Executive Summary
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective
The objective of this health technology assessment was to determine the effectiveness, cost-effectiveness, and safety of long-term oxygen therapy (LTOT) for chronic obstructive pulmonary disease (COPD).
Clinical Need: Condition and Target Population
Oxygen therapy is used in patients with COPD with hypoxemia, or very low blood oxygen levels, because they may have difficulty obtaining sufficient oxygen from inspired air.
Technology
Long-term oxygen therapy is extended use of oxygen. Oxygen therapy is delivered as a gas from an oxygen source. Different oxygen sources are: 1) oxygen concentrators, electrical units delivering oxygen converted from room air; 2) liquid oxygen systems, which deliver gaseous oxygen stored as liquid in a tank; and 3) oxygen cylinders, which contain compressed gaseous oxygen. All are available in portable versions. Oxygen is breathed in through a nasal cannula or through a mask covering the mouth and nose. The treating clinician determines the flow rate, duration of use, method of administration, and oxygen source according to individual patient needs. Two landmark randomized controlled trials (RCTs) of patients with COPD established the role of LTOT in COPD. Questions regarding the use of LTOT, however, still remain.
Research Question
What is the effectiveness, cost-effectiveness, and safety of LTOT compared with no LTOT in patients with COPD, who are stratified by severity of hypoxemia?
Research Methods
Literature Search
Search Strategy
A literature search was performed on September 8, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, the Cochrane Library, and INAHTA for studies published from January 1, 2007 to September 8, 2010.
A single clinical epidemiologist reviewed the abstracts, obtained full-text articles for studies meeting the eligibility criteria, and examined reference lists for additional relevant studies not identified through the literature search. A second clinical epidemiologist and then a group of epidemiologists reviewed articles with an unknown eligibility until consensus was established.
Inclusion Criteria
patients with mild, moderate, or severe hypoxemia;
English-language articles published between January 1, 2007 and September 8, 2010;
journal articles reporting on effectiveness, cost-effectiveness, or safety for the comparison of interest;
clearly described study design and methods;
health technology assessments, systematic reviews, RCTs, or prospective cohort observational studies;
any type of observational study for the evaluation of safety.
Exclusion Criteria
no hypoxemia
non-English papers
animal or in vitro studies
case reports, case series, or case-case studies
studies comparing different oxygen therapy regimens
studies on nocturnal oxygen therapy
studies on short-burst, palliative, or ambulatory oxygen (supplemental oxygen during exercise or activities of daily living)
Outcomes of Interest
mortality/survival
hospitalizations
readmissions
forced expiratory volume in 1 second (FEV1)
forced vital capacity (FVC)
FEV1/FVC
pulmonary hypertension
arterial partial pressure of oxygen (PaO2)
arterial partial pressure of carbon dioxide (PaCO2)
end-exercise dyspnea score
endurance time
health-related quality of life
Note: Outcomes of interest were formulated according to existing studies, with arterial pressure of oxygen and carbon dioxide as surrogate outcomes.
Summary of Findings
Conclusions
Based on low quality of evidence, LTOT (~ 15 hours/day) decreases all-cause mortality in patients with COPD who have severe hypoxemia (PaO2 ~ 50 mm Hg) and heart failure.
The effect for all-cause mortality had borderline statistical significance when the control group was no LTOT: one study.
Based on low quality of evidence, there is no beneficial effect of LTOT on all-cause mortality at 3 and 7 years in patients with COPD who have mild-to-moderate hypoxemia (PaO2 ~ 59-65 mm Hg)1
Based on very low quality of evidence, there is some suggestion that LTOT may have a beneficial effect over time on FEV1 and PaCO2 in patients with COPD who have severe hypoxemia and heart failure: improved methods are needed.
Based on very low quality of evidence, there is no beneficial effect of LTOT on lung function or exercise factors in patients with COPD who have mild-to-moderate hypoxemia, whether survivors or nonsurvivors are assessed.
Based on low to very low quality of evidence, LTOT does not prevent readmissions in patients with COPD who have severe hypoxemia. Limited data suggest LTOT increases the risk of hospitalizations.
Limited work has been performed evaluating the safety of LTOT by severity of hypoxemia.
Based on low to very low quality of evidence, LTOT may have a beneficial effect over time on health-related quality of life in patients with COPD who have severe hypoxemia. Limited work using disease-specific instruments has been performed.
Ethical constraints of not providing LTOT to eligible patients with COPD prohibit future studies from examining LTOT outcomes in an ideal way.
Background
In July 2010, the Medical Advisory Secretariat (MAS) began work on a Chronic Obstructive Pulmonary Disease (COPD) evidentiary framework, an evidence-based review of the literature surrounding treatment strategies for patients with COPD. This project emerged from a request by the Health System Strategy Division of the Ministry of Health and Long-Term Care that MAS provide them with an evidentiary platform on the effectiveness and cost-effectiveness of COPD interventions.
After an initial review of health technology assessments and systematic reviews of COPD literature, and consultation with experts, MAS identified the following topics for analysis: vaccinations (influenza and pneumococcal), smoking cessation, multidisciplinary care, pulmonary rehabilitation, long-term oxygen therapy, noninvasive positive pressure ventilation for acute and chronic respiratory failure, hospital-at-home for acute exacerbations of COPD, and telehealth (including telemonitoring and telephone support). Evidence-based analyses were prepared for each of these topics. For each technology, an economic analysis was also completed where appropriate. In addition, a review of the qualitative literature on patient, caregiver, and provider perspectives on living and dying with COPD was conducted, as were reviews of the qualitative literature on each of the technologies included in these analyses.
The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made up of the following reports, which can be publicly accessed at the MAS website at: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Chronic Obstructive Pulmonary Disease (COPD) Evidentiary Framework
Influenza and Pneumococcal Vaccinations for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Smoking Cessation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Pulmonary Rehabilitation for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Long-term Oxygen Therapy for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Acute Respiratory Failure Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Hospital-at-Home Programs for Patients With Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Home Telehealth for Patients With Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis
Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model
Experiences of Living and Dying With COPD: A Systematic Review and Synthesis of the Qualitative Empirical Literature
For more information on the qualitative review, please contact Mita Giacomini at: http://fhs.mcmaster.ca/ceb/faculty_member_giacomini.htm.
For more information on the economic analysis, please visit the PATH website: http://www.path-hta.ca/About-Us/Contact-Us.aspx.
The Toronto Health Economics and Technology Assessment (THETA) collaborative has produced an associated report on patient preference for mechanical ventilation. For more information, please visit the THETA website: http://theta.utoronto.ca/static/contact.
Objective of Analysis
The objective of this health technology assessment was to determine the effectiveness, cost-effectiveness, and safety of long-term oxygen therapy (LTOT) for chronic obstructive pulmonary disease (COPD).
Clinical Need and Target Population
Patients With COPD and Hypoxemia and Respiratory Failure: Need for LTOT
Airflow limitation in COPD may cause very low arterial blood oxygen levels, or hypoxemia. (1) Hypoxemia increases respiratory drive to maintain adequate oxygen delivery to tissues. Prolonged hypoxemia may lead to tissue hypoxia and permanent damage as a result of adverse effects on organ function and structure. Short-term effects of hypoxemia include increased breathing difficulty, peripheral vascular dilation with increased heart rate and cardiac output, regional pulmonary vasoconstriction, high erythropoietin levels, and increased blood viscosity. Long-term effects include pulmonary hypertension, right ventricular failure, and polycythemia. (2)
Respiratory failure is found only in stage 4, very severe COPD, where the arterial partial pressure of oxygen (PaO2) is less than 60 mm Hg, and it may be accompanied by a forced expiratory volume in 1 second (FEV1) less than 30% predicted. Respiratory failure in the absence of such severely decreased lung function is a criterion for very severe COPD. Respiratory failure may lead to secondary effects on the heart, known as cor pulmonale, or right heart failure. The clinical signs of cor pulmonale include jugular venous pressure elevation and pitting ankle edema. Patients at this very severe stage have endorgan dysfunction related to COPD, and exacerbations may be life-threatening. (3)
Respiratory failure is classified as type I (hypoxemic) respiratory failure or type II (hypercapnic) respiratory failure. In hypoxemic respiratory failure, PaO2 is decreased to less than 60 mm Hg, and the arterial pressure of carbon dioxide (PaCO2) is normal or low. (4) Clinical signs of hypoxemia include restlessness, confusion, and coma. In hypercapnic respiratory failure, PaO2 is also low, but PaCO2 is increased. Clinical signs of hypercapnia include drowsiness, flapping tremor, warm peripheries, headaches, and a bounding pulse. (5) About 10 to 15% of patients with COPD have type II respiratory failure. (4)
The normal range for PaO2 is 80 to 100 mm Hg, and the normal range for PaCO2 is 35 to 45 mm Hg. (5) Normally, elevated PaCO2 stimulates respiratory drive to reduce these levels through increased breathing. This stimulation is diminished, however, in type II respiratory failure, and low PaO2 triggers hypoxic drive instead. (4)
The management goal in treating respiratory failure is to reverse hypoxemia while preventing further increases in hypercapnia, which can be fatal in people who retain carbon dioxide (CO2). Hypoxic drive is needed to maintain respiration in CO2 retainers. (5) A clinical safety dilemma arises because oxygen therapy may decrease respiration in type II respiratory failure, but withholding oxygen from a patient with COPD who is hypoxemic may be detrimental. (4)
Oxygen therapy can reverse hypoxemia. (2) Indications for oxygen therapy include respiratory failure and an increased respiratory rate. The following conditions, which are associated with hypoxemia, may require oxygen therapy: cardiac respiratory arrest, acute myocardial infarction with reduced cardiac output, severe trauma, anemia, infection, general anesthesia, and surgical procedures. (4) Patients with COPD may have difficulty obtaining enough oxygen from inspired air and may benefit from oxygen therapy. (1) The purpose of oxygen therapy is to correct the deficiency of oxygen in arterial blood and prevent tissue hypoxia. (6)
Long-term oxygen therapy is extended use of oxygen. Oxygen therapy needs vary depending on activity levels. Patients with daytime resting hypoxemia may need LTOT during sedentary periods, when they are typically resting at home or performing nonstressful domiciliary activities of daily living. Increased activity levels, such as casual walking, may require ambulatory or supplemental oxygen to meet higher systemic demands. Patients with COPD have decreased sensitivity to the normal neurochemical control of breathing during sleep, which results in nocturnal oxygen desaturation. As a result, nocturnal oxygen therapy may be needed. Some guidelines recommend increasing the oxygen dose during periods of extended exercise and during sleep. (7;8)
At higher altitudes, decreased atmospheric pressure reduces the partial pressure of oxygen in the air. Patients living at or travelling to higher altitudes may also require supplemental oxygen. Episodes of breathlessness in patients with COPD may require short-burst oxygen therapy, or palliative oxygen therapy. (7;8) Long-term oxygen therapy may be given after an acute exacerbation of COPD, and the need for LTOT should be reassessed after an exacerbation. Home LTOT is a potential risk factor for relapse after acute exacerbation. (9) When acute exacerbations are treated in hospital, oxygen therapy is titrated to achieve a PaO2 above 60 mm Hg (> 8 kilopascals [kPa]) or oxygen saturation measured by pulse oximetry (SpO2) greater than 90%. The goal is to achieve adequate oxygenation without promoting CO2 retention or acidosis. (3)
The role of LTOT in COPD is based on 2 landmark randomized controlled trials (RCTs) of patients with COPD. In the first trial, (10) LTOT for 15 hours per day, including nocturnal therapy, was compared with no LTOT in patients with COPD, who had severe airflow limitation (FEV1: 0.58–0.76 L), severe hypoxemia (PaO2: 49–52 mm Hg), hypercapnia (PaCO2: 53–60 mm Hg), and mild pulmonary hypertension. The oxygen flow rate was at least 2 L/minute but sufficient to achieve a PaO2 above 60 mm Hg. Study findings demonstrated that LTOT improved survival, a primary outcome. No between-group differences were seen in pulmonary hemodynamics, among secondary outcomes of the trial. The 15-hour period was based on its ability to reduce pulmonary arterial pressure. (11) In a second trial, (12) continuous use of LTOT was compared with nocturnal oxygen therapy in patients with COPD who had severe hypoxemia (PaO2 < 56 mm Hg) or moderate hypoxemia (PaO2 < 60 mm Hg) with edema, polycythemia (hematocrit > 54%), or P pulmonale, an electrocardiographic finding. The relative risk (RR) of death for nocturnal oxygen was about twice that for continuous LTOT (RR, 1.94: 95% confidence interval [CI], 1.17–3.24). Continuous LTOT was associated with a beneficial clinical profile, decreased hematocrit levels, and reduced pulmonary vascular resistance compared with nocturnal use. Mean daily duration of oxygen use was 17.7 hours in the continuous LTOT group and 12 hours in the nocturnal oxygen therapy group. (13)
The results of these trials indicate that some oxygen is better than none, and that when oxygen is given, continuous use is better than nocturnal use. The way in which oxygen prolongs survival is not known. (2) A remaining question is whether LTOT confers a survival advantage in mild-to-moderate hypoxemia2 (PaO2: 56-65 mm Hg) and during exercise or sleep. (13)
Canadian Context
The Canadian Thoracic Society recommends patients with stable COPD and severe hypoxemia (PaO2 ≤ 55 mm Hg), or less severe hypoxemia (PaO2 < 60 mm Hg) with at least 1 additional factor of bilateral ankle edema, cor pulmonale, or hematocrit above 56%, receive LTOT for at least 15 hours per day to achieve an oxygen saturation of at least 90%. (14) The number of people using LTOT in Canada is not known.
