Executive Summary
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective
The objective of this evidence-based review was to determine the effectiveness and cost-effectiveness of pulmonary rehabilitation in the management of chronic obstructive pulmonary disease (COPD).
Technology
Pulmonary rehabilitation refers to a multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary rehabilitation programs, though they may also include components such as patient education and psychological support. Pulmonary rehabilitation is recommended as the standard of care in the treatment and rehabilitation of patients with COPD who remain symptomatic despite treatment with bronchodilators.
For the purpose of this review, the Medical Advisory Secretariat focused on pulmonary rehabilitation programs as defined by the Cochrane Collaboration—that is, any inpatient, outpatient, or home-based rehabilitation program lasting at least 4 weeks that includes exercise therapy with or without any form of education and/or psychological support delivered to patients with exercise limitations attributable to COPD.
Research Questions
What is the effectiveness and cost-effectiveness of pulmonary rehabilitation compared with usual care (UC) for patients with stable COPD?
Does early pulmonary rehabilitation (within 1 month of hospital discharge) in patients who had an acute exacerbation of COPD improve outcomes compared with UC (or no rehabilitation)?
Do maintenance or postrehabilitation programs for patients with COPD who have completed a pulmonary rehabilitation program improve outcomes compared with UC?
Research Methods
Literature Search
Search Strategy
For Research Questions 1and 2, a literature search was performed on August 10, 2010 for studies published from January 1, 2004 to July 31, 2010. For Research Question 3, a literature search was performed on February 3, 2011 for studies published from January 1, 2000 to February 3, 2011. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists and health technology assessment websites were also examined for any additional relevant studies not identified through the systematic search.
Inclusion Criteria
Research questions 1 and 2:
published between January 1, 2004 and July 31, 2010
randomized controlled trials, systematic reviews, and meta-analyses
COPD study population
studies comparing pulmonary rehabilitation with UC (no pulmonary rehabilitation)
duration of pulmonary rehabilitation program ≥ 6 weeks
pulmonary rehabilitation program had to include at minimum exercise training
Research question 3:
published between January 1, 2000 and February 3, 2011
randomized controlled trials, systematic reviews, and meta-analyses
COPD study population
studies comparing a maintenance or postrehabilitation program with UC (standard follow-up)
duration of pulmonary rehabilitation program ≥ 6 weeks
initial pulmonary rehabilitation program had to include at minimum exercise training
Exclusion Criteria
Research questions 1, 2, and 3:
grey literature
duplicate publications
non-English language publications
study population ≤ 18 years of age
studies conducted in a palliative population
studies that did not report primary outcome of interest
Additional exclusion criteria for research question 3:
studies with ≤ 2 sessions/visits per month
Outcomes of Interest
The primary outcomes of interest for the stable COPD population were exercise capacity and health-related quality of life (HRQOL). For the COPD population following an exacerbation, the primary outcomes of interest were hospital readmissions and HRQOL. The primary outcomes of interest for the COPD population undertaking maintenance programs were functional exercise capacity and HRQOL.
Quality of Evidence
The quality of each included study was assessed taking into consideration allocation concealment, randomization, blinding, power/sample size, withdrawals/dropouts, and intention-to-treat analyses.
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria. The following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Summary of Findings
Research Question 1: Effect of Pulmonary Rehabilitation on Outcomes in Stable COPD
Seventeen randomized controlled trials met the inclusion criteria and were included in this review.
The following conclusions are based on moderate quality of evidence.
Pulmonary rehabilitation including at least 4 weeks of exercise training leads to clinically and statistically significant improvements in HRQOL in patients with COPD.1
Pulmonary rehabilitation also leads to a clinically and statistically significant improvement in functional exercise capacity2 (weighted mean difference, 54.83 m; 95% confidence interval, 35.63–74.03; P < 0.001).
Research Question 2: Effect of Pulmonary Rehabilitation on Outcomes Following an Acute Exacerbation of COPD
Five randomized controlled trials met the inclusion criteria and are included in this review. The following conclusion is based on moderate quality of evidence.
Pulmonary rehabilitation (within 1 month of hospital discharge) after acute exacerbation significantly reduces hospital readmissions (relative risk, 0.50; 95% confidence interval, 0.33–0.77; P = 0.001) and leads to a statistically and clinically significant improvement in HRQOL.3
Research Question 3: Effect of Pulmonary Rehabilitation Maintenance Programs on COPD Outcomes
Three randomized controlled trials met the inclusion criteria and are included in this review. The conclusions are based on a low quality of evidence and must therefore be considered with caution.
Maintenance programs have a nonsignificant effect on HRQOL and hospitalizations.
Maintenance programs have a statistically but not clinically significant effect on exercise capacity (P = 0.01). When subgrouped by intensity and quality of study, maintenance programs have a statistically and marginally clinically significant effect on exercise capacity.
Background
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective of Analysis
The objective of this evidence-based review was to determine the effectiveness and cost-effectiveness of pulmonary rehabilitation in the management of chronic obstructive pulmonary disease (COPD).
Technology
Pulmonary rehabilitation refers to a multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Pulmonary rehabilitation is recommended as the standard of care in the treatment and rehabilitation of patients with COPD who remain symptomatic despite treatment with bronchodilators.
Exercise training, the cornerstone of pulmonary rehabilitation programs, may include both aerobic and strength training. Other possible components of pulmonary rehabilitation include psychological support, patient education, nutritional counselling, occupational therapy, medication information, and smoking cessation.
While pulmonary rehabilitation can be delivered in multiple settings for varying durations, the optimal delivery site, components, duration, target populations, and timing remain in question.
For the purpose of this review, the Medical Advisory Secretariat focused on pulmonary rehabilitation programs as defined by the Cochrane Collaboration (1)—that is, any inpatient, outpatient, or home-based rehabilitation program lasting at least 4 weeks that includes exercise therapy with or without any form of education and/or psychological support delivered to patients with exercise limitations attributable to COPD.
Evidence-Based Analysis
Research Question(s)
What is the effectiveness and cost-effectiveness of pulmonary rehabilitation compared with usual care (UC) for patients with stable COPD?
Does early pulmonary rehabilitation (within 1 month of hospital discharge) in patients who had an acute exacerbation of COPD (AECOPD) improve outcomes compared with UC (or no rehabilitation)?
Do maintenance or postrehabilitation programs for patients with COPD who have completed a pulmonary rehabilitation program improve outcomes compared with UC?
Research Methods
Literature Search
Search Strategy
Research Questions 1 and 2: A literature search was performed on August 10, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 to July 31, 2010 (Appendix 1).
Research Question 3: A literature search was performed on February 3, 2011 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2000 to February 3, 2011 (Appendix 2).
Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles of uncertain eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established. The quality of evidence was assessed as high, moderate, low, or very low according to GRADE methodology.
Definition of a Pulmonary Rehabilitation Program
As noted previously, there is much clinical heterogeneity in the literature with respect to the duration, intensity, components, and delivery of pulmonary rehabilitation programs. In order to reduce the heterogeneity across studies included in this review we adopted the definition of pulmonary rehabilitation used in a Cochrane review (1) of pulmonary rehabilitation: any inpatient, outpatient, or home based-rehabilitation program lasting at least 4 weeks that includes exercise therapy with or without any form of education and/or psychological support delivered to patients with exercise limitations attributable to COPD.
Inclusion Criteria
Research Questions 1 and 2:
published between January 1, 2004 and July 31, 2010
randomized controlled trials (RCTs), systematic reviews, and meta-analyses
COPD study population
studies comparing pulmonary rehabilitation with UC (no pulmonary rehabilitation)
duration of pulmonary rehabilitation program ≥ 6 weeks
pulmonary rehabilitation program had to include at minimum exercise training
Research Question 3:
published between January 1, 2000 and February 3, 2011
RCTs, systematic reviews, and meta-analyses
COPD study population
studies comparing a maintenance or postrehabilitation program with UC (standard follow-up)
duration of pulmonary rehabilitation program ≥ 6 weeks
initial pulmonary rehabilitation program had to include at minimum exercise training
Exclusion Criteria
Research Questions 1, 2, and 3:
grey literature
duplicate publications
non-English language publications
study population ≤ 18 years of age
studies conducted in a palliative population
studies that did not report primary outcome of interest
Additional Exclusion Criteria for Research Question 3:
studies with ≤ 2 sessions/visits a month
Outcomes of Interest
The primary outcomes of interest for the stable COPD population were exercise capacity and health-related quality of life (HRQOL). For the COPD population following an exacerbation, the primary outcomes of interest were hospital readmissions and HRQOL. Other health outcomes examined in this population were mortality, emergency department visits, and exercise capacity. The primary outcomes of interest for the COPD population undertaking maintenance programs were functional exercise capacity and HRQOL. Other outcomes examined were hospital admissions and length of hospital stay.
Statistical Analysis
Statistical Challenges: Meta-analysis
Meta-analyzing continuous measurements, such as functional exercise capacity using the 6 Minute Walking Test (6MWT), presents statistical challenges, as studies quite often report only baseline (pre) and final values (post) for intervention and control groups without reporting change-from-baseline values. While the absolute difference between pre and post values is easy to obtain (final value minus baseline value), the standard deviation (SD) necessary for meta-analysis is often lacking.
To clarify the statistical challenges relevant to this report, it is important to define some terms:
The intra-group change from baseline to final refers to the mean difference between baseline and final values within intervention or within control groups (i.e., the difference in pre and post measurements within groups).
The inter-group difference refers to the mean difference in intra-group change from baseline to final values (as defined above) between intervention and control (i.e., the difference in change from baseline values between groups).
Solutions to Challenges
To solve the problem of missing SDs, the Cochrane Handbook for Systematic Reviews has identified 2 solutions (http://www.cochrane-handbook.org/), both of which are usually explored in any one meta-analysis:
Meta-analyze only the inter-group difference in mean final values between intervention and control. This approach assumes that, if baseline values do not significantly differ between intervention and control, the inter-group difference in mean final values will be similar to the inter-group difference of the intra-group change from baseline to final. One can test for significant differences at baseline; if they do not differ, this approach is valid.
Use statistical calculations to derive the standard deviations for the intra-group change from baseline to final, then meta-analyze these data. Repeated (pre and post) measurements made on the same participants tend to be correlated, thus lowering standard errors and creating tighter confidence intervals in comparison to single measurements. A correlation coefficient quantifies the correlation between measurements. This explains why meta-analyzing the change from baseline to final is preferable to meta-analyzing final values only, particularly if there are significant differences between intervention and control at baseline.
There are 2 ways to derive the standard deviations for the intra-group change from baseline to final when information is lacking:
Derive the standard deviation of the intra-group change from baseline to final using P values, confidence intervals, or standard errors reported from a t-test for the intra-group change from baseline to final. It should be noted, however, that if a study does not report standard deviations for the intra-group change from baseline to final, it is unlikely (though not impossible) that the study will report relevant t-test values. This approach is thus rare.
Calculate the standard deviation of the intra-group change from baseline to final by imputing a correlation coefficient. Correlation coefficients can be calculated from studies that report all relevant data (baseline ± SD, final ± SD, difference ± SD). These correlation coefficients can then be applied to studies lacking relevant information to derive appropriate SDs. Alternatively, one can impute varying correlation coefficients and run multiple sensitivity meta-analyses to observe any changes in effect. It should be noted, however, that imputation has been historically shown to have little effect on the summary estimates and conclusions of a meta-analysis. (2;3)
For this particular analysis, changes from baseline values were meta-analyzed. Standard deviations for these changes were generated by imputing a correlation coefficient of 0.5.
Quality of Evidence
The quality of each included study was assessed taking into consideration the following 7 study design characteristics:
adequate allocation concealment,
randomization (study must include a description of the randomization procedure used and this must be a proper method),
power/sample size (adequate sample size based on a priori calculations; underpowered studies were identified, when possible, using post-hoc sample size power calculations),
blinding (if double blinding was not possible, a single-blind study with unbiased assessment of outcome was considered adequate for this criterion),
Fewer than 20% withdrawals/dropouts,
intention-to-treat (ITT) analysis conducted and done properly (withdrawals/dropouts considered in analysis), and
other criteria as appropriate for the particular research question and study design.
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (4) as presented below.
Quality refers to criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Results of Evidence-Based Analysis
Research Question 1: Effect of Pulmonary Rehabilitation on Outcomes in Stable COPD
The database search yielded 2,069 citations published between January 2004 and July 2010. Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when citations were excluded in the analysis.
Figure 1: Citation Flow Chart.
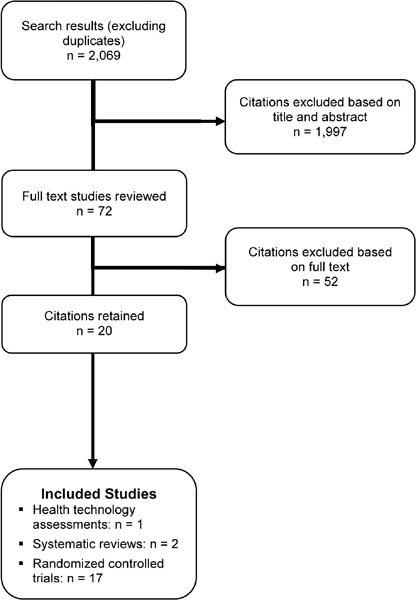
Twenty studies met the inclusion criteria described above; of these, 1 paper was a health technology assessment, 2 studies were systematic reviews, and the remaining 17 studies were RCTs (Table 1).
Table 1: Body of Evidence Examined According to Study Design*.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studies | |
| Systematic review of RCTs | 3 |
| Large RCT† | 3 |
| Small RCT | 14 |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with contemporaneous controls | |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | |
| Database, registry, or cross-sectional study | |
| Case series | |
| Retrospective review, modelling | |
| Studies presented at an international conference or other sources of grey literature | |
| Expert opinion | |
| Total | 20 |
Abbreviation: RCT, randomized controlled trial.
Large RCT is defined as having a sample size of at least 100.
For each included study, the study design was identified and is summarized below in Table 1, which is a modified version of a hierarchy of study design by Goodman. (5) The additional designation “g” was added for preliminary reports of studies that had been presented to international scientific meetings. Table 1 lists the body of evidence examined according to study design and the number of studies identified.
The literature search identified 3 reviews focusing on pulmonary rehabilitation for COPD. A summary of the reviews can be found below (Table 2). Two of them were narrative reviews, (6;7) of which 1 focused solely on home-based pulmonary rehabilitation. The remaining review, conducted in 2006 by Lacasse et al, (8) included a meta-analysis of the effects of pulmonary rehabilitation on exercise capacity and HRQOL based on 31 studies from the years 1966 to 2004. The authors concluded that pulmonary rehabilitation featuring at least 4 weeks of exercise training leads to clinically and statistically significant improvements in important domains of quality of life including dyspnea, fatigue, emotional function, and mastery. For exercise capacity, the results favoured the pulmonary rehabilitation group over the UC group, with a weighted mean 6MWT difference of 48 m (95% confidence interval [CI], 32–65 m). (Sixteen studies were included in this pooled estimate.) Subgroup analyses based on a priori reasons for clinical heterogeneity did not have an effect on study results.
