They identified 7 trials, representing 7,907 high-risk patients (i.e., patients suffering from diabetes mellitus, chronic renal, hepatic, or respiratory disease, or cancer). Although there was a trend towards a lower risk of definite pneumococcal pneumonia (OR 0.58; 95% CI, 0.18−1.0) and mortality due to pneumonia (OR 0.69; 95% CI, 0.28−1.27), these effects were not statistically significant due to low power for this subgroup analysis and low events rates.
Studies of Pneumococcal Vaccine Efficacy in COPD Patients
A prospective cohort study (13) investigated the clinical effectiveness of the 23-valent pneumococcal vaccine (PPSV23) in older adults (mean age 75 years) with chronic respiratory disease (bronchitis, emphysema, and asthma). A total of 1,298 persons were observed for 3 years (a total of 3,676 person-years). The study found that PPSV23 did not significantly reduce the risk of overall CAP, outpatient CAP, 30-day mortality from CAP, or all-cause mortality. Hospitalization due to overall CAP or due to pneumococcal pneumonia was not significantly different between the 2 groups (Table 1).
Table 1: Results of a Prospective Cohort Study of the Pneumococcal Vaccine in Patients With Respiratory Disease*.
| Incidence per 1,000 Person-Years |
Age Adjusted HR for All Subjects (95% CI) |
P Value | Multivariable HR for All Subjects (95% CI) |
P Value | ||
|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | |||||
| Overall CAP | 46.96 | 45.77 | 0.93 (0.68−1.28) | 0.68 | 0.77 (0.56−1.07) | 0.12 |
| Outpatient CAP | 10.53 | 7.15 | 1.29 (0.61−2.72) | 0.5 | 1.15 (0.48−2.72) | 0.75 |
| Hospitalization for overall CAP | 36.43 | 38.62 | 0.87 (0.61−1.23) | 0.43 | 0.7 (0.48−1.00) | 0.05 |
| Hospitalization for CAP due to Pneumococcal pneumonia | 5.26 | 5.72 | 0.87 (0.35−2.17) | 0.78 | 0.76 (0.30−1.90) | 0.56 |
| 30-day mortality due to CAP | 6.14 | 5.72 | 0.91 (0.35−2.37) | 0.84 | 0.87 (0.33−2.28) | 0.78 |
| All-cause mortality | 81.19 | 64.36 | 1.22 (0.92−1.62) | 0.16 | 1.2 (0.91−1.59) | 0.2 |
Abbreviations: CI, confidence interval; CAP, community-acquired pneumonia; HR, hazard ratio.
Source: Ochoa-Gondar et al, 2008 (13)
Prevalence and Incidence of Pneumococcal Pneumonia
The rate of pneumococcal pneumonia in developed countries is still not known due to the lack of accurate diagnostic tests. In the United States Veterans’ Administration Trial among participants aged 55 years and older, the incidence of pneumococcal pneumonia per 1,000 person-years was 1.7 in people with no underlying disease, 3.4 in those with 1 underlying disease, and 15 for those with 3 underlying diseases. (14)
Technology
Current Vaccines
Influenza Vaccine
The selection of influenza viruses for the seasonal influenza vaccine is based on the type of influenza viruses that circulated during the previous year. Every year, the World Health Organization (WHO) convenes at technical meetings in February and September and makes recommendations about the selection of virus strains. The WHO recommended the following strains of viruses for use in the influenza vaccines in the 2010 to 2011 northern hemisphere influenza season: (8)
A/California/7/2009 (H1N1)-like virus,
A/Perth/16/2009 (H3N2)-like virus, and
B/Brisbane/60/2008-like virus.
In Canada, there are currently 5 trivalent influenza vaccines authorized for use by injection (15). Four of these are formulated for intramuscular use and the fifth product (Intanza®) is formulated for intradermal use.
The 4 vaccines for intramuscular use are:
Fluviral (GlaxoSmithKline), split virus, inactivated vaccine, for use in adults and children ≥ 6 months;
Vaxigrip (Sanofi Pasteur), split virus inactivated vaccine, for use in adults and children ≥ 6 months;
Agriflu (Novartis), surface antigen inactivated vaccine, for use in adults and children ≥ 6 months; and
Influvac (Abbott), surface antigen inactivated vaccine, for use in persons ≥ 18 years of age.
FluMist is a live attenuated virus in the form of a nasal spray for persons aged from 2 to 59 years. Immunization (with current available influenza vaccines) is not recommended for infants less than 6 months of age.
The Public Health Agency of Canada (PHAC) (15) provided recommendations for the use of the influenza vaccine for the following groups of people:
people at high risk for influenza-related complications or those likely to require hospitalization for the conditions indicated in the report, which includes cardiac or pulmonary disorders3;
people capable of transmitting influenza to those at high risk4;
people who provide essential community services; and
people in direct contact during culling operations with poultry infected with the avian influenza.
Special groups considered for influenza vaccination in 2010 to 2011 include:
persons who are morbidly obese (body mass index ≥ 40),
Aboriginal peoples, and
healthy children from 2 to 4 years of age.
Pneumococcal Vaccine
Pneumococcal polysaccharide vaccines were developed more than 50 years ago and have progressed from 2-valent vaccines to the current 23-valent vaccines to prevent diseases caused by 23 of the most common serotypes of S pneumoniae. Canada-wide estimates suggest that approximately 90% of cases of pneumococcal bacteremia and meningitis are caused by these 23 serotypes. (16) Health Canada has issued licenses for 2 types of 23-valent vaccines to be injected intramuscularly or subcutaneously:
Pneumovax 23® (Merck & Co Inc. Whitehouse Station, NJ, USA) (17), and
Pneumo 23® (Sanofi Pasteur SA, Lion, France) for people 2 years of age and older. (16)
Other types of pneumococcal vaccines licensed in Canada are for pediatric use. Pneumococcal polysaccharide vaccine is injected only once. A second dose is applied only in some conditions.
The Centers for Disease Control and Prevention (CDC) provided recommendations for the use of PPSV23 among all adults aged 65 years and older, and those aged 19 to 64 years with underlying medical conditions that put them at a greater risk for serious pneumococcal infection. (18)
The underlying medical conditions for the administration of PPSV23 include the following:
Immunocompetent persons
chronic heart disease including congestive heart failure and cardiomyopathies (excluding hypertension),
chronic lung disease including COPD, emphysema, and asthma,
diabetes mellitus,
cerebrospinal fluid leaks,
cochlear implant,
alcoholism,
chronic liver disease including cirrhosis, and
cigarette smoking;
Persons with functional or anatomical asplenia
sickle cell disease and other hemoglobinopathies, and
congenital or acquired asplenia, splenic dysfunction, or splenectomy;
Immunocompromised persons
congenital or acquired immunodeficiency,
HIV infection,
chronic renal failure,
nephrotic syndrome,
leukemia,
lymphomas,
Hodgkin’s disease,
generalized malignancy,
disease requiring treatment with immunosuppressive drugs including long-term systemic corticosteroids or radiation therapy,
solid organ transplantation, and
multiple myeloma.
Evidence-Based Analysis
Research Questions
What is the effectiveness of the influenza vaccination and the pneumococcal vaccination compared with no vaccination in COPD patients?
What is the safety of these 2 vaccines in COPD patients?
What is the budget impact and cost-effectiveness of these 2 vaccines in COPD patients?
Research Methods
Literature Search
Search Strategy
A literature search was performed on July 5, 2010 using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2000 to July 5, 2010. The search was updated monthly through the AutoAlert function of the search up to January 31, 2011.
Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Articles with an unknown eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established. Data extraction was carried out by the author.
Inclusion Criteria
studies comparing clinical efficacy of the influenza vaccine or pneumococcal vaccine with no vaccine or placebo;
RCTs published between January 1, 2000 and January 31, 2011;
studies including patients with COPD only ;
studies investigating the efficacy of the types of vaccines approved by Health Canada;
English language studies.
Exclusion Criteria
non-RCTs;
studies investigating vaccines for other diseases;
studies comparing different variations of vaccines;
studies in which patients received 2 or more types of vaccines;
studies comparing different routes of administering vaccines;
studies not reporting clinical efficacy of the vaccine or reporting immune response only;
studies investigating the efficacy of vaccines not approved by Health Canada.
Outcomes of Interest
Primary Outcomes
Influenza vaccination: Episodes of acute respiratory illness due to influenza virus.
Pneumococcal vaccination: Time to the first episode of community-acquired pneumonia due to pneumococcus or of unknown etiology.
Secondary Outcomes
rate of hospitalization and mechanical ventilation
mortality rate
adverse events
Statistical Analysis
Review Manager 5 Version 5.1 software was used for graphical presentation of data. However, only the P values reported by the authors were used for this report.
Quality of Evidence
The quality of each included study was assessed taking into consideration the following 7 study design characteristics:
adequate allocation concealment,
randomization (study must include a description of the randomization procedure used and this must be a proper method),
power/sample size (adequate sample size based on a priori calculations; underpowered studies were identified, when possible, using post hoc sample size power calculations),
blinding (if double blinding is not possible, a single blind study with unbiased assessment of outcome was considered adequate for this criterion),
< 20% withdrawals/dropouts,
intention-to-treat analysis conducted and done properly (withdrawals/dropouts considered in analysis), and
other criteria as appropriate for the particular research question and study design.
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (19) as presented below.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding, and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
The database search identified 1,286 citations including several existing systematic reviews and health technology assessments. Two systematic reviews performed by the Cochrane Collaboration were identified; one (20) was for the influenza vaccine and the other (21) for the pneumococcal vaccine. The systematic reviews of the influenza vaccine included 11 RCTs published up to May 2009. The systematic reviews of the pneumococcal vaccine included 7 RCTs published up to March 2010.
Tables 2 and 3 list RCTs identified through literature search or published systematic reviews for influenza vaccination and pneumococcal vaccination in patients with respiratory illness conducted since 1961.
Table 2: Randomized Controlled Trials of the Influenza Vaccine in Patients with Respiratory Illness*.
| Author, Year | Patients | Population | Comparison | Outcomes/Objectives |
|---|---|---|---|---|
| Howells and Tyler, 1961 (22) | Chronic bronchitis with severity grade |
|
||
| Cate et al, 1977 (23) | > 50 or high risk (5% lung disease) |
|
||
| Fell et al,1977 (24) | Chronic bronchitis, severity unclear |
|
||
| Medical Research Council,1980 (25) | Chronic bronchitis and airway obstruction |
|
||
| Treanor et al,1992 (26) | 523 | Nursing home residents | IM trivalent +/− IN |
|
| Treanor et al,1994 (27) | 81 | High risk (18% COPD) | IM vs. IN |
|
| Govaert et al,1993 (28) | 1,838 | People > 60 years old | IM trivalent vs. placebo |
|
| Govaert et al,1994 (29) | 1,838 | People > 60 years old | IM trivalent vs. placebo |
|
| Gorse et al, 1995 (30) | 50 | Nursing home residents | IM trivalent +/− IN |
|
| Gorse et al, 1997 (31) | 29 | Veterans Affairs Volunteers with a history of COPD | IM trivalent +/− IN |
|
| Gorse et al,2003 (32) | 2,215 | Veterans Affairs Volunteers with a history of COPD | IM trivalent +/− IN |
|
| Neuzil et al, 2003 (33) | 2,215 (n = 585) |
Veterans Affairs Volunteers with a history of COPD | IM trivalent +/− IN |
|
| Gorse et al,2004 (34) | 2,215 | Veterans Affairs Volunteers with a history of COPD | IM trivalent +/− IN |
|
| Wongsurakiat et al, 2004 (35;36) | 125 | COPD | IM trivalent vs. placebo |
|
| Gorse et al,2006 (37) | 2,215 | Veterans Affairs Volunteers with a history of COPD | IM trivalent +/− IN |
|
| Chuaychoo et al, 2010 (38) | 156 | COPD | IM trivalent vs. IN trivalent |
|
| Clancy, 2010 (39) | 64 | Smokers | Oral NTHi vaccine vs. placebo |
|
| Tendon et al, 2010 (40) | 38 | COPD | Oral NTHi vaccine vs. placebo |
|
Abbreviations: COPD, chronic obstructive pulmonary disease; IM, intramuscular; IN, intranasal; NTHi, nontypeable haemophilus influenza.