Ontario Context
The Assistive Devices Program administers oxygen therapy in Ontario. Eligibility criteria for LTOT under the Ministry of Health and Long-Term Care are consistent with Canadian guidelines (PaO2 ≤ 55 mm Hg or PaO2 < 60 mm Hg with comorbidities). Patients with persistent hypoxemia (PaO2: 56–60 mm Hg), exercise-limiting hypoxemia documented to improve with supplemental oxygen, or nocturnal hypoxemia, are also eligible for LTOT, as are patients with exertional hypoxemia without hypoxemia at rest. In summary, the eligibility criteria for LTOT in Ontario are: 1) severe hypoxemia, 2) mild-to-moderate hypoxemia with specific comorbidities, 3) mild-to-moderate hypoxemia plus exercise-limiting hypoxemia or nocturnal hypoxemia, 4) exertional hypoxemia without hypoxemia at rest. (15;16)
To confirm eligibility for LTOT, oximetry testing is required for applicants younger than 18 years, and arterial blood gas determination is required for applicants older than 19 years. Oximetry testing for application renewal for Ontario’s home oxygen program is required at the 90-day period and 12-month period to determine whether eligibility criteria are being met. Oximetry test results are required for patients renewing their funding annually. The oximetry tests must monitor the oxygen level for at least 5 continuous minutes and demonstrate hypoxemia for at least 2 continuous minutes. Uncertain oximetry test results must be confirmed with arterial blood gases. (15;16)
Oxygen prescription in Ontario is based on oxygen titration at a pulmonary function laboratory or assessment in the person’s home by the oxygen vendor. The method used depends on the prescribing physician and availability of the pulmonary function laboratory (Personal communication, expert, November 7, 2011). Titration determines oxygen needed at rest and while walking to achieve an SpO2 of about 90% with a flow rate that prevents CO2 build-up in people prone to retaining CO2. The flow rate at rest is used as the nocturnal flow rate. Either the respirologist or the family physician completes the required prescription, and the respirologist may contact the oxygen company. Reassessment occurs as necessary (Personal communication, clinical expert, November 4, 2010).
Patient need determines the choice of oxygen delivery system. Until April 2010, the Home Oxygen Program funded any combination of oxygen supply delivery systems, such as home oxygen concentrator and portable compressed gas cylinder, or home oxygen concentrator and portable liquid oxygen, without tracking the system that patients received. After April 2010, the vendor was required to provide the details of the oxygen delivery system that patients used. As a result, the provincial government is now able to track the systems patients in Ontario are using. Most patients receive a home oxygen concentrator and a second portable system (Personal communication, expert, January 19, 2011).
Technology
An oxygen source delivers oxygen as a gas. The different oxygen sources are: 1) oxygen concentrators, electrical units delivering oxygen that has been converted from room air; 2) liquid oxygen systems, delivering gaseous oxygen stored as liquid in a tank; and 3) oxygen cylinders, which contain compressed gaseous oxygen. All have portable versions. Oxygen is breathed in through a nasal cannula or through a mask covering the mouth and nose. The treating physician determines the flow rate, duration of use, method of administration, and oxygen source according to individual needs. A critical point with oxygen therapy is that the therapy is only effective while it is being used. (17)
Safety
Limited work has been performed on the safety of oxygen therapy. Oxygen itself is not flammable, but it accelerates a fire source, such as a lit cigarette. Patient safety education includes the hazards of oxygen use and initial training on equipment, including demonstration of skills needed to operate the equipment safely and independently. Another hazard is falls due to oxygen tubing. (18;19) Underuse of oxygen is also a safety issue, and it is responsible for deaths and permanent disability. A small risk is associated with high-dose oxygen among CO2 retainers. (5)
Evidence-Based Analysis
Research Questions
What is the effectiveness, cost-effectiveness, and safety of LTOT compared with no LTOT in patients with COPD, who are stratified by severity of hypoxemia?
Research Methods
Literature Search
Search Strategy
A literature search was performed on September 8, 2010 using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 1, 2007 until September 8, 2010.
A single clinical epidemiologist reviewed the abstracts, obtained full-text articles for studies meeting the eligibility criteria, and examined reference lists for additional relevant studies not identified through the literature search. A second clinical epidemiologist and then a group of epidemiologists reviewed articles with an unknown eligibility until consensus was established.
Inclusion Criteria
patients with mild, moderate, or severe hypoxemia;
English-language articles published between January 1, 2007 and September 8, 2010;
journal articles reporting on effectiveness, cost-effectiveness, or safety for the comparison of interest;
clearly described study design and methods;
health technology assessments, systematic reviews, randomized controlled trials, or prospective cohort observational studies;
any type of observational study for the evaluation of safety.
Exclusion Criteria
no hypoxemia
non-English papers
animal or in vitro studies
case reports, case series, or case-case studies
studies comparing different oxygen therapy regimens
studies on nocturnal oxygen therapy
studies on short-burst, palliative, or ambulatory oxygen (supplemental oxygen during exercise or activities of daily living)
Outcomes of Interest
mortality/survival
hospitalizations
readmissions
FEV1
forced vital capacity (FVC)
FEV1/FVC
pulmonary hypertension
PaO2
PaCO2
end-exercise dyspnea score
endurance time
health-related quality of life (HRQOL)
Note: Outcomes of interest were formulated according to existing studies, with arterial pressure of oxygen and carbon dioxide as surrogate outcomes.
Statistical Analysis
An analysis of individual studies was performed using Review Manager version 5. The analysis section describes details of the analyses. No formal meta-analysis was performed. Mean difference was calculated for continuous data, and RR was calculated for dichotomous RCT data. A change value was calculated for continuous variables with available mean baseline and follow-up data as the difference between these 2 mean values. Standard deviation (SD) accounting for baseline and follow-up SD was calculated from 3 parameters: baseline SD, follow-up SD, and a correlation coefficient, which represents the strength of the relationship between the 2 SDs. A correlation coefficient of 0.5 was used for this analysis. Forest plots were also examined.
Quality of Evidence
The quality of each included study was assessed taking into consideration the following 7 study design characteristics:
adequate allocation concealment,
randomization (study must include a description of the randomization procedure used and must be a proper method),
power/sample size (adequate sample size based on a priori calculations, underpowered studies were identified, when possible, using post hoc sample size power calculations),
blinding (if double blinding is not possible, a single blind study with unbiased assessment of outcome was considered adequate for this criterion),
< 20% withdrawals/dropouts,
intention-to-treat analysis conducted and done properly (withdrawals/dropouts considered in analysis), and
other criteria as appropriate for the particular research question and study design.
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (20) as presented below.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding, and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Results of Evidence-Based Analysis
The database search yielded 1,096 citations published between January 1, 2007 and September 8, 2010 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded in the analysis. Three systematic reviews met the inclusion criteria.
Figure 1: Citation Flow Chart*.
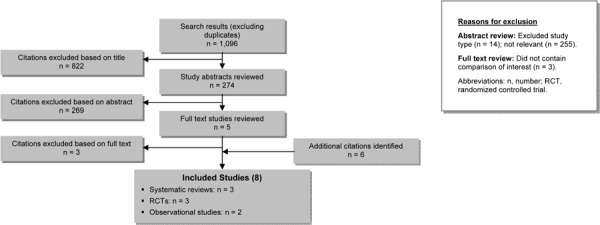
For each included study, the study design was identified and is summarized below in Table 1, which is a modified version of a hierarchy of study design by Goodman. (21)
Table 1: Body of Evidence Examined According to Study Design*.
| Study Design | Number of Eligible Studies |
|---|---|
| Randomized Controlled Trials | |
| Systematic review of RCTs | 2 |
| Large RCT† | - |
| Small RCT | - |
| Observational Studies | |
| Systematic review of non-RCTs with contemporaneous controls | 1 |
| Non-RCT with contemporaneous controls | - |
| Systematic review of non-RCTs with historical controls | - |
| Non-RCT with historical controls | - |
| Database, registry, or cross-sectional study | - |
| Case series | - |
| Retrospective review, modelling | - |
| Studies presented at an international conference or other sources of grey literature | - |
| Expert opinion | - |
| Total | 3 |
Abbreviation: RCT, randomized controlled trial.
Large RCT ≥ 150 subjects.
Randomized Controlled Trials
Long-Term Oxygen Treatment Trial
The Long-Term Oxygen Treatment Trial sponsored by the National Heart, Lung, and Blood Institute and Centers for Medicare & Medicaid Services is an ongoing phase 3 trial. This multicentre RCT is being performed in the United States and is following 1,134 patients with moderate resting hypoxemia for up to 4.5 years and comparing continuous LTOT (24 hours/day) with no LTOT to determine whether continuous LTOT prolongs time to all-cause mortality or hospitalization. Among other secondary outcome measures, the trial is evaluating HRQOL.
The intervention includes oxygen at rest and during sleep at 2 L/min via nasal cannula. Supplemental oxygen is used for people with normal blood oxygen levels at rest but low or very low blood oxygen levels during exercise. The supplemental oxygen dose aims to achieve an SpO2 of at least 90% for at least 2 minutes during walking. Participants with resting hypoxemia are instructed to use oxygen 24 hours per day, whereas those with normal resting blood oxygen levels but low or very low blood oxygen levels during exercise are instructed to use oxygen during physical activity and sleep. Eligible individuals are older than 39 years, have COPD defined as postbronchodilator FEV1 less than 66% predicted or FEV1/FVC less than 0.70, and have dyspnea. It is estimated the study will be completed by May 2013. (13;22) Results of this trial may provide information about safety, efficacy, and cost-effectiveness of LTOT.
Cochrane Review
One of the largest systematic reviews and meta-analyses of LTOT RCTs was published as a Cochrane review. (23) The objective of the review was to examine the effect of domiciliary LTOT on survival, quality of life, and physiological measures. The review included articles published up to January 2007. Six identified RCTs are summarized individually and in Appendix 3, Tables A8 and A9, and reviewed by severity of hypoxemia according to standard definitions (Existing Guidelines for Long-term Oxygen Therapy). The studies on nocturnal oxygen therapy and the study on patients with COPD without hypoxemia were not eligible for this evidence-based analysis and are not included.
Severe Hypoxemia
Survival
A multicentre RCT performed by the Medical Research Council (MRC) Working Group (10) in the United Kingdom compared the effect of LTOT with no LTOT on survival in 87 patients with stable COPD (chronic bronchitis or emphysema with irreversible airway obstruction) and severe hypoxemia (40–60 mm Hg). Inclusion criteria included patient age less than 70 years and at least 1 episode of heart failure with ankle edema. Patients taking drug therapy and current smokers were included. Exclusion criteria included fibrotic or infiltrative lung disease, pneumoconiosis, severe kyphoscoliosis, pulmonary embolism, hypertension, coronary artery disease, or other unspecified life-threatening diseases. A random numbers table randomly allocated participants. Oxygen sources were oxygen from a concentrator, liquid oxygen, and oxygen from cylinders. The 3 study sites measured adherence differently: weighing cylinders, recording time of use, or performing random visits.
Duration of follow-up was 5 years. One participant in the treatment group withdrew from the study. Baseline age and clinical and physiological factors were comparable between groups. Overall, 19 of 42 (45.2%) patients in the treatment (LTOT) group died and 30 of 45 (66.7%) patients in the control (CT) group died (P not given). Examination of the results by sex for the 66 men and 21 women in the trial found a lower risk of death for women in the LTOT group (5.7%) compared with the CT group (36.5%, P < 0.05). A mortality difference between the groups for men emerged only after 500 days (LTOT 12% vs. CT 29%, P = 0.04). Rates of change for PaO2, PaCO2, and pulmonary vascular resistance were favourable for survivors receiving LTOT. The authors concluded that LTOT confers a survival advantage for both men and women with severe hypoxemia and cor pulmonale.
Mild-to-Moderate Hypoxemia
Survival
A multicentre RCT performed by Gorecka et al (24) in Poland compared the effect of LTOT with no LTOT on survival in 135 patients with stable COPD and moderate hypoxemia (56–65 mm Hg). Participants were aged 40 to 80 years, were not smokers, and received usual or conventional medical treatment. Exclusion criteria included serious organ disease other than lung disease. Centrally developed randomization schedules randomly allocated patients to treatment assignments using computer-generated random numbers. An oxygen concentrator provided oxygen, and a built-in meter monitored adherence.
Follow-up duration was at least 3 years or until death. There were no dropouts. Baseline data were comparable between the treatment (LTOT) and CT groups, except for mean PaO2, which was slightly lower in the LTOT group (mean PaO2 59.5 mm Hg, SD 2.7) than in the CT group (PaO2 61.3 mm Hg, SD 2.7, P < 0.05). The preliminary sensitivity analysis, however, found no effect of PaO2 on survival. With up to 7 years’ follow-up, no difference was seen in survival between the LTOT group (38/68, 55.9%) and the CT group (32/67, 47.8%, P = 0.89). Among surviving participants, mean PaO2 levels were lower in the LTOT group (mean PaO2 59.6 mm Hg, SD 2.9) than in the CT group (PaO2 61.2 mm Hg, SD 2.7, P < 0.05). No differences were seen among surviving participants between the LTOT and CT groups in PaCO2, FEV1 percent predicted, vital capacity (VC) percent predicted, or FEV1/VC. The authors concluded that LTOT does not provide a survival advantage in patients with COPD who have chronic airflow obstruction and moderate hypoxemia.
Survival and Exercise Parameters
A randomized single-institution study by Haidl et al (25) in Germany compared the effect of LTOT with no LTOT on survival and exercise parameters in 28 patients with stable COPD and mild hypoxemia (PaO2 > 55 mm Hg). Patients had been admitted for an acute exacerbation of COPD that included reversible hypercapnia (PaCO2 > 45 mm Hg). Exclusion criteria were malignant disease, left heart failure, or other severe comorbidities, such as advanced renal failure or severe diabetes. Patients were randomly allocated to treatment groups, but randomization details were not provided. An oxygen concentrator provided oxygen. Patients’ self-reported duration of oxygen use from the built-in meter determined adherence.
Duration of follow-up was up to 3 years. Only 13 of the original 28 patients (46.4%) remained at the end of 3 years. Baseline data were comparable in both groups except for mean body mass index (BMI), which was slightly higher in the LTOT group (BMI 26.2 kg/m2, SD 3.7) than in the CT group (BMI 23.7 kg/m2, SD 3.8, P = 0.05). At the start of the study, each group included 3 smokers (21.4%). Survival was comparable in both groups: over 3 years, 4 of the original 14 patients (28.6%) in the LTOT group and 3 of the 14 patients (21.4%, P not given) in the CT group died.
At 1 year, mean endurance time in the LTOT group (7.1 minutes, SD 4.1) was greater than in the CT group (4.9 min, SD 3.8 minutes, P = 0.04) and mean perceived end-exercise dyspnea was lower in the LTOT group (4.5 minutes, SD 1.5) than in the CT group (5.7 minutes, SD 1.9, P = 0.03). No differences were seen for PaCO2, PaO2, or FEV1 percent predicted between the groups at 1 year. The authors concluded that LTOT in patients with COPD who have mild hypoxemia and reversible hypercapnia helped to stop the natural decline in exercise performance and reduced dyspnea. The biological basis of this effect is not known.