Table 2: Summary of Existing Evidence on Pulmonary Rehabilitation Interventions for Stable COPD*.
| Study (Type) | Number of Trials Search Years |
Conclusions |
|---|---|---|
| CADTH, 2010 (HTA) (6) | 102 1998 onwards |
Pulmonary rehabilitation improves short-term exercise capacity, HRQOL, and mental health outcomes for patients with COPD. |
| Lacasse et al, 2006 (MA) (8) | 31 1966–2004 |
Pulmonary rehabilitation including at least 4 weeks of exercise training leads to clinically and statistically significant improvements in important domains of quality of life including dyspnea, fatigue, emotional function, and mastery. |
| Viera et al, 2010 (SR) (7) | 8 | Self-monitored, home-based pulmonary rehabilitation is useful and, if properly done, may be an equivalent alternative to outpatient pulmonary rehabilitation. Many programs with endurance training have been found beneficial in improving HRQOL and exercise capacity. |
Abbreviations: CADTH, Canadian Agency for Technologies and Health; COPD, chronic obstructive pulmonary disease; HRQOL, health-related quality of life; HTA, health technology assessment; MA, meta-analysis; SR, systematic review.
Randomized Controlled Trials
A total of 17 RCTs that met the inclusion criteria were identified and included in this review. (9-24) The sample size of the studies ranged from 28 to 200, with a total of 1,155 participants in the 17 studies. The mean reported age of the participants was 66 years. All studies reported gender, and the mean percentage of females was 67 percent. The percent predicted forced expiratory volume in 1 second (% predicted FEV1) in the study populations ranged from 27 to 72. Few studies characterised the study sample in terms of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD stage criteria (see below) based on FEV1 and forced vital capacity (FVC). Using these criteria, the population of the remaining studies was assessed. In total, 77% of studies were conducted in a severe COPD population, 18% in a moderate COPD population, and 5% in a very severe COPD population.
The GOLD COPD stage criteria are as follows:
Stage I (Mild COPD): Mild airflow limitation (FEV1/FVC < 70%; FEV1 ≥ 80% predicted) and sometimes, but not always, chronic cough and sputum production. (At this stage, the individual may not be aware that his or her lung function is abnormal.)
Stage II (Moderate COPD): Worsening airflow limitation (FEV1/FVC < 70%; 50% > FEV1 < 80% predicted), with shortness of breath typically developing on exertion. (This is the stage at which patients typically seek medical attention because of chronic respiratory symptoms or exacerbations.)
Stage III (Severe COPD): Further worsening of airflow limitation (FEV1/FVC < 70%; 30% > FEV1 < 50% predicted), greater shortness of breath, reduced exercise capacity, and repeated exacerbations that have an impact on patients’ quality of life.
Stage IV (Very Severe COPD): Severe airflow limitation (FEV1/FVC < 70%; FEV1 < 30% predicted) or FEV1 < 50% predicted plus chronic respiratory failure. When this complication is present, patients may have very severe (Stage IV) COPD even if the FEV1 is greater than 30% predicted. (At this stage, quality of life is very appreciably impaired and exacerbations may be life-threatening.)
Nine studies excluded patients with comorbidities that precluded participation in a rehabilitation program or that could limit exercise training. Some of these trials specifically excluded patients with neurological or musculoskeletal disease, cancer and/or diabetes. Eight trials specifically excluded patients with heart failure, ischemic heart disease, or a history of heart disease.
Study Characteristics
Studies were conducted between 1990 and 2009. Two studies were conducted in Canada, with the remainder from the United Kingdom, Europe, India, and Australia. Sample sizes ranged from 28 to 200 participants. A detailed description of the studies can be found in Appendix 2. The individual quality of the studies varied, with differences in quality mainly due to methodological issues such as inadequate description of randomization, sample size calculation, allocation concealment, blinding, and uncertainty around the use of ITT analysis (Appendix 3). Pulmonary rehabilitation programs were delivered through a variety of settings, although the majority of studies (71%) were conducted in an outpatient setting of a hospital. All 17 studies reported a UC control group and 3 reported a wait-list control group.
Intervention Characteristics
All the interventions examined in the studies included a minimum of exercise training. Exercise programs consisted of aerobic training and in many cases included a strength-training component. Some interventions also featured disease education, dietary education/advice, self-care, smoking cessation advice, endurance training, self-management skills, breathing and relaxation exercises, referrals to social services, and/or psychological support. Many of the programs also included an individualized home training program that participants were encouraged to follow. All the studies examined the outcomes of HRQOL and exercise capacity. Despite homogeneity in outcome assessment, clinical heterogeneity was evident in intervention characteristics such as duration, intensity, setting, and interventionist.
Duration and Intensity
Intervention durations ranged from 4 weeks to 1 year. The majority of interventions lasted 6 to 12 weeks (13 studies), while the rest fell into categories of 4 weeks (1 study), 6 months (2 studies), or 1 year (1 study). The intensity of the interventions varied between trials, although the majority of studies had pulmonary rehabilitation programs that were 3 to 6 hours per week.
Interventions and Setting
The majority of interventions were carried out by a multidisciplinary team of physiotherapists, occupational therapists, dieticians, and nurses. However, a physiotherapist and/or physical therapist alone carried out the intervention in 5 studies, and a sole nurse in 1 other study. In 3 studies, a primary care physician was involved in supervision of the rehabilitation group during outpatient care. Three studies had an unclear description of who delivered the intervention. The majority of interventions occurred in an outpatient setting (71%).
Outcomes
Duration of follow-up ranged from 8 weeks to 2 years, with the most common reported length being 12 weeks. In addition, 41% of studies followed patients at a minimum of 2 time points.
All studies reported 6MWT results as a measure of exercise capacity. (Two studies reporting functional exercise capacity in terms of the shuttle walk test were not included in the meta-analysis.) Eighty-two percent of trials measured HRQOL using the Chronic Respiratory Questionnaire (CRQ) or St. George’s Respiratory Questionnaire (SGRQ). Additional outcomes examined in the trials included patient satisfaction, fatigue, lung function, anxiety and depression, functional dyspnea, psychological general well-being, health status, exacerbations, and hospitalizations.
The results of the meta-analyses identified in the literature search are summarized below in Table 3. Forest plots are found in Appendix 4.
Table 3: Summary of Findings of Meta-Analyses of Studies Investigating the Effectiveness of Pulmonary Rehabilitation on HRQOL and Functional Exercise Capacity in Patients With COPD*.
| Outcome | Number of Studies | Number of Participants | Effect Size Mean Difference (95% CI) |
GRADE |
|---|---|---|---|---|
| Quality of Life – Change in SGRQ | ||||
| Total Score | 8 | 514 | −8.40 (−13.30,−3.50) | |
| Symptoms | 8 | 514 | −3.40 (−7.85, 1.04) | Moderate |
| Impacts | 8 | 514 | −3.41 (11.03, 4.21) | |
| Activity | 8 | 514 | −7.73 (−14.24,−1.22) | |
| Quality of Life – Change in CRQ | ||||
| Fatigue | 8 | 507 | 0.83 (0.62, 1.04) | |
| Emotional Function | 8 | 507 | 0.70 (0.45, 0.95) | Moderate |
| Mastery | 8 | 507 | 0.85 (0.63, 1.06) | |
| Dyspnea | 8 | 507 | 0.97 (0.77, 1.17) | |
| Functional Exercise Capacity (6MWT) | 15 | 659 | 54.83 (35.63, 74.03) | Moderate |
Abbreviations: 6MWT, 6 Minute Walking Test; CI, confidence interval; CRQ, Chronic Respiratory Questionnaire; SGRQ, St. George’s Respiratory Questionnaire.
Health-Related Quality of Life
Eight studies reported results of an HRQOL assessment based on the SGRQ. (12-15;17;20;22;23) All studies compared the difference in the mean change scores from baseline to follow-up between the pulmonary rehabilitation and UC groups. A mean decrease in the SGRQ indicates an improvement in quality of life, while a mean increase indicates a deterioration in quality of life. The minimal clinically important difference (MCID)—that is, the smallest difference in score corresponding to the smallest difference perceived by the average patient that would mandate, in the absence of troublesome side effects and excessive costs, a change in patient management—for the SGRQ is 4 units. As seen above (Table 3), there was a statistically and clinically significant improvement in quality of life for the pulmonary rehabilitation group compared with the UC group as reflected in the total score (P < 0.001) and activity scores (P = 0.02) of the SGRQ.
The GRADE quality of evidence was assessed as moderate for this outcome. Details of this assessment, including reasons for downgrading the quality of evidence, are reported in Appendix 3.
Eight studies reported results of the quality-of-life assessment based on the CRQ. (16-19;24-27) All studies compared the difference in the mean change scores from baseline to follow-up between the pulmonary rehabilitation and UC groups. A mean increase in CRQ indicates an improvement in quality of life, while a mean decrease indicates a deterioration in quality of life. The MCID for the CRQ has been established as 0.5 units. Taking this figure into consideration, pulmonary rehabilitation (including all CRQ domains) was associated with a statistically and clinically significant improvement in quality of life (P < 0.001) (Table 3).
Exercise Capacity
Eighty-eight percent of studies reported results of functional exercise capacity assessments based on the 6MWT. All studies compared the difference in the mean change in scores from baseline to follow-up between the pulmonary rehabilitation and UC groups. The MCID for the 6MWT has been reported to be from 25 to 35 meters. (28;29) As seen above (Table 3), there was a statistically and clinically significant improvement in functional exercise capacity for the pulmonary rehabilitation group compared with the UC group, with an estimated pooled difference of 54.83 meters (P < 0.001). The GRADE quality of evidence was assessed as moderate for this outcome.
Details of this assessment, including reasons for downgrading the quality of evidence, are reported in Appendix 3.
Conclusion
Based on moderate-quality evidence, pulmonary rehabilitation including at least 4 weeks of exercise training leads to clinically and statistically significant improvements in HRQOL in patients with COPD.1
Pulmonary rehabilitation also leads to a clinically and statistically significant improvement in functional exercise capacity2 (weighted mean difference, 54.83 m; 95% CI, 35.63–74.03; P < 0.001).
Research Question 2: Effect of Pulmonary Rehabilitation on Outcomes Following an Acute Exacerbation of COPD
The database search yielded 2,069 citations published between January 2004 and July 2010. Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 2 shows the breakdown of when citations were excluded in the analysis.
Figure 2: Citation Flow Chart.
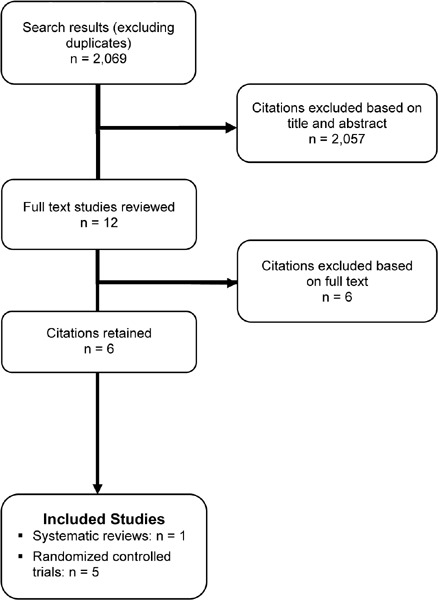
Six studies met the inclusion criteria for this research question; of these, 1 paper was a meta-analysis and the remainder were RCTs (Table 4).
Table 4: Body of Evidence Examined According to Study Design*.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studies | |
| Systematic review of RCTs | 1 |
| Large RCT† | |
| Small RCT | 5 |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with contemporaneous controls | |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | |
| Database, registry, or cross-sectional study | |
| Case series | |
| Retrospective review, modelling | |
| Studies presented at an international conference or other sources of grey literature | |
| Expert opinion | |
| Total | 6 |
Abbreviation: RCT, randomized controlled trial
Large RCT is defined as having a sample size of at least 100.
For each included study, the study design was identified and is summarized below in Table 4, which is modified version of a hierarchy of study design by Goodman. (5)
One systematic review, conducted in 2010 by Puhan et al,(30) focused on pulmonary rehabilitation following an AECOPD and included a meta-analysis. The review aimed to evaluate the effects of pulmonary rehabilitation on future hospital admissions (primary outcome) and other important outcomes (mortality, health-related quality of life, and exercise capacity) after COPD exacerbations. Six studies from 1966 to 2008 were included in the review.
The authors concluded that these studies suggest that pulmonary rehabilitation is highly effective and safe in reducing hospital admissions and mortality and improving HRQOL in COPD patients following an exacerbation. There were highly clinically and statistically significant differences between the rehabilitation group and the UC group for all domains of the CRQ and for the total, impact, and activity scores of the SGRQ. Pulmonary rehabilitation also improved exercise capacity measured by the 6MWT or shuttle test.
In assessing the Puhan et al review, (31) the Medical Advisory Secretariat excluded 3 of the 6 RCTs because:
2 studies had pulmonary rehabilitation programs lasting no longer than 10 days.
1 study excluded patients with an exacerbation in the previous month.
Randomized Controlled Trials
The database search identified citations published between 2004 and August 2010, but the literature was searched from 2008 forward. Five RCTs met the inclusion criteria and were thus included in this review. (9;32-35) The sample size of the studies ranged from 31 to 97, with a total of 276 participants in the 5 studies. The mean age of the participants was about 68 years. All studies reported gender, and the mean percentage of females was about 46 percent. The percent predicted FEV1 in the study populations ranged from 35 to 59. None of the studies characterised the study sample in terms of the GOLD COPD stage criteria. Using these criteria, 60% of studies included patients with severe COPD while the remaining studies included patients with moderate COPD.
Study Characteristics
Studies were conducted between 2000 and 2010. A detailed description of the studies can be found in Appendix 2. Two studies were conducted in the United Kingdom and the remainder in Germany, Ireland, and New Zealand. Sample sizes ranged from 31 to 97 participants. The individual quality of the studies varied, with differences in quality mainly due to methodological issues such as inadequate description of randomization, sample size calculation, allocation concealment, blinding, and uncertainty around the use of ITT analysis (Appendix 3). Pulmonary rehabilitation programs were delivered through a variety of settings. Two studies had outpatient pulmonary rehabilitation programs (35;36), 2 studies began with an inpatient program followed by an outpatient program (home-based in 1 case) (9;32), and the remaining study had a home-based program for patients discharged from hospital (34). All studies reported a UC control group.
Intervention Characteristics
All the interventions examined in the studies included a minimum of aerobic exercise training, with a strength-training component also included in many cases. Some interventions also featured disease education, dietary education/advice, self-care, smoking cessation advice, endurance training, self-management skills, breathing and relaxation exercises, referrals to social services, and psychological support. All the studies examined the outcomes of hospital readmissions, HRQOL, and exercise capacity. Despite homogeneity in outcome assessment, there was some clinical heterogeneity in intervention characteristics such as duration, intensity, setting, and individuals delivering the intervention.