Table 3: Randomized Controlled Trials of the Pneumococcal Vaccine in Patients with Respiratory Illness*.
| Author, Year | Patients | Population | Comparison | Outcomes/Objectives |
|---|---|---|---|---|
| Leech et al, 1987 (41) | 189 | COPD | IF + PN Vaccine vs. PN+Placebo |
|
| Davis et al, 1987 (42) | 103 | COPD | 14-valent vaccine vs. placebo |
|
| Steentoft et al, 2006 (43) | 49 In 4 groups (Steroid +/- vaccine/placebo) |
COPD | Effect of steroid on antibody levels Clinical variables |
|
| Meyer et al, 2006 (44) | 30 (3 arms) IM vs. alveolar ventilation vs. bronchial ventilation | COPD | Comparison between 3 arms |
|
| Alfagemeet al, 2006 (45) | 596 | COPD | 23-valent vaccine vs. no vaccine |
|
| Ya Tseimakh et al, 2006 (46) | Abstract | |||
| Teramotoet al, 2007 (47) | Abstract | |||
| Furumoto et al, 2008 (48) | 167 | Chronic lung disease | Group 1: IF + PN Group 2: IF |
|
| Dransfield et al, 2009 (49) | 120 | COPD (Moderate to severe) | 7-valent vaccine vs. 23-valent |
|
Abbreviations: CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; IF, influenza vaccine; PN, pneumococcal vaccine.
From the above lists, one RCT (35) met the inclusion/exclusion criteria for the influenza vaccination. Adverse effects of the influenza vaccination were reported in a separate citation. (36) One RCT (45) met the criteria for the pneumococcal vaccination (Table 4). The literature search updated to January 31, 2011 did not identify any further RCTs.
Table 4: Body of Evidence Examined According to Study Design*.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studies | |
| Systematic review of RCTs | |
| Large RCT | Pneumococcal vaccination 1 |
| Small RCT | Influenza vaccination 1 |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with contemporaneous controls | |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | |
| Database, registry, or cross-sectional study | |
| Case series | |
| Retrospective review, modeling | |
| Studies presented at an international conference or other sources of grey literature | |
| Expert opinion | |
| Total | 2 |
Abbreviation: RCT, randomized controlled trial.
For each included study, the study design was identified and is summarized below in Table 4, which is a modified version of a hierarchy of study design by Goodman. (50)
Clinical Efficacy of the Influenza Vaccination in Immunocompetent Patients with COPD
Study Design and Method
One small RCT (35) investigated the effectiveness of the influenza vaccination on influenza-related acute respiratory illness (ARI) and total ARIs. The study was conducted in Thailand between June 1997 and October 1998 in a single university hospital. The design of the study was double-blinded, placebo controlled with a power of 80%.
Study Population
Patients must have had a clinical diagnosis of COPD, together with a forced expiratory volume in 1 second (FEV1) less than 70% of the forced vital capacity and a less than 15% increase in FEV1 predicted, after inhalation of the bronchodilator. Patients under immunosuppressive therapy (except corticosteroids), immunocompromised patients, and those having a malignancy or an expected survival of less than 1 year were excluded. Patients were excluded if they had a history of an allergy to eggs.
The study sample was 125 participants with COPD who were recruited from the COPD clinic. Sixty-two patients were assigned to the vaccine group and 63 patients were assigned to the placebo group. The medical management of all patients was based on the Thai guideline for the management of COPD.
Three patients, 1 in the vaccine group and 2 in the placebo group, dropped out of the study. Eight patients, 5 in the vaccine group and 3 in the placebo group, died from diseases or conditions not related to ARI, but data for these patients were retained in the analysis where possible.
Randomization
All patients were stratified based on the degree of airflow obstruction: mild (FEV1 ≥ 70% predicted), moderate (FEV1 50%−69% predicted), and severe (FEV1 < 50% predicted). Patients were numbered consecutively within their severity stratum. Numbers were previously randomized to either the vaccine or the placebo groups. Patients’ numbers were identified at the vaccination session and the process of checking the assigned numbers and whether vaccine or placebo would be injected was performed by a nurse who did not participate in the care of these patients.
Intervention
Patients in the vaccine group were injected with 0.5 mL of purified, trivalent, split virus vaccine (Pasteur Merieux: Lyon, France). Each dose contained influenza A/Texas/36/91 (H1N1), A/Nanchang/933/95 (H3N2), and B/Harbin/07/94, all with 15 μg of hemagglutinin according to the WHO recommendation. Patients in the placebo group received 0.5 mL of vitamin B1. In both groups, a second dose of the vaccine or placebo was injected 4 weeks after the first dose.
Primary Outcome
The primary outcome of the study was the number of episodes of influenza-related ARI and its relationship to the degree of airflow obstruction.
Classification of Acute Respiratory Illness
Patients were told to notify the study centre immediately if they developed symptoms of ARI. All patients were seen at the COPD clinic at 4-week intervals. At each visit, they were also asked about episodes of respiratory illness during the past month. The clinical characteristics of each ARI were recorded as one of the following 4 types:
Common cold:
Infection of the upper respiratory tract with predominating rhinitis and pharyngitis;
Influenza-like illness:
At least 2 of the 3 following symptoms with or without upper respiratory symptoms:
generalized aches,
fever,
headache;
Or, 1 of the above 3 symptoms in addition to at least 1 of the following symptoms:
upper respiratory tract infection (sore throat, nasal discharge) within the past 5 days,
fever without any other cause,
increased wheezing,
increased cough, and
a 20% or more increase in respiratory rate or heart rate;
Acute exacerbation of COPD:
Increased dyspnea, sputum volume, or sputum purulence;
Pneumonia:
Compatible symptoms plus new infiltrates shown on a chest x-ray.
Laboratory Measurements
Blood samples were taken from each patient for the HI test during the following visits:
the day of vaccination or placebo injection,
at 4 weeks,
at 6 months, and
at 1 year.
Diagnostic Criteria for Influenza Infection
For each ARI, the HI antibody titre was determined twice: at the first visit (acute serum) and at 4 to 6 weeks afterwards (convalescent serum). If the duration of ARI was less than 6 days, a throat or nasal swab, and a sputum specimen were also collected for viral culture. A fourfold HI titre increase in convalescent serum compared with the acute serum (with a titre ≥ 40) and/or demonstration of influenza antigen with or without a positive culture finding was considered as meeting the criteria for the influenza virus infection.
Classification of the Severity of Acute Respiratory Illness
For each ARI, the severity was classified as one of the 3 following categories:
treated in an outpatient clinic,
needed hospitalization, or
needed mechanical ventilation.
Method of Data Analysis
The incidence of ARI in each group was calculated using an incidence density (number of episodes of ARI over the number and time of follow-up [person-years]), estimated by a Poisson model. The effectiveness of the influenza vaccine was calculated as 1 minus relative risk. The Kaplan-Meier survival analysis was used to demonstrate the probability of not acquiring influenza-related ARI and overall ARI during the study period.
Baseline Characteristics of the Study Population
There were no significant differences between the 2 groups in baseline characteristics of the patients. In each group about 30% of the patients had comorbid diseases such as hypertension, coronary artery disease, and diabetes. Among patients who had an HI titre greater than or equal to 10 at the baseline, about one half had had a previous infection by at least 1 type of influenza virus type A and about one fifth had been infected with influenza virus type B. However, their geometrical means titres were at a low level. There were no significant differences between the 2 groups in baseline HI antibody titre.
Crude Incidence and Incidence Density of Influenza-Related Acute Respiratory Illness
During the study period, a total of 21 patients (4 in the vaccine group and 17 in the placebo group) acquired influenza-related ARI as evidenced by a fourfold rise in the HI antibody titre. Thirteen of these patients had symptoms of acute exacerbation, 6 had symptoms of the common cold, and 2 had symptoms of an influenza-like illness. Two of the 21 cases were caused by influenza type A and only 1 case was caused by influenza type B. There was another patient in the vaccine group who had a fourfold increase in his HI titre against influenza A without ARI symptoms. From a total of 165 specimens collected from the patients’ throats, noses, and sputum, only 3 showed positive results on the viral culture.
The incidence density of influenza-related ARI was 6.8 episodes per 100 person-years in the vaccine group and 28.1 episodes per 100 person-years in the placebo group (RR, 0.2; 95% CI, 0.06−0.7; P = 0.005).
Acute exacerbation was the most common presentation of influenza-related ARI (13/21 episodes, 61.9%), as well as the most common presentation of ARI (161/269 episodes, 59.8%). The incidence rate of influenza-like illness was significantly lower in the vaccine group than the placebo group (vaccine group 0.08 episodes per 100 person-years; placebo group 0.2 episodes per 100 person-years; RR, 0.34; 95% CI, 0.1−0.99; P = 0.03).
Vaccine Effectiveness
The crude incidence rate of influenza-related ARIs of the vaccine group over the placebo group was 0.24 (95% CI, 0.09−0.67; P = 0.007) (Figure 1), and the overall effectiveness of vaccination against the influenza virus was 76%. The effectiveness of vaccination against the influenza virus in patients with mild, moderate, and severe COPD was 84%, 45%, and 85% respectively.
Figure 1: Incidence and Severity of Influenza-Related ARI in Patients with COPD*.
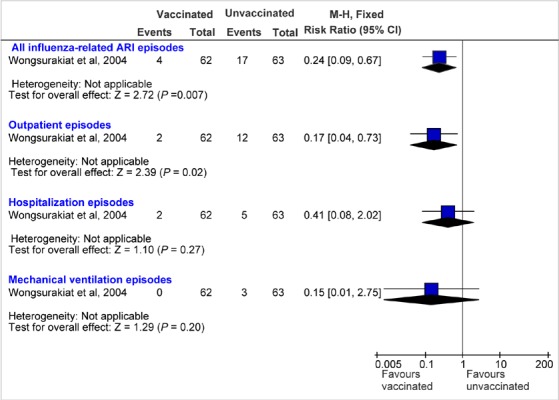
Abbreviations: ARI, acute respiratory illness; CI, confidence interval; COPD, chronic obstructive pulmonary disease; M-H, Mantel-Haenszel.
The incidence rate ratio of the vaccine group over the placebo group for influenza-related ARIs adjusted for age, sex, smoking status, comorbid diseases, and severity of COPD was 0.24, 0.24, 0.24, 0.22, and 0.24 respectively, and none of the P values for the effect modification were statistically significant.