Results of the Cochrane Review
The objective of the Cochrane review was to determine the effect of domiciliary LTOT on survival and quality of life in patients with COPD and hypoxemia. The literature search identified 6 studies for inclusion. (10;12;24−27) The authors scored the methodological quality of 5 of the 6 studies as moderate (10;12;24;26;27) and of the remaining study as low. (25) Data analysis was performed by degree of hypoxemia. Data from 2 studies on mild-to-moderate hypoxemia and nocturnal oxygen therapy were analyzed together (26;27), and data from 2 studies on mild-to-moderate hypoxemia and LTOT were analyzed together. (24;25) Although the 2 remaining studies both evaluated patients with severe hypoxemia, they were analyzed separately, due to differences in interventions and study populations. (10;12)
Standard meta-analysis was performed, including calculating a Peto odds ratio (OR) for dichotomous data. Detailed results are presented (Appendix 3, Table A9) only for studies relevant for this evidence-based analysis. Study design characteristics included in the Cochrane review and relevant for this evidence-based analysis are summarized in Appendix 3, Tables A8 to A11. A discussion of results for studies from the Cochrane review that are relevant for this evidence-based analysis follows.
Severe Hypoxemia: Mortality and Physiological Factors
Analysis of the MRC study (10) compared the effect of LTOT on mortality and physiological factors with no LTOT in highly selected patients with severe hypoxemia and possible episodes of heart failure and ankle edema. After 5 years of follow-up, patients receiving LTOT were less likely to die than patients receiving no LTOT (OR 0.42, 95% CI, 0.18–0.98; P = 0.045).
Rates of change for a subset of physiological factors were analyzed for men who died at 500 days or less and for men surviving more than 500 days, as in the original article. Factors discussed here include weight, FEV1, FVC, PaO2, and PaCO2. Rates of change for FEV1 (mean difference [MD] 0.08 L, 95% CI 0.04, 0.12; P < 0.001), FVC (MD 0.56 L, 95% CI 0.12, 1.00; P < 0.012), and PaCO2 (MD −2.16 mm Hg, 95% CI: −4.04 to −0.28; P < 0.03) favoured LTOT. Therefore, among patients surviving more than 500 days, patients receiving LTOT had increased FEV1 and decreased PaCO2 compared with patients not receiving LTOT. An improvement in FVC was shown among nonsurvivors. The remaining physiological factors were similar in both groups. Change data were determined from 2 monthly values in the original article; the timing of the values was not described. Pulmonary arterial pressure was not analyzed in the Cochrane review, as no patients with data on pulmonary arterial pressure died. No data were available for FEV1/FVC.
Mild-to-Moderate Hypoxemia: Mortality and Exercise Factors
The studies by Gorecka et al (24) and Haidl et al (25) were analyzed together, because both included patients with mild-to-moderate hypoxemia and compared LTOT with no LTOT. Analysis identified no difference between groups for mortality (OR, 1.39; 95% CI, 0.74–2.59), with an index of heterogeneity of 0%. Only Gorecka et al (24) performed a survival analysis and only Haidl et al (25) compared the effect of continuous LTOT with no LTOT on exercise factors in patients with mild-to-moderate hypoxemia. At 1 year, the groups were similar in end-exercise dyspnea score (MD, −1.20; 95% CI, −2.47 to 0.07) and endurance time (MD, 2.20 minutes; 95% CI, −0.73 to 5.13), in contrast to the original study, which showed small differences at 1-year follow-up in mean dyspnea between the LTOT group (4.5, SD 1.5) and the CT group (5.7, SD 1.9, P = 0.03) and in mean endurance time between the LTOT group (7.1 minutes, SD 4.1) and the CT group (4.9 minutes, SD: 3.8, P = 0.04).
The authors of the Cochrane review concluded that LTOT improved survival in selected patients with COPD with severe hypoxemia but did not improve survival in patients with COPD with mild-to-moderate hypoxemia. (23)
Additional Studies, Systematic Reviews, and Meta-Analyses
Mortality
Wilt et al (28) performed a second systematic review and meta-analysis of RCTs of LTOT that identified 8 RCTs and 1 systematic review published up to March 2007. This review included articles that have already been discussed in the Cochrane review. (10;12;24;27) Sin et al (29) performed an earlier systematic review that identified 7 RCTs, most of which were also discussed in the Cochrane review (10;12;24;26;27) and the Wilt et al review. (10;12;24;27;30−32) The other studies included in the Wilt et al review investigated ambulatory oxygen therapy (30-33) and are not relevant for this evidence-based analysis.
Wilt et al concluded that LTOT for at least 15 hours daily to maintain a PaO2 greater than 60 mm Hg reduces mortality among patients with COPD, who have an FEV1 less than 30% predicted and a mean resting PaO2 less than or equal to 55 mm Hg. This conclusion is based on good evidence with a Mantel-Haenszel relative risk ratio summary estimate of 0.61 (95% CI, 0.46–0.82) for the 2 studies on severe hypoxemia combined. (10;12) The 2 studies of patients with PaO2 greater than 60 mm Hg demonstrated no benefit for LTOT (RR, 1.16; 95% CI, 0.85–1.58). (24;27)
Hospitalization and Readmissions
A systematic review of observational studies (34) of risk factors for hospital admission or readmission among patients experiencing COPD exacerbations identified and included 17 studies published up to October 2006. Two prospective cohort studies, 2 retrospective cohort studies, 1 case-control study, and 1 cross-sectional study examined LTOT. The authors of the systematic review concluded that the evidence related to hospital admission and readmission is equivocal and requires further study. The 2 prospective studies were not analyzed together because they described different outcomes (readmission vs. hospitalization), nor were they individually analyzed, as suitable data were lacking.
Among the cohort studies, only 1 prospective cohort study included an adjusted analysis for hospital readmissions. (35) Analysis found no statistically significant difference between LTOT and no LTOT for the risk of hospital readmission (hazard ratio, 1.26; 95% CI, 0.87–1.84; P = 0.22). This multicentre prospective study, conducted in Barcelona, examined the association between readmission for a COPD exacerbation and several modifiable risk factors. The sampling scheme, diagnosis of COPD, exacerbation, readmission, death, analysis, and follow-up were well defined. The population was mostly men with a mean age of 69 years, severe COPD (mean FEV1 36% predicted), and mild-to-moderate hypoxemia (mean PaO2 64 mm Hg). Mean follow-up was 1.1 years. Sensitivity analyses had no effect on the results. The authors concluded that no association existed between readmission and factors relating to medical care. The main limitations of the study are the potential for confounding by unmeasured factors in observational studies and the potential for heterogeneity in the comparison, with individuals using LTOT having severe hypoxemia and those not using LTOT having mild-to-moderate hypoxemia, although this was not well described.
The second prospective cohort study was a single-centre study (36) that used well-defined parameters to examine predictive factors for hospitalization for acute exacerbation in a stable COPD population. The study recruited consecutive patients and followed them for an exacerbation, defined by American Thoracic Society criteria and using quarterly visits and hospitalization. The population was mostly men with a mean age of 64 years, severe COPD (mean FEV1 39% predicted), and mild-to-moderate hypoxemia (mean PaO2 66 mm Hg). Ten of 64 patients with COPD, who had severe hypoxemia, were receiving LTOT. This study found that the cumulative proportion of patients using home LTOT at 1-year follow-up, who were free of hospitalization due to an exacerbation (38.5%), was lower than the proportion of patients not using LTOT (77%, P = 0.01). Limitations of the study include its small sample size, lack of a random sampling scheme, unmeasured confounders, absence of multivariable analysis for home LTOT, and heterogeneity in the comparison.
An additional prospective cohort study identified by the systematic search, which examined factors associated with revisiting the emergency department for an exacerbation, was excluded because the authors considered all patients to be using oxygen therapy and did not describe the nature of the oxygen therapy. (37)
Safety
Only 1 study included in the Cochrane review described a lack of evidence for toxicity of LTOT. (10) No other individual study mentioned adverse effects of LTOT by severity of hypoxemia.
Analysis
Examination of the research question of effectiveness of LTOT compared with no LTOT in patients with COPD by severity of hypoxemia analyzed mortality, lung function, and exercise factors. Analysis of lung function and exercise factors uses change values, which include the maximum amount of data compared with analysis of follow-up data only and show the difference between mean baseline and follow-up values.
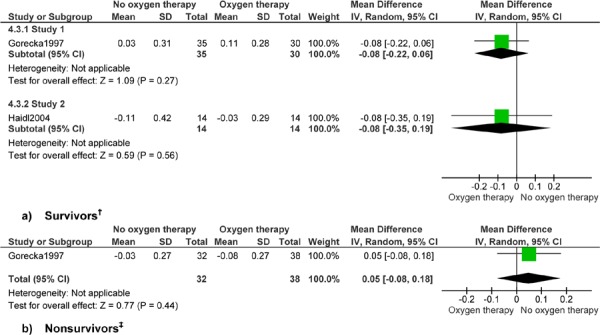
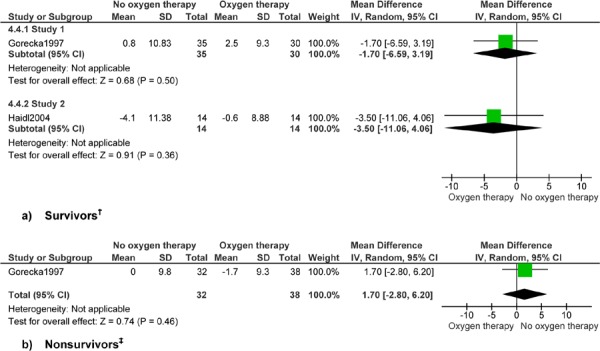
No data were available for exercise factors in patients with COPD who had severe hypoxemia. (10) Gorecka et al (24) followed patients for 7 years but did not specify the time at which lung function factors were measured. Haidl et al (25) measured lung function and exercise factors at 1 year. Lung function data are presented by survivors and nonsurvivors separately, as shown in the study by Gorecka et al, (24) and consistent with the presentation of data in the Cochrane review. (23) Presentation of results by survivors and nonsurvivors is a limitation of the published data on lung function. The results on lung function presented here are not combined with data from the Cochrane review, as the study populations differed in degree of hypoxemia. Exercise data in the study by Haidl et al (25) are not presented by survivors and nonsurvivors, but for survivors only.
Data from the 2 studies evaluating mild-to-moderate hypoxemia patients with COPD are not combined. A formal meta-analysis was not performed, nor was a summary estimate calculated, due to different follow-up lengths (7 vs. 3 years for mortality, and up to 7 years vs. 1 year for lung function), and the potential for clinical heterogeneity. Estimates for lung function and exercise factors are interpreted as the change over time for a given factor. Interpretation of the results differs based on the direction of change and the factor. A positive change over time is favourable for FEV1, FEV1/FVC, FVC, PaO2, and endurance time, suggesting an increase in lung function or exercise capacity. A negative change over time is favourable for PaCO2 and the dyspnea score, suggesting a decrease in adverse factors.
Results of the mortality analysis define a beneficial effect of LTOT compared with no LTOT as decreased risk, or an RR less than 1.0. Results of the lung function and exercise analysis results define a beneficial effect of LTOT compared with no LTOT as a mean difference that is a negative number less than 1.0. Authors were contacted for additional data as necessary. Measures of PaO2 and PaCO2 are considered indirect surrogate measures. The analyses are presented consistently in Figures 2-11 below.
Figure 2: Mortality (Number of Events)*.
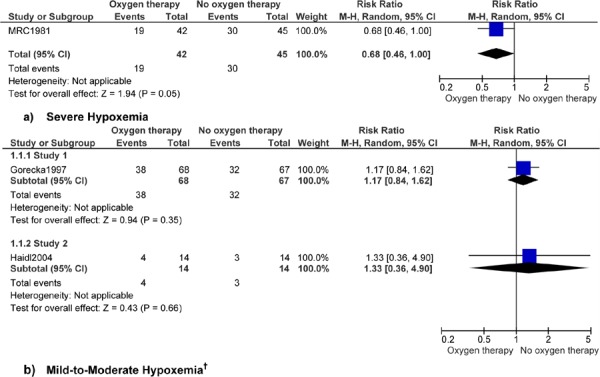
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Study 1 reports 7 years’ follow-up and study 2 reports 3 years’ follow-up.
Figure 3: Forced Expiratory Volume in One Second (Litres)*.
Abbreviations: CI, confidence interval; SD, standard deviation.
Study 1 reports up to 7 years’ follow-up and study 2 reports 1 year’s follow-up.
Gorecka et al (24) reports 7 years’ follow-up.
Figure 4: Forced Expiratory Volume in One Second (% Predicted)*.
Abbreviations: CI, confidence interval; SD, standard deviation.
Study 1 reports up to 7 years’ follow-up and study 2 reports 1 year’s follow-up.
Gorecka et al (24) reports 7 years’ follow-up.
Figure 5: Forced Expiratory Volume in One Second by Forced Vital Capacity (%)*.
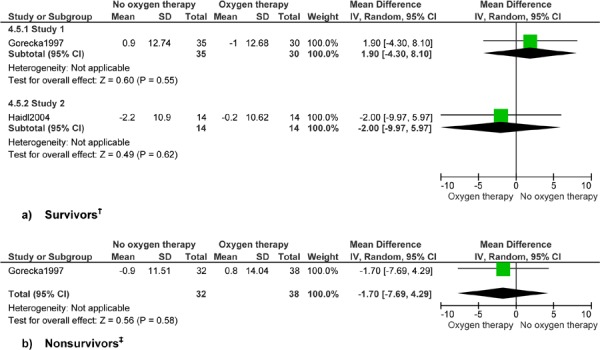
Abbreviations: CI, confidence interval; SD, standard deviation.
Study 1 reports up to 7 years’ follow-up and study 2 reports 1 year’s follow-up.
Gorecka et al reports 7 years’ follow-up.
Figure 6: Forced Vital Capacity (Litres)*.
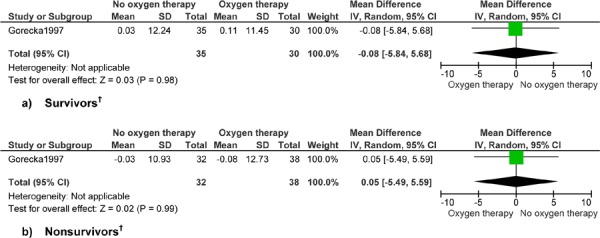
Abbreviations: CI, confidence interval; SD, standard deviation.