Duration and Intensity
Intervention durations ranged from 6 weeks to 6 months. Eighty percent of studies had interventions lasting from 6 to 8 weeks. The intensities of the interventions were comparable in the studies, typically involving 2 to 3 two-hour sessions per week for the duration of the rehabilitation program.
Interventions and Setting
Two interventions were carried out by a multidisciplinary team that included 2 or more of the following health care professionals: COPD nurse, physiotherapist, occupational therapist, and dietician. The remaining studies either used a single physiotherapist to carry out the intervention or did not clearly describe who carried out the intervention.
Outcomes
Duration of follow-up ranged from 1 month to 6 months from baseline. Two of the 5 studies followed patients at a minimum of 2 time points. (9;34)
All studies reported hospital readmissions (9;32-35) and 3 reported COPD-specific readmissions. (9;32;35) All 5 trials measured quality of life using the CRQ or the SGRQ. Exercise capacity, (9;32) mortality, (9;33) and emergency department visits, (33;35) were each reported in 2 of the 5 studies. Other outcomes reported in some of the trials included dyspnea, lung function, body mass index (BMI), and fatigue. The results of the meta-analyses identified in the literature search are summarized below in Tables 5 and 6. Forest plots are found in Appendix 4.
Table 5: Summary of Findings of a Meta-Analysis of Studies Investigating the Effectiveness of Pulmonary Rehabilitation on Hospital Readmission in Patients with COPD Following an Acute Exacerbation*.
| Outcome | Number of Studies |
Number of Participants |
Pooled Rate Ratio (95% CI) |
GRADE |
|---|---|---|---|---|
| All hospital readmissions | 5 | 251 | 0.50 (0.33−0.77) | |
| COPD-related readmission | 3 | 183 | 0.41 (0.18−0.93) | Moderate |
| General readmission | 2 | 68 | 0.54 (0.29−1.03) |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Table 6: Summary of Findings of Meta-Analyses of Studies Investigating the Effectiveness of Pulmonary Rehabilitation on HRQOL in Patients with COPD Following an Acute Exacerbation*.
| Outcome | Number of Studies |
Number of Participants |
Effect Size Mean Difference (95% CI) |
GRADE |
|---|---|---|---|---|
| Quality of Life − Change in SGRQ | ||||
| Total Score | 3 | 109 | −11.44 (−16.71 to −6.17) | |
| Symptoms | 3 | 109 | −1.59 (−5.16 to 8.35) | Moderate |
| Impact | 3 | 109 | −14.51 (−21.52 to −7.51) | |
| Activity | 3 | 109 | −11.44 (−18..3 to −4.52) | |
| Quality of Life − Change in CRQ | ||||
| Fatigue | 4 | 196 | 2.54 (2.11, 2.97) | |
| Emotional Function | 4 | 196 | 2.11 (1.63, 2.60) | Moderate |
| Mastery | 4 | 196 | 3.17 (2.70, 3.64) | |
| Dyspnea | 4 | 196 | 3.42 (2.99, 3.85) |
Abbreviations: CI, confidence interval; CRQ, Chronic Respiratory Questionnaire; HRQOL, health-related quality of life; SGRQ, St. George’s Respiratory Questionnaire.
Hospital Readmissions
All studies reported hospital readmissions as an outcome. (9;32-35) Three of the studies reported COPD-related readmissions (9;32;35), while 2 of the studies reported general admissions. (33;34) There was a decrease in all hospital readmissions as seen by the pooled relative risk of 0.50 (95% CI, 0.33–0.77; P = 0.001) favouring pulmonary rehabilitation versus UC. When admissions were subgrouped by type, the effect observed was greater for COPD-related readmissions than for general readmissions.
Health-Related Quality of Life
Three studies reported results of HRQOL assessments based on the SGRQ. (33-35) All studies compared the difference in the mean change scores from baseline to follow-up between the pulmonary rehabilitation and UC groups. (9;32-35) Based on the MCID, there was a statistically and clinically significant improvement in quality of life for the pulmonary rehabilitation group as compared to the UC group reflected in the total (P < 0.001), impact (P < 0.001), and activity scores (P = 0.001) of the SGRQ (Table 6).
The GRADE quality of evidence was assessed as moderate for this outcome. Details of this assessment, including reasons for downgrading the quality of evidence, are reported in Appendix 3.
Four studies reported results of the quality-of-life assessment based on the CRQ. (9;32;33;35) Based on the MCID, there was a statistically and clinically significant improvement in quality of life for the pulmonary rehabilitation group compared with the UC group reflected in all domains of the CRQ (P < 0.001) (Table 6).
The GRADE quality of evidence was assessed as moderate for this outcome. Details of this assessment, including reasons for downgrading the quality of evidence, are reported in Appendix 3.
Additional Outcomes
Additional relevant outcomes were reported in several of the studies. Functional exercise capacity as measured by the 6MWT was reported in 2 studies. (9;32) There was a statistically and clinically significant improvement in exercise capacity as measured by the 6MWT favouring the pulmonary rehabilitation group as compared to the UC group (weighted mean difference, 203.14 m; 95% CI, 185.17– 221.11; P < 0.001). Two studies reported emergency department visits (33;35) and 2 studies reported mortality, (9;33) but no statistically significant differences were found for any of these outcomes between the pulmonary rehabilitation and UC groups.
Conclusion
Based on moderate-quality evidence, pulmonary rehabilitation (within 1 month of hospital discharge) after an AECOPD significantly reduces hospital readmissions (relative risk, 0.34; 95% CI, 0.25– 0.46; P < 0.001) and leads to a statistically and clinically significant improvement in HRQOL.3
Research Question 3: Effect of Maintenance Programs on COPD Outcomes
The database search yielded 1,000 citations published between January 2000 and February 2011. Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 3 shows the breakdown of when citations were excluded in the analysis.
Figure 3: Citation Flow Chart.
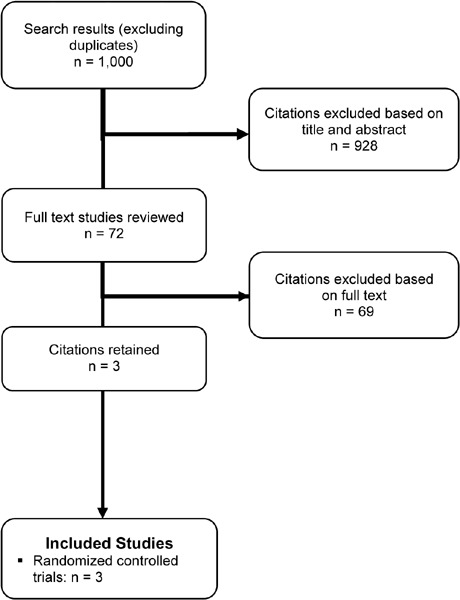
Three studies met the inclusion criteria for this research question. All studies included were RCTs (Table 7).
Table 7: Body of Evidence Examined According to Study Design*.
| Study Design | Number of Eligible Studies |
|---|---|
| RCT Studies | |
| Systematic review of RCTs | |
| Large RCT† | 1 |
| Small RCT | 2 |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls | |
| Non-RCT with contemporaneous controls | |
| Systematic review of non-RCTs with historical controls | |
| Non-RCT with historical controls | |
| Database, registry, or cross-sectional study | |
| Case series | |
| Retrospective review, modelling | |
| Studies presented at an international conference or other sources of grey literature | |
| Expert opinion | |
| Total | 3 |
Abbreviation: RCT, randomized controlled trial
Large RCT is defined as a sample size of at least 100.
For each included study, the study design was identified and is summarized below in Table 7, which is a modified version of a hierarchy of study design by Goodman. (5)
The search did not identify any systematic reviews or meta-analyses focused on maintenance programs following pulmonary rehabilitation for COPD patients.
Randomized Controlled Trials
The database search identified 1,000 citations published between January 2000 and February 2011. Of the 72 full-text articles reviewed, only 3 studies met the inclusion criteria described earlier, of which one was a large RTC and 2 were small RTCs (Table 7). (37-39) Sample sizes in the studies ranged from 48 to 140, with a total of 284 participants in the 3 studies. The mean reported age of the participants was 67 years. All studies reported gender, and the mean percentage of females ranged from 44 to 64 percent. The percent predicted FEV1 in the study populations ranged from 35 to 59. None of the studies characterised the study sample in terms of the GOLD COPD stage criteria. Using these criteria, 2 studies included patients with moderate COPD (37;39) and 1 included patients with severe COPD. (38)
The majority of studies mentioned exclusion criteria. Criteria included subjects who had experienced an AECOPD in the previous month, required supplemental oxygen, or had comorbidities precluding participation in exercise training, and subjects who had a medical condition limiting their ability to participate in exercise training.
Study Characteristics
Studies were conducted between 2000 and 2010. A detailed description of the studies can be found in Appendix 2. One study was carried out in Australia and the other 2 in Denmark and the United States. Sample sizes ranged from 48 to 140 participants. The individual quality of the studies was generally poor due to methodological issues such as inadequate description of randomization, sample size calculation, allocation concealment, blinding, and uncertainty around the use of ITT analysis (Appendix 3). All the maintenance programs were delivered in an outpatient setting. All studies reported a UC control group.
Intervention Characteristics
All the interventions examined in the studies included a minimum of aerobic exercise training, while some also included a strength-training component. Two studies included unsupervised home exercise as part of the interventions (38;39), and one of them also supplemented the exercise training with weekly educational sessions. (38) Some clinical heterogeneity was evident in the intervention characteristics, such as duration of the initial program, duration of the maintenance program, and intensity of the maintenance program.
Duration of Initial Pulmonary Rehabilitation Program
The duration of the initial pulmonary rehabilitation programs ranged from 7 to 12 weeks.
Duration and Intensity of Maintenance Programs
The duration of the maintenance programs ranged from 12 to 18 months. In 2 of the studies these maintenance programs had comparable intensities, typically involving one 1-to-2-hour session per week plus unsupervised home exercise training. (38;39) The remaining study was more intense, with maintenance sessions carried out 3 times per week. (37)
Outcomes
Outcomes were measured at various time points. One study assessed outcomes at 3, 6, and 12 months post-randomization, (38) another followed patients 3, 6, and 12 months following the intervention, (39) and the remaining study evaluated outcomes 3 months after the intervention as well as at 9, 15, and 18 months from baseline. (37)
Two of the 3 studies reported on exercise capacity as measured by the 6MWT (37;39) and 2 studies also reported on HRQOL. (38;39) The latter 2 studies also included hospitalizations and length of stay as outcomes.
The results of the findings for the maintenance programs identified in the literature search are summarized below in Tables 8 through 10. For studies in which results were meta-analyzed, forest plots can be found in Appendix 4.
Table 8: Summary of Findings of Meta-Analyses of Studies Investigating the Effectiveness of Maintenance Programs on Functional Exercise Capacity*.
| Outcome | Number of Studies |
Number of Participants |
Mean Difference (95% CI) |
P Value | GRADE |
|---|---|---|---|---|---|
| Functional Exercise Capacity (6MWT) | 2 | 166 | 22.93 (5.16−40.71)† | 0.01 | LOW |
Abbreviations: 6MWT, Six Minute Walking Test; CI, confidence interval.
Minimally clinically important difference ~25-35 m.
Table 10: Summary of Findings of Studies Investigating the Effectiveness of Maintenance Programs on Hospitalizations and Length of Stay*.
| Outcome | N | Maintenance | Usual Care | P Value |
|---|---|---|---|---|
| Mean Number of Hospital Admissions per Patient Over 12 Months (Mean) | ||||
| Ringbaek et al, 2010 (38) | 96 | 0.8 | 0.8 | 0.83 |
| Spencer et al, 2010 (39) | 48 | 0.3 | 0.5 | NR† |
| Mean Number of Days Spent in Hospital per Patient Over 12 Months (Mean) | ||||
| Ringbaek et al, 2010 (38) | 96 | 2.8 | 3.0 | 0.78 |
| Spencer et al, 2010 (39) | 48 | Reported no difference in the length of hospital stay between | ||
Abbreviations: N, sample size; NR, not reported.
Data not reported.
Table 9: Summary of Findings of Studies Investigating the Effectiveness of Maintenance Programs on Health-Related Quality of Life*.
| Outcome | N | Effect Size Mean Difference (95% CI) |
P Value | GRADE |
|---|---|---|---|---|
| HRQOL - Change in SGRQ | ||||
| Spencer et al, 2010 (39) | 48 | 5 (−2, 11) | NR | |
| Ringbaek et al, 2010 (38) | 96 | NR† | NR | LOW |
Abbreviations; CI, confidence interval; HRQOL, health-related quality of life; N, sample size; NR, not reported; SGRQ, St. George’s Respiratory Questionnaire.
Data not reported; authors concluded there was no significant difference between groups.
Exercise Capacity
Two studies reported results of a functional exercise capacity assessment based on the 6MWT.(37;39) Both studies compared the difference in the mean change in scores from baseline to follow-up between the maintenance and UC groups. Based on the MCID, there was a statistically but not clinically significant improvement in functional exercise capacity for the maintenance group compared with the UC group, with an estimated pooled difference of 22.93 m (95% CI, 5.16–40.71; P = 0.01) (Table 8). When higher-intensity maintenance programs were considered individually, the pooled difference reached marginal clinical significance at 25.88 m (95% CI, 25.27–26.49). The GRADE quality of evidence was assessed as low for this outcome. Details of this assessment, including reasons for downgrading the quality of evidence, are reported in Appendix 3.
Health-Related Quality of Life
Two studies reported results of HRQOL assessments based on the SGRQ. (38;39) Both studies compared the difference in the mean change scores from baseline to follow-up between the maintenance program and UC groups. Based on the MCID, one study failed to show a statistically or clinically significant improvement in quality of life for patients receiving the maintenance program compared with those receiving UC. (39) The other study failed to report data for this outcome, although the authors noted that there was no significant difference between the groups. (38) The GRADE quality of evidence was assessed as low for this outcome.
Hospitalizations and Length of Stay
Two studies reported hospitalizations and length of stay as an outcome. (38;39) There was no difference in the mean number of hospital admissions per patient over a 12-month period between patients receiving a maintenance program and those receiving UC (Table 10). There was also no difference in the mean number of days spent in hospital per patient over the 12 months between these 2 groups (Table 10). The GRADE quality of evidence was assessed as low for this outcome.
Conclusion
Based on low-quality evidence, pulmonary rehabilitation maintenance programs have a nonsignificant effect on HRQOL and hospitalizations.
Based on low-quality evidence, pulmonary rehabilitation maintenance programs for COPD patients have a statistically but not clinically significant effect on exercise capacity (P = 0.01). When studies are subgrouped by intensity and quality, the difference becomes marginally clinically significant.