Severity of Influenza-Related Acute Respiratory Illness
Fewer patients in the vaccine group compared with the placebo group required hospitalization; 2 patients in the vaccine group and 5 patients in the placebo group became hospitalized because of influenza-related ARIs (P = 0.3). Three of the hospitalized patients, all in the placebo group, underwent mechanical ventilation. None of the patients in the vaccine group underwent mechanical ventilation and the difference did not reach statistical significance due to the low event rate.
All patients in the subgroups of moderate and severe COPD who did not receive the vaccine and were hospitalized because of influenza-related ARIs underwent mechanical ventilation. This included 2 patients in the severe category and 1 patient in the moderate category. One of the patients with severe COPD in the placebo group who required mechanical ventilation died because of ventilation-associated pneumonia.
The crude incidence rate of hospitalization from influenza-related ARIs of the vaccine group over the placebo group was 0.41 (95% CI, 0.08−2.02; P = 0.27). The incidence rate of hospitalization due to influenza-related ARIs of the vaccine group over the placebo group adjusted for age, sex, smoking status, comorbid diseases, and severity of COPD was 0.38, 0.42, 0.41, 0.38, and 0.4 respectively, and none of the P values for the effect modification were statistically significant.
Figure 1 shows the number of patients who acquired influenza-related ARIs in the 2 groups and the severity of their illness, and relative risk ratios calculated by the Mantel-Haenszel test method using Review Manager 5 Version 5.1 software.
The incidence density of the vaccine group versus the placebo group for influenza-related ARI and for hospitalization from influenza-related ARI for categories of disease severity is shown in Table 5.
Table 5: Incidence Density for Episodes of Influenza-Related ARI and Episodes Requiring Hospitalization – Vaccine Versus Placebo*.
| COPD Severity | All Episodes of Influenza-Related ARI/100 Person-Years RR (95% CI) |
P Value | All Episodes of Influenza-Related ARI That Required Hospitalization/100 Person-Years RR (95% CI) |
P value |
|---|---|---|---|---|
| All patients | Vaccine: 6.8 Placebo: 28.1 0.2 (0.06−0.70) |
0.005 | Vaccine: 3.4 Placebo: 8.3 0.4 (0.04−2.50) |
0.3 |
| Mild | Vaccine: 4.5 Placebo: 28.2 0.2 (0.003−1.30) |
0.06 | Vaccine: 4.5 Placebo: 9.4 0.5 (0.01−9.30) |
0.6 |
| Moderate | Vaccine: 13.2 Placebo: 23.8 0.5 (0.05−3.80) |
0.5 | Vaccine: 0 Placebo: 5.9 0 (0.00−43.10) |
0.5 |
| Severe | Vaccine: 4.6 Placebo: 31.2 0.1 (0.003−1.10) |
0.04 | Vaccine: 3.4 Placebo: 8.3 0.5 (0.01−10.00) |
0.6 |
Abbreviations: ARI, acute respiratory illness; CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk. Source: Wongsurakiat et al, 2004 (35)
The incidence of influenza-related ARIs requiring mechanical ventilation was 0 per 100 person-years and 5 per 100 person-years in the vaccine group and the placebo group, respectively (RR, 0; 95% CI, 0−2.5; P = 0.1).
Survival Analysis
The Kaplan-Meier survival analysis showed a significantly higher probability of not acquiring influenza-related ARIs in favour of the vaccine group (P = 0.003 by log-rank test). There was no significant difference in the probability of not acquiring ARIs between the 2 groups.
There was no significant difference between the 2 groups in the probability of not being hospitalized as well as the probability of not receiving mechanical ventilation due to influenza-related ARIs (P = 0.2 and P = 0.4 respectively by log-rank tests).
Explanatory Factors
The effectiveness of the influenza vaccine was not related to age, sex, severity of COPD, smoking status, or comorbid diseases. The incidence rate ratio of the vaccine group over the placebo group for influenza-related ARIs and hospitalization from influenza-related ARIs adjusted for the above factors are shown in Table 6.
Table 6: Adjusted Incidence Rate Ratios for Vaccine Versus Placebo*.
| Influenza-Related ARI | Influenza-Related Hospitalization | ||||
|---|---|---|---|---|---|
| Adjusted | Categories | Risk Ratio | P Value | Risk Ratio | P Value |
| Age | < 70/ ≥ 70 years | 0.24 | 0.3 | 0.38 | 0.3 |
| Sex | Male/Female | 0.24 | 0.8 | 0.42 | 0.8 |
| Current smoking status | Yes/No | 0.24 | 0.6 | 0.41 | 0.8 |
| Severity of COPD | Mild/Moderate/ Severe | 0.22 | 0.1 | 0.38 | 0.9 |
| Comorbid disease | Yes/No | 0.24 | 0.5 | 0.40 | 1.0 |
Abbreviations: ARI, acute respiratory illness; COPD, chronic obstructive pulmonary disease.
Source: Wongsurakiat et al, 2004 (35)
Adverse Events
Frequency of Acute Exacerbation
There were 269 episodes of ARIs (124 in the vaccine group and 145 in the placebo group). Acute exacerbations accounted for 161 of the total ARIs (76 in the vaccine group and 85 in the placebo group). There was no significant difference between the 2 groups in the incidence of acute exacerbation during the first week and 4 weeks following injection (Table 7). Thirteen (8%) of the acute exacerbations were influenza-related.
Table 7: Development of ARI During the First Week and the First Four Weeks*.
| First Week, N (%) | P Value | First 4 Weeks, N (%) | P Value |
|---|---|---|---|
| Vaccine: 4 (6.40) | 1.0 | Vaccine:15 (24.20) | 0.5 |
| Placebo: 4 (6.30) | Placebo: 20 (31.70) |
Abbreviations: ARI, acute respiratory illness; N, number.
Source: Wongsurakiat et al, 2004 (35)
Local Reaction
Vaccinated patients had significantly more local adverse reactions compared with the placebo group (17 [27%] in the vaccine group vs. 4 [6%] in the placebo group; P = 0.002). The most common local reactions among vaccinated patients were swelling, itching, and pain when touched. Significantly more patients in the vaccine group than the placebo group experienced swelling and itching (vaccine 4, placebo 0; P = 0.04 for either). The duration of local symptoms was usually less than 48 hours and did not require specific treatment.
Systemic Reaction
Systemic reactions were headache, myalgia, fever, and skin rash. No significant differences in systemic reactions between the 2 groups were observed (47 [76%] in the vaccine group vs. 51 [81%] in the placebo group; P = 0.5).
Effects of Vaccination on Lung Function, Dyspneic Symptoms, and Exercise Capacity
Lung function was measured by spirometry, oxygen saturation level in arterial blood was measured by pulse oximetry, dyspneic symptoms were measured by the visual analogue scale, and exercise capacity was measured by the 6 Minute Walking Test. There were no significant differences between the 2 groups for changes in lung function, dyspneic symptoms, and exercise capacity at 1 week and at 4 weeks (Table 8).
Table 8: P Value for the Difference in Changes in Lung Function, Dyspneic Symptoms, and Exercise Capacity – Vaccine Versus Placebo*.
| Measures | P Value for Vaccine vs. Placebo | |
|---|---|---|
| 1 Week | 4 Weeks | |
| FEV1 | 1.0 | 0.7 |
| PImax | 0.9 | 0.5 |
| SpO2-pre exercise | 0.8 | 0.2 |
| SpO2-post exercise | 0.7 | 0.2 |
| VAS-pre exercise | 0.9 | 0.3 |
| VAS-post exercise | 0.7 | 0.7 |
| 6 minute walk | 0.2 | 0.5 |
Abbreviations: FEV1, Forced expiratory volume in 1 second; PImax, maximum inspiratory pressure at residual volume; SpO2, oxygen saturation level of arterial blood; VAS, visual analogue scale.
Source: Wongsurakiat et al, 2004 (35)
Summary of Efficacy of the Influenza Vaccination in Immunocompetent Patients With COPD
This study was conducted in a year that was not an epidemic influenza period, therefore the incidence of influenza was low.
Clinical Effectiveness
Influenza vaccination was associated with significantly fewer episodes of influenza-related ARIs. The incidence density of influenza-related ARIs was:
All patients: vaccine group: (total of 4 cases) = 6.8 episodes per 100 person-years; placebo group: (total of 17 cases) = 28.1 episodes per 100 person-years, (RR, 0.2; 95% CI, 0.06−0.70; P = 0.005);
Patients with severe airflow obstruction (FEV1 < 50% predicted): vaccine group: (total of 1 case) = 4.6 episodes per 100 person-years; placebo group: (total of 7 cases) = 31.2 episodes per 100 person-years, (RR, 0.1; 95% CI, 0.003−1.1; P = 0.04);
Patients with moderate airflow obstruction (FEV1 50%−69% predicted): vaccine group: (total of 2 cases) = 13.2 episodes per 100 person-years; placebo group: (total of 4 cases) = 23.8 episodes per 100 person-years, (RR, 0.5; 95% CI, 0.05−3.8; P = 0.5);
Patients with mild airflow obstruction (FEV1 ≥ 70% predicted): vaccine group: (total of 1 case) = 4.5 episodes per 100 person-years; placebo group: (total of 6 cases) = 28.2 episodes per 100 person-years, (RR, 0.2; 95% CI, 0.003−1.3; P = 0.06).
The Kaplan-Meier survival analysis showed a significant difference between the vaccinated group and the placebo group regarding the probability of not acquiring influenza-related ARIs (log-rank test P value = 0.003).
Overall, the vaccine effectiveness was 76%. For categories of mild, moderate, or severe COPD the vaccine effectiveness was 84%, 45%, and 85% respectively.
With respect to hospitalization, fewer patients in the vaccine group compared with the placebo group were hospitalized due to influenza-related ARIs, although these differences were not statistically significant. The incidence density of influenza-related ARIs that required hospitalization was 3.4 episodes per 100 person-years in the vaccine group and 8.3 episodes per 100 person-years in the placebo group (RR, 0.4; 95% CI, 0.04−2.5; P = 0.3; log-rank test P value = 0.2). No statistically significant differences between the 2 groups were observed for the 3 categories of severity of COPD.
Fewer patients in the vaccine group compared with the placebo group required mechanical ventilation due to influenza-related ARI. However, these differences were not statistically significant. The incidence density of influenza-related ARIs that required mechanical ventilation was 0 episodes per 100 person-years in the vaccine group and 5 episodes per 100 person-years in the placebo group (RR, 0.0; 95% CI, 0−2.5; P = 0.1; log-rank test P value = 0.4). In addition, no statistically significant differences between the 2 groups were observed for the 3 categories of severity of COPD.
The effectiveness of the influenza vaccine in preventing influenza-related ARIs and influenza-related hospitalization was not related to age, sex, severity of COPD, smoking status, or comorbid diseases.
Safety
Overall, significantly more patients in the vaccine group than the placebo group experienced local adverse reactions (vaccine: 17 [27%], placebo: 4 [6%]; P = 0.002). Significantly more patients in the vaccine group than the placebo group experienced swelling (vaccine 4, placebo 0; P = 0.04) and itching (vaccine 4, placebo 0; P = 0.04).
Systemic reactions included headache, myalgia, fever, and skin rash, and there were no significant differences between the 2 groups with regard to these reactions (vaccine: 47 [76%], placebo: 51 [81%]; P = 0.5).
With respect to lung function, dyspneic symptoms, and exercise capacity, there were no significant differences between the 2 groups in FEV1, maximum inspiratory pressure at residual volume, oxygen saturation level of arterial blood, visual analogue scale for dyspneic symptoms, and the 6Minute Walking Test for exercise capacity at 1 week and at 4 weeks.