Gorecka et al (24) reports up to 7 years’ follow-up.
Figure 7: Forced Vital Capacity (% Predicted)*.
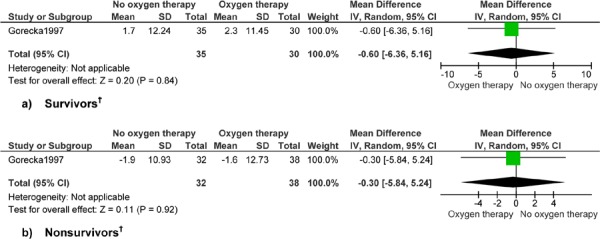
Abbreviations: CI, confidence interval; SD, standard deviation.
Gorecka et al (24) reports up to 7 years’ follow-up.
Figure 8: Arterial Pressure of Oxygen (mm Hg)*,†.
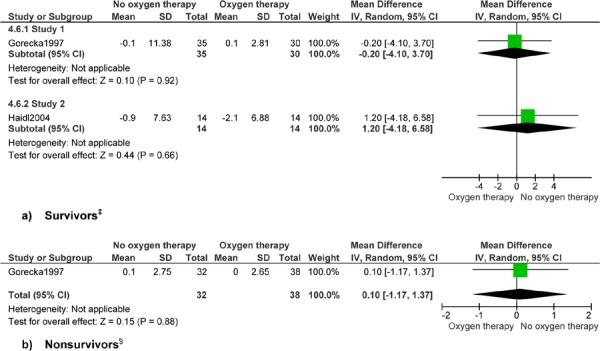
Abbreviations: CI, confidence interval; SD, standard deviation.
Surrogate outcome.
Study 1 reports up to 7 years’ follow-up and study 2 reports 1 year’s follow-up.
Gorecka et al reports (24) 7 years’ follow-up.
Figure 9: Arterial Pressure of Carbon Dioxide (mm Hg)*.
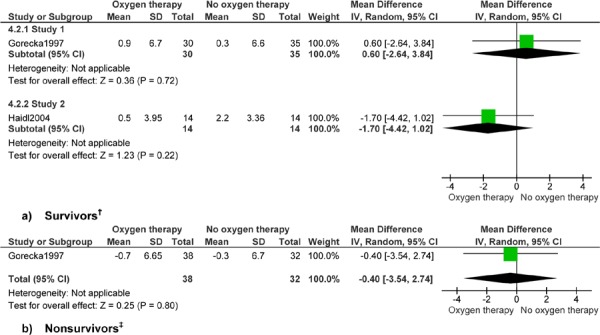
Abbreviations: CI, confidence interval; SD, standard deviation.
Study 1 reports up to 7 years’ follow-up and study 2 reports 1 year’s follow-up.
Gorecka et al (24) reports 7 years’ follow-up.
Figure 10: Dyspnea (Borg Scale)*†.
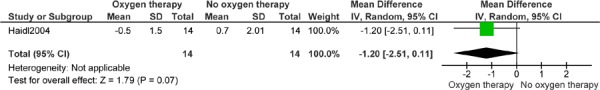
Abbreviations: CI, confidence interval; SD, standard deviation.
Haidl et al (25) reports 1 year’s follow-up.
Figure 11: Endurance Time (Minutes)*,†.
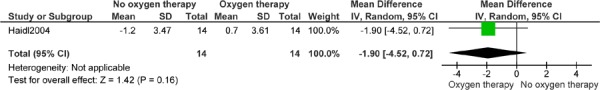
Abbreviations: CI, confidence interval; SD, standard deviation.
Haidl et al (25) reports 1 year’s follow-up.
Results of Analysis
Analysis of mortality data found a 32% decreased risk of mortality for patients with COPD who had severe hypoxemia and heart failure and used LTOT compared with patients not using LTOT (RR, 0.68; 95% CI, 0.46–1.00; P = 0.05). It is important to note, however, that the CI includes 1.0 and the statistical significance level is 0.05, suggesting no effect.
Analysis of data on lung function and exercise factors found no difference in change values over time for patients with COPD who had mild-to-moderate hypoxemia and received LTOT compared with those who did not receive LTOT. No clinical benefit of LTOT for patients with COPD and mild-to-moderate hypoxemia was seen for FEV1, FEV1/FVC, FVC, PaO2, PaCO2, dyspnea, or endurance time. Significant results for FEV1 and PaCO2 for patients with COPD and severe hypoxemia and heart failure have been discussed previously.
Summary of Literature Review
The methods used in the Cochrane review helped to address the research question in this evidence-based analysis. (23) Of the 2 studies of patients with severe hypoxemia, only 1 study examined continuous LTOT compared with no LTOT. (10) This study was analyzed separately. (10) Two studies comparing patients with mild-to-moderate hypoxemia who received LTOT with those who received no LTOT were analyzed separately from the 2 studies on nocturnal oxygen use. (24-27) Overall, 3 studies provided data that were useful for the analysis. (10;24;25) The results from the systematic review by Wilt et al (28) were consistent with the Cochrane review, (23) but the analysis aggregated the 2 studies of patients with severe hypoxemia. (10;12) In this evidence-based analysis, the 3 eligible studies identified in the Cochrane review were analyzed separately, due to heterogeneity in length of follow-up and severity of hypoxemia. (10;24;25)
Mortality
The Cochrane review (23) found a beneficial effect of continuous LTOT on survival compared with no LTOT in patients with severe hypoxemia and heart failure when considering the MRC (10) study. Study strengths were the RCT design, successful randomization with no baseline differences, definition of irreversible airway obstruction consistent with a diagnosis of COPD, and low attrition rate over the 5-year follow-up. Study limitations were the absence of survival analysis for the main comparison of interest (LTOT vs. no LTOT), non-standardized measurement of adherence across the 3 study sites, lack of information on the mean number of hours of oxygen therapy used, and the non-blinded nature of the study.
Adherence in the treatment group is difficult to assess and may have affected the results. It is not known if patients received at least 15 hours of oxygen. If adherence was less than ideal, the magnitude of effect may be greater than shown in the meta-analysis. Alternatively, given adequate adherence and no control group receiving LTOT, the effect shown in the Cochrane meta-analysis is a true effect, and continuous LTOT produced a 60% reduction in the risk of all-cause mortality for continuous LTOT compared with no LTOT (OR, 0.42; 95% CI, 0.18–0.98). (23)
Similarly, the analysis in this report showed a decreased risk of mortality, but the magnitude of effect was attenuated and the result was not significant, with a CI that included 1.0 and a statistical significance value of 0.05. Closer examination of the result from the Cochrane review indicates a borderline statistically significant result. A post hoc power calculation shows that a type 2 error occurred, as the study had only 46% power to detect a 20% difference between treatment and control groups with 43 patients per arm. Conversely, had there been 100 patients per group, the study would have had 81% power to detect the same difference.
Smoking is related to COPD mortality, (38;38) but a post hoc analysis found no difference between the number of smokers in the treatment and control groups at baseline and at the end of the study, where some patients had quit smoking (P > 0.05). (10) Similarly, acute exacerbations are related to accelerated decline in lung function and increased mortality, with the typical patient with COPD experiencing 2 exacerbations per year. (14) The authors reported no between-group differences in the number of days hospitalized due to exacerbations. (10)
Meta-analysis results of the Cochrane review found no difference in mortality between continuous LTOT and no LTOT in patients with mild-to-moderate hypoxemia, based on data from the Gorecka et al (24) and Haidl et al (25) studies. Individual analysis of these studies similarly found no between-group differences in mortality, although the study by Haidl et al (25) had a small sample size (N = 28), the randomization process was less detailed than in Gorecka et al (24), and 5 of 14 subjects in the control group required LTOT over the 3 years of follow-up. Mean use of LTOT was less than 15 hours/day in both studies, and exclusion criteria were not adequately detailed. Gorecka et al (24) did not state whether any patients in the control group began using LTOT over the 7-year follow-up period. Although study participants were not smoking at the start of the study, some patients resumed smoking by the end of the study; this information was known only for participants in the treatment group. A post hoc power calculation indicates a type 2 error due to small sample sizes.
An important difference between the study of severe hypoxemia (10) and the studies of mild-to-moderate hypoxemia (24;25) is the inclusion of patients with heart failure in the severe hypoxemia study. The severe hypoxemia study included patients with a severe cardiovascular comorbidity, whereas the mild-to-moderate hypoxemia studies may have included patients with less severe comorbidities. In addition, the mild-to-moderate hypoxemia studies included patients that were less severe than those defining eligibility for LTOT according to existing guidelines. The biological cause-effect link between some comorbidities, COPD, and mortality is not clear, and the inaccurate recording of cause of death in patients with COPD may be a limiting factor. (39)
The cause of death of most patients in the MRC (10) study who died was respiratory failure. The benefit of LTOT can therefore perhaps be described as preventing COPD-related deaths. (38) Most deaths in the study by Gorecka et al (24) were due to COPD. Analysis for all-cause and COPD-related mortality, with well-defined exclusion criteria, would help to clarify pulmonary versus extrapulmonary benefits of LTOT in COPD. In addition, studies should include well-defined mortality endpoints and methods of ascertainment, such as use of death certificates or number and type of International Classification of Diseases codes used. (40) Appendix 3 summarizes quality assessment according to GRADE Evidence. The evidence for mortality among patients with COPD who had severe hypoxemia and among those with mild-to-moderate hypoxemia was graded as low quality.
Lung Function and Exercise Factors
Pathological changes characteristic of COPD include chronic inflammation and structural changes from repeated injury and repair. Affected sites include proximal and peripheral airways, lung parenchyma, and pulmonary vasculature. (3) Standard spirometry measures of pulmonary function can be used to assess the efficacy of treatment on lung function. Changes in arterial blood gases are important measures for interventions that affect respiratory drive, such as oxygen therapy, but arterial blood gases are considered surrogate outcomes. It is suggested that a change of 10 mm Hg for PaO2 and PaCO2 is clinically significant (Personal communication, clinical expert, April 13, 2011).
The analyses for change in lung function factors FEV1 and PaCO2 showed improvement over time in patients with COPD who had severe hypoxemia and survived more than 500 days. (10) No other differences among survivors were shown. No differences were shown for lung function factors among those dying within 500 days of the start of the study, except for FVC.
Analysis found no differences in lung function or exercise factors for patients with COPD with mild-to-moderate hypoxemia among survivors or nonsurvivors. (24) The main limitations in interpreting these analyses are the nonspecific time point at which lung function was measured and the subset analysis, which does not maintain successful randomization. Only the study by Haidl et al, (25) which assessed lung function and exercise factors at 1 year’s follow-up for all subjects, maintained randomization. In addition, measurement of exercise variables was not described in detail. Dyspnea was measured using the Borg scale, which is a validated and reproducible 10-point scale that assesses either perceived dyspnea or effort required during a formal exercise test. The exercise test in Haidl et al (25) was a formal laboratory-based test using a stationary bicycle. Ascertainment of maximal workload was not described in detail. (41) In Appendix 3, GRADE Quality Assessment graded the evidence for all lung function factors among patients with COPD who had severe hypoxemia as very low quality. No data were available for exercise factors for patients with COPD who had severe hypoxemia. The evidence for all lung function and exercise factors among patients with COPD who had mild-to-moderate hypoxemia was graded as very low quality.
Hospitalizations and Readmissions
Two prospective studies, 1 study for readmission and 1 study for hospitalization, were evaluated. The readmission study, (35) a well-designed prospective cohort study, found no effect of LTOT on risk of readmission. The observational nature of the study resulted in grading as low-quality evidence (Appendix 3). The prospective study on hospitalization, (36) an adequately designed study, found that LTOT increased the risk of hospitalization. The observational nature of the study, heterogeneity in the comparison (10 of 64 patients with severe hypoxemia used LTOT), and limited analysis resulted in grading as very low quality evidence (Appendix 3).
Health-Related Quality of Life
The concern about LTOT and quality of life in COPD patients is that home LTOT equipment, such as oxygen concentrators, may reduce quality of life by restricting mobility and producing noise. The relation between dyspnea, exercise limitation, anxiety and depression, muscle wasting, quality of life, and disability is complex in COPD patients with severe hypoxemia. Long-term oxygen therapy may have little effect on health status but may reduce anxiety. Reduced independence in patients with COPD may also be related to the degree of airflow obstruction, depression, and poor health status. (42)
Analysis of HRQOL is also an objective of this report. A modified literature search of MEDLINE only with no limits on date or study design identified 91 articles on quality of life outcomes in patients with COPD who used LTOT. Hand-searching reference lists also identified potentially appropriate studies. One health technology assessment on a related topic, portable oxygen therapy, was also identified.
Studies were included in the analysis if:
study design and methods were clearly described,
the study assessed HRQOL using the St. George’s Respiratory Questionnaire (SGRQ) or the Chronic Respiratory Questionnaire (CRQ), and
the study was a health technology assessment, systematic review, RCT, or observational study.
Studies that did not meet the inclusion criteria were excluded. Studies were also excluded from analysis for the following reasons:
nonrelevant outcome measures, such as psychiatric measures or non-standardized measures (n = 4),
nonrelevant comparison, such as different oxygen delivery systems or nocturnal oxygen (n = 2),
heterogeneity in the comparison, such as patients with COPD, severe hypoxemia, and LTOT compared with patients with mild-to-moderate hypoxemia and no LTOT (n = 4),
no information on LTOT (n = 1),
previously used LTOT (n = 1).
Summary of the Evidence
Nine studies were reviewed in detail and 3 observational studies were eligible and appropriate for review. One health technology assessment of portable oxygen therapy was also identified, but it was not considered relevant. (43) A review of the references of this study did not provide any additional studies. None of the 91 citations identified in the modified literature search were eligible and included. From 2 of the 3 observational studies identified, relevant LTOT information was abstracted, providing a before-and-after comparison for patients with COPD using LTOT, which were reviewed and are summarized in Appendix 3, Table A12.
Chronic Respiratory Questionnaire
A prospective study of 68 consecutive patients with COPD, who were referred to a regional oxygen service in New Zealand for assessment for LTOT, evaluated changes in HRQOL with a 6-month follow-up. (44) The study compared patients who were eligible for LTOT to those who were not eligible (no LTOT) for changes in HRQOL. Eligible patients were clinically stable for at least 2 months. Patients were ineligible if they had major but unspecified comorbidities, were smokers, or were unable to complete the questionnaire. Ambulatory oxygen was not provided. A total of 43 patients used LTOT for a mean 14.6 hours daily by meter reading. Mean baseline PaO2 was 51.8 mm Hg for the LTOT group and 66 mm Hg for the no-LTOT group (P < 0.001). The percent predicted FEV1 was 31.7% for the LTOT group and 29.6% for the no-LTOT group.