Economic Analysis
The results of the economic analysis are summarized in issue 12 of the COPD series entitled Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model. This report can be accessed at: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Economic_March.pdf.
Glossary
- 6 Minute Walking Test (6MWT)
A measure of exercise capacity which measures the distance that a patient can quickly walk on a flat, hard surface in a period of 6 minutes. A widely used outcome measure in respiratory rehabilitation of patients with COPD.
- Acute exacerbations of chronic obstructive pulmonary disease (AECOPD)
A change in baseline symptoms that is beyond day-to-day variation, particularly increased breathlessness, cough, and/or sputum, which has an abrupt onset.
- Admission avoidance hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and avoid admission to hospital. After patients are assessed in the emergency department for an acute exacerbation, they are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home where they receive regular visits from a medical professional until the exacerbation has resolved.
- Ambulatory oxygen therapy
Provision of oxygen therapy during exercise and activities of daily living for individuals who demonstrate exertional desaturation.
- Bilevel positive airway pressure (BiPAP)
A continuous positive airway pressure mode used during noninvasive positive pressure ventilation (see definition below) that delivers preset levels of inspiratory and expiratory positive airway pressure. The pressure is higher when inhaling and falls when exhaling, making it easier to breathe.
- Cost-effectiveness acceptability curve (CEAC)
A method for summarizing uncertainty in estimates of cost-effectiveness.
- Cor pulmonale
Right heart failure, as a result of the effects of respiratory failure on the heart.
- Dyspnea
Difficulty breathing or breathlessness.
- Early discharge hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and decrease their length of stay in hospital. After being assessed in the emergency department for acute exacerbations, patients are admitted to the hospital where they receive the initial phase of their treatment. These patients are discharged early into a hospital-at-home program where they receive regular visits from a medical professional until the exacerbation has resolved.
- Forced expiratory volume in 1 second (FEV1)
A measure of lung function used for COPD severity staging; the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
- Forced vital capacity (FVC)
The amount of air that can be forcibly exhaled from the lungs after taking the deepest breath possible.
- Fraction of inspired oxygen (FiO2)
The percentage of oxygen participating in gas exchange.
- Hypercapnia
Occurs when there is too much carbon dioxide in the blood (arterial blood carbon dioxide > 45 to 60 mm Hg).
- Hypopnea
Slow or shallow breathing.
- Hypoxemia
Low arterial blood oxygen levels while breathing air at rest. May be severe (PaO2 ≤ 55 mm Hg), moderate (56 mm Hg ≤ PaO2 < 65 mm Hg), or mild-to-moderate (66 mm Hg < PaO2 ≤ 74 mm Hg).4
- Incremental cost-effectiveness ratio (ICER)
Ratio of the change in costs of a therapeutic intervention to the change in effects of the intervention compared to the alternative (often usual care).
- Intention-to-treat analysis (ITT)
An analysis based on the initial treatment the participant was assigned to, not on the treatment eventually administered.
- Invasive mechanical ventilation (IMV)
Mechanical ventilation via an artificial airway (endotracheal tube or tracheostomy tube).
- Long-term oxygen therapy (LTOT)
Continuous oxygen use for about 15 hours per day. Use is typically restricted to patients fulfilling specific criteria.
- Multidisciplinary care
Defined as care provided by a team (compared to a single provider). Typically involves professionals from a range of disciplines working together to deliver comprehensive care that addresses as many of the patient’s health care and psychosocial needs as possible.
- Nicotine replacement therapy (NRT)
The administration of nicotine to the body by means other than tobacco, usually as part of smoking cessation.
- Noninvasive positive pressure ventilation (NPPV)
Noninvasive method of delivering ventilator support (without the use of an endotracheal tube) using positive pressure. Provides ventilatory support through a facial or nasal mask and reduces inspiratory work.
- Partial pressure of carbon dioxide (PaCO2)
The pressure of carbon dioxide dissolved in arterial blood. This measures how well carbon dioxide is able to move out of the body.
- Partial pressure of oxygen (PaO2)
The pressure of oxygen dissolved in arterial blood. This measures how well oxygen is able to move from the airspace of the lungs into the blood.
- Palliative oxygen therapy
Use of oxygen for mildly hypoxemic or nonhypoxemic individuals to relieve symptoms of breathlessness. Used short term. This therapy is “palliative” in that treatment is not curative of the underlying disease.
- Pulmonary rehabilitation
Multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary rehabilitation programs.
- Pulse oximetry
A noninvasive sensor, which is attached to the finger, toe, or ear to detect oxygen saturation of arterial blood.
- Quality-adjusted life-years (QALYs)
A measure of disease burden that includes both the quantity and the quality of the life lived that is used to help assess the value for money of a medical intervention.
- Respiratory failure
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. It can be either acute (acute respiratory failure, ARF) or chronic, and is classified as either hypoxemic (type I) or hypercapnic (type II) respiratory failure. Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD.
- Short-burst oxygen therapy
Short-duration, intermittent, supplemental oxygen administered either before or after exercise to relieve breathlessness with exercise.
- Sleep apnea
Interruption of breathing during sleep due to obstruction of the airway or alterations in the brain. Associated with excessive daytime sleepiness.
- Smoking cessation
The process of discontinuing the practice of inhaling a smoked substance.
- Spirometry
The gold standard test for diagnosing COPD. Patients breathe into a mouthpiece attached to a spirometer which measures airflow limitation.
- SpO2
Oxygen saturation of arterial blood as measured by a pulse oximeter.
- Stable COPD
The profile of COPD patients which predominates when patients are not experiencing an acute exacerbation.
- Supplemental oxygen therapy
Oxygen use during periods of exercise or exertion to relieve hypoxemia.
- Telemedicine (or telehealth)
Refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services.
- Telemonitoring (or remote monitoring)
Refers to the use of medical devices to remotely collect a patient’s vital signs and/or other biologic health data and the transmission of those data to a monitoring station for interpretation by a health care provider.
- Telephone only support
Refers to disease/disorder management support provided by a health care provider to a patient who is at home via telephone or videoconferencing technology in the absence of transmission of patient biologic data.
- Ventilator-associated pneumonia (VAP)
Pneumonia that occurs in patients undergoing mechanical ventilation while in a hospital.
Acknowledgements
Medical Information Officer
Kellee Kaulback
Editorial Staff
Gabrielle Bauer
Irina Alecu
COPD Expert Advisory Panel
The role of the expert panel was to provide direction on the scope of the project and the relevant outcomes measures of effectiveness, to review the evidence-based analyses and to identify any societal or systemic issues that are relevant to intervention effectiveness. However, the statements, conclusions and views expressed in this report do not necessarily represent the views of the expert panel members.
Jeremy Grimshaw, MD, MBChB, PhD (Chair)
Senior Scientist, Ottawa Hospital Research Institute
Professor, Department of Medicine, University of Ottawa
Dina Brooks, PhD
Professor, Department of Physical Therapy, University of Toronto
Debbie Coutts, RRT, CRE
Andrea Gershon, MD, MSc, FRCP(C)
Scientist, Institute for Clinical Evaluative Sciences
Respirologist, Sunnybrook Health Sciences Centre
Assistant Professor, Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto
Mita Giacomini, BSc, MPH, MA, PhD
Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Ron Goeree, BA, MA
Director, PATH Research Institute, St. Joseph’s Hospital (Hamilton)
Associate Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Roger Goldstein, MBCHB, FRCP(C), FRCP(UK)
NSA Chair in Respiratory Rehabilitation Research
Director, Respiratory Services, and Senior Scientist, West Park Healthcare Centre
Professor, Medicine and Physical Therapy, University of Toronto
Alan G Kaplan, MD, CCFP(EM), FCFP
Chairperson, Family Physician Airways Group of Canada
Chairperson, Special Interest Focused Care Group in Respiratory Medicine, College of Family Physicians of Canada
Clinical Lecturer, Department of Family and Community Medicine, University of Toronto
DE O’Donnell, MD, FRCP(C)
Director, COPD Centre, Kingston General Hospital
Professor, Department of Medicine, Queen’s University
Asad Razzaque, MD
Family Physician
Holger Schünemann, MD, PhD, MSc, FRCP(C)
Michael Gent Chair in Healthcare Research
Chair, Department of Clinical Epidemiology & Biostatistics, McMaster University
Professor, Department of Clinical Epidemiology & Biostatistics and Medicine, McMaster University
Tasnim Sinuff, MD, PhD, FRCP(C)
Clinician Scientist, Sunnybrook Health Sciences Centre
Assistant Professor, Department of Medicine, University of Toronto
Laura Watling, RRT, BSc(HK)
Clinical Practice Leader/Clinical Coordinator, Respiratory Therapy, West Park Healthcare Centre
Appendices
Appendix 1: Literature Search Strategies
Initial Literature Search on Pulmonary Rehabilitation for COPD
Search date: August 10, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1950 to July Week 4 2010>
Search Strategy:
--------------------------------------------------------------------------------
exp Pulmonary Disease, Chronic Obstructive/ (14057)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (20996)
(copd or coad).ti,ab. (15985)
chronic airflow obstruction.ti,ab. (486)
exp Emphysema/ (6925)
((chronic adj2 bronchitis) or emphysema).ti,ab. (22569)
or/1-6 (53015)
exp Rehabilitation/ (120272)
exp Physical Therapy Modalities/ (98967)
((pulmonary or lung* or respirat*) adj2 (physiotherap* or therap* or rehabilitat*)).ti,ab. (8251)
rh.fs. (135769)
or/8-11 (297725)
7 and 12 (3342)
limit 13 to (english language and humans and yr=“2004 - 2010”) (1206)
limit 14 to (case reports or comment or editorial or letter) (124)
14 not 15 (1082)
Database: EMBASE <1980 to 2010 Week 31>
Search Strategy:
--------------------------------------------------------------------------------
exp chronic obstructive lung disease/ (47119)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (25414)
(copd or coad).ti,ab. (20656)
chronic airflow obstruction.ti,ab. (548)
exp emphysema/ (25316)
exp chronic bronchitis/ (6517)
((chronic adj2 bronchitis) or emphysema).ti,ab. (25290)
or/1-7 (86799)
exp pulmonary rehabilitation/ (993)
exp physical medicine/ (288069)
((pulmonary or lung* or respirat*) adj2 (physiotherap* or therap* or rehabilitat*)).ti,ab. (9992)
rh.fs. (108684)
or/9-12 (387063)
8 and 13 (5140)
limit 14 to (human and english language and yr=“2004 -Current”) (1758)
limit 15 to (editorial or letter or note) (195)
15 not 16 (1563)
case report/ (1665922)
17 not 18 (1459)
| # | Query | Results |
|---|---|---|
| S13 | S12 Limiters - Published Date from: 20040101-20101231 |
546 |
| S12 | S6 and S11 | 942 |
| S11 | (S7 or S8 or S9 or S10) | 54560 |
| S10 | pulmonary rehabilitat* or pulmonary therap* or lung rehabilitat* or respiratory therap* | 2776 |
| S9 | (MH “Home Rehabilitation+”) | 945 |
| S8 | (MH “Physical Therapy+”) | 50558 |
| S7 | (MH “Rehabilitation, Pulmonary+”) | 1644 |
| S6 | S1 or S2 or S3 or S4 or S5 | 7306 |
| S5 | chronic bronchitis or emphysema | 1562 |
| S4 | (MH “Emphysema+”) | 954 |
| S3 | copd or coad | 4032 |
| S2 | (chronic obstructive and (lung* or pulmonary or airway* or airflow or respiratory) and (disease* or disorder*)) | 5524 |
| S1 | (MH “Pulmonary Disease, Chronic Obstructive+”) | 4266 |
Final Revised Search for COPD-Rehabilitation Revised to Include 2000-2011
Search date: February 3, 2011
Databases Searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1948 to January Week 3 2011>
Search Strategy:
--------------------------------------------------------------------------------
exp Pulmonary Disease, Chronic Obstructive/ (14676)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (21256)
(copd or coad).ti,ab. (16373)
chronic airflow obstruction.ti,ab. (478)
exp Emphysema/ (9400)
((chronic adj2 bronchitis) or emphysema).ti,ab. (22372)
or/1-6 (54975)
exp Rehabilitation/ (120569)
exp Physical Therapy Modalities/ (99270)
((pulmonary or lung* or respirat*) adj2 (physiotherap* or therap* or rehabilitat*)).ti,ab. (8341)
rh.fs. (135921)
or/8-11 (298194)
7 and 12 (3404)
limit 13 to (english language and humans and yr=”2000 -Current”) (1730)
limit 14 to (case reports or comment or editorial or letter) (164)
14 not 15 (1566)
Database: EMBASE <1980 to 2011 Week 04>
Search Strategy:
--------------------------------------------------------------------------------
exp chronic obstructive lung disease/ (49584)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (26878)
(copd or coad).ti,ab. (22292)
chronic airflow obstruction.ti,ab. (553)
exp emphysema/ (25972)
exp chronic bronchitis/ (6645)
((chronic adj2 bronchitis) or emphysema).ti,ab. (25749)
or/1-7 (90366)
exp pulmonary rehabilitation/ (1194)
exp physical medicine/ (300551)
((pulmonary or lung* or respirat*) adj2 (physiotherap* or therap* or rehabilitat*)).ti,ab. (10482)
rh.fs. (110909)
or/9-12 (401639)
8 and 13 (5385)
limit 14 to (human and english language and yr=”2000 -Current”) (2478)
CINAHL
| # | Query | Results |
|---|---|---|
| S12 | S6 AND S11 Limiters - Published Date from: 20000101-20111231 English Language | 787 |
| S11 | S7 or S8 or S9 or S10 | 60613 |
| S10 | pulmonary rehabilitat* or pulmonary therap* or lung rehabilitat* or respiratory therap* | 7241 |
| S9 | (MH “Home Rehabilitation+”) | 976 |
| S8 | (MH “Physical Therapy+”) | 52603 |
| S7 | (MH “Rehabilitation, Pulmonary+”) | 1682 |
| S6 | S1 or S2 or S3 or S4 or S5 | 7702 |
| S5 | chronic bronchitis or emphysema | 1623 |
| S4 | (MH “Emphysema+”) | 994 |
| S3 | copd or coad | 4205 |
| S2 | (chronic obstructive and (lung* or pulmonary or airway* or airflow or respiratory) and (disease* or disorder*)) | 5860 |
| S1 | (MH “Pulmonary Disease, Chronic Obstructive+”) | 4568 |
Appendix 2: Study Characteristics of Included Studies
Table A1: Description of Studies Examining Pulmonary Rehabilitation in the Stable COPD Population* (n = 18).