There was no significant difference between the 2 groups with regard to the probability of not acquiring total ARI (influenza-related and/or non-influenza-related), (log-rank test P value = 0.6).
Clinical Efficacy of the Pneumococcal Vaccination in Immunocompetent Patients with COPD
Study Design and Method
A large RCT (45) investigated the clinical efficacy of PPSV23 in patients with COPD. Although the study had a large sample size, the details about the power calculation were not reported. This study was conducted in Spain between October 1999 and July 2004.
Study Population
All patients had a spirometric diagnosis of COPD and were not previously vaccinated. Pregnant patients and those diagnosed with any of the following conditions were excluded from the study:
immunodeficiency
neoplasia
renal insufficiency in dialysis
HIV infection
hypogammaglobulinemia
anatomical or functional asplenia
Initially, 600 patients with a diagnosis of COPD were included in the study (300 in each group). Four patients (2 in each group) were lost to follow-up and were excluded from the final analysis. The analysis was therefore performed for 596 patients. Thirty-four patients were diagnosed with neoplasia during the follow-up period. The mean age of the patients was 65.8 (standard deviation 9.7) years. Baseline characteristics of the patients in the 2 groups were similar.
Randomization
Randomization of patients was performed through computerized generation of random numbers in block lengths of 20 (10 in each group). Patients were randomly assigned to receive the PPSV23 or no vaccine and both groups were checked routinely every 6 months for 3 years. Physicians participating in the study and performing follow-ups were unaware of the patients’ assignment. Patients were instructed to contact their physician if they developed symptoms that might suggest pneumonia.
Intervention
Patients who were assigned to the vaccine group received PPSV23 (Pneumo 23; Aventis Pasteur MSD) together with a clinical follow-up examination. Patients in the control arm of the study did not receive the vaccine but had a clinical follow-up examination. The vaccine was given to the patients free of charge at the centre where each patient was recruited.
Diagnosis of Pneumonia
The diagnosis of pneumonia was based on chest x-ray findings, presence of fever, and patients’ symptoms suggesting lower respiratory tract infection. The diagnosis of pneumococcal pneumonia was based on the presence of pneumonia and the isolation of streptococcal pneumonia from the patient’s sputum, bronchial aspirate, pleural fluid, blood, or cerebrospinal fluid.
Primary Outcome
The main outcome of the study was time to the first episode of developing CAP, either due to pneumococcus or of unknown etiology.
Follow-up
All patients were followed for a period of 3 years except for patients who died before the end of the follow-up period (115 patients). Patients who were diagnosed with pneumonia had a follow-up radiograph 2 to 4 weeks after the first visit.
Method of Data Analysis
The effectiveness of the pneumococcal vaccine was calculated as 1 minus relative risk of acquiring CAP, either due to pneumococcus or of unknown etiology. The Kaplan-Meier survival analysis was used to demonstrate the probability of not acquiring pneumococcal pneumonia or pneumonia of unknown etiology. In this analysis, the effectiveness of the vaccine was investigated in the entire group as well as in subgroups of patients stratified by age and severity of the airflow obstruction (age < 65 vs. ≥ 65; FEV1 < 40% of expected vs. ≥ 40% of expected). The authors indicated that the threshold for subgroup analysis was based on the previously published data (11;51;52), suggesting that younger patients (< 65 years) and those with severe airflow obstruction (FEV1 < 40% predicted) would benefit most from the vaccine administration.
The multivariate Cox proportional hazards regression model was used to evaluate the association between vaccine administration and time to the first outcome event. In the general model, age, severity of airflow obstruction (as defined above), and the interaction of the age with vaccine were used as covariates. In another model, the interaction term was not used but the model was run separately for ages less than 65years and greater than or equal to 65 years.
Results
Incidence and Episodes of Pneumonia
Overall, the incidence of global pneumonia (CAP and nosocomial) was 55.1 per 1,000 patients with COPD per year. A total of 75 patients developed pneumonia, from which 38 (12.7%) were in the vaccine group and 37 (12.4%) were in the control group. During the study period, no difference in the incidence of pneumonia was observed between the 2 groups.
A total of 88 episodes of pneumonia occurred during the study period (43 in the vaccine group and 45 in the control group), from which 73 (83%) were treated in hospital and the remaining 15 (17%) were treated as outpatients (Figure 2). Determination of the etiology and method of treatment was based on the decision of the treating physicians. Therefore, an etiological diagnosis was obtained for 23 patients diagnosed with pneumonia and the remaining 65 patients had unknown etiologies. There were no cases of bacteremic pneumococcal infection.
Figure 2: Episodes of Global Pneumonia in Patients with COPD: Vaccinated Versus Unvaccinated*.
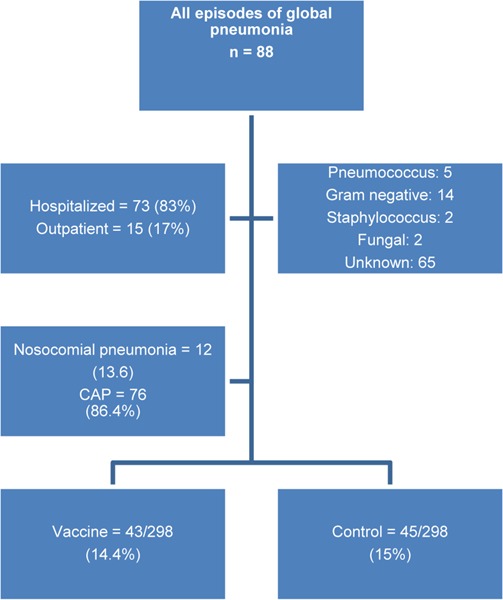
Abbreviations: CAP, community-acquired pneumonia; n, number.
Incidence and Episodes of Community-Acquired Pneumonia
Incidence of CAP was 47.6 per 1,000 COPD patients per year (vaccine 46.3, control 49). There was a total of 76 episodes of CAP (vaccine 37, control 39) and 67 first episodes of CAP (vaccine 33, control 34) (Figure 3). Fifty-eight of these were either due to pneumococcus or of unknown etiology (vaccine 25, control 33). Kaplan-Meier survival analysis did not show significant differences between the 2 groups (log-rank test = 1.15; P = 0.28). The efficacy of PPSV23 in preventing the first episode of CAP in the whole group of COPD patients was 24% (95% CI, −24 to 54; P = 0.3).
Figure 3: First Episode of Community-Acquired Pneumonia*.
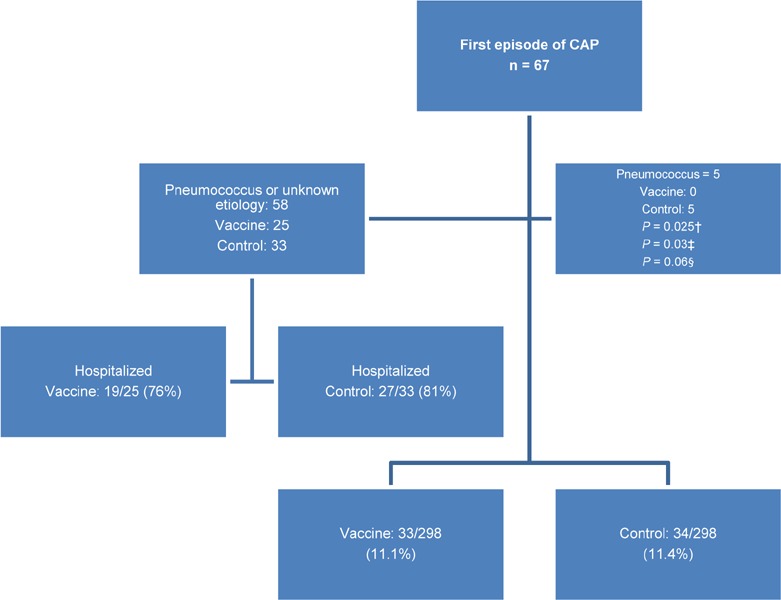
Abbreviation: n, number.
P value by log-rank test
P value by single-sided Fisher’s exact test
P value by two-sided Fisher’s exact test
There were 5 cases of pneumococcal pneumonia (all were first episodes) among unvaccinated patients. No cases of pneumococcal pneumonia were observed in the vaccinated group. Analysis by Fisher’s exact test showed no significant difference between the 2 groups by the 2-sided test (P = 0.06), while a single-sided test provided a significant result (P = 0.03). In 2 of these patients, H influenza or P aeroginosa was detected along with pneumococcus bacteria.
Figure 4 shows the number of patients with first episode of CAP of unknown etiology or pneumococcal pneumonia in the 2 groups of patients, and the relative risk ratios calculated by the Mantel-Haenszel test method using Review Manager 5 Version 5.1 software.
Figure 4: First Episode of Community-Acquired Pneumonia of Unknown Etiology and Pneumococcal Pneumonia in Vaccinated and Unvaccinated Patients*.
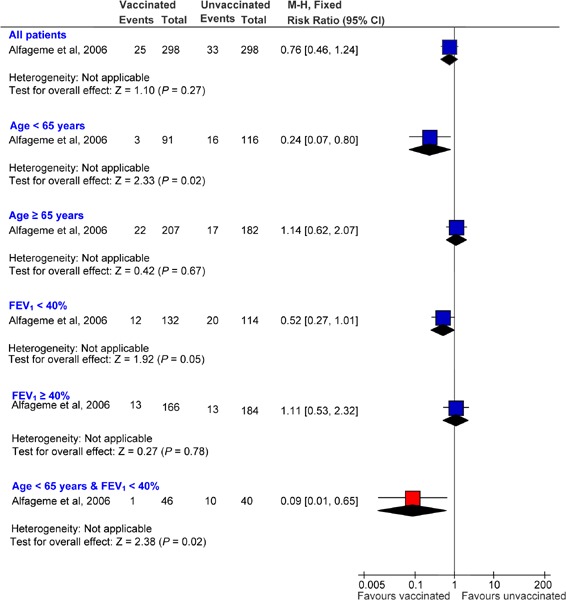
Abbreviations: CI, confidence interval; FEV1, Forced expiratory volume in one second; M-H, Mantel-Haenszel.
Vaccine Efficacy
By univariate analysis there was no significant difference between the vaccinated group and the control group in regard to the vaccine efficacy of 24% (95% CI,−24 to 54; P = 0.3). Subgroup analysis showed that while there was no significant difference in the vaccine efficacy between the 2 groups (in the age group ≥ 65 years), with a vaccine efficacy of −14% (95% CI, −107 to 38; P = 0.8), the difference was statistically significant for those who were younger than 65 years of age, with a vaccine efficacy of 76% (95% CI, 20−93; P = 0.01).
Vaccine efficacy was not significantly different between the 2 groups when analysis was performed separately for patients with a FEV1 less than 40% predicted, with a vaccine efficacy of 48% (95% CI, −7 to 80; P = 0.08), or those with a FEV1 greater than or equal to 40% predicted, with a vaccine efficacy of −11% (95% CI, −132 to 47; P = 0.95). However, vaccine efficacy was highest among patients who were both under the age of 65 years and had severe airflow obstruction, with a vaccine efficacy of 91% (95% CI, 35−99; P = 0.002).