The Chronic Respiratory Questionnaire measured HRQOL at baseline and at 2 and 6 months. The mean change of the total score (possible score 20-140) from baseline was calculated. Increasing CRQ scores indicate improvements in HRQOL. Patients using LTOT had statistically significant improvements in HRQOL at 2 and 6 months. Mean change scores at 2 months were 2.36 (95% CI, 0.48−4.23) for dyspnea, 2.00 (95% CI, 0.57−3.43) for fatigue, 2.43 (95% CI, 0.36−4.50) for emotional function, and 1.55 (95% CI, 0.21−2.88) for mastery. Mean change scores at 6 months were similar, with only emotional function lacking statistical significance. The mean change total CRQ score for the LTOT group was 8.10 (95% CI, 3.02−13.17) at 2 months and 9.26 (95% CI, 2.37−16.15) at 6 months. Health-related quality of life improved with LTOT. The authors also concluded that the benefits of LTOT should be expanded to include HRQOL. (44)
A prospective study in Australia (45) followed 114 patients (59 men and 55 women) with COPD, who used LTOT including ambulatory oxygen, and assessed changes in CRQ at 3, 6, and 12 months. No exclusions were made for concomitant disease. Patients used LTOT for a mean 19 hours per day (Personal communication, January 17, 2011). At baseline, mean PaO2 was 54 mm Hg in men and 53.3 mm Hg in women, and mean FEV1 was 0.5 L in men and 0.4 L in women.
The minimal clinically important difference (MCID) in CRQ scores is 0.5. (46) In men, fatigue improved by at least 0.5 from baseline at 3, 6, and 12 months, a statistically significant improvement. A trend toward improvement was also seen for emotional function and mastery, but the results were not statistically significant. In women, mastery improved by at least 0.5 from baseline at 3, 6, and 12 months, which are statistically significant changes. Emotional function and fatigue also significantly improved by at least 0.5 from baseline at 3 and 6 months. (45) Improvements in emotional function and fatigue at 12 months were not significant, possibly due to reductions in sample size.
During the first 12 months of the study, 17 patients had not completed the 12-month follow-up, and 36 patients were lost to follow-up. Of the 36 total patients lost to follow-up, 16 men (44.4%) and 8 women (22.2%) were reported to have died, with remaining differences in loss to follow-up not reported by sex. These reasons included cessation of LTOT use, mental deterioration, refusal to continue, and transfer to another hospital. Overall, men and women using LTOT experienced statistically significant and clinically relevant improvements in HRQOL. (45)
St. George’s Respiratory Questionnaire
A prospective study in the United Kingdom (47) examined changes in HRQOL among 36 patients with COPD who were referred to outpatient chest clinics for assessment for LTOT, comparing patients who were eligible for LTOT with those who were not eligible (no LTOT). Exclusion criteria were age less than 45 years and inability to understand or complete the quality-of-life questionnaires. Included patients were free from acute exacerbations for at least 3 weeks. Ambulatory oxygen was not provided. Follow-up duration was 6 months. Mean PaO2 at baseline was 52.5 mm Hg for the LTOT group and 62.3 mm Hg for the no-LTOT group (P < 0.001). The percent predicted FEV1 was 40% for the LTOT group and 43% for the no-LTOT group. The 19 patients in the LTOT group used oxygen for a mean 16.7 hours per day according to meter readings.
The St. George’s Respiratory Questionnaire measured HRQOL at baseline, at 2 weeks, and at 3 and 6 months. A higher SGRQ score indicates poorer HRQOL (Table 2). A negative mean change in SGRQ score from baseline to follow-up indicates better HRQOL (Table 3). For the SGRQ, the MCID is 4 (Table 3). (48)
Table 2: Comparison of Health-Related Quality of Life Between LTOT and No-LTOT Groups (St. George’s Respiratory Questionnaire)*,†.
| LTOT Group | No-LTOT Group | P Value | |||
|---|---|---|---|---|---|
| Mean Total SGRQ Score |
Standard Deviation |
Mean Total SGRQ Score |
Standard Deviation |
||
| Baseline | 61.8 | 18.3 | 45.8 | 15.5 | 0.008 |
| 2 Weeks | 55.0 | 13.7 | 41.8 | 17.7 | ? |
| 3 Months | 55.9 | 12.1 | 44.3 | 17.4 | ? |
| 6 Months | 60.5 | 16.5 | 48.7 | 17.3 | ? |
Abbreviations: LTOT, long-term oxygen therapy; SGRQ, St. George’s Respiratory Questionnaire; ?, unknown information.
Source: Okubadejo et al, 1996 (47)
Table 3: Change in Health-Related Quality of Life Results From Baseline (St. George’s Respiratory Questionnaire)*,†.
| Change From Baseline to Follow-Up: Mean (SD) | |||
|---|---|---|---|
| SGRQ | 2 Weeks | 3 Months | 6 Months |
| SGRQ Total | −6.8 (16.5) | −5.9 (16.1) | −1.3 (17.5) |
| Symptoms | 3.3 (18.3) | 3.0 (17.5) | 4.1 (18.1) |
| Activities | 1.7 (18.3) | −4.2 (16.9) | 1.8 (18.1) |
| Impacts | −14.1 (22.7) | −9.0 (22.8) | −4.1 (23.9) |
Abbreviations: SGRQ, St. George’s Respiratory Questionnaire; SD, standard deviation.
Source: Okubadejo et al, 1996 (47)
The LTOT group had statistically significantly higher SGRQ total scores at all time points than did the no-LTOT group (Table 2), indicating poorer quality of life. At 2 weeks, there was no statistically significant difference in improvement from baseline in SGRQ score between the LTOT group (6.8, SD 12.7) and the no-LTOT group (4.0, SD 10.7, P = 0.48). Similarly, at 6 months, the improvement from baseline in HRQL in the LTOT group (1.3, SD 14.5) did not differ significantly from that in the no-LTOT group (2.9, SD 13.4, P = 0.38). The authors concluded from the nonsignificant differences in changes in SGRQ total over time between LTOT and no-LTOT that LTOT does not adversely affect quality of life. (47)
The analysis in this report uses a before-and-after design to examine the LTOT cohort by itself and to calculate the mean change in SGRQ total score; mean change in the SGRQ domains of symptoms, activities, and impacts; and mean change SD, using a correlation of 0.5 (Table 3).
When examining change scores and SGRQ, a negative mean change from baseline to follow-up indicates better HRQL, and for SGRQ, the MCID is four. (48) The analysis demonstrates that LTOT use produces clinically important and statistically significant improvements in the SGRQ domain of impacts at 2 weeks, based on calculation of 95% CIs, which are not shown. Use of LTOT is also associated with a trend for clinically important improvement in at least 1 of the SGRQ domains of symptoms, activities, or impacts at 2 weeks and 3 and 6 months, and for SGRQ total score at 2 weeks and 3 months.
In summary, HRQOL results for observational studies are graded as low quality of evidence for CRQ and as very low quality of evidence for SGRQ. Quality assessment uses GRADE Evidence (Appendix 3). It is important to note that ethical constraints of not providing LTOT to eligible patients with COPD prohibit future studies from examining LTOT outcomes in an ideal way.
Economic Analysis
The results of the economic analysis are summarized in issue 12 of the COPD series entitled Cost-Effectiveness of Interventions for Chronic Obstructive Pulmonary Disease Using an Ontario Policy Model. This report can be accessed at: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_COPD_Economic_March.pdf.
Conclusions
Based on low quality of evidence, LTOT (~ 15 hours/day) decreases all-cause mortality in patients with COPD who have severe hypoxemia (PaO2 ~ 50 mm Hg) and heart failure.
The effect for all-cause mortality had borderline statistical significance when the control group was no LTOT: one study.
Based on low quality of evidence, there is no beneficial effect of LTOT on all-cause mortality at 3 and 7 years in patients with COPD who have mild-to-moderate hypoxemia (PaO2 ~ 59-65 mm Hg).
Based on very low quality of evidence, there is some suggestion that LTOT may have a beneficial effect over time on FEV1 and PaCO2 in patients with COPD who have severe hypoxemia and heart failure: improved methods are needed.
Based on very low quality of evidence, there is no beneficial effect of LTOT on lung function or exercise factors in patients with COPD who have mild-to-moderate hypoxemia, whether survivors or nonsurvivors are assessed.
Based on low to very low quality of evidence, LTOT does not prevent readmissions in patients with COPD who have severe hypoxemia. Limited data suggest LTOT increases the risk of hospitalization.
Limited work has been performed evaluating the safety of LTOT by severity of hypoxemia.
Based on low to very low quality of evidence, LTOT may have a beneficial effect over time on HRQOL in patients with COPD who have severe hypoxemia. Limited work using disease-specific instruments has been performed.
Ethical constraints of not providing LTOT to eligible patients with COPD prohibit future studies from examining LTOT outcomes in an ideal way.
Existing Guidelines for Long-Term Oxygen Therapy
International guidelines for use of LTOT for stable COPD, (2) which are based on the severity of hypoxemia, differ (Table 4).
Table 4: International Guidelines for Use of Long-term Oxygen Therapy in Patients with Stable Chronic Obstructive Pulmonary Disease*.
| Guideline | Severe Hypoxemia |
Moderate Hypoxemia | No Hypoxemia |
|---|---|---|---|
| Ministry of Health and Long-Term Care (15) | PaO2 ≤ 55 mm Hg or SpO2 ≤ 88% | PaO2 56–60 mm Hg plus cor pulmonale, pulmonary hypertension, persistent erythrocytosis, exercise-limiting hypoxemia documented to improve with supplemental oxygen, or nocturnal hypoxemia | Funding assistance is provided to individuals who are not hypoxemic at rest but who exhibit exertional hypoxemia on room air and improved exercise tolerance with oxygen |
| ATS-ERS (49) | PaO2 < 55 mm Hg or SpO2 < 88% | PaO2 55–59 mm Hg or SpO2 of 89% plus at least one of cor pulmonale, peripheral edema, or hematocrit > 55% | PaO2 ≥ 60 mm Hg or SpO2 > 90% with severe nocturnal desaturation and lung-related dyspnea responsive to oxygen |
| GOLD (50) | PaO2 ≤ 55 mm Hg or SpO2 ≤ 88%, with or without hypercapnia | PaO2 56–59 mm Hg or SpO2 of 88% with evidence of pulmonary hypertension, peripheral edema suggesting congestive cardiac failure, or polycythemia (hematocrit > 55%) | No recommendation |
| NICE (51) | PaO2 < 55 mm Hg | PaO2 56–59 mm Hg plus secondary polycythemia, nocturnal hypoxemia (SpO2 < 90% for >30% of the time), peripheral edema, or pulmonary hypertension | No recommendation |
| TSA-NZ (52) | PaO2<55 mm Hg | PaO2 56–59 mm Hg, plus evidence of hypoxic organ damage including right heart failure, pulmonary hypertension, or polycythemia | Nocturnal oxygen may be indicated if SpO2 ≤ 88% for > 30% sleep time, or hypoxia-related sequelae |
| AIPO (2) | PaO2 < 55 mm Hg | PaO2 55–60 mm Hg, plus at least one of hematocrit > 55%, signs of pulmonary hypertension, signs of hypoxia such as peripheral edema or right heart failure or mental decline, and ischemic heart failure | Intermittent oxygen may be indicated for SpO2 < 90% for > 30% sleep time or exercise-related desaturation |
Abbreviations: AIPO, Associazione Italiana Pneumologi Ospedalieri; ATS-ERS, American Thoracic Society and European Respiratory Society; GOLD, Global Initiative for Obstructive Lung Disease; mm Hg, millimetres of mercury; NICE, National Institute for Health and Clinical Excellence; PaCO2, arterial pressure of carbon dioxide; PaO2, arterial pressure of oxygen; SpO2, oxygen saturation level measured by pulse oximetry TSA-NZ, Thoracic Society of Australia and New Zealand.
Glossary
- 6 Minute Walking Test (6MWT)
A measure of exercise capacity which measures the distance that a patient can quickly walk on a flat, hard surface in a period of 6 minutes. A widely used outcome measure in respiratory rehabilitation of patients with COPD.
- Acute exacerbations of chronic obstructive pulmonary disease (AECOPD)
A change in baseline symptoms that is beyond day-to-day variation, particularly increased breathlessness, cough, and/or sputum, which has an abrupt onset.
- Admission avoidance hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and avoid admission to hospital. After patients are assessed in the emergency department for an acute exacerbation, they are prescribed the necessary medications and additional care needed (e.g., oxygen therapy) and then sent home where they receive regular visits from a medical professional until the exacerbation has resolved.
- Ambulatory oxygen therapy
Provision of oxygen therapy during exercise and activities of daily living for individuals who demonstrate exertional desaturation.
- Bilevel positive airway pressure (BiPAP)
A continuous positive airway pressure mode used during noninvasive positive pressure ventilation (see definition below) that delivers preset levels of inspiratory and expiratory positive airway pressure. The pressure is higher when inhaling and falls when exhaling, making it easier to breathe.
- Cost-effectiveness acceptability curve (CEAC)
A method for summarizing uncertainty in estimates of cost-effectiveness.
- Cor pulmonale
Right heart failure, as a result of the effects of respiratory failure on the heart.
- Dyspnea
Difficulty breathing or breathlessness.
- Early discharge hospital-at-home program
Treatment program for patients experiencing acute exacerbations of COPD which allows patients to receive treatment in their home and decrease their length of stay in hospital. After being assessed in the emergency department for acute exacerbations, patients are admitted to the hospital where they receive the initial phase of their treatment. These patients are discharged early into a hospital-at-home program where they receive regular visits from a medical professional until the exacerbation has resolved.
- Forced expiratory volume in 1 second (FEV1)
A measure of lung function used for COPD severity staging; the amount of air that can be forcibly exhaled from the lungs in the first second of a forced exhalation.
- Forced vital capacity (FVC)
The amount of air that can be forcibly exhaled from the lungs after taking the deepest breath possible.
- Fraction of inspired oxygen (FiO2)
The percentage of oxygen participating in gas exchange.