| Study | Population | Setting | Groups | Delivered By | Length of Intervention | Description | Follow-up | Outcomes of Interest | Other Outcomes | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Theander et al (23) 2009 weden |
N = 30 Rehab: 15 Control: 15 Subject characteristics: ~65 years FEV1 % predicted ~33 BMI ~25 Optimized on pharmacological treatment prior to study initiation |
Pulmonary outpatient department Optimized on pharmacological treatment prior to study initiation |
Control group Rehab group Control group did not receive any of the rehab program or care from professionals who performed the program |
Physiotherapist, dietician, occupational therapist, nurse | 12 weeks physiotherapy 1 hr, 2–5 days/week dietician/ occupational therapist 1 hr, 3x during program |
Multi-disciplinary Aerobic training, strength training; after 1 month, pts received an individualized home training program Dietary education/ advice, self-care, smoking cessation advice |
12 weeks from baseline | QOL (SGRQ) Functional exercise capacity (6MWT) |
Fatigue; fatigue- related functional limitations; functional performance and satisfaction; lung function; grip strength | Not adequately powered; significant difference in gender distribution between groups; no blinding in outcome assessment; calculated sample size based on CRQ but used SGRQ; Dropout: 3/15 in rehab group, 1/15 in control group |
| Elci et al (13) 2008 Turkey |
N = 78 Rehab: 39 Control: 39 Subject characteristics: ~59 years ~85% male FEV1 % predicted ~47 ~51% GOLD stage III |
Outpatient department of community hospital Lacked specialist pulmonary rehab services |
Control group Rehab group Control group received standard medical care including instructions on the use of respiratory medicines |
Nurses Received training in the pulmonary rehab program 2 weeks prior 1 session supervised by nurse (the remaining sessions supervised by a family member) Standard telephone questionnaire once weekly |
12 weeks | Educational activities aimed at improving self-management skills Individualized rehab plan Exercises at home: 24 sessions of up to 90 min 2x/week Endurance training: abdominal, upper and lower limb muscle strengthening |
4, 8, 12 weeks from baseline | QOL (SGRQ) Functional exercise capacity (6MWT) |
Lung function; anxiety and depression; functional dyspnea No difference in lung function between control and rehab groups |
Outcome assessors not blinded; unclear allocation concealment; no sample size calculation; no mention of droupouts Emailed author for individual data from SGRQ, 4- week data assumed to be baseline (according to author); potential underestimation of effect |
| Karapolat etal (20) 2007 Turkey |
N = 49 Rehab: 27 Control: 22 Subject characteristics: ~65 years ~87% male FEV1% predicted ~72, ~57% GOLD stage II |
Outpatient program | Control group Rehab group |
Physiotherapist | 8 weeks | Educational component (1hour session weekly for 16 weeks): respiratory physiology, disease education, dietary advice, relaxation, etc. Exercise training component (3x/week): aerobic, strength training, breathing and relaxation, etc. |
8 and 12 weeks from baseline | QOL (SGRQ) Functional exercise capacity (6MWT) |
Lung function; arterial blood gas analysis; dyspnea | Outcome assessors not blinded; unclear allocation concealment; no sample size calculation; no mention of dropouts Emailed author for individual data from SGRQ, 4-week data assumed to be baseline (according to author); potential underestimation of effect |
| Guell et al(18) 2000 Spain |
N = 60 Rehab: 30 Control: 30 Subject characteristics: ~65 years ~100% male FEV1 % predicted ~35 |
Outpatient clinic of hospital | Control group Rehab group |
Unclear | 6 months | Breathing retraining: chest wall exercises, abdominal exercises, etc. Exercise training: aerobic, home exercise Maintenance group sessions: breathing exercises, no formal exercise program |
3, 6, 9, 12, 18, and 24 months from baseline | QOL (CRQ) Functional exercise capacity (6MWT) Maximal exercise capacity |
Lung function; dyspnea; breathlessness; exacerbations; hospitalizations | Generalizable to men only; no allocation concealment; formal exercise component began after 3 months |
| Engstrom etal (14) 1999 Sweden |
N = 55 Rehab: 28 Control: 27 Subject characteristics: ~ 66 years ~52% male FEV1% predicted: Rehab, 35.8 (11.9) Control, 34.1 (10.2) |
Outpatient | Usual outpatient care Rehabilitation program |
Physiotherapist, occupational therapist, dietician Information program (self-care, smoking cessation): COPD outpatient team (respiratory nurse, physician) |
12 months 45-min sessions 2x/week for 6 weeks; 1x every 2nd week for 6 weeks; 1x/month thereafter |
Physiotherapy program: breathing techniques, aerobic exercise, arm training, muscle strength training, instruction to walk daily, individual 30-min home training program, education | 12 months from baseline | QOL (SGRQ) Functional exercise capacity (6MWT) Days in hospital |
Sickness impact profile; Mood Adjective Check List; maximum symptoms; limited incremental exercise test | Unclear randomization and allocation concealment; blinded outcome assessors with the exception of the 6MWT; no sample size calculation |
| Ringbaek etal (22) 2000 Denmark |
N = 45 Rehab: 24 Control: 21 Subject characteristics: ~ 63 years ~15% male FEV1% predicted: Rehab: 49.5 (17.4) Control: 44.3 (13.7) |
Outpatient | Control group Rehabilitation program |
Physiotherapist, nutritional therapist, occupational therapist Patient education: physician and nurse |
8 weeks 2-hour sessions 2x/week |
Aerobic and strength training; dietary counselling; relaxation; breathing techniques; patient education; nutritional counselling | 8 weeks from baseline | QOL (SGRQ) Functional exercise capacity (6MWT) |
Psychological general well-being index; Borg Dyspnea Score | Unclear randomization and allocation concealment; no sample size calculation; high dropout rate |
| Borghi-Silvaet al (11) 2009 |
N = 40 Rehab: 20 Control: 20 Subject characteristics: ~ 67 years ~74% male FEV1% predicted: Treatment: 64 (16.0) Control: 64 (18) Moderate to severe COPD |
Outpatient | Usual care Supervised aerobic training program *All pts received regular treatment consisting of inhaled bronchodilators and steroids. Patients in UC received no physical training |
Physiotherapist, physical therapist | 6 weeks 3x/week for 6 weeks |
Supervised program with: stretching of lower and upper limbs and treadmill ambulation (30 min); stretching exercises (back, hamstrings, shoulders, neck, etc.); breathing exercises | 6 weeks from baseline | Functional exercise capacity (6MWT) | Borg Dyspnea Score; lung function; cardio-pulmonary exercise testing | Unclear randomization and allocation concealment; no sample size calculation; high dropout in control group |
| Singh (26) 2002 India |
N = 40 Rehab: 20 Control: 20 Subject Characteristics: ~ 59 years ~80% male FEV1% predicted: Treatment: 28 (7.5) Control: 26 (7.1) Severe airway obstruction |
Outpatient | Pulmonary rehab Usual care Usual care patients were asked to continue their activities as usual |
Not reported Supervised weekly |
4 weeks 30 min 2x /day |
Removal of secretions; lower extremity exercises (walking 2x/day); breathing strategies; energy conservation and work simplification | 4 weeks from baseline | Functional exercise capacity (6MWT) QOL (CRQ) |
FEV1 | Unclear randomization and allocation concealment; no sample size calculation; no mention of blinding |
| Lake et al (21) 1990 Australia |
N = 28 Treatment: 20 Control: 8 Subject characteristics: ~ 66 years ~85% male FEV1: Treatment 1: 0.97 (±0.29) Treatment 2: 0.73 (±0.24) Treatment 3: 0.83 (±0.25) Control: 0.97 (±0.29) FEV1 % predicted < 55 |
Outpatient | Training group 1 (upper limb) Training group 2 (lower limb) Training group 3 (combined) Control group Control group offered active program at end of study |
Supervised by physiotherapist | 8 weeks 1 hour 3x/week |
Upper limb group: 10-min warm-up, 20-min circuit training, 10-min cool-off Lower-limb group: 10-min warm-up, 20-min walking, and 10-min cool-off Combined group:10-min warm-up, 15-min circuit training, 12-min walking, and 10-min cool-off |
8 weeks from baseline | Functional exercise capacity (6MWT) QOL (Self-Efficacy Scale) |
FEV1; FVC; maximal exercise tolerance | Unclear randomization and allocation concealment; no sample size calculation; no mention of blinding; population may not be representative of COPD |
| Goldstein etal (16) 1994 Canada |
N = 89 Rehab: 45 Control: 44 Subject characteristics: ~ 66 years ~49 % male FEV1% predicted: Treatment: 34.8(14.5); Control: 34.6 (11.8) Severe stable COPD FEV1 < 40% |
Inpatient rehab followed by outpatient care | Conventional community care Rehabilitation Conventional care group received care from their family doctors and respiratory specialists |
Multidisciplinary, medically supervised team | 24 weeks Inpatient: 8 weeks, ~3x/week Outpatient care: 16 weeks; home training routine; graduated discharge program supervised by a member of rehab staff; periodic visits by a home care physiotherapist) |
Stretching; breathing techniques; interval training (40 min 3x/week); treadmill (2–3 x/week); upper-extremity training; leisure walking (30 min 1x/week) | 12, 18, and 24 weeks from baseline | Functional exercise capacity (6MWT) QOL (CRQ) Incremental exercise capacity |
Pulmonary function | Unclear randomization and allocation concealment; no sample size calculation; no mention of blinding; high dropout rate |
| Simpson and Rocker (25) 1992 Canada |
N = 34 Rehab: 17 Control: 17 Subject characteristics: ~ 72 years ~54% male FEV1% predicted: Treatment: 39.5(18.96); Control: 39.2 (21.39) |
Community-based, local physiotherapy practices | Control Rehabilitation |
Physiotherapist | 8 weeks 3x/week |
Weightlifting program: arm curls, leg extensions, leg press exercises, resistance training | 8 weeks from baseline | QOL (CRQ) Functional exercise capacity (6MWT) |
Borg Dyspnea Scale | Unclear allocation concealment; no sample size calculation; no ITT |
| Wijkstra etal (27) 1994 Netherlands |
N = 45 Rehab: 30 Control: 15 Subject characteristics: FEV1% pred: Treatment: 34.8 (14.5) Control: 34.6 (11.8) Severe airflow limitation: FEV1% pred < 60% |
Community-based (home-based program) | Control Rehabilitation |
Supervised by multidisciplinary team: physiotherapist, nurse, pulmonologist, family doctor |
12 weeks 2x/week visit to physiotherapist |
Conventional physiotherapy: relaxation exercises, breathing retraining, upper limb training, exercise training (1 hr/day) Exercise training: 30 min 2x/day according to an individual protocol Patient education: supervised monthly by nurse Supervised clinical status: monthly GP visits |
12 weeks after rehab | QOL (CRQ) Cycle ergometer test |
FEV1, IVC | Unclear randomization and allocation concealment; no sample size calculation; no mention of blinding |
| Boxall et al (12) 2005 Australia |
N = 60 Rehab: 30 Control: 30 Subject Characteristics ~ 76 years ~56% male FEV1% predicted: Treatment: 40.5 (15.9) Control: 37.7 (15.0) House-bound elderly COPD patients > 60 years |
Community-based (home-based program) | Control Rehabilitation Control group offered program after initial 12 weeks |
Nurse, occupational therapist, physiotherapist | 12 weeks 11 total home visits |
Graduated walking and arm exercises (1x daily): resistance; strengthening upper limb muscles used for respiration; exercise diaries; weekly physiotherapy visits for the first 6 weeks, then every 2 weeks until end of program; education sessions (~6 total) | 12 weeks from baseline | QOL (SGRQ) Exercise tolerance (6MWT) |
Dyspnea; hospital admission rates with exacerbation of COPD; average length of stay at readmission | No blinding of outcome assessors; sample size was calculated but study was underpowered due to dropouts |
| Hernandezet al (19) 2000 Spain |
N = 60 Rehab: 30 Control: 30 Subject Characteristics: ~ 64.3 years FEV1% predicted: Treatment: 41.7(15.6) Control: 40 (16.4) All patients were medically optimized |
Community-based (home-based program) | Control Rehabilitation Control: standard medical treatment alone, but visited hospital every 2 weeks for a clinical checkup and supervision of treatment |
Unclear | 12 weeks 1 hour sessions, 6 days/week |
Lower extremity training, walking Patients also went to hospital every 2 weeks for supervision of clinical status, treatment, and exercise-training compliance |
12 weeks from baseline | QOL (CRQ) Exercise capacity (SWT) |
Pulmonary function Resistance Test Dyspnea |
Unclear randomization and allocation concealment; no sample size calculation; no/unclear ITT; high dropout rate; unclear description of who delivered rehabilitation |
| Griffiths etal (17) 2000 United Kingdom |
N = 200 Rehab: 99 Control: 101 Subject characteristics: ~ 68 years 60 % male FEV1% predicted: Treatment: 39.7 (16.2) Control: 39.4 (16.4) |
Outpatient | Control Rehabilitation Control: outpatients or primary care patients followed for 1 year and then offered pulmonary rehab |
Multidisciplinary: occupational therapists, physiotherapists, dieticians, respiratory nurse, smoking cessation counsellor | 6 weeks 3 half-days/week, 2-hour sessions |
1/3 time: educational activities: understanding of disease, nutrition, medicines, exercise Individualized sessions; aerobic training, circuit training Patients encouraged to follow a home exercise routine Psychological support and education At end of rehab program, patients were invited to join a patient-run group that met weekly at a local recreation centre for social activities and exercise |
6 weeks and 1 year from baseline | QOL (SGRQ and CRQ) Exercise capacity (SWT) |
Health status (SF-36); hospital anxiety and depression score; number of admissions and days spent in hospital | Well-described randomization and allocation concealment; ITT analysis completed; sample size calculated |
| Troosters etal (24) 2000 Belgium |
N = 100 Rehab: 50 Control: 50 Subject characteristics: ~ 62 years ~85 % male FEV1 % predicted: Treatment: 41 (16) Control: 43 (12) Severe COPD |
Outpatient | Control Rehabilitation |
Supervised by physiotherapists | 6 weeks Outpatient sessions: 1.5 hours 3x/week in first 3 months, 2x/week in subsequent 3 months |
Training: aerobic (cycling, treadmill, etc.) and muscle strength training | 6 and 18 months from baseline | QOL (CRQ) Functional exercise capacity (6MWT) |
Maximal exercise capacity | Unclear randomization and allocation concealment; no sample size calculation; no/unclear ITT |
| Finnerty etal (15) 2001 |
N = 100 Rehab: 50 Control: 50 Subject characteristics: ~ 69 years ~68% male FEV1% predicted: Rehab: 41.2 (19.2) Control: 41.2 (16.2) |
Outpatient | Routine outpatient attendance Rehabilitation program |
Physiotherapist, occupational therapist, respiratory specialist nurse, dietician | 6 weeks | 2 visits/week: 2-houreducation visit and 1-hour exercise (aerobic) visit Patients asked to exercise 1 to 2x daily 5x/week Dietary advice, referrals to social services, coping strategies, psychological input |
12 and 24 weeks from baseline | QOL (SGRQ) Functional exercise capacity (6MWT) |
None | Blinded outcome assessors; no sample size calculation; outcome assessed 6 weeks after program completed |
| Behnke etal, 2000 (9) | N = 46 Rehab: 15 Control: 15 Baseline characteristics given for 30 patients who completed the study Subject characteristics: ~66 years 77% male FEV1% predicted: Treatment: 34.1±7.4 Control: 37.5 ±6.6 |
Inpatient pulmonary rehab and home-based program | Control group Training group Control group: standard in-patient care with no exercise and standard community care with respirologist |
Unclear Investigators visited pts every 2 weeks for the first 3 months, then monthly telephone contact |
10 day hospital-based program and 6-month home-based program after discharge Started 4–7 days after admission |
10-day hospital-based walking program (5 walking sessions/day for 10 days) + supervised home-based training program (3 walking sessions/day for 6 months) Monthly patient diaries |
1, 2, 3, and 6 months after discharge | Lung function QOL (CRQ) Functional Exercise capacity (6MWT) Dyspnea scores Hospital readmission |
Unclear randomization; allocation concealment; no ITT; high dropout rate; primary outcome not identified; no sample size calculation | |
| Behnke etal, 2003 (10) | N = 3026 analyzed | 12 month follow-up | Follow-up at 12 months on only 26/46 original patients |
Abbreviations: 6MWT, Six Minute Walking Test; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; hr, hour; min, minutes; ITT, intention-to-treat analysis; IVC, inspiratory vital capacity; N, sample size; QOL, quality of life; pts, patients; rehab, rehabilitation; SGRQ, St. George’s Respiratory Questionnaire; SF-36, Short form 36; SWT, Shuttle Walking Test; UC, usual care.