Table 9 summarizes the efficacy of the vaccine in reducing the incidence of a first episode of CAP of unknown etiology and due to pneumococcus in subgroups of patients.
Table 9: Efficacy of the 23-Serotype Pneumococcal Vaccine in Reducing the Incidence of Community-Acquired Pneumonia of Unknown Etiology and due to Pneumococcus*.
| Subgroups | Vaccine Efficacy (%) | P value |
|---|---|---|
| All patients | 24 (−24 to 54) | 0.333 |
| Age < 65 yeas | 76 (20 to 93) | 0.013 |
| Age ≥ 65 years | −14 (−107 to 38) | 0.801 |
| FEV1 < 40% | 48 (−7 to 80) | 0.076 |
| FEV1 ≥ 40% | −11 (−132 to 47) | 0.945 |
| Age < 65 years & FEV1 < 40% | 91 (35 to 99) | 0.002 |
Abbreviation: FEV1, forced expiratory volume in 1 second.
Source: Alfageme et al, 2006 (45)
Survival Analysis
The Kaplan-Meier survival analysis showed no significant differences between the vaccinated group and the control group for time to the first episode of CAP (log-rank test = 1.15; P = 0.28) (Figure 5).
Figure 5: Kaplan-Meier Survival Curve Demonstrating the Cumulative Proportion of Patients Without Pneumonia Over the Follow-up Period.
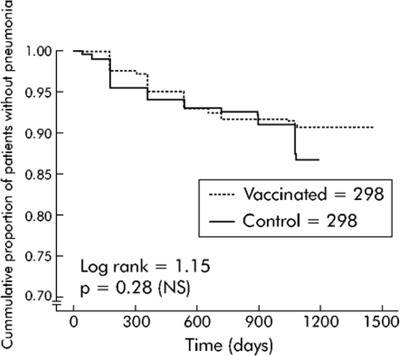
There were significant differences between the 2 groups for pneumonia of unknown etiology and pneumonia due to pneumococcus when data was analyzed according to subgroups of patients for those under age 65 or those who had severe airflow obstruction (Figures 6 and 7).
Figure 6: Kaplan-Meier Survival Curve Demonstrating the Cumulative Proportion of Patients Less Than 65 Years of Age Without Pneumonia Over the Follow-up Period.
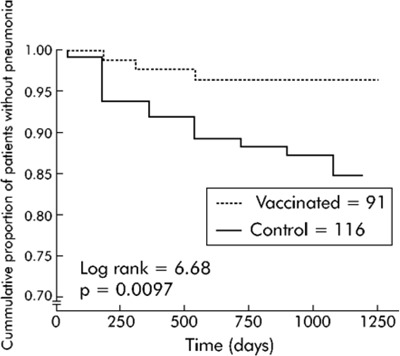
Figure 7: Kaplan-Meier Survival Curve Demonstrating the Cumulative Proportion of Patients with Severe COPD Without Pneumonia Over the Follow-up Period*.
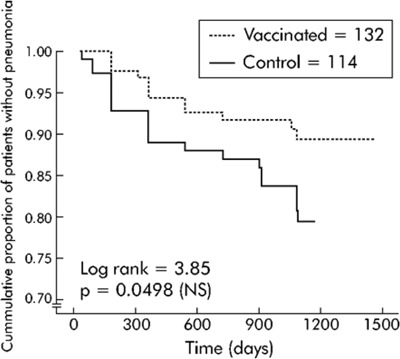
Abbreviation: COPD, chronic obstructive pulmonary disease.
Reproduced from Thorax; Alfageme I, Vazquez R, Reyes N, Munoz J, Fernandez A, Hernandez M et al. 61(3):189−95, 2006, with permission from BMJ Publishing Group Limited. (45)
Influence of Modifying Factors
In the Cox proportional hazards regression model analyses, the hazard ratio (HR) for developing pneumonia was adjusted for the effect of selected factors including age (< 65 years vs. ≥ 65 years), the severity of airflow obstruction (FEV1 < 40% vs. ≥ 40% predicted), and the interaction of the age with vaccine. The results of this analysis showed that the age of the patients influenced the efficacy of the vaccine. The HR in the global model was 0.2 (95% CI, 0.6−0.68; P = 0.01).
Separate models for the 2 age groups showed that the vaccine was not effective in older patients in reducing the incidence of pneumonia, but younger patients in the vaccine group were less likely to develop pneumonia compared with the unvaccinated patients in the same age group. The 2 models also showed that patients with more severe airflow obstruction could benefit from pneumococcal vaccination, and the benefit was statistically significant in the model for younger patients. Results are summarized in Table 10.
Table 10: Cox Proportional Hazards Regression Model*.
| Model | Factor† | HR (95% CI) | P Value |
|---|---|---|---|
| Global model | Vaccine | 0.2 (0.06−0.68) | 0.01 |
| Age | 0.66 (0.33−1.31) | 0.23 | |
| Severe airflow obstruction | 2.03 (1.21−3.41) | 0.01 | |
| Interaction (Age x vaccine) | 5.82 (1.45−23.34) | 0.01 | |
| Model for age < 65 years | Vaccine | 0.19 (0.06−0.66) | 0.01 |
| Severe airflow obstruction | 2.62 (1.04−6.55) | 0.04 | |
| Model for age ≥ 65 years | Vaccine | 1.53 (0.61−2.17) | 0.66 |
| Severe airflow obstruction | 1.81 (0.96−3.39) | 0.07 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Vaccine (0 = no, 1 = yes); Age (0 equals < 65 years, 1 equals ≥ 65 years); Severe airflow obstruction (0 = no, 1 = yes); Source: Alfageme et al, 2006 (45)
Hospital Admission Rate
There were 67 first episodes of CAP (33 [11.1%] in the vaccine group, 34 [11.8%] in the control group). Most of the episodes of CAP required hospital admission, but the hospital admission rate did not differ between the 2 groups (vaccine group 19/25 [76%], control group 27/33 [81%]; P = 0.59).
The total number of days in the hospital due to CAP was 242 in the vaccine group and 412 in the control group. The median length of hospital stay was lower in the vaccine group than the control group, but the difference did not reach statistical significance (vaccine 9.5 days, control 12 days; P = 0.16).
Number Needed to Treat
The number needed to treat to prevent 1 patient from acquiring pneumonia was calculated as 10 (95% CI, 6−31) for vaccinating patients less than 65 years of age and as 3 (95% CI, 2−4) for these patients if they also had severe airflow obstruction.
Mortality
Vaccinated and unvaccinated patients had a similar mortality rate (19%). Mortality rates per 1,000 per year are shown in Table 11. The cause of death was: respiratory failure (n = 34), cardiovascular disease (n = 29), cancer (n = 21), infection (n = 13), gastrointestinal causes (n = 11), other (n = 5), and unknown (n = 2).
Table 11: Mortality Rates Due to Pneumonia*.
| CAP and Nosocomial Pneumonia | CAP only | |
|---|---|---|
| Mortality rates per 1,000 per year | 50.80 | 34.40 |
Abbreviation: CAP, community-acquired pneumonia. Source: Alfageme et al, 2006 (45)
Table 12 shows factors influencing mortality among patients.
Table 12: Factors Influencing Mortality*.
| Factors | RR (95% CI) | P Value |
|---|---|---|
| Age | 1.05 (1.03−1.08) | < 0.001 |
| FEV1 | 0.97 (0.95−0.98) | < 0.001 |
| Current smoker | 1.67 (1.08−2.60) | 0.022 |
| Presence of neoplasia | 6.54 (4.15−10.23) | < 0.001 |
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in one second; RR, relative risk. Source: Alfageme et al, 2006 (45)
Adverse Events
No patients reported a local or systemic reaction to the vaccine.
Summary of Efficacy of the Pneumococcal Vaccination in Immunocompetent Patients with COPD
Clinical Effectiveness
The Kaplan-Meier survival analysis showed no significant differences between the vaccinated group and the control group for time to the first episode of community-acquired pneumonia due to pneumococcus or of unknown etiology (log-rank test = 1.15; P = 0.28). Overall, the vaccine efficacy was 24% (95% CI, −24 to 54; P = 0.33).
With respect to the incidence of pneumococcal pneumonia, the Kaplan-Meier survival analysis showed a significant difference between the 2 groups (vaccine: 0/298, control: 5/298; log-rank test 5.03; P = 0.03).
Hospital admission rates and median lengths of hospital stay were lower in the vaccine group, but the difference was not statistically significant. The mortality rate was not different between the 2 groups.
Subgroup Analysis
The Kaplan-Meier survival analysis showed significant differences between the vaccine and control groups for pneumonia of unknown etiology and due to pneumococcus when data were analyzed according to the subgroups of patients (age < 65 years, and severe airflow obstruction of FEV1 < 40% predicted). The accumulated percentage of patients without pneumonia (of unknown etiology and due to pneumococcus) across time was significantly lower in the vaccine group than in the control group in patients younger than 65 years of age (log-rank test 6.68; P = 0.0097) and patients with an FEV1 less than 40% predicted (log-rank test 3.85; P = 0.0498).
Vaccine effectiveness was 76% (95% CI, 20−93; P = 0.01) for patients who were younger than 65 years of age and −14% (95% CI, −107 to 38; P = 0.8) for those who were aged 65 years or older. Vaccine effectiveness for patients with an FEV1 less than 40% predicted and those who had an FEV1 greater than or equal to 40% predicted was 48% (95% CI, −7 to 80; P = 0.08) and −11% (95% CI, −132 to 47; P = 0.95), respectively. For patients who were less than 65 years of age and had an FEV1 less than 40% predicted, vaccine effectiveness was 91% (95% CI, 35−99; P = 0.002).
Cox modelling showed that the effectiveness of the vaccine was dependent on the age of the patient. The vaccine was not effective in patients greater than or equal to 65 years old (HR, 1.53; 95% CI, 0.61−2.17; P = 0.66), but it reduced the risk of acquiring pneumonia by 80% in patients less than 65 years old (HR, 0.19; 95% CI, 0.06−0.66; P = 0.01).
Safety
No patients reported any local or systemic adverse reactions to the vaccine.
Economic Analysis
The results of the economic analysis are summarized in issue 12 of the COPD series entitled Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model. This report can be accessed at:
www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Economic_March.pdf.
The results from the systematic review of the clinical evidence for influenza and pneumococcal vaccinations for patients with COPD were not included in the economic model because the appropriate model inputs were not identified in the literature.
Conclusions
Influenza vaccination significantly reduces the risk of acquiring influenza-related ARIs in patients with COPD, especially in patients with severe airflow obstruction. Although it was shown that the rates of hospitalization and subsequent mechanical ventilation due to episodes of influenza-related ARI were lower in patients who received the vaccine compared with those who did not, the study did not have sufficient power to demonstrate the presence of a statistically significant difference.
The study showed that patients’ age, sex, severity of COPD, smoking status, or comorbid diseases do not modify the effectiveness of the vaccine. Adverse effects of the influenza vaccination included both systemic reactions (headache, myalgia, fever, and skin rash) and local reactions (swelling and itching) at the site of vaccination. The influenza vaccination was regarded as safe since systemic reactions and measures of lung function, dyspneic symptoms, exercise capacity, and total ARI (influenza-related and non-influenza-related) were not significantly different between the vaccinated group and the control group up to 4 weeks following the vaccination.