- Hypercapnia
Occurs when there is too much carbon dioxide in the blood (arterial blood carbon dioxide > 45 to 60 mm Hg).
- Hypopnea
Slow or shallow breathing.
- Hypoxemia
Low arterial blood oxygen levels while breathing air at rest. May be severe (PaO2 ≤ 55 mm Hg), moderate (56 mm Hg ≤ PaO2 < 65 mm Hg), or mild-to-moderate (66 mm Hg < PaO2 ≤ 74 mm Hg).3
- Incremental cost-effectiveness ratio (ICER)
Ratio of the change in costs of a therapeutic intervention to the change in effects of the intervention compared to the alternative (often usual care).
- Intention-to-treat analysis (ITT)
An analysis based on the initial treatment the participant was assigned to, not on the treatment eventually administered.
- Invasive mechanical ventilation (IMV)
Mechanical ventilation via an artificial airway (endotracheal tube or tracheostomy tube).
- Long-term oxygen therapy (LTOT)
Continuous oxygen use for about 15 hours per day. Use is typically restricted to patients fulfilling specific criteria.
- Multidisciplinary care
Defined as care provided by a team (compared to a single provider). Typically involves professionals from a range of disciplines working together to deliver comprehensive care that addresses as many of the patient’s health care and psychosocial needs as possible.
- Nicotine replacement therapy (NRT)
The administration of nicotine to the body by means other than tobacco, usually as part of smoking cessation.
- Noninvasive positive pressure ventilation (NPPV)
Noninvasive method of delivering ventilator support (without the use of an endotracheal tube) using positive pressure. Provides ventilatory support through a facial or nasal mask and reduces inspiratory work.
- Partial pressure of carbon dioxide (PaCO2)
The pressure of carbon dioxide dissolved in arterial blood. This measures how well carbon dioxide is able to move out of the body.
- Partial pressure of oxygen (PaO2)
The pressure of oxygen dissolved in arterial blood. This measures how well oxygen is able to move from the airspace of the lungs into the blood.
- Palliative oxygen therapy
Use of oxygen for mildly hypoxemic or nonhypoxemic individuals to relieve symptoms of breathlessness. Used short term. This therapy is “palliative” in that treatment is not curative of the underlying disease.
- Pulmonary rehabilitation
Multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy. Exercise training is the cornerstone of pulmonary
- Pulse oximetry
A noninvasive sensor, which is attached to the finger, toe, or ear to detect oxygen saturation of arterial blood.
- Quality-adjusted life-years (QALYs)
A measure of disease burden that includes both the quantity and the quality of the life lived that is used to help assess the value for money of a medical intervention.
- Respiratory failure
Respiratory failure occurs when the respiratory system cannot oxygenate the blood and/or remove carbon dioxide from the blood. It can be either acute (acute respiratory failure, ARF) or chronic, and is classified as either hypoxemic (type I) or hypercapnic (type II) respiratory failure. Acute hypercapnic respiratory failure frequently occurs in COPD patients experiencing acute exacerbations of COPD.
- Short-burst oxygen therapy
Short-duration, intermittent, supplemental oxygen administered either before or after exercise to relieve breathlessness with exercise.
- Sleep apnea
Interruption of breathing during sleep due to obstruction of the airway or alterations in the brain. Associated with excessive daytime sleepiness.
- Smoking cessation
The process of discontinuing the practice of inhaling a smoked substance.
- Spirometry
The gold standard test for diagnosing COPD. Patients breathe into a mouthpiece attached to a spirometer which measures airflow limitation.
- SpO2
Oxygen saturation of arterial blood as measured by a pulse oximeter.
- Stable COPD
The profile of COPD patients which predominates when patients are not experiencing an acute exacerbation.
- Supplemental oxygen therapy
Oxygen use during periods of exercise or exertion to relieve hypoxemia.
- Telemedicine (or telehealth)
Refers to using advanced information and communication technologies and electronic medical devices to support the delivery of clinical care, professional education, and health-related administrative services.
- Telemonitoring (or remote monitoring)
Refers to the use of medical devices to remotely collect a patient’s vital signs and/or other biologic health data and the transmission of those data to a monitoring station for interpretation by a health care provider.
- Telephone only support
Refers to disease/disorder management support provided by a health care provider to a patient who is at home via telephone or videoconferencing technology in the absence of transmission of patient biologic data.
- Ventilator-associated pneumonia (VAP)
Pneumonia that occurs in patients undergoing mechanical ventilation while in a hospital.
Acknowledgements
Medical Information Officer
Kellee Kaulback
Editorial Staff
Joanna Gorski
Irina Alecu
COPD Expert Advisory Panel
The role of the expert panel was to provide direction on the scope of the project and the relevant outcomes measures of effectiveness, to review the evidence-based analyses and to identify any societal or systemic issues that are relevant to intervention effectiveness. However, the statements, conclusions and views expressed in this report do not necessarily represent the views of the expert panel members.
Jeremy Grimshaw, MD, MBChB, PhD (Chair)
Senior Scientist, Ottawa Hospital Research Institute Professor, Department of Medicine, University of Ottawa
Dina Brooks, PhD
Professor, Department of Physical Therapy, University of Toronto
Debbie Coutts, RRT, CRE
Andrea Gershon, MD, MSc, FRCP(C)
Scientist, Institute for Clinical Evaluative Sciences Respirologist, Sunnybrook Health Sciences Centre Assistant Professor, Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto
Mita Giacomini, BSc, MPH, MA, PhD
Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Ron Goeree, BA, MA
Director, PATH Research Institute, St. Joseph’s Hospital (Hamilton)
Associate Professor, Department of Clinical Epidemiology & Biostatistics, McMaster University
Roger Goldstein, MBCHB, FRCP(C), FRCP(UK)
NSA Chair in Respiratory Rehabilitation Research
Director, Respiratory Services, and Senior Scientist, West Park Healthcare Centre
Professor, Medicine and Physical Therapy, University of Toronto
Alan G Kaplan, MD, CCFP(EM), FCFP
Chairperson, Family Physician Airways Group of Canada
Chairperson, Special Interest Focused Care Group in Respiratory Medicine, College of Family Physicians
of Canada Clinical Lecturer, Department of Family and Community Medicine, University of Toronto
DE O’Donnell, MD, FRCP(C)
Director, COPD Centre, Kingston General Hospital Professor, Department of Medicine, Queen’s University
Asad Razzaque, MD
Family Physician
Holger Schünemann, MD, PhD, MSc, FRCP(C)
Michael Gent Chair in Healthcare Research
Chair, Department of Clinical Epidemiology & Biostatistics, McMaster University
Professor, Department of Clinical Epidemiology & Biostatistics and Medicine, McMaster University
Tasnim Sinuff, MD, PhD, FRCP(C)
Clinician Scientist, Sunnybrook Health Sciences Centre Assistant Professor, Department of Medicine, University of Toronto
Laura Watling, RRT, BSc(HK)
Clinical Practice Leader/Clinical Coordinator, Respiratory Therapy, West Park Healthcare Centre
Appendices
Appendix 1: Literature Search Strategies
Search date: September 8, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1996 to August Week 4 2010> Search Strategy:
-------------------------------------------------------------
exp Pulmonary Disease, Chronic Obstructive/ (13896)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (14772)
(copd or coad).ti,ab. (13084)
chronic airflow obstruction.ti,ab. (110)
exp Emphysema/ (2921)
((chronic adj2 bronchitis) or emphysema).ti,ab. (8434)
or/1-6 (29825)
exp Oxygen Inhalation Therapy/ (6948)
exp Oxygen/ (56003)
(oxygen adj2 (therap* or supplement* or portab* or ambulatory)).ti,ab. (5161)
or/8-10 (63799)
7 and 11 (1875)
limit 12 to (english language and humans and yr=”2007 -Current”) (399)
Database: EMBASE <1980 to 2010 Week 35> Search Strategy:
-------------------------------------------------------------
exp chronic obstructive lung disease/ (47610)
(chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (25725)
(copd or coad).ti,ab. (20981)
chronic airflow obstruction.ti,ab. (549)
exp emphysema/ (25443)
exp chronic bronchitis/ (6546)
((chronic adj2 bronchitis) or emphysema).ti,ab. (25378)
or/1-7 (87489)
exp oxygen therapy/ (26313)
exp OXYGEN/ (112232)
(oxygen adj2 (therap* or supplement* or portab* or ambulatory)).ti,ab. (10687)
or/9-11 (135284)
8 and 12 (5682)
limit 13 to (human and english language and yr=”2007 -Current”) (946)
CINAHL
| # | Query | Results |
| S12 | S6 and S10 Limiters - Published Date from: 20070101-20101231; | 151 |
| S11 | S6 and S10 | 573 |
| S10 | S7 or S8 or S9 | 6119 |
| S9 | oxygen therap* or supplement* oxygen or therapeutic oxygen or portab* oxygen or ambulatory oxygen | 3585 |
| S8 | (MH ”Oxygen+”) | 2379 |
| S7 | (MH ”Oxygen Therapy+”) | 3501 |
| S6 | S1 or S2 or S3 or S4 or S5 | 7364 |
| S5 | chronic bronchitis or emphysema | 1575 |
| S4 | (MH ”Emphysema+”) | 964 |
| S3 | copd or coad | 4065 |
| S2 | (chronic obstructive and (lung* or pulmonary or airway* or airflow or respiratory) and (disease* or disorder*)) | 5571 |
| S1 | (MH ”Pulmonary Disease, Chronic Obstructive+”) | 4315 |
Appendix 2: GRADE Evidence Tables
Table A1: GRADE Evidence Table for Long-Term Oxygen Therapy and Mortality in Patients With Severe Hypoxemia*.
| LTOT Versus No LTOT: Severe Hypoxemia for Patients with COPD | |||||
|---|---|---|---|---|---|
| Outcomes | Illustrative Comparative Risks (95% CI) | Relative Effect (95% CI) |
No. of Participants (Studies) |
Quality of Evidence (GRADE)† |
|
| Assumed Risk | Corresponding Risk | ||||
|
Mortality MRC (10) |
Study Population | RR 0.68 (0.46−1) |
87 (1 study) | ⊕⊕ Low‡,§,║,¶,# | |
| 667 per 1000 | 454 per 1000 (307–667) | ||||
| Medium-Risk Population | |||||
| 667 per 1000 | 454 per 1000 (307–667) | ||||
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen therapy; MRC, Medical Research Council; no., number; RR, relative risk.
Specific weighting, for all (−2 overall).
Study design limitations include measurement and reporting of adherence, such as lack of standardized assessment of oxygen therapy and no information on mean hours of use. (−1/2)
This study included a highly selected group of patients with COPD, heart failure, and severe hypoxemia. Ascertainment of death was not described in detail. (did not contribute to GRADE)
Allocation concealment was not addressed. (−1/2)
Not blinded. (did not contribute to GRADE for all-cause mortality)
Sparse data. Type 2 error not excluded. (−1)
Table A2: GRADE Evidence Table for Long-Term Oxygen Therapy and Mortality in Patients With Mild-to-Moderate Hypoxemia*.
| LTOT Versus No LTOT: Mild-to-Moderate Hypoxemia for Patients with COPD | |||||
|---|---|---|---|---|---|
| Outcomes | Illustrative Comparative Risks (95% CI) | Relative Effect (95% CI) |
No of Participants (studies) |
Quality of Evidence (GRADE)† |
|
| Assumed Risk | Corresponding Risk | ||||
| Mortality: Study 1 | Study Population | RR 1.17 (0.84−1.62) |
135 (1 study) |
⊕⊕Low‡,§,║,¶,#,††,‡‡ | |
| 478 per 1000 | 559 per 1000 (402–774) | ||||
| 7 years’ follow-up | Medium-Risk Population | ||||
| Gorecka et al (24) | 478 per 1000 | 559 per 1000 (402–774) | |||
| Mortality: Study 2 | Study Population | RR 1.33 (0.36- 4.9) |
28 (1 study) |
⊕⊕Low‡,§,║,¶,**,††,‡‡, §§ | |
| 214 per 1000 | 285 per 1000 (77–1000) | ||||
| 3 years’ follow-up | Medium-Risk Population | ||||
| Haidl et al (25) | 214 per 1000 | 285 per 1000 (77–1000) | |||
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen therapy; no., number; RR, relative risk.
Specific weighting, for all (−2 overall).
Oxygen use was inadequate according to study protocol. (−1/4 or −1/3)
Allocation concealment was not addressed. (−1/4 or −1/3)
Exclusion criteria were not adequately detailed. (did not contribute to GRADE)
Ascertainment of mortality was not described in detail. (did not contribute to GRADE)
It was not known whether or not individuals in the control arm received LTOT during follow-up. (−1/3)
High numbers crossing over to LTOT in the control arm. (−1/4)
Process of generating randomization not adequately described. (−1/4)
Not blinded. (did not contribute to GRADE for all-cause mortality)
Sparse data. (−1)
Table A3: GRADE Evidence Table for Long-Term Oxygen Therapy and Lung Function in Patients With Severe Hypoxemia (Survivors and Nonsurvivors)*,†.
| Outcomes | Illustrative Comparative Risks (95% CI) | No. of Participants (Studies) |
Quality of Evidence (GRADE)‡ |
|
|---|---|---|---|---|
| Corresponding Risk: Intervention Groups | ||||
| Mean FEV1 (L) | Nonsurvivors | 0.11 lower (0.27 lower to 0.05 higher) | 21 (1 study) | ⊕Very low§,║,¶,#,**,†† |
| Survivors | 0.08 higher (0.04 to 0.12 higher) | 40 (1 study) | ⊕Very low§,║,¶,#,**,†† | |
| Mean FVC (L) | Nonsurvivors | 0.56 higher (0.12 to 1.00 higher) | 21 (1 study) | ⊕Very low§,║,¶,#,**,†† |
| Survivors | 0.05 higher (0.89 lower to 0.99 higher) | 40 (1 study) | ⊕Very low§,║,¶,#,**,†† | |
| Mean PaO2 (mm Hg) | Nonsurvivors | 4.56 higher (1.04 lower to 10.16 higher) | 19 (1 study) | ⊕Very low§,║,¶,#,**,†† |
| Survivors | 1.07 higher (1.24 lower to 3.38 higher) | 40 (1 study) | ⊕Very low§,║,¶,#,**,†† | |
| Mean PaCO2 (mm Hg) | Nonsurvivors | 0.96 lower (8.53 lower to 6.61 higher) | 19 (1 study) | ⊕Very low§,║,¶,#,**,†† |
| Survivors | 2.16 lower (4.04 to 0.28 lower) | 39 (1 study) | ⊕Very low§,║,¶,#,**,†† | |
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; mm Hg, millimetres of mercury; no., number; PaCO2, arterial pressure of carbon dioxide; PaO2, arterial pressure of oxygen; RR, relative risk.