Table A2: Description of Studies Examining Pulmonary Rehabilitation Within One Month of an Acute Exacerbation of COPD (n = 4 Plus Behnke et al, 2000).
| Study | Population | Setting | Groups | Delivered By | Length of Intervention | Description | Follow-up | Outcomes of Interest | Other Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Man et al (36) 2004 United Kingdom |
N = 42 Rehab: 21 Control: 21 Subject characteristics: ~70 years ~41% male FEV1 %predicted ~39% after inpatient treatment for acute exacerbation |
Outpatient | Control group Rehab group Control: standard community care with respirologist |
Multidisciplinary team: physiotherapists, respiratory nurses, occupational therapist, dietician, respiratory doctor, smoking cessation adviser, social worker, pharmacist, lay member of a patients’ group | 8 weeks | Outpatient PR (within 10 days of discharge) Aerobic and strength exercise and patient education for 12 weeks (two 2-hour sessions/week) |
12 weeks | Incremental shuttle walk distance (SGRQ, CRQ, and SF-36) | Hospital readmission; hospital days; emergency admissions; mortality |
| Murphy et al (34) 2005 Ireland |
N = 31 Rehab: 16 Control: 15 Subject characteristics: ~66 years ~65% male Moderate COPDFEV1% predicted < 60% Long history of smoking |
Supervised home exercise program Admitted to a COPD home-from-hospital treatment program |
Control group Rehab group Control group: Standard medical treatment without any form of rehabilitation exercises or lifestyle change advice |
Physiotherapist | 6 weeks | 2x/week for 6 weeks 12 supervised exercise sessions (30–40 min each) Patients were instructed to exercise for at least 15 min on other days Use of diaries Aerobic exercise, upper limb exercises |
6 weeks from baseline 3-month follow-up for exacerbations |
Functional exercise capacity (shuttle walk test, 3-min step test) | Dyspnea QOL: SGRQ admissions exacerbations |
| Eaton et al (32) 2009 |
N = 97 Rehab: 47 Control: 50 Subject characteristics: ~70 years ~44% male FEV1% predicted ~35 Elderly Severe impairment of pulmonary function, poor HRQOL, and high COPD-related morbidity |
Inpatient followed by outpatient Mean of 2.6 days after admission |
Usual Care Rehabilitation program Usual care: received standardized advice on benefits of exercise; COPD nurse administered standardized care |
Inpatient program: COPD nurse Outpatient education: multidisciplinary |
~8 weeks + inpatient program | Inpatient program: structured, supervised exercise regimen (at least 30 min/day) with aerobic and upper/lower limb strengthening Outpatient program:1-hour sessions of supervised exercise training 2x/week for 8 weeks Educational sessions At end of program, prescribed 30 min daily activity with government-funded opportunity to attend local gym |
3 months from baseline | No. COPD-related readmissions; time to first COPD-related readmission; no. inpatient days; unscheduled ED visits | BMI, airflow obstruction, dyspnea, exercise capacity, functional capacity (6MWT), and HRQOL (CRQ) |
| Seymour et al (35) 2010 |
N = 60 Rehab: 30 Control: 30 Subject characteristics: ~ 66 years ~45% male FEV1 %predicted ~52 |
Outpatient Initiated within 1 week after discharge |
Usual Care Rehabilitation program UC and rehab arms offered general information about COPD prior to randomization and outpatient appointments with patients’ family doctor or respiratory team |
2 physiotherapists | 8 weeks | 2x /week exercise and education sessions (each lasting 2 hours) for 8 weeks Individually tailored aerobic and limb strengthening |
3 months after admission | Primary outcome: hospital admission for exacerbation Hospital or emergency department attendance for exacerbation |
Quadriceps strength; exercise capacity (paced incremental and endurance shuttle walking tests); fatigue; HRQOL(SGRQ, CRQ) |
Abbreviations: 6MWT, 6 Minute Walking Test; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; Ctrl, control; ED, emergency department; FEV1, forced expiratory volume in 1 second; HRQOL, health-related quality of life; min, minutes; N, sample size; no., number; PR, pulmonary rehabilitation; rehab, rehabilitation; SF-36, Short form 36, SGRQ, St. George’s Respiratory Questionnaire; UC, usual care.
Table A3: Description of Studies Examining Pulmonary Rehabilitation Maintenance Programs (n = 3)*.
| Study | Population | Setting | Groups | Delivered By | Length/ Description of Initial PR |
Length of Maintenance Program | Description | Follow-up | Outcomes of Interest |
|---|---|---|---|---|---|---|---|---|---|
| Ringbaek et al (38) 2010 |
N = 96 MT: 55 Control: 41 Subject characteristics: ~ 68 years ~33% male FEV1% predicted: Treatment: 35.6 (14.0) Control: 36.9 (16.0) Stable COPD Excluded: patients with significant cardiac, musculoskeletal, or cognitive problems |
Outpatient | Maintenance training Control group Both groups requested to continue |
No mention | 7-week outpatient program Supervised walking and cycling 2x/week combined with unsupervised daily training at home Educational sessions 1x/week Patients instructed to continue the unsupervised training at home after program end |
1 year supervised training (every week in first 6 months and every second week in next 6 months) + 6 months unsupervised | Supervised training sessions (assumed to be similar to initial rehab) | Prior to rehab, at randomization, 3, 6, 12,18 months after randomization | Functional exercise capacity (SWT) QOL (SGRQ) Other outcomes: adherence to supervised training, dropout rates, hospitalization |
| Spencer et al (39) 2010 |
N = 48 MT: 24 Control: 24 Subject characteristics: ~ 67 years ~46% male FEV1% predicted: Treatment: 51 ±11 Control: 54 ±11 Moderate COPD Excluded: cardiovascular, neurological, musculoskeletal comorbidities; exacerbation in previous month |
Outpatient | Intervention group Control group Control group performed unsupervised home exercise 5 days/ week and received home exercise booklet and diary |
No mention | 8-week program 20 min walking, 20 min cycling, 10 min arm cycling and upper and lower limb strength training exercises using weight equipment and free weights |
1 year | Supervised exercise 1 day/week plus unsupervised home exercise on 4 other days Supervised exercise same as initial rehab program Unsupervised home exercise: 30 min walking + 30 min upper and lower limb strengthening exercises using free weights and body weight; included exercise booklet and diary |
3, 6, and 12 months following pulmonary rehab | Functional exercise capacity (6MWT) QOL (SGRQ) Other outcomes: lung function; incremental shuttle walk test; hospital anxiety and depression scale; hospital admission; length of stay; exacerbations |
| Berry et al (37) 2003 |
N = 140 MT: 70 Control: 70 Subject characteristics: ~ 67 years ~56% male FEV1% predicted: Treatment: 57.6 (53.2–62.0) Control: 59.1 (55.0–63.2) Excluded: cardiac and peripheral vascular disease, concurrent cancer treatment, uncontrolled diabetes or hypertension |
Community and university centre | Long-term intervention Short-term intervention (control group) Control group: All participants completed a 3-month pulmonary rehabilitation program prior to randomization Patients in control group were encouraged to continue exercising on their own |
Not reported | 3-month supervised centre-based program Exercise sessions with both aerobic and upper-extremity resistance training: included walking, biceps curls, triceps extension, shoulder exercises Sessions were 3x weekly for 1 hour |
15 months | After initial 3-month rehab, and 9, 15, and 18 months from this point | Functional exercise capacity (6MWT) Other outcomes: physical disability; physical function (stairs climbed); peak oxygen uptake; pulmonary function; physical activity scale; compliance; training intensity |
Abbreviations: 6MWT, 6 Minute Walking Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; min, minutes; MT, maintenance training; N, sample size; PR, pulmonary rehabilitation; QOL, quality of life; rehab, rehabilitation; SGRQ, St. George’s Respiratory Questionnaire; SWT, Shuttle Walking Test.
Appendix 3: Methodological Quality and GRADE Profile of Studies
Table A4: Methodological Quality of Studies Examining Stable COPD* (n = 18).
| Study | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding of Outcome Assessors for Primary Outcome | Sample Size Calculation | Losses to Follow-up | ITT |
|---|---|---|---|---|---|---|---|---|
| Theander et al, 2009 (23) | 30 | ? | ✓ | ✓ | No | ✓† | 21% T: 25%; C: 16.5% |
No |
| Elci et al, 2008 (13) | ✓ | ✓ | Unclear | No | X | NR | NR | |
| Karapolat et al, 2007 (20) | 49 | ? | ✓ | ✓ | NR | X | 6% patients | No |
| Borghi-Silva et al, 2009 (11) | 40 | Unclear | ✓ | Unclear | NR | ✓║ | 15% T:0%; C: 30% |
No |
| Guell et al, 2000 (18) | 60 | Unclear | ✓ | no | Yes | X | None at end of program | No |
| Finnerty et al, 2001 (15) | 100 | ✓ | ✓ | Unclear | Yes | X | 27% T: 20%; C: 34% |
No |
| Engstrom et al, 1999 (14) | 55 | Unclear | ✓ | Unclear | Yes‡ | X | 9% T: 7%; C: 11% |
No |
| Ringbaek et al, 2000 (22) | 45 | Unclear | X§ | Unclear | NR | X | 16% T: 29%; C: 0% |
No |
| Wijkstra et al, 1994 (27) | 45 | Unclear | ✓ | Unclear | NR | X | 4.4% T: 6.7%; C: 0% |
No |
| Troosters et al, 2000 (24) | 100 | Unclear | ✓ | Unclear | NR | X | 38% T: 32%; C: 44%* |
No |
| Behnke et al, 2000 (9) | 46 | Unclear | ✓ | ✓ | X | X | 35% T: 35%; C: 35% |
No |
| Boxall et al, 2005 (12) | 60 | ✓ | ✓ | ✓ | X | ✓# | 23% T: 23%; C: 23% |
X |
| Simpson and Rocker, 1992 (25) | 34 | ✓ | ✓ | NR | ✓ | X | 17.6% T: 17.6%; C:17.6 % |
X |
| Lake et al, 1990 (21) | 28 | Unclear | ✓ | NR | NR | X | 7.1% T: 5%; C: 12.5% |
X |
| Griffiths et al, 2000 (17) | 200 | ✓ | ✓ | ✓ | ✓ | ✓ | 10% T: 7%; C: 12.9% |
✓ |
| Singh, 2003 (26) | 40 | Unclear | ✓ | NR | NR | X | NR | ? |
| Hernandez et al, 2000 (19) | 60 | Unclear | ✓ | NR | NR | X | 38.3% T: 33.3%; C: 43.3% |
? |
| Goldstein et al, 1994 (16) | 89 | Unclear | ✓ | NR | NR | ✓¶ | 20.2% T: 15.5%; C: 9% |
? |
Abbreviations: 6 MWT, 6 Minute Walking Test; C, control; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; ITT, intention-to-treat analysis; n, number of studies; N: sample size; NR, not reported; SGRQ, St. Georger’s Respiratory Questionnaire; T, treatment.
Theander et al, 2009 (12) was underpowered. The sample size was calculated based on the CRQ, but the SGRQ was used in the study. Also, the baseline gender distribution between the 2 groups was not comparable.
Engstrom et al, 1999 (13) had all outcomes blinded with the exception of the 6MWT.
Ringbaek et al, 2000 (16) had more females in the control group at baseline and more smokers in the treatment group at baseline.
Borghi-Silva et al, 2009 (11) was underpowered.
Goldstein et al, 1994 (17) was not well-described in terms of power.
Boxall et al, 2005 (9) was underpowered.
Table A5: Methodological Quality of Studies Following an Acute Exacerbation of COPD* (n = 5).
| Study | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding of Outcome Assessors for Primary Outcome | Sample Size Calculation | Losses to Follow-up | ITT |
|---|---|---|---|---|---|---|---|---|
| Behnke et al, 2000 (9) | 46 | Unclear | ✓ | Unclear | Yes | X | 35% | No |
| Man et al, 2004 (36) | 42 | ✓ | ✓ | Unclear | No | ✓ | 19% T: 14%; C: 24% |
✓ |
| Murphy et al, 2005 (34) | 31 | Unclear | ✓ | Yes | Yes | X | 16% T:19%; C: 13% |
No |
| Eaton et al, 2009 (32) | 97 | ✓ | ✓ | Unclear | No | ✓† | 13% T:17%; C: 10% |
✓ |
| Seymour et al, 2010 (35) | 60 | Unclear | ✓ | Unclear | No | ✓ | 18% T:23%; C: 13% |
✓ |
Abbreviations: C, control; COPD, chronic obstructive pulmonary disease; ITT, intention-to-treat analysis; n, number of studies; N, sample size; T, treatment.
Eaton et al, 2009 (28) was underpowered to show a difference in primary outcome.
Table A6: Methodological Quality of Studies Examining Maintenance Programs* (n = 3).
| Study | N | Adequate Randomization Methods | Baseline Comparable | Adequate Allocation Concealment | Blinding of Outcome Assessors for Primary Outcome | Sample Size Calculation | Losses to Follow-up | ITT |
|---|---|---|---|---|---|---|---|---|
| Spencer et al, 2010 (39) | 48 | Unclear | ✓ | Unclear | No | ✓ | 19% T: 23%; C: 14% |
Yes |
| Ringbaek et al, 2010 (38) | 96 | Unclear | ✓† | Unclear | Unclear | X | At 12 months:13% T: 15%: C: 24% |
Unclear |
| At 18 months:29% T: 24%; C: 34% |
||||||||
| Berry et al, 2003 (37) | 14 0 |
Unclear | ✓ | Yes | Yes | X | 16% T:19%; C: 13% |
No |
Abbreviations: C, control; ITT, intention-to-treat analysis; n, number of studies; N, sample size; T, treatment.