The pneumococcal vaccination does not result in a significant reduction in the risk of acquiring CAP due to pneumococcus or of unknown etiology, but it significantly reduces the risk of acquiring pneumococcal pneumonia in patients with COPD. However, for pneumonia due to pneumococcus and of unknown etiology, there were significant findings when data were analyzed according to subgroups of patients (age < 65 years) and severe airflow obstruction (FEV1 < 40% predicted).
The accumulated percentage of patients without pneumonia due to pneumococcus and of unknown etiology across time was significantly lower in the vaccine group than in the control group in patients younger than 65 years of age and also in patients with severe airflow obstruction (FEV1 < 40% predicted). The study showed that the efficacy of the vaccine is dependent on the age of the patient. The vaccine was not effective in patients 65 years of age or older, but it reduced the risk of acquiring pneumonia by 80% in patients younger than 65 years.
Hospital admission rates and median lengths of hospital stay were lower in the vaccine group than the control group, but the difference was not statistically significant. No patients reported any local or systemic adverse reactions to the vaccine, and the mortality rate was not different between patients who received the vaccine and patients who did not.
Glossary
- 6 Minute Walking Test (6MWT)
A measure of exercise capacity which measures the distance that a patient can quickly walk on a flat, hard surface in a period of 6 minutes. A widely used outcome measure in respiratory rehabilitation of patients with COPD.
- Acute exacerbations of chronic obstructive pulmonary disease (AECOPD)
A change in baseline symptoms that is beyond day-to-day variation, particularly increased breathlessness, cough, and/or sputum, which has an abrupt onset.
- Admission avoidance hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and avoid admission to hospital. After patients are assessed in the emergency department for an acute exacerbation, they are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home where they receive regular visits from a medical professional until the exacerbation has resolved.
- Ambulatory oxygen therapy
Provision of oxygen therapy during exercise and activities of daily living for individuals who demonstrate exertional desaturation.
- Bilevel positive airway pressure (BiPAP)
A continuous positive airway pressure mode used during noninvasive positive pressure ventilation (see definition below) that delivers preset levels of inspiratory and expiratory positive airway pressure. The pressure is higher when inhaling and falls when exhaling, making it easier to breathe.
- Cost-effectiveness acceptability curve (CEAC)
A method for summarizing uncertainty in estimates of cost-effectiveness.
- Cor pulmonale
Right heart failure, as a result of the effects of respiratory failure on the heart.
- Dyspnea
Difficulty breathing or breathlessness.
- Early discharge hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and decrease their length of stay in hospital. After being assessed in the emergency department for acute exacerbations, patients are admitted to the hospital where they receive the initial phase of their treatment. These patients are discharged early into a hospital-at-home program where they receive regular visits from a medical professional until the exacerbation has resolved.
- Forced expiratory volume in 1 second (FEV1)
A measure of lung function used for COPD severity staging; the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
- Forced vital capacity (FVC)
The amount of air that can be forcibly exhaled from the lungs after taking the deepest breath possible.
- Fraction of inspired oxygen (FiO2)
The percentage of oxygen participating in gas exchange.
- Hypercapnia
Occurs when there is too much carbon dioxide in the blood (arterial blood carbon dioxide > 45 to 60 mm Hg).
- Hypopnea
Slow or shallow breathing.
- Hypoxemia
Low arterial blood oxygen levels while breathing air at rest. May be severe (PaO2 ≤ 55 mm Hg), moderate (56 mm Hg ≤ PaO2 < 65 mm Hg), or mild-to-moderate (66 mm Hg < PaO2 ≤ 74 mm Hg).5
- Incremental cost-effectiveness ratio (ICER)
Ratio of the change in costs of a therapeutic intervention to the change in effects of the intervention compared to the alternative (often usual care).
- Intention-to-treat analysis (ITT)
An analysis based on the initial treatment the participant was assigned to, not on the treatment eventually administered.
- Invasive mechanical ventilation (IMV)
Mechanical ventilation via an artificial airway (endotracheal tube or tracheostomy tube).
- Long-term oxygen therapy (LTOT)
Continuous oxygen use for about 15 hours per day. Use is typically restricted to patients fulfilling specific criteria.
- Multidisciplinary care
Defined as care provided by a team (compared to a single provider). Typically involves professionals from a range of disciplines working together to deliver comprehensive care that addresses as many of the patient’s health care and psychosocial needs as possible.
- Nicotine replacement therapy (NRT)
The administration of nicotine to the body by means other than tobacco, usually as part of smoking cessation.
- Noninvasive positive pressure ventilation (NPPV)
Noninvasive method of delivering ventilator support (without the use of an endotracheal tube) using positive pressure. Provides ventilatory support through a facial or nasal mask and reduces inspiratory work.
- Partial pressure of carbon dioxide (PaCO2)
The pressure of carbon dioxide dissolved in arterial blood. This measures how well carbon dioxide is able to move out of the body.
- Partial pressure of oxygen (PaO2)
The pressure of oxygen dissolved in arterial blood. This measures how well oxygen is able to move from the airspace of the lungs into the blood.
- Palliative oxygen therapy
Use of oxygen for mildly hypoxemic or nonhypoxemic individuals to relieve symptoms of breathlessness. Used short term. This therapy is “palliative” in that treatment is not curative of the underlying disease.
- Pulmonary rehabilitation
Multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary rehabilitation programs.
- Pulse oximetry
A noninvasive sensor, which is attached to the finger, toe, or ear to detect oxygen saturation of arterial blood.
- Quality-adjusted life-years (QALYs)
A measure of disease burden that includes both the quantity and the quality of the life lived that is used to help assess the value for money of a medical intervention.
- Respiratory failure
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. It can be either acute (acute respiratory failure, ARF) or chronic, and is classified as either hypoxemic (type I) or hypercapnic (type II) respiratory failure. Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD.
- Short-burst oxygen therapy
Short-duration, intermittent, supplemental oxygen administered either before or after exercise to relieve breathlessness with exercise.
- Sleep apnea
Interruption of breathing during sleep due to obstruction of the airway or alterations in the brain. Associated with excessive daytime sleepiness.
- Smoking cessation
The process of discontinuing the practice of inhaling a smoked substance.
- Spirometry
The gold standard test for diagnosing COPD. Patients breathe into a mouthpiece attached to a spirometer which measures airflow limitation.
- SpO2
Oxygen saturation of arterial blood as measured by a pulse oximeter.
- Stable COPD
The profile of COPD patients which predominates when patients are not experiencing an acute exacerbation.
- Supplemental oxygen therapy
Oxygen use during periods of exercise or exertion to relieve hypoxemia.
- Telemedicine (or telehealth)
Refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services.
- Telemonitoring (or remote monitoring)
Refers to the use of medical devices to remotely collect a patient’s vital signs and/or other biologic health data and the transmission of those data to a monitoring station for interpretation by a health care provider.
- Telephone only support
Refers to disease/disorder management support provided by a health care provider to a patient who is at home via telephone or videoconferencing technology in the absence of transmission of patient biologic data.
- Ventilator-associated pneumonia (VAP)
Pneumonia that occurs in patients undergoing mechanical ventilation while in a hospital.
Acknowledgements
Medical Information Officer
Kellee Kaulback
Editorial Staff
Jan Collins
Irina Alecu
COPD Expert Advisory Panel
The role of the expert panel was to provide direction on the scope of the project and the relevant outcomes measures of effectiveness, to review the evidence-based analyses and to identify any societal or systemic issues that are relevant to intervention effectiveness. However, the statements, conclusions and views expressed in this report do not necessarily represent the views of the expert panel members.
Jeremy Grimshaw, MD, MBChB, PhD (Chair)
Senior Scientist, Ottawa Hospital Research Institute
Professor, Department of Medicine, University of Ottawa
Dina Brooks, PhD
Professor, Department of Physical Therapy, University of Toronto
Debbie Coutts, RRT, CRE
Andrea Gershon, MD, MSc, FRCP(C)
Scientist, Institute for Clinical Evaluative Sciences
Respirologist, Sunnybrook Health Sciences Centre
Assistant Professor, Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto
Mita Giacomini, BSc, MPH, MA, PhD
Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Ron Goeree, BA, MA
Director, PATH Research Institute, St. Joseph’s Hospital (Hamilton)
Associate Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Roger Goldstein, MBCHB, FRCP(C), FRCP(UK)
NSA Chair in Respiratory Rehabilitation Research
Director, Respiratory Services, and Senior Scientist, West Park Healthcare Centre
Professor, Medicine and Physical Therapy, University of Toronto
Alan G Kaplan, MD, CCFP(EM), FCFP
Chairperson, Family Physician Airways Group of Canada
Chairperson, Special Interest Focused Care Group in Respiratory Medicine, College of Family Physicians of Canada
Clinical Lecturer, Department of Family and Community Medicine, University of Toronto
DE O’Donnell, MD, FRCP(C)
Director, COPD Centre, Kingston General Hospital
Professor, Department of Medicine, Queen’s University
Asad Razzaque, MD
Family Physician
Holger Schünemann, MD, PhD, MSc, FRCP(C)
Michael Gent Chair in Healthcare Research
Chair, Department of Clinical Epidemiology & Biostatistics, McMaster University
Professor, Department of Clinical Epidemiology & Biostatistics and Medicine, McMaster University
Tasnim Sinuff, MD, PhD, FRCP(C)
Clinician Scientist, Sunnybrook Health Sciences Centre
Assistant Professor, Department of Medicine, University of Toronto
Laura Watling, RRT, BSc(HK)
Clinical Practice Leader/Clinical Coordinator, Respiratory Therapy, West Park Healthcare Centre
Appendices
Appendix 1: Literature Search Strategies
Search date: July 5, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID
EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1996 to June Week 4 2010>
Search Strategy:
--------------------------------------------------------------------------------
1 exp Pulmonary Disease, Chronic Obstructive/ (13537)
2 (chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (14459)
3 (copd or coad).ti,ab. (12785)
4 chronic airflow obstruction.ti,ab. (110)
5 exp Emphysema/ (2879)
6 ((chronic adj2 bronchitis) or emphysema).ti,ab. (8342)
7 or/1-6 (29288)
8 exp Vaccines/ (75124)
9 exp Immunotherapy/ (80295)
10 exp Influenza, Human/im [Immunology] (1354)
11 exp Orthomyxoviridae/im [Immunology] (4209)
12 (vaccin* or immuni* or immunotherap* or Flulaval or FluMist or Fluarix or Fluvirin or AgriFlu or Fluzone or Afluria or Prevnar or Pneumovax).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (212365)
13 exp Pneumococcal Infections/im [Immunology] (1064)
14 or/8-13 (233246)
15 7 and 14 (665)
16 limit 15 to (english language and humans and yr=”2000 -Current”) (430)
Database: EMBASE <1980 to 2010 Week 26>
Search Strategy:
--------------------------------------------------------------------------------
1 exp chronic obstructive lung disease/ (35960)
2 (chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (19439)
3 (copd or coad).ti,ab. (15823)
4 chronic airflow obstruction.ti,ab. (453)
5 exp emphysema/ (14553)
6 exp chronic bronchitis/ (6199)
7 ((chronic adj2 bronchitis) or emphysema).ti,ab. (14573)
8 or/1-7 (58597)
9 exp immunization/ (107323)
10 exp vaccine/ (125618)
11 (vaccin* or immuni* or immunotherap* or Flulaval or FluMist or Fluarix or Fluvirin or AgriFlu or Fluzone or Afluria or Prevnar or Pneumovax).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (353106)
12 or/9-11 (356099)
13 8 and 12 (1811)
14 limit 13 to (human and english language and yr=”2000 -Current”) (1078)
| # | Query | Results |
|---|---|---|
| S14 | (S7 or S8 or S9 or S10 or S11 or S12) and (S6 and S13) | 126 |
| S13 | S7 or S8 or S9 or S10 or S11 or S12 | 31539 |
| S12 | vaccin* or immuni* or immunotherap* or Flulaval or FluMist or Fluarix or Fluvirin or AgriFlu or Fluzone or Afluria or Prevnar or Pneumovax | 30134 |
| S11 | (MH ”Pneumococcal Infections/IM”) | 57 |
| S10 | (MH ”Influenza, Human+/IM”) | 46 |
| S9 | (MH ”Orthomyxoviridae+/IM”) | 202 |
| S8 | (MH ”Vaccines+”) | 16768 |
| S7 | (MH ”Immunotherapy+”) | 12124 |
| S6 | S1 or S2 or S3 or S4 or S5 | 7203 |
| S5 | chronic bronchitis or emphysema | 1550 |
| S4 | (MH ”Emphysema+”) | 942 |
| S3 | copd or coad | 3982 |
| S2 | (chronic obstructive and (lung* or pulmonary or airway* or airflow or respiratory) and (disease* or disorder*)) | 5434 |
| S1 | (MH ”Pulmonary Disease, Chronic Obstructive+”) | 4195 |
Appendix 2: Quality of Studies and GRADE Tables
Quality of Studies for Each Outcome
Table A1: Influenza Vaccination: Episodes of Influenza-Related Acute Respiratory Illness*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Wongsurakiat et al, 2004 (35) | 125 | ✓ | ✓ | ✓ | ✓ Double blinded |
80% | 3 | ✓ | High |
Abbreviations: ITT, intention-to-treat; N, number of participants.