MRC et al (10).
Specific weighting, for all (−3 overall).
Study design limitations include the measurement and reporting of compliance such as the lack of standardized assessment of oxygen therapy and no information on the mean hours of use. (−1/3)
Subgroup analysis on men lacks the benefits of randomization. (−1)
Time point of lung function measurements not specified in detail. (−1/3)
Standard spirometry not described in detail. (did not contribute to GRADE)
Not blinded. (−1/3)
Sparse data. (−1)
Table A4: GRADE Evidence Table for Long-Term Oxygen Therapy and Lung Function in Survivors, Mild-to-Moderate Hypoxemia*.
| Outcomes | Illustrative Comparative Risks (95% CI) | No. of Participants (Studies) |
Quality of Evidence (GRADE)† |
|
|---|---|---|---|---|
| Corresponding Risk: Intervention Groups | ||||
| Mean FVC % | Study 1 | 0.6 lower (6.36 lower to 5.16 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Mean PaCO2 (mm Hg) | Study 1 | 0.6 higher (2.64 lower to 3.84 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Study 2 | 1.7 lower (4.42 lower to 1.02 higher) | 28 (1 study) | ⊕ Very low#,**,††,§§,║║ | |
| Mean FEV1 (L) | Study 1 | 0.08 lower (0.22 lower to 0.06 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Study 2 | 0.08 lower (0.35 lower to 0.19 higher) | 28 (1 study) | ⊕ Very low#,**,††,§§,║║ | |
| Mean FEV1 % Predicted | Study 1 | 1.7 lower (6.59 lower to 3.19 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Study 2 | 3.5 lower (11.06 lower to 4.06 higher) | 28 (1 study) | ⊕ Very low#,**,††,§§,║║ | |
| Mean FEV1/FVC | Study 1 | 1.9 higher (4.3 lower to 8.1 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Study 2 | 2 lower (9.97 lower to 5.97 higher) | 28 (1 study) | ⊕ Very low#,**,††,§§,║║ | |
| Mean PaO2 (mm Hg) | Study 1 | 0.2 lower (4.1 lower to 3.7 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Study 2 | 1.2 higher (4.18 lower to 6.58 higher) | 28 (1 study) | ⊕ Very low#,**,††,§§,║║ | |
| Mean FVC (L) | Study 1 | 0.08 lower (5.84 lower to 5.68 higher) | 65 (1 study) | ⊕ Very low║,¶,#,**,††,‡‡,§§ |
| Mean Endurance Time (minutes) | Study 2 | 1.9 lower (4.52 lower to 0.72 higher) | 28 (1 study) | ⊕ Very low#,††, ║║,¶¶ |
| Mean Dyspnea (Borg Scale) | Study 2 | 1.2 lower (2.51 lower to 0.11 higher) | 28 (1 study) | ⊕ Very low#,††, ║║,¶¶ |
Abbreviations: CI, confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; mm Hg, millimetres of mercury; no., number; PaCO2, arterial pressure of carbon dioxide; PaO2, arterial pressure of oxygen.
Specific weighting, for all (−3 overall).
Study 1, 7 years’ follow-up in Gorecka et al (24).
Study 2, 1-year follow-up in Haidl et al (25).
Randomization not maintained. (−1 and with the remaining items below contributing equal amounts)
Time point of lung function measurement not described in detail.
Oxygen use was inadequate according to study protocol.
Not blinded.
Sparse data. (−1 and with the remaining items below contributing equal amounts)
It is unknown if individuals in the control arm began using oxygen therapy.
Measurement of spirometry was not described in detail. (did not contribute to GRADE)
Allocation concealment was not addressed.
Measurement of exercise data was not described in detail. (did not contribute to GRADE)
Table A5: GRADE Evidence Table for Long-Term Oxygen Therapy and Lung Function in Nonsurvivors, Mild-to-Moderate Hypoxemia*.
| Outcomes | Illustrative Comparative Risks (95% CI) | Quality of Evidence (GRADE)† |
|---|---|---|
| Corresponding Risk: Intervention Groups | ||
| Mean FVC % | 0.3 lower (5.84 lower to 5.24 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
| Mean PaCO2 (mm Hg) | 0.4 lower (3.54 lower to 2.74 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
| Mean FEV1 (L) | 0.05 higher (0.08 lower to 0.18 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
| Mean FEV1 % Predicted | 1.7 higher (2.8 lower to 6.2 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
| Mean FEV1/FVC | 1.7 lower (7.69 lower to 4.29 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
| Mean PaO2 (mm Hg) | 0.1 higher (1.17 lower to 1.37 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
| Mean FVC (L) | 0.05 higher (5.49 lower to 5.59 higher) | ⊕ Very low‡,§,║,¶,#,**,†† |
Abbreviations: CI, confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; mm Hg, millimetres of mercury; PaCO2, arterial pressure of carbon dioxide; PaO2, arterial pressure of oxygen.
Gorecka et al (24); 70 participants; specific weighting, for all (−3 overall).
Randomization not maintained. (−1)
Time point of lung function measurement not described in detail. (−1/4)
Oxygen use was inadequate according to study protocol. (−1/4)
It is unknown if individuals in the control arm began using oxygen therapy. (−1/4)
Measurement of spirometry not described in detail. (did not contribute to GRADE)
Not blinded. (−1/4)
Sparse data. (−1)
Table A6: GRADE Evidence Table for Long-Term Oxygen Therapy and Hospital Readmissions and Hospitalizations, Severe Hypoxemia*†,‡.
| LTOT Versus No LTOT: Hospital Readmission and Hospitalization for COPD | |||
|---|---|---|---|
| Outcomes | Relative Effect (95% CI) | No. of Participants (studies) |
Quality of Evidence (GRADE)§ |
| Hospital Readmission | HR 1.26 (0.87–1.84) | 312 (1 study) | ⊕ ⊕ Low ║,# |
| Hospitalization | RR 0 (0−0) | 64 (1 study) | ⊕ Very low ║,¶,#,** |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LTOT, long-term oxygen therapy; no., number; RR, relative risk.
No meta-analysis.
Specific weighting −1 overall; already low quality of evidence for observational studies.
Unmeasured confounders for non-RCT studies. (did not contribute to GRADE)
Sparse data. (did not contribute to GRADE, already very low quality of evidence)
Heterogeneity in the comparison. (did not contribute to GRADE)
Unadjusted analysis only. (−1)
Table A7: GRADE Evidence Table for Long-Term Oxygen Therapy and Health-Related Quality of Life, Severe Hypoxemia*.
| LTOT Only: Health-Related Quality of Life | |||||
|---|---|---|---|---|---|
| Population: Patients With COPD | |||||
| Outcomes | Illustrative Comparative Risks (95% CI) |
Relative Effect (95% CI) |
No. of Participants (studies) |
Quality of Evidence (GRADE) |
Comments |
| Assumed Risk Corresponding Risk | |||||
| CRQ† | Meta-analysis not performed | Not estimable | 157 (2 studies) | ⊕ ⊕ Low§,║,¶ | LTOT only Observational |
| SGRQ‡ | Meta-analysis not performed | Not estimable | 19 (1 study) | ⊕ Very low# | LTOT only Observational |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; LTOT, long-term oxygen therapy; no., number; SGRQ, St. George’s Respiratory Questionnaire.
Okubadejo et al (47).
Ambulatory oxygen provided in 1 study. (did not contribute to GRADE)
Confounders not accounted for. (did not contribute to GRADE)
CRQ was characterized differently for each study. (did not contribute to GRADE)
Sparse data. (−1)
Appendix 3: Summary Tables
The following studies were included in the Cochrane Review (23) and are relevant for this evidence-based analysis. The objective of the Cochrane Review was to determine the effect of domiciliary oxygen therapy on survival and quality of life in patients with COPD.
Table A8: Summary of Study Design Characteristics*.
| Author | Study Design: Comparison |
Study Population | Intervention | Main Results (Original Study) | Comments |
|---|---|---|---|---|---|
| MRC (10) | MC RCT: LTOT vs. no LTOT | 87 patients with CB/E, CS FEV1 < 1.2 L, PaO2 40–60 mm Hg ≥ 1 episode of heart failure + ankle edema FU: up to 5 years |
LTOT (n = 42) vs. no LTOT (n = 45) FR 2 L/minute ≥ 15 hours/day including sleeping hours |
Mean age ~58 years, mean PaO2 ~50 mm Hg, FEV1 ~0.65 L LTOT 19/42 (45.2%) vs. CT 30/45 (66.7%), P = ?, Men (n = 66) > 500 days, risk of death: LTOT 12% vs. CT 29% (P = 0.04) Women (n = 21), LTOT 5.7% vs. CT 36.5% (P < 0.05) |
Severe hypoxemia, not blinded, differences in O2 source, usage check not standardized (adherence), hours? 1 DO (O2) |
| Gorecka et al (24) | MC RCT: LTOT vs. no LTOT | 135 patients with COPD FEV1/VC < 70%, PaO2 56–65 mm Hg FU ≥ 3 years or death, NS |
LTOT + UC (n = 68) vs. no LTOT + UC (n = 67) FR adjusted to > 65 mm Hg at rest ≥ 17 hours/day |
Mean age ~60 years, mean PaO2 60.4 mm Hg, mean O2 use 13.5 hours/day, FEV1 29.8%, 0 DO LTOT 38/68 (55.9%) vs. CT 32/67 (47.8%), no difference in survival (P = 0.982) |
Moderate hypoxemia, not blinded, UC included drug therapies, concentrator, adherence check, crossover? |
| Haidl et al (25) | RCT: LTOT vs. no LTOT | 28 patients with COPD + PaCO2 > 45 mm Hg at rest or exercise test, CS FEV1/VC < 70%, PaO2 > 55 mm Hg FU 3 years |
LTOT (n = 14) vs. no LTOT (n = 14) FR 2 L/minute, 15 hours/day |
Mean age ~64 years, mean PaO2 ~66 mm Hg, mean O2 use 10.4 hours/day, FEV1 ~40%, ↑ endurance time at 1 year (P = 0.04), ↓ dyspnea score at 1 year (P = 0.03), no difference in deaths after 3 years LTOT 4/14 (28.6%) vs. CT 3/14 (21.4%), P = ? | Mild hypoxemia, not blinded, concentrator, adherence check by O2 meter, 5 CTs given LTOT |
Abbreviations: CB/E, chronic bronchitis and emphysema; CO2, continuous oxygen therapy; COPD, chronic obstructive pulmonary disease; CS, current smokers; CT, control group; DO, dropouts; FEV1, forced expiratory volume in 1 second; FR, flow rate; FS, former smokers; FU, follow-up; L, litres; LTOT, long-term oxygen therapy; MC, multicentre; mm Hg, millimetres of mercury; MRC, Medical Research Council; NS, not significant; O2, oxygen; PaO2, arterial pressure of oxygen; RCT, randomized controlled trial; UC, usual care.
Table A9: Summary of Relevant Outcomes*.
| Comparison | Studies Included | Outcome† | Results (Pooled Analysis)‡ | Comments | ||
|---|---|---|---|---|---|---|
| LTOT vs. No LTOT: Severe Hypoxemia | MRC (10) | Mortality | 60 months | OR: 0.42 (0.18, 0.98) | + | |
| FEV1 change | ≤ 500 days | MD: −0.11 (−0.27, 0.05) | NS | |||
| > 500 days | MD: 0.08 (0.04, 0.12) | + | ||||
| FVC change | ≤ 500 days | MD: 0.56 (0.12, 1.00) | + | |||
| > 500 days | MD: 0.05 (−0.89, 0.99) | NS | ||||
| PaO2 change | ≤ 500 days | MD: 4.56 (−1.04, 10.16) | NS | |||
| > 500 days | MD: 1.07 (−1.24, 3.38) | NS | ||||
| PaO2 change | ≤ 500 days | MD: −0.96 (−8.53, 6.61) | NS | |||
| > 500 days | MD: −2.16 (−4.04, −0.28) | + | ||||
| LTOT vs. No LTOT: Mild-to-moderate Hypoxemia | Gorecka et al (24) Haidl et al (25) |
Mortality | OR: 1.39 (0.74, 2.59) | NS | ||
| LTOT vs. No LTOT: Mild-to-moderate Hypoxemia | Haidl et al (25) | End-exercise dyspnea score | MD: −1.20 (−2.47, 0.07) | NS | ||
| Endurance time | MD: 2.20 (−0.73, 5.13) | NS | ||||
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LTOT, long-term oxygen therapy; MD, mean difference; MRC, Medical Research Council; NS, not significant; OR, Peto odds ratio; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide.
500 days, for men dying between 180 and 500 days; > 500 days, for men surviving more than 500 days. Excluded were results for changes in packed cell volume (≤ 500 and > 500 days), and mortality for oxygen use of >15 hours per day and <15 hours per day. Units for FEV1 and FVC are litres, and units for PaO2 and PaCO2 are mm Hg. Borg scale was used for dyspnea and minutes for endurance time.
OR < 1 favours LTOT (+). A positive MD value favours LTOT (+). The reverse is true for PaCO2 in which a negative MD favours LTOT (+).
Source: Cranston et al, 2005 (23)
Table A10: Study Design Strengths and Limitations by Severity of Hypoxemia for Relevant Studies From the Cochrane Review*.
| Study | COPD Study Population | Adequate Sample Size | Exclusions Detailed | Randomization Achieved | Blinding | Adequately Measured Adherence | All-cause Mortality | Survival Analysis | Intent-to- Treat Analysis† | Minimal Attrition |
|---|---|---|---|---|---|---|---|---|---|---|
| Severe Hypoxemia | ||||||||||
| MRC (10), ‡,§, ║ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Mild-to-moderate Hypoxemia | ||||||||||
| Gorecka et al (24), ‡ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Haidl et al (25), ‡ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
Abbreviations: COPD, chronic obstructive pulmonary disease; MRC, Medical Research Council; checkmark (•) refers to the presence of study design strengths.
Considering mortality/survival as the main comparison of interest.