Ringbaek et al, 2010 (34): Baseline characteristics comparable with the exception of % of heart diseases: treatment group 41.8% versus 9.8% in the Control arm, P < 0.01.
Table A7: GRADE Quality of Evidence for Studies Examining Stable COPD*.
| Number of Studies | Design | Study Quality | Consistency | Directness | Imprecision | Other Modifying Factors | Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Exercise Capacity – 6MWT | |||||||
| 15 | RCT | Serious limitations† | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
| Outcome: HRQL – CRQ | |||||||
| 8 | RCT | Serious limitations‡ | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
| Outcome: HRQL – SGRQ | |||||||
| 8 | RCT | Serious limitations§ | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
Abbreviations: 6MWT, Six Minute Walking Test; CRQ, chronic respiratory questionnaire; HRQOL, health-related quality of life; n/a, not applicable; RCT, randomized controlled trial; SGRQ, St. George’s Respiratory Questionnaire
Study quality was downgraded for the exercise capacity because of serious limitations in many of the studies including: unknown or inadequate allocation concealment (12 of 15 studies); unclear randomization process based on published trials (12 of 15 studies); unclear whether assessor was blinded (single blind) (11 of15 studies); lack of a priori power calculations (11 of 15 studies); inadequately powered studies based on post hoc sample size calculations (3 of 15 studies), withdrawals/dropouts > 20% (5 of 15 studies); and intention-to-treat (ITT) analysis not used or unknown (15 of 15 studies).
Study quality was downgraded for health-related quality of life (CRQ) because of serious limitations in many of the studies including: unknown or inadequate allocation concealment (12 of 15 studies); unclear randomization process based on published trials (7 of 8 studies); unclear whether assessor was blinded (single blind) (5 of 8 studies); lack of a priori power calculations (6 of 8 studies); withdrawals/dropouts > 20% (3 of 8 studies); and intention-to-treat (ITT) analysis not used or unknown (8 of 8 studies).
Study quality was downgraded for health-related quality of Life (SGRQ) because of serious limitations in many of the studies including: unknown or inadequate allocation concealment (3 of 8 studies); unclear randomization process based on published trials (4 of 8 studies); unclear whether assessor was blinded (single blind) (4 of 8 studies); lack of a priori power calculations (4 of 8 studies); inadequately powered studies based on post hoc sample size calculations (2 of 8 studies); withdrawals/dropouts > 20% (2 of 8 studies); and intention-to-treat (ITT) analysis not used or unknown (8 of 8 studies).
Table A8: GRADE Quality of Evidence for Studies Examining Pulmonary Rehabilitation Following an Acute Exacerbation of COPD*.
| Number of Studies | Design | Study Quality | Consistency | Directness | Imprecision | Other Modifying Factors | Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Hospital Readmissions | |||||||
| 5 | RCT | Serious limitations† | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
| Outcome: HRQL – CRQ | |||||||
| 4 | RCT | Serious limitations‡ | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
| Outcome: HRQL – SGRQ | |||||||
| 3 | RCT | Serious limitations‡ | No serious limitations | No serious limitations | No serious limitations | n/a | Moderate |
Abbreviations: 6MWT, Six Minute Walking Test; CRQ, Chronic Respiratory Questionnaire; HRQOL, health-related quality of life; n/a, not applicable; RCT, randomized controlled trial; SGRQ, St. George’s Respiratory Questionnaire.
Study quality was downgraded for the hospital readmissions outcomes because of serious limitations in many of the studies including: unknown or inadequate allocation concealment (4 of 5 studies); unclear randomization process based on published trials (3 of 5 studies); unclear or no blinded outcome assessors (single blind) (3 of 5 studies); lack of a priori power calculations (2 of 5 studies); inadequately powered studies (1 of 5 studies); withdrawals/dropouts > 20% (1 of 5 studies); and intention-to-treat (ITT) analysis not used (2 of 5 studies).
Study quality was downgraded for the HRQL outcome because of serious limitations in many of the studies including: unknown or inadequate allocation concealment (CRQ, 4 of 4 studies; SGRQ, 2 of 3 studies); unclear randomization process based on published trials (CRQ, 2 of 4 studies; SGRQ, 2 of 3 studies); unclear or no blinded outcome assessors (single blind) (CRQ, 3 of 4 studies; SGRQ, 2 of 3 studies); lack of a priori power calculations (CRQ, 1 of 4 studies; SGRQ, 1 or 3 studies); inadequately powered studies (CRQ, 1 of 4 studies); withdrawals/dropouts > 20% (CRQ, 1 of 4 studies); and intention-to-treat (ITT) analysis not used (CRQ, 1 of 4 studies; SGRQ, 1 of 3 studies).
Table A9: GRADE Quality of Evidence for Studies Examining Pulmonary Rehabilitation Maintenance Programs*.
| Number of Studies | Design | Study Quality | Consistency | Directness | Imprecision | Other Modifying Factors | Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Exercise Capacity – 6MWT | |||||||
| 2 | RCT | Serious limitations† | Serious limitations† | No serious limitations | No serious limitations | n/a | Low |
| Outcome: HRQL – SGRQ | |||||||
| 2 | RCT | Very serious limitations‡ | No serious limitations | No serious limitations | No serious limitations | n/a | Low |
Abbreviations: 6MWT, Six Minute Walking Test; HRQOL, health-related quality of life; n/a, not applicable; RCT, randomized controlled trial; SGRQ, St. George’s Respiratory Questionnaire; WMD, weighted mean difference.
Study quality was downgraded for the exercise capacity outcome because of serious limitations in many of the studies including: unknown or inadequate allocation concealment (1 of 2 studies); unclear randomization process based on published trials (2 of 2 studies); unclear whether assessor was blinded (single blind) (1 of 2 studies); lack of a priori power calculations (1 of 2 studies); inadequately powered studies (2 of 2 studies); and intention-to-treat (ITT) analysis not used (1 of 2 studies). The study quality was also downgraded because of inconsistent findings for the WMD of the 6MWT.
Study quality was downgraded for the HRQOL outcome because of very serious limitations in many of the studies including: unknown or inadequate allocation concealment (2 of 2 studies); unclear randomization process based on published trials (2 of 2 studies); unclear whether assessor was blinded (single blind) (1 of 2 studies); lack of a priori power calculations (1 of 2 studies); and intention-to-treat (ITT) analysis not used (1 of 2 studies). The study quality was also downgraded because of missing data reported for the effect size and associated P values.
Appendix 4: Forest Plots
Studies of Stable COPD
Quality of Life (St. George’s Respiratory Questionnaire)
Figure A1: Forest Plot of Pooled Quality of Life Data Measured by the Total Score of the St. George’s Respiratory Questionnaire.
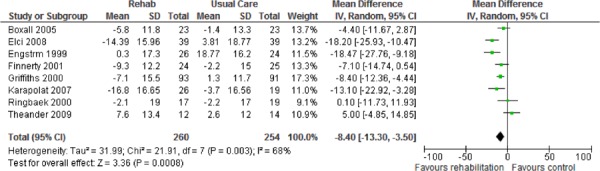
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Figure A2: Forest Plot of Pooled Quality of Life Data Measured by the Symptom Score of the St. George’s Respiratory Questionnaire.
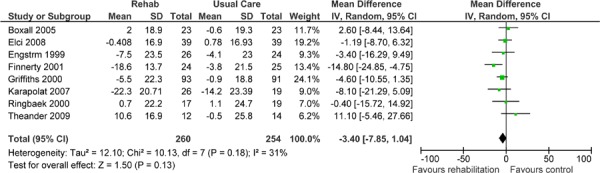
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Figure A3: Forest Plot of Pooled Quality of Life Data Measured by the Impacts Score of the St. George’s Respiratory Questionnaire.
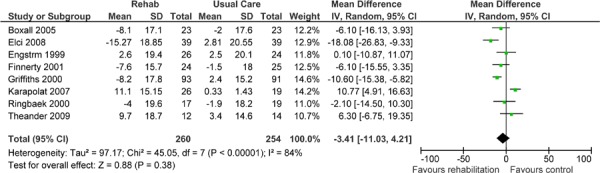
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Figure A4: Forest Plot of Pooled Quality of Life Data Measured by the Activity Score of the St. George’s Respiratory Questionnaire.
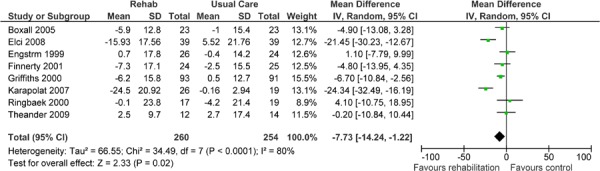
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Quality of Life (Chronic Respiratory Questionnaire)
Figure A5: Forest Plot of Pooled Quality of Life Data Measured by the Mastery Score of the Chronic Respiratory Questionnaire.
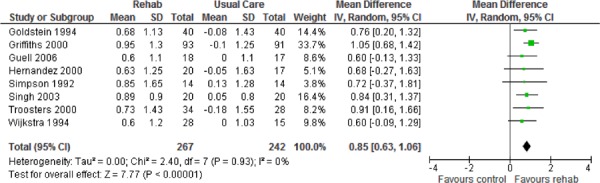
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Figure A6: Forest Plot of Pooled Quality of Life Data Measured by the Emotional Function Score of the Chronic Respiratory Questionnaire.
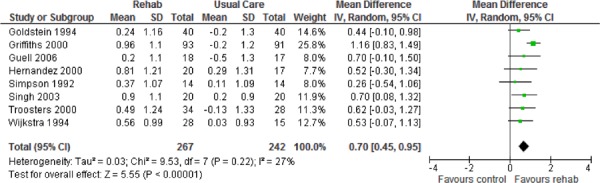
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Figure A7: Forest Plot of Pooled Quality of Life Data Measured by the Dyspnea Score of the Chronic Respiratory Questionnaire.
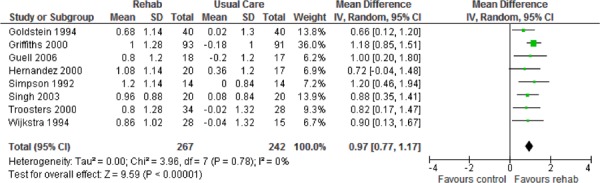
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Figure A8: Forest Plot of Pooled Quality of Life Data Measured by the Fatigue Score of the Chronic Respiratory Questionnaire.
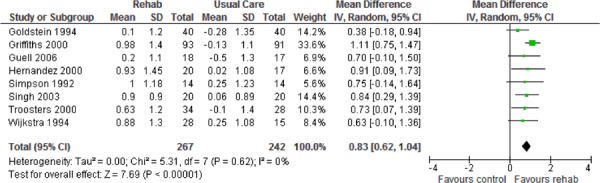
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Functional Exercise Capacity (6 Minute Walking Test)
Figure A9: Forest Plot of Pooled Data on Functional Exercise Capacity Measured by the Six Minute Walking Test.
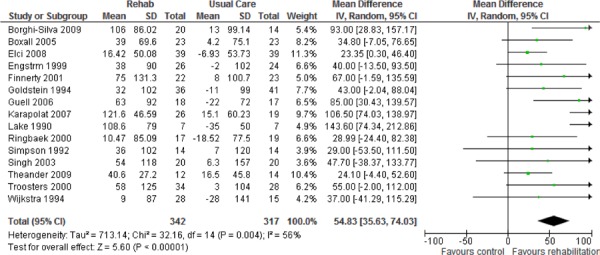
Abbreviations: CI, confidence interval; rehab, rehabilitation; SD, standard deviation.
Studies Examining Pulmonary Rehabilitation Within One Month of an Acute Exacerbation
Hospital Readmissions
Figure A10: Forest Plot of Pooled Data on Hospital Readmissions Subgrouped by Type of Readmission.
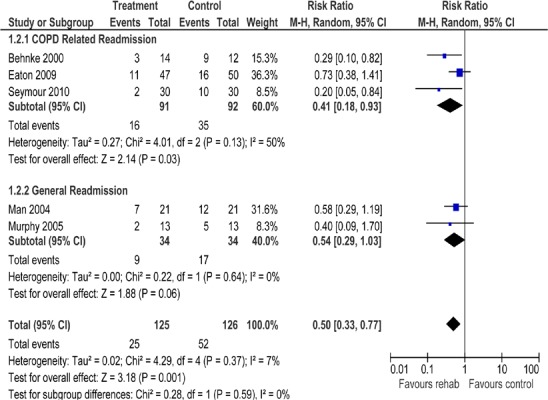
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; M–H, Mantel–Haenszel.
Health-Related Quality of Life (Chronic Respiratory Questionnaire)
Figure A11: Forest Plot of Pooled Data on Health-Related Quality of Life as Measured by the CRQ, Subgrouped by Components of the CRQ.
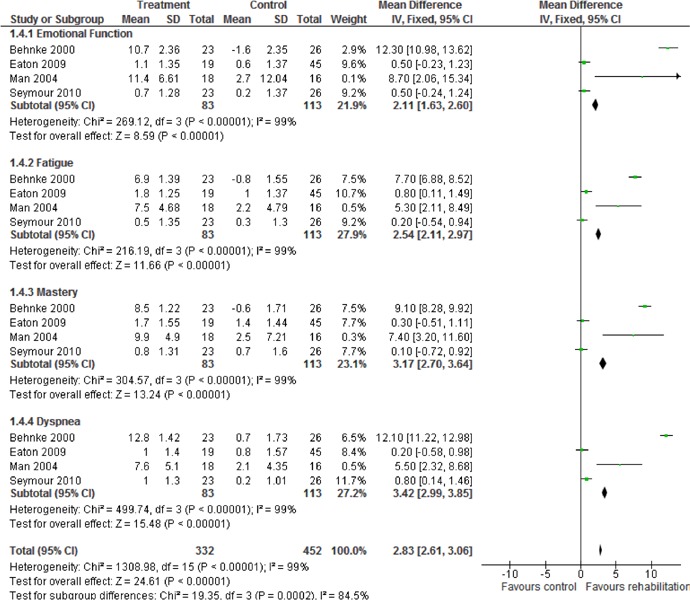
Abbreviations: CI, confidence interval; CRQ, Chronic Respiratory Questionnaire; SD, standard deviation.
Health-Related Quality of Life (St. George’s Respiratory Questionnaire)
Figure A12: Forest Plot of Pooled Data on Health-Related Quality of Life as Measured by the SGRQ, Subgrouped by Components of SGRQ.