Table A2: Influenza Vaccination: Hospitalization*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Wongsurakiat et al, 2004 (35) | 125 | ✓ | ✓ | ✓ | ✓ Double blinded |
X Inadequate power (Low event rate) |
3 | ✓ | Low |
Abbreviations: ITT, intention-to-treat; N, number of participants.
Table A3: Influenza Vaccination: Mechanical Ventilation*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Wongsurakiat et al, 2004 (35) | 125 | ✓ | ✓ | ✓ | ✓ Double blinded |
X Inadequate power (Low event rate) |
3 | ✓ | Low |
Abbreviations: ITT, intention-to-treat; N, number of participants.
Table A4: Influenza Vaccination: Safety Outcomes*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Wongsurakiat et al, 2004 (35) | 125 | ✓ | ✓ | ✓ | ✓ Double blinded |
X Inadequate power (Low event rate) |
3 | ✓ | Low |
Abbreviations: ITT, intention-to-treat, N, number of participants.
Table A5: Pneumococcal Vaccination: Time to the First Episode of Community-Acquired Pneumonia Either Due to Pneumococcus or of Unknown Etiology*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Alfageme et al, 2006 (45) | 596 | ✓ | ✓ | Not reported | ✓ Treating physicians blinded |
Not reported Large RCT | ✓ None |
✓ | High |
Abbreviations: ITT, intention-to-treat; N, number of participants; RCT, randomized controlled trial.
Table A6: Pneumococcal Vaccination: Hospital Admission*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Alfageme et al, 2006 (45) | 596 | ✓ | ✓ | Not reported | ✓ Treating physicians blinded |
X Inadequate power (Low event rate) |
✓ None |
✓ | Low |
Abbreviations: ITT, intention-to-treat; N, number of participants.
Table A7: Pneumococcal Vaccination: Safety Outcomes*.
| Author, Year | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding | Power | Loss to Follow-up | ITT | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|
| Alfageme et al, 2006 (45) | 596 | ✓ | ✓ | Not reported | ✓ Treating physicians blinded |
X Inadequate power (Low event rate) |
✓ None |
✓ | Low |
Abbreviations: ITT, intention-to-treat; N, number of participants.
GRADE Tables
Table A8: GRADE of Evidence for Influenza Vaccination*.
| No. of Studies | Design | Study Quality | Consistency | Directness | Imprecision | Other Modifying Factors |
Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Episodes of Influenza-Related ARI | |||||||
| 1 | RCT | High | No serious limitations | No serious limitations | No serious limitations | n/a | High |
| Outcome: Hospitalization | |||||||
| 1 | RCT | Low | No serious limitations | No serious limitations | No serious limitations | n/a | Low |
| Outcome: Mechanical Ventilation | |||||||
| 1 | RCT | Low | No serious limitations | No serious limitations | No serious limitations | n/a | Low |
| Outcome: Safety Measures | |||||||
| 1 | RCT | Low | No serious limitations | No serious limitations | No serious limitations | n/a | Low |
Abbreviations: ARI, acute respiratory illness; no., number; RCT, randomized controlled trial.
Table A9: GRADE of Evidence for Pneumococcal Vaccination*.
| No. of Studies | Design | Study Quality | Consistency | Directness | Imprecision | Other Modifying Factors |
Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Time to the First Episode of Community- Acquired Pneumonia | |||||||
| 1 | RCT | High | No serious limitations | No serious limitations | No serious limitations | n/a | High |
| Outcome: Hospital Admission | |||||||
| 1 | RCT | Low | No serious limitations | No serious limitations | No serious limitations | n/a | Low |
| Outcome: Safety Measures | |||||||
| 1 | RCT | Low | No serious limitations | No serious limitations | No serious limitations | n/a | Low |
Abbreviations: no., number; RCT, randomized controlled trial.
Suggested Citation
This report should be cited as follows:
Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2012 Mar; 12(3) 1−64. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Vaccinations_March.pdf.
Indexing
The Ontario Health Technology Assessment Series is currently indexed in Excerpta Medica/EMBASE and the Center for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: MASinfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All analyses in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: MASinfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-7009-1 (PDF)
© Queen’s Printer for Ontario, 2012
About the Medical Advisory Secretariat
Effective April 5, 2011, the Medical Advisory Secretariat (MAS) became a part of Health Quality Ontario (HQO), an independent body funded by the Ministry of Health and Long-Term Care. The mandate of MAS is to provide evidence-based recommendations on the coordinated uptake of health services and health technologies in Ontario to the Ministry of Health and Long-Term Care and to the health care system. This mandate helps to ensure that residents of Ontario have access to the best available and most appropriate health services and technologies to improve patient outcomes.
To fulfill its mandate, MAS conducts systematic reviews of evidence and consults with experts in the health care services community. The resulting evidence-based analyses are reviewed by the Ontario Health Technology Advisory Committee—to which MAS also provides a secretariat function—and published in the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, MAS systematically reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, the Secretariat collects and analyzes information about how a new technology fits within current practice and existing treatment alternatives. Details about the technology’s diffusion into current health care practices add an important dimension to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist decision-makers in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals wishing to comment on an analysis prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by MAS for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data and information provided by experts and applicants to MAS to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the MAS website for a list of all evidence-based analyses: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
List of Tables
| Table 1: Results of a Prospective Cohort Study of the Pneumococcal Vaccine in Patients With Respiratory Disease* |
| Table 2: Randomized Controlled Trials of the Influenza Vaccine in Patients with Respiratory Illness* |
| Table 3: Randomized Controlled Trials of the Pneumococcal Vaccine in Patients with Respiratory Illness* |
| Table 4: Body of Evidence Examined According to Study Design* |
| Table 5: Incidence Density for Episodes of Influenza-Related ARI and Episodes Requiring Hospitalization – Vaccine Versus Placebo* |
| Table 6: Adjusted Incidence Rate Ratios for Vaccine Versus Placebo* |
| Table 7: Development of ARI During the First Week and the First Four Weeks* |
| Table 8: P Value for the Difference in Changes in Lung Function, Dyspneic Symptoms, and Exercise Capacity – Vaccine Versus Placebo* |
| Table 9: Efficacy of the 23-Serotype Pneumococcal Vaccine in Reducing the Incidence of Community-Acquired Pneumonia of Unknown Etiology and due to Pneumococcus* |
| Table 10: Cox Proportional Hazards Regression Model* |
| Table 11: Mortality Rates Due to Pneumonia* |
| Table 12: Factors Influencing Mortality* |
| Table A1: Influenza Vaccination: Episodes of Influenza-Related Acute Respiratory Illness* |
| Table A2: Influenza Vaccination: Hospitalization* |
| Table A3: Influenza Vaccination: Mechanical Ventilation* |
| Table A4: Influenza Vaccination: Safety Outcomes* |
| Table A5: Pneumococcal Vaccination: Time to the First Episode of Community-Acquired Pneumonia Either Due to Pneumococcus or of Unknown Etiology* |
| Table A6: Pneumococcal Vaccination: Hospital Admission* |
| Table A7: Pneumococcal Vaccination: Safety Outcomes* |
| Table A8: GRADE of Evidence for Influenza Vaccination* |
| Table A9: GRADE of Evidence for Pneumococcal Vaccination* |
List of Figures
| Figure 1: Incidence and Severity of Influenza-Related ARI in Patients with COPD* |
| Figure 2: Episodes of Global Pneumonia in Patients with COPD: Vaccinated Versus Unvaccinated* |
| Figure 3: First Episode of Community-Acquired Pneumonia* |
| Figure 4: First Episode of Community-Acquired Pneumonia of Unknown Etiology and Pneumococcal Pneumonia in Vaccinated and Unvaccinated Patients* |
| Figure 5: Kaplan-Meier Survival Curve Demonstrating the Cumulative Proportion of Patients Without Pneumonia Over the Follow-up Period |
| Figure 6: Kaplan-Meier Survival Curve Demonstrating the Cumulative Proportion of Patients Less Than 65 Years of Age Without Pneumonia Over the Follow-up Period |
| Figure 7: Kaplan-Meier Survival Curve Demonstrating the Cumulative Proportion of Patients with Severe COPD Without Pneumonia Over the Follow-up Period* |
List of Abbreviations
- ARI
Acute respiratory illness
- CAP
Community-acquired pneumonia
- CI
Confidence interval(s)
- CDC
Centers for Disease Control and Prevention
- CIRID
Centre for Immunization and Respiratory Infectious Diseases
- COPD
Chronic obstructive pulmonary disease
- FEV1
Forced expiratory volume in 1 second
- H
Hemagglutinin
- HI
Hemagglutination inhibition
- HR
Hazard Ratio
- MAS
Medical Advisory Secretariat
- N
Neuraminidase
- OR
Odds Ratio
- PHAC
Public Health Agency of Canada
- PPSV
Pneumococcal polysaccharide vaccine
- RCT
Randomized controlled trial
- RR
Relative risk
- WHO
World Health Organization
Footnotes
Defined as clinically and radiographically confirmed pneumonia with S pneumoniae isolated from a culture of blood or any other usually sterile fluid
Defined as clinically and radiographically confirmed pneumonia with S pneumoniae isolated from a culture of sputum or a nasal swab
Details are provided in the PHAC report (15)
Details are provided in the PHAC report (15)
The mild-to-moderate classification was created for the purposes of the report.