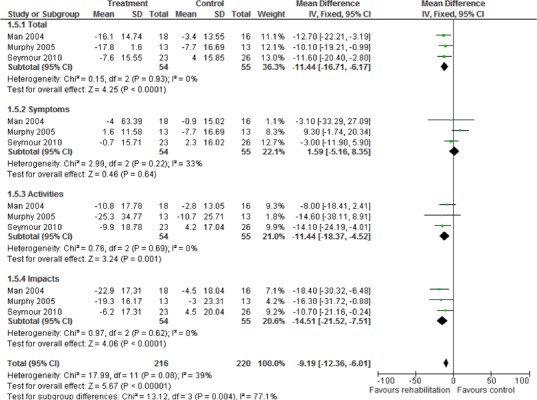
Abbreviations: CI, confidence interval; SD, standard deviation; SQRQ, St. George’s Respiratory Questionnaire.
Exercise Capacity
Figure A13: Forest Plot of Pooled Data on Exercise Capacity as Measured by the Six Minute Walking Test.
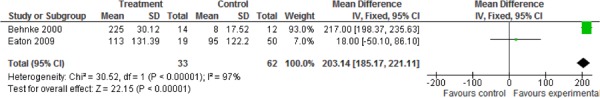
Abbreviations: CI, confidence interval; SD, standard deviation.
Emergency Department Visits
Figure A14: Forest Plot of Pooled Data on Emergency Department Visits.
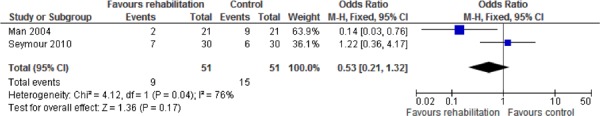
Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
Mortality
Figure A15: Forest Plot of Pooled Data on Mortality.
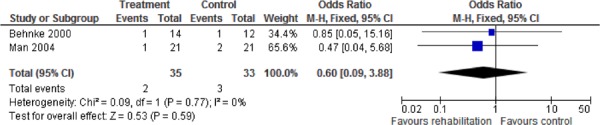
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Studies Examining Pulmonary Rehabilitation Maintenance Programs
Exercise Capacity
Figure A16: Forest Plot of Pooled Data on Exercise Capacity as Measured by the Six Minute Walking Test.
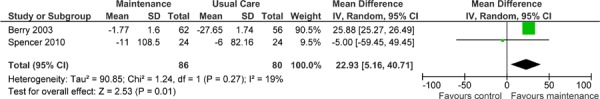
Abbreviations: CI, confidence interval; SD, standard deviation.
Suggested Citation
This report should be cited as follows:
COPD Working Group. Pulmonary rehabilitation for patients with chronic pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2012 Mar; 12(6) 1–75. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_Pulmomary_Rehab_March.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in Excerpta Medica/EMBASE and the Center for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: MASinfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All analyses in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: MASinfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-7012-1 (PDF)
© Queen’s Printer for Ontario, 2012
About the Medical Advisory Secretariat
Effective April 5, 2011, the Medical Advisory Secretariat (MAS) became a part of Health Quality Ontario (HQO), an independent body funded by the Ministry of Health and Long-Term Care. The mandate of MAS is to provide evidence-based recommendations on the coordinated uptake of health services and health technologies in Ontario to the Ministry of Health and Long-Term Care and to the health care system. This mandate helps to ensure that residents of Ontario have access to the best available and most appropriate health services and technologies to improve patient outcomes.
To fulfill its mandate, MAS conducts systematic reviews of evidence and consults with experts in the health care services community. The resulting evidence-based analyses are reviewed by the Oario Health Technology Advisory Committee—to which MAS also provides a secretariat function—and published in the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, MAS systematically reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, the Secretariat collects and analyzes information about how a new technology fits within current practice and existing treatment alternatives. Details about the technology’s diffusion into current health care practices add an important dimension to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist decision-makers in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals wishing to comment on an analysis prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by MAS for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data and information provided by experts and applicants to MAS to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the MAS website for a list of all evidence-based analyses: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
List of Tables
| Table 1: Body of Evidence Examined According to Study Design* |
| Table 2: Summary of Existing Evidence on Pulmonary Rehabilitation Interventions for Stable COPD* |
| Table 3: Summary of Findings of Meta-Analyses of Studies Investigating the Effectiveness of Pulmonary Rehabilitation on HRQOL and Functional Exercise Capacity in Patients With COPD* |
| Table 4: Body of Evidence Examined According to Study Design* |
| Table 5: Summary of Findings of a Meta-Analysis of Studies Investigating the Effectiveness of Pulmonary Rehabilitation on Hospital Readmission in Patients with COPD Following an Acute Exacerbation* |
| Table 6: Summary of Findings of Meta-Analyses of Studies Investigating the Effectiveness of Pulmonary Rehabilitation on HRQOL in Patients with COPD Following an Acute Exacerbation* |
| Table 7: Body of Evidence Examined According to Study Design* |
| Table 8: Summary of Findings of Meta-Analyses of Studies Investigating the Effectiveness of Maintenance Programs on Functional Exercise Capacity* |
| Table 9: Summary of Findings of Studies Investigating the Effectiveness of Maintenance Programs on Health-Related Quality of Life* |
| Table 10: Summary of Findings of Studies Investigating the Effectiveness of Maintenance Programs on Hospitalizations and Length of Stay* |
| Table A1: Description of Studies Examining Pulmonary Rehabilitation in the Stable COPD Population* (n = 18) |
| Table A2: Description of Studies Examining Pulmonary Rehabilitation Within One Month of an Acute Exacerbation of COPD (n = 4 Plus Behnke et al, 2000) |
| Table A3: Description of Studies Examining Pulmonary Rehabilitation Maintenance Programs (n = 3)* |
| Table A4: Methodological Quality of Studies Examining Stable COPD* (n = 18) |
| Table A5: Methodological Quality of Studies Following an Acute Exacerbation of COPD* (n = 5) |
| Table A6: Methodological Quality of Studies Examining Maintenance Programs* (n = 3) |
| Table A7: GRADE Quality of Evidence for Studies Examining Stable COPD* |
| Table A8: GRADE Quality of Evidence for Studies Examining Pulmonary Rehabilitation Following an Acute Exacerbation of COPD* |
| Table A9: GRADE Quality of Evidence for Studies Examining Pulmonary Rehabilitation Maintenance Programs* |
List of Figures
| Figure 1: Citation Flow Chart |
| Figure 2: Citation Flow Chart |
| Figure 3: Citation Flow Chart |
| Figure A1: Forest Plot of Pooled Quality of Life Data Measured by the Total Score of the St. George’s Respiratory Questionnaire |
| Figure A2: Forest Plot of Pooled Quality of Life Data Measured by the Symptom Score of the St. George’s Respiratory Questionnaire |
| Figure A3: Forest Plot of Pooled Quality of Life Data Measured by the Impacts Score of the St. George’s Respiratory Questionnaire |
| Figure A4: Forest Plot of Pooled Quality of Life Data Measured by the Activity Score of the St. George’s Respiratory Questionnaire |
| Figure A5: Forest Plot of Pooled Quality of Life Data Measured by the Mastery Score of the Chronic Respiratory Questionnaire |
| Figure A6: Forest Plot of Pooled Quality of Life Data Measured by the Emotional Function Score of the Chronic Respiratory Questionnaire |
| Figure A7: Forest Plot of Pooled Quality of Life Data Measured by the Dyspnea Score of the Chronic Respiratory Questionnaire |
| Figure A8: Forest Plot of Pooled Quality of Life Data Measured by the Fatigue Score of the Chronic Respiratory Questionnaire |
| Figure A9: Forest Plot of Pooled Data on Functional Exercise Capacity Measured by the Six Minute Walking Test |
| Figure A10: Forest Plot of Pooled Data on Hospital Readmissions Subgrouped by Type of Readmission |
| Figure A11: Forest Plot of Pooled Data on Health-Related Quality of Life as Measured by the CRQ, Subgrouped by Components of the CRQ |
| Figure A12: Forest Plot of Pooled Data on Health-Related Quality of Life as Measured by the SGRQ, Subgrouped by Components of SGRQ |
| Figure A13: Forest Plot of Pooled Data on Exercise Capacity as Measured by the Six Minute Walking Test |
| Figure A14: Forest Plot of Pooled Data on Emergency Department Visits |
| Figure A15: Forest Plot of Pooled Data on Mortality |
| Figure A16: Forest Plot of Pooled Data on Exercise Capacity as Measured by the Six Minute Walking Test |
List of Abbreviations
- 6MWT
6 Minute Walking Test
- AECOPD
Acute exacerbation of COPD
- COPD
Chronic obstructive pulmonary disease
- CI
Confidence interval(s)
- CRQ
Chronic Respiratory Questionnaire
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HRQOL
Health-related quality of life
- ITT
Intention-to-treat
- MCID
Minimal clinically important difference
- RCT
Randomized controlled trial
- SD
Standard deviation
- SGRQ
St. George’s Respiratory Questionnaire
- UC
Usual care
Footnotes
As measured by all domains of the Chronic Respiratory Questionnaire
As measured by the 6 Minute Walking Test
As measured by all domains of the Chronic Respiratory Questionnaire and total, impact, and activity scores of the St. George’s Respiratory Questionnaire
As measured by all domains of the CRQ
As measured by the 6MWT
As measured by all domains of the CRQ and total, impact, and activity scores of the SGRQ
The mild-to-moderate classification was created for the purposes of the report.
References
- 1.Lacasse Y, Maltais F, Goldstein RS. Smoking cessation in pulmonary rehabilitation: goal or prerequisite? J Cardpulm Rehabil. 2002 May;22(3):148–53. doi: 10.1097/00008483-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Wantanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006 Jan 1;59(1):7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Thiessen PH, Barrowman N, Garg AX. Imputing variance estimates do not alter the conclusions of a meta-analysis with continuous outcomes: a case study of changes in renal function after living kidney donation. J Clin Epidemiol. 2007 Jan 1;60(3):228–40. doi: 10.1016/j.jclinepi.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care. SBU Report No. 119E. 1996 doi: 10.1017/s0266462300008321. [DOI] [PubMed] [Google Scholar]
- 6.Hailey D, Jacobs P, Stickland M, Chuck A, Marciniuk DD, Mayers I, Mierzwinsky-Urban M. Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease: Clinical, Economic, and Budget Impact Analysis [Technology report number 126] Ottawa: Canadian Agency for Drugs and Technologies in Health. 2010 [Google Scholar]
- 7.Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010 Mar;16(2):134–43. doi: 10.1097/MCP.0b013e32833642f2. [DOI] [PubMed] [Google Scholar]
- 8.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006;4 doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Behnke M, Taube C, Kirsten D, Lehnigk B, Jorres RA, Magnussen H. Home-based exercise is capable of preserving hospital-based improvements in severe chronic obstructive pulmonary disease. Respir Med. 2000 Dec;94(12):1184–91. doi: 10.1053/rmed.2000.0949. [DOI] [PubMed] [Google Scholar]
- 10.Behnke M, Jorres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home-based exercise programme over 18 months in patients with severe COPD. Monaldi Arch Chest Dis. 2003;59(1):44–51. [PubMed] [Google Scholar]
- 11.Borghi-Silva A, Arena R, Castello V, Simoes RP, Martins LE, Catai AM, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med. 2009 Oct;103(10):1503–10. doi: 10.1016/j.rmed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Boxall A, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community: a randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardpulm Rehabil. 2005 Nov;25(6):378–85. doi: 10.1097/00008483-200511000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Elci A, Borekci S, Ovayolu N, Elbek O. The efficacy and applicability of a pulmonary rehabilitation programme for patients with COPD in a secondary-care community hospital. Respirology. 2008 Sep;13(5):703–7. doi: 10.1111/j.1440-1843.2008.01327.x. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom CP, Persson LO, Larsson S, Sullivan M. Health-related quality of life in COPD: why both disease-specific and generic measures should be used. Eur Respir J. 2001 Jul;18(1):69–76. doi: 10.1183/09031936.01.00044901. [DOI] [PubMed] [Google Scholar]
- 15.Finnerty JP, Keeping I, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease: a randomized controlled trial. Chest. 2001 Jun;119(6):1705–10. doi: 10.1378/chest.119.6.1705. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein RS, Gort ES, Stubbing D, Avendano MA, Guyatt GH. Randomised controlled trial of respiratory rehabilitation. Lancet. 1994 Jan 19;344(8934):1394. doi: 10.1016/s0140-6736(94)90568-1. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000 Jan 29;355(9201):362–8. doi: 10.1016/s0140-6736(99)07042-7. [DOI] [PubMed] [Google Scholar]
- 18.Guell R, Resqueti V, Sangenis M, Morante F, Martorell B, Casan P, et al. Impact of pulmonary rehabilitation on psychosocial morbidity in with severe COPD. Chest. 2006;129(4):899–904. doi: 10.1378/chest.129.4.899. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez MT, Rubio TM, Ruiz FO, Riera HS, Gil RS, Gomez JC. Results of a home-based training program for patients with COPD. Chest. 2000 Jul;118(1):106–14. doi: 10.1378/chest.118.1.106. [DOI] [PubMed] [Google Scholar]
- 20.Karapolat H, Atasever A, Atamaz F, Kirazli Y, Elmas F, Erdinc E. Do the benefits gained using a short-term pulmonary rehabilitation program remain in COPD patients after participation? Lung. 2007 Jul;185(4):221–5. doi: 10.1007/s00408-007-9011-4. [DOI] [PubMed] [Google Scholar]
- 21.Lake FR, Henderson K, Briffa T, Openshaw J, Musk AW. Upper-limb and lower-limb exercise training in patients with chronic airflow obstruction. Chest. 1990;97:1077–82. doi: 10.1378/chest.97.5.1077. [DOI] [PubMed] [Google Scholar]
- 22.Ringbaek TJ, Broendum E, Hemmingsen L, Lybeck K, Nielsen D, Andersen C, et al. Rehabilitation of patients with chronic obstructive pulmonary disease Exercise twice a week is not sufficient. Respir Med. 2000 Feb;94(2):150–4. doi: 10.1053/rmed.1999.0704. [DOI] [PubMed] [Google Scholar]
- 23.Theander K, Jakobsson P, Jorgensen N, Unosson M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clin Rehabil. 2009 Feb;23(2):125–36. doi: 10.1177/0269215508096174. [DOI] [PubMed] [Google Scholar]
- 24.Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000 Aug 15;109(3):207–12. doi: 10.1016/s0002-9343(00)00472-1. [DOI] [PubMed] [Google Scholar]
- 25.Simpson AC, Rocker GM. Advanced chronic obstructive pulmonary disease: rethinking models of care. Qjm. 2008 Sep;101(9):697–704. doi: 10.1093/qjmed/hcn087. [DOI] [PubMed] [Google Scholar]
- 26.Singh S. Chronic obstructive pulmonary disease. Curr Anaesth Crit Care. 2003;14(2):74–80. [Google Scholar]
- 27.Wijkstra PJ, Altena RV, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J. 1994 Jan 1;7:269–73. doi: 10.1183/09031936.94.07020269. [DOI] [PubMed] [Google Scholar]
- 28.Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010 Feb;91(2):221–5. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]