References
- 1.Wesseling G. Occasional review. Influenza in COPD: pathogenesis, prevention, and treatment. Int J Chron Obstruct Pulmon Dis. 2007;2(1):5–10. doi: 10.2147/copd.2007.2.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plans-Rubio P. Prevention and control of influenza in persons with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(1):41–53. doi: 10.2147/copd.2007.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hovden A-O, Cox RJ, Haaheim LR. Influenza: The virus and prophylaxis with inactivated influenza vaccine in ”at risk” groups, including COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(3):229–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Zuckerman AJ, Banatvala JE, Griffiths P, Schoub B, Mortimer P. Principles and practice of clinical virology. 6th edition. London, UK: Wiley-Blackwell; 2009. Chapter 16, Influenza; pp. 373–408. [Google Scholar]
- 5.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007 Oct 4;357(14):1373–81. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 7.Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, et al. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001 Sep 14;19(32):4610–7. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. [[updated 2011 Feb 13; cited 201 Feb 13]];Recommended viruses for influenza vaccines for use in the 2010-2011 northern hemisphere influenza season [Internet] Available from: http://www.flutrackers.com/forum/showthread.php?t=141403 .
- 9.Schenkein JG, Nahm MH, Dransfield MT. Pneumococcal vaccination for patients with COPD: current practice and future directions. Chest. 2008 Mar;133(3):767–74. doi: 10.1378/chest.07-0996. [DOI] [PubMed] [Google Scholar]
- 10.Fedson DS, Liss C. Precise answers to the wrong question: prospective clinical trials and the metaanalyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine. 2004 Feb 25;22(8):927–46. doi: 10.1016/j.vaccine.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003 May 1;348(18):1747–55. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 12.Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine. 2001;19(32):4780–90. doi: 10.1016/s0264-410x(01)00217-1. [DOI] [PubMed] [Google Scholar]
- 13.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine. 2008 Apr 7;26(16):1955–62. doi: 10.1016/j.vaccine.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986 Nov 20;315(21):1318–27. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 15.Public Health Agency of Canada. [[updated 2010; cited 2011 Feb 17]];Statement on seasonal trivalent inactivated influenza vaccine (TIV) for 2010-2011 [Internet] Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/acs-6/index-eng.php#toc4 .
- 16.Sanofi Pasteur. [[updated 2008; cited 2011 Feb 17]];Product Monograph, Pneumo 23 [Internet] Available from: https://www.vaccineshoppecanada.com/secure/pdfs/ca/Pneumo23E.pdf .
- 17.Merck & Co. [[updated 2011; cited 2011 Feb 13].];Pneumovax 23: Pneumococcal vaccine polyvalent [Internet] Available from: http://www.merck.com/product/usa/picirculars/p/pneumovax23/pneumovax_pi.pdf .
- 18.Centers for Disease Control and Prevention. [[updated 2010; cited 2011 Feb 17]];Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23 valent pneumococcal polysaccharide vaccine (PPSV23) [Internet] Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5934a3.htm#tab .
- 19.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole P, Chacko EE, Wood-Baker R, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(Issue 1) doi: 10.1002/14651858.CD002733.pub2. Art. No.: CD002733. DOI: 10.1002/14651858.CD002733.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Walters JA, Smith S, Poole P, Granger RH, Wood-Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;(Issue 11) doi: 10.1002/14651858.CD001390.pub3. Art. No.: CD001390. DOI: 10.1002/14651858.CD001390.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Howells C.H, Tyler LE. Prophylactic use of influenza vaccine in patients with chronic bronchitis. A pilot trial. Lancet. 1961 Dec 30;2(7218):1428–32. doi: 10.1016/s0140-6736(61)91248-x. [DOI] [PubMed] [Google Scholar]
- 23.Cate TR, Kasel JA, Couch RB, Six HR, Knight V. Clinical trials of bivalent influenza A/New Jersey/76-A/Victoria/75 vaccines in the elderly. J Infect Dis. 1977 Dec;136(Suppl):S518–S525. doi: 10.1093/infdis/136.supplement_3.s518. [DOI] [PubMed] [Google Scholar]
- 24.Fell PJ, Watson NP, O’Donnell HF, Simmons RL, Hasell SK. Longer term effects of live influenza vaccine in patients with chronic pulmonary disease. Lancet. 1977 Jun 18;1(8025):1282–4. doi: 10.1016/s0140-6736(77)91319-8. [DOI] [PubMed] [Google Scholar]
- 25.A study of live influenza virus vaccine in patients with chronic bronchitis. Report to Medical Research Council’s Committee on Influenza and Other Respiratory Virus Vaccines. Advisory Group on pulmonary function tests in relation to live influenza virus vaccines. Br J Dis Chest. 1980 Apr;74(2):121–7. [PubMed] [Google Scholar]
- 26.Treanor JJ, Mattison HR, Dumyati G, Yinnon A, Erb S, O’Brien D, et al. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann Intern Med. 1992 Oct 15;117(8):625–33. doi: 10.7326/0003-4819-117-8-625. [DOI] [PubMed] [Google Scholar]
- 27.Treanor J, Dumyati G, O’Brien D, Riley MA, Riley G, Erb S, et al. Evaluation of cold-adapted, reassortant influenza B virus vaccines in elderly and chronically ill adults. J Infect Dis. 1994 Feb;169(2):402–7. doi: 10.1093/infdis/169.2.402. [DOI] [PubMed] [Google Scholar]
- 28.Govaert TM, Dinant GJ, Aretz K, Masurel N, Sprenger MJ, Knottnerus JA. Adverse reactions to influenza vaccine in elderly people: randomised double blind placebo controlled trial. BMJ. 1993 Oct 16;307(6910):988–90. doi: 10.1136/bmj.307.6910.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994 Dec 7;272(21):1661–5. [PubMed] [Google Scholar]
- 30.Gorse GJ, Campbell MJ, Otto EE, Powers DC, Chambers GW, Newman FK. Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J Infect Dis. 1995 Jul;172(1):1–10. doi: 10.1093/infdis/172.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Gorse GJ, Otto EE, Daughaday CC, Newman FK, Eickhoff CS, Powers DC, et al. Influenza virus vaccination of patients with chronic lung disease. Chest. 1997 Nov 5;112(5):1221–33. doi: 10.1378/chest.112.5.1221. [DOI] [PubMed] [Google Scholar]
- 32.Gorse GJ, O’Connor TZ, Young SL, Mendelman PM, Bradley SF, Nichol KL, et al. Efficacy trial of live, cold-adapted and inactivated influenza virus vaccines in older adults with chronic obstructive pulmonary disease: a VA cooperative study. Vaccine. 2003 May 16;21(17-18):2133–44. doi: 10.1016/s0264-410x(02)00748-x. [DOI] [PubMed] [Google Scholar]
- 33.Neuzil KM, O’Connor TZ, Gorse GJ, Nichol KL. Recognizing influenza in older patients with chronic obstructive pulmonary disease who have received influenza vaccine. Clin Infect Dis. 2003 Jan 15;36(2):169–74. doi: 10.1086/345668. [DOI] [PubMed] [Google Scholar]
- 34.Gorse GJ, O’Connor TZ, Newman FK, Mandava MD, Mendelman PM, Wittes J, et al. Immunity to influenza in older adults with chronic obstructive pulmonary disease. J Infect Dis. 2004 Jul 1;190(1):11–9. doi: 10.1086/421121. [DOI] [PubMed] [Google Scholar]
- 35.Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004 Jun;125(6):2011–20. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- 36.Wongsurakiat P, Maranetra KN, Gulprasutdilog P, Aksornint M, Srilum W, Ruengjam C, et al. Adverse effects associated with influenza vaccination in patients with COPD: a randomized controlled study. Respirology. 2004 Nov;9(4):550–6. doi: 10.1111/j.1440-1843.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 37.Gorse GJ, O’Connor TZ, Young SL, Habib MP, Wittes J, Neuzil KM, et al. Impact of a winter respiratory virus season on patients with COPD and association with influenza vaccination. Chest. 2006 Oct;130(4):1109–16. doi: 10.1378/chest.130.4.1109. [DOI] [PubMed] [Google Scholar]
- 38.Chuaychoo B, Wongsurakiat P, Nana A, Kositanont U, Maranetra KN. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine. 2010 May 28;28(24):4045–51. doi: 10.1016/j.vaccine.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Clancy RL, Dunkley ML. Oral non-typable Haemophilus influenzae enhances physiological mechanism of airways protection. Clin Exp Immunol. 2010;161(1):127–33. doi: 10.1111/j.1365-2249.2010.04142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tandon MK, Phillips M, Waterer G, Dunkley M, Comans P, Clancy R. Oral immunotherapy with inactivated nontypeable Haemophilus influenzae reduces severity of acute exacerbations in severe COPD. Chest. 2010 Apr;137(4):805–11. doi: 10.1378/chest.09-1382. [DOI] [PubMed] [Google Scholar]
- 41.Leech JA, Gervais A, Ruben FL. Efficacy of pneumococcal vaccine in severe chronic obstructive pulmonary disease. CMAJ. 1987 Feb 15;136(4):361–5. [PMC free article] [PubMed] [Google Scholar]
- 42.Davis AL, Aranda CP, Schiffman G, Christianson LC. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest. 1987 Aug;92(2):204–12. doi: 10.1378/chest.92.2.204. [DOI] [PubMed] [Google Scholar]
- 43.Steentoft J, Konradsen HB, Hilskov J, Gislason G, Andersen JR. Response to pneumococcal vaccine in chronic obstructive lung disease—the effect of ongoing, systemic steroid treatment. Vaccine. 2006 Feb 27;24(9):1408–12. doi: 10.1016/j.vaccine.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Meyer P, Menzel M, Muellinger B, Weber N, Haeussinger K, Ziegler-Heitbrock L. Inhalative vaccination with pneumococcal polysaccharide in patients with chronic obstructive pulmonary disease. Vaccine. 2006 Jul 26;24(31-32):5832–8. doi: 10.1016/j.vaccine.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Alfageme I, Vazquez R, Reyes N, Munoz J, Fernandez A, Hernandez M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006 Mar;61(3):189–95. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ya Tseimakh I, Martynenko I, Paraeva S. Prophylactic efficacy of pneumococcal vaccination for chronic obstructive pulmonary disease (COPD) [Abstract] Eur Respir J. 2006 Sep 3;28(suppl 50):178s. [Google Scholar]
- 47.Teramoto S, Yamamoto H, Yamaguchi Y, Hanaoka Y, Ishil M, Ouchi YY. Clinical efficacy of anti-pneumococcal vaccination in elderly patients with COPD [Abstract] American Thoracic Society International Conference, May 18-23, 2007, San Francisco, California, USA [Google Scholar]
- 48.Furumoto A, Ohkusa Y, Chen M, Kawakami K, Masaki H, Sueyasu Y, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine. 2008 Aug 5;26(33):4284–9. doi: 10.1016/j.vaccine.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 49.Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, et al. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009 Sep 15;180(6):499–505. doi: 10.1164/rccm.200903-0488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: The Swedish Council on Technology Assessment in Health Care. 1993:81. doi: 10.1017/s0266462300008321. SBU-Report No 119E. [DOI] [PubMed] [Google Scholar]
- 51.Fine MJ, Smith MA, Carson CA, Meffe F, Sankey SS, Weissfeld LA, et al. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch Intern Med. 1994 Dec 12;154(23):2666–77. doi: 10.1001/archinte.1994.00420230051007. [DOI] [PubMed] [Google Scholar]
- 52.Melegaro A, Edmunds WJ. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol. 2004;19(4):353–63. doi: 10.1023/b:ejep.0000024701.94769.98. [DOI] [PubMed] [Google Scholar]