Allocation concealment was adequate for none of the above studies and the process of generating randomized schedules was adequate for MRC (10) and Gorecka et al (24).
Survival analysis was not shown for the primary comparison of interest.
A larger proportion of patients in the treatment group smoked; however a post hoc analysis showed that this difference was not statistically significant (P > 0.05).
Source: Cranston et al, 2005 (23)
Table A11: Summary of Key Study Characteristics*.
| Study | Baseline FEV1 % Predicted (L) (Mean)† | Baseline PaO2 (mm Hg)(Mean)‡ | Baseline PaCO2 (mm Hg) (Mean)§ | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total║ | LTOT | Control | Total║ | LTOT | Control | Total║ | LTOT | Control | Mean (SD) or Range (Years) | |
| Severe Hypoxemia | ||||||||||
| MRC (10) | 0.66 | 0.67 | 0.64 | 50.8 | 49.9 | 51.7 | 54.4 | 54.9 | 53.9 | 0−5 |
| Mild-to-moderate Hypoxemia | ||||||||||
| Gorecka et al (24) | 29.8 | 29.7 | 29.8 | 60.4 | 59.5 | 61.3 | 44.1 | 45.3 | 42.8 | 0.2−7 |
| Haidl et al (25) | 40.8 | 38.8 | 42.7 | 66.5 | 65.6 | 67.3 | 40.8 | 41.9 | 39.7 | 1−3 |
| Garcia-Aymerich et al (35), ¶ | 36 | - | - | 64 | ? | - | - | - | - | 1.1 (0.5) |
| Kessler et al (36), ¶ | 39 | - | - | 66 | <60 | - | 46 | - | - | 0−1 |
Abbreviations: FEV1, forced expiratory volume in 1 second; LTOT, long-term oxygen therapy treatment group; mm Hg, millimetres of mercury; O2, oxygen; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide, SD, standard deviation.
COPD Stage: mild, FEV1 > 80% predicted; moderate, FEV1 > 50% and < 80% predicted; severe, FEV1 > 30% and < 50% predicted; very severe, FEV1 < 30%. Severe COPD defined as FEV1 < 1.5 litres.
Hypoxemia: severe, < 50 mm Hg; mild-to-moderate, ~ 50-65 mm Hg.
Hypercapnia: > 45-60 mm Hg.
Total: study population as a whole including treatment and control groups, either taken from the original paper or calculated as the mean from the 2 arms of the trial.
Heterogeneity in the comparison: COPD patients using O2 have severe hypoxemia and COPD patients not using O2 (e.g., controls) have mild-to-moderate hypoxemia.
Table A12: Summary of Key Study Characteristics for the Three Studies on Health-related Quality of Life*.
| Author | Study Design |
Study Population | Intervention | Main Results† | Comments |
|---|---|---|---|---|---|
| Eaton et al (44) | Prospective follow-up: baseline to 2 and 6 months | 68 patients with COPD, NS LTOT need assessed by standard criteria: severe hypoxemia or mild-to-moderate hypoxemia with a COPD-related condition Clinically stable ≥ 2 months |
LTOT vs. no LTOT (43/25) |
Baseline: significant differences‡ in PaO2 (LTOT 51.8 vs. no LTOT 66 mm Hg), PaCO2 (LTOT 48.8 vs. no LTOT 42.8 mm Hg), and CRQ (fatigue, emotional function, mastery, total); FEV1 % predicted (LTOT: 31.7; no LTOT, 29.6) Mean O2 use 14.6 hours At 2 months CRQ total: LTOT 8.10 (3.02–13.17); no LTOT −0.28 (−5.98 to 5.42), significant increases for domains in LTOT At 6 months, CRQ total: LTOT 9.26 (2.37–16.15); no LTOT −2.56 (−8.31 to 3.19), significant increases for domains in LTOT |
>50% men Age ~70 years CRQ analyzed as total scores Other factors affecting HRQOL? Ambulatory O2 not provided |
| Crockett et al (45) | Prospective follow-up: baseline to 3, 6, and 12 months | 114 COPD patients LTOT need determined by standard criteria |
LTOT only (M 59/W 55) |
Baseline, PaO2 ~53.7 mm Hg, PaCO2 49.2 mm Hg, FEV1 0.5 L (men and women combined); mean O2 use ~19 hours Men: CRQ at all time points, emotional function and mastery: nonsignificant increase; fatigue: significant increase > 0.5 Women: CRQ mastery, significant increase > 0.5 at all time points; emotional function and fatigue: significant increase > 0.5 at 3 and 6 months |
~52% men Age ~70 years CRQ analysis mean score, 7-point scale Other factors affecting HRQOL? Ambulatory O2 provided MCID 0.5 units |
| Okubadejo et al (47) | Prospective follow-up: baseline to 2 weeks, and 3 and 6 months | 36 patients with COPD LTOT need assessed by standard criteria: severe hypoxemia or mild-to-moderate hypoxemia with a COPD-related condition Clinically stable ≥ 3 weeks |
LTOT vs. no LTOT (19/17) |
Baseline: LTOT, PaO2 ~52.5 mm Hg, PaCO2 50.3 mm Hg, FEV1 40% predicted; mean O2 use ~17 hours; no LTOT, PaO2 ~62.3 mm Hg, PaCO2 ~45 mm Hg, FEV1 43% predicted Significant differences between LTOT and no LTOT for SGRQ total at baseline (61.8 vs. 45.8, P = 0.008), no significant differences in improvement from baseline at 2 weeks and 3 and 6 months |
% by sex unknown Age ~71 years Other factors affecting HRQOL Ambulatory O2 not provided MCID 4 units |
Abbreviations: CRQ, Chronic Respiratory Questionnaire; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; HRQOL, health-related quality of life; LTOT, long-term oxygen therapy; M, men; MCID, minimal clinically important difference; mm Hg, millimetres of mercury; NS, nonsmokers; O2, oxygen; PaCO2, arterial pressure of carbon dioxide; PaO2, arterial pressure of oxygen; SGRQ, St. George’s Respiratory Questionnaire; W, women.
Data are reported as means and 95% confidence intervals, or mean alone.
Significant at < 0.05.
Suggested Citation
This report should be cited as follows:
COPD Working Group. Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2012 Mar;12(7):1–64. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/rev_LTOT_March.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in Excerpta Medica/EMBASE and the Center for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: MASinfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All analyses in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: MASinfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-7013-8 (PDF)
© Queen’s Printer for Ontario, 2012
About the Medical Advisory Secretariat
Effective April 5, 2011, the Medical Advisory Secretariat (MAS) became a part of Health Quality Ontario (HQO), an independent body funded by the Ministry of Health and Long-Term Care. The mandate of MAS is to provide evidence-based recommendations on the coordinated uptake of health services and health technologies in Ontario to the Ministry of Health and Long-Term Care and to the health care system. This mandate helps to ensure that residents of Ontario have access to the best available and most appropriate health services and technologies to improve patient outcomes.
To fulfill its mandate, MAS conducts systematic reviews of evidence and consults with experts in the health care services community. The resulting evidence-based analyses are reviewed by the Ontario Health Technology Advisory Committee—to which MAS also provides a secretariat function—and published in the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, MAS systematically reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, the Secretariat collects and analyzes information about how a new technology fits within current practice and existing treatment alternatives. Details about the technology’s diffusion into current health care practices add an important dimension to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist decision-makers in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals wishing to comment on an analysis prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by MAS for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data and information provided by experts and applicants to MAS to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the MAS website for a list of all evidence-based analyses: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
List of Tables
| Table 1: Body of Evidence Examined According to Study Design* |
| Table 2: Comparison of Health-Related Quality of Life Between LTOT and No-LTOT Groups (St. George’s Respiratory Questionnaire)*,† |
| Table 3: Change in Health-Related Quality of Life Results From Baseline (St. George’s Respiratory Questionnaire)*,† |
| Table 4: International Guidelines for Use of Long-term Oxygen Therapy in Patients with Stable Chronic Obstructive Pulmonary Disease* |
| Table A1: GRADE Evidence Table for Long-Term Oxygen Therapy and Mortality in Patients With Severe Hypoxemia* |
| Table A2: GRADE Evidence Table for Long-Term Oxygen Therapy and Mortality in Patients With Mild-to-Moderate Hypoxemia* |
| Table A3: GRADE Evidence Table for Long-Term Oxygen Therapy and Lung Function in Patients With Severe Hypoxemia (Survivors and Nonsurvivors)*,† |
| Table A4: GRADE Evidence Table for Long-Term Oxygen Therapy and Lung Function in Survivors, Mild-to-Moderate Hypoxemia* |
| Table A5: GRADE Evidence Table for Long-Term Oxygen Therapy and Lung Function in Nonsurvivors, Mild-to-Moderate Hypoxemia* |
| Table A6: GRADE Evidence Table for Long-Term Oxygen Therapy and Hospital Readmissions and Hospitalizations, Severe Hypoxemia*,†,‡ |
| Table A7: GRADE Evidence Table for Long-Term Oxygen Therapy and Health-Related Quality of Life, Severe Hypoxemia* |
| Table A8: Summary of Study Design Characteristics* |
| Table A9: Summary of Relevant Outcomes* |
| Table A10: Study Design Strengths and Limitations by Severity of Hypoxemia for Relevant Studies From the Cochrane Review* |
| Table A11: Summary of Key Study Characteristics* |
| Table A12: Summary of Key Study Characteristics for the Three Studies on Health-related Quality of Life* |
List of Figures
| Figure 1: Citation Flow Chart* |
| Figure 2: Mortality (Number of Events)* |
| Figure 3: Forced Expiratory Volume in One Second (Litres)* |
| Figure 4: Forced Expiratory Volume in One Second (% Predicted)* |
| Figure 5: Forced Expiratory Volume in One Second by Forced Vital Capacity (%)* |
| Figure 6: Forced Vital Capacity (Litres)* |
| Figure 7: Forced Vital Capacity (% Predicted)* |
| Figure 8: Arterial Pressure of Oxygen (mm Hg)*,† |
| Figure 9: Arterial Pressure of Carbon Dioxide (mm Hg)* |
| Figure 10: Dyspnea (Borg Scale)*,† |
| Figure 11: Endurance Time (Minutes)*,† |
List of Abbreviations
- CI
Confidence interval(s)
- CO2
Carbon dioxide
- CRQ
Chronic Respiratory Questionnaire
- CT
Control group
- FVC
Forced vital capacity (also referred to as vital capacity in this report)
- FEV1
Forced expiratory volume in 1 second
- HRQOL
Health-related quality of life
- kPa
Kilopascal (1 kPa = 7.5 mm Hg)
- LTOT
Long-term oxygen therapy
- MAS
Medical Advisory Secretariat
- MCID
Minimal clinically important difference
- MD
Mean difference
- mm Hg
Millimetre of mercury
- O2
Oxygen
- OR
Odds ratio
- PaCO2
Arterial partial pressure of carbon dioxide
- PaO2
Arterial partial pressure of oxygen
- RCT
Randomized controlled trial
- RR
Relative risk
- SpO2
Oxygen saturation level of arterial blood measured by pulse oximetry
- SD
Standard deviation
- SGRQ
St. George’s Respiratory Questionnaire
- VC
Vital capacity (used interchangeably with forced vital capacity in this report)
Footnotes
The mild-to-moderate classification was created for the purposes of the report.
The mild-to-moderate classification was created for the purposes of the report.
The mild-to-moderate classification was created for the purposes of the report.
References
- 1.Public Health Agency of Canada. Chronic Obstructive Pulmonary Disease (COPD) [Internet] [[updated 2008 Oct 7; cited 2010 Oct 27]]; Available from: http://www.phac-aspc.gc.ca/cd-mc/crd-mrc/copd-mpoc-eng.php . [Google Scholar]
- 2.Corrado A, Renda T, Bertini S. Long-term oxygen therapy in COPD: evidences and open questions of current indications. Monaldi Arch Chest Dis. 2010;73(1):34–43. doi: 10.4081/monaldi.2010.311. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Pulmonary Disease. [[cited: 2010 Oct 27]]; Global Initiative for Chronic Obstructive Pulmonary Disease [Internet]. Unknown: Medical Communications Resources Inc. 2009 Jan 1. Available from: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=2003 .
- 4.McGloin S. Administration of oxygen therapy. Nurs Stand. 2008;22(21):46–8. doi: 10.7748/ns2008.01.22.21.46.c6416. [DOI] [PubMed] [Google Scholar]
- 5.Henderson Y. Delivering oxygen therapy to acutely breathless adults. Nurs Stand. 2008;22(35):46–8. doi: 10.7748/ns2008.05.22.35.46.c6535. [DOI] [PubMed] [Google Scholar]
- 6.Lynes D, Kelly C. Domiciliary oxygen therapy: assessment and management. Nurs Stand. 2009;23(20):50–6. doi: 10.7748/ns2009.01.23.20.50.c6747. [DOI] [PubMed] [Google Scholar]
- 7.Dunne PJ. The clinical impact of new long-term oxygen therapy technology. Respir Care. 2009;54(8):1100–11. [PubMed] [Google Scholar]
- 8.Uronis H, McCrory DC, Samsa G, Currow D, Abernethy A. Palliative oxygen for non-hypoxaemic chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 2. 2007 doi: 10.1002/14651858.CD006429.pub2. Art. No.: CDD006429. DOI: 10.1002/14651858.CD006429. [DOI] [PubMed] [Google Scholar]
- 9.Osthoff M, Leuppi JD. Management of chronic obstructive pulmonary disease patients after hospitalization for acute exacerbation. Respiration. 2010;79(3):255–61. doi: 10.1159/000235721. [DOI] [PubMed] [Google Scholar]
- 10.Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–6. [PubMed] [Google Scholar]
- 11.Stark RD, Finnegan P, Bishop JM. Long-term domiciliary oxygen in chronic bronchitis with pulmonary hypertension. Br Med J. 1973;3(5878):467–70. doi: 10.1136/bmj.3.5878.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391–8. doi: 10.7326/0003-4819-93-3-391. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. [DOI] [PubMed] [Google Scholar]
- 13.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: Current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179–87. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease - 2008 update - Highlights for primary care. Can Respir J. 2008;15(SUPPL. A):1A–8A. doi: 10.1155/2008/641965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health and Long-Term Care. Home Oxygen Program - Physician [Internet] [[cited: 2010 Oct 28].];[Toronto, ON]: Government of Ontario. 2004 Jul 1; Available from: http://www.health.gov.on.ca/english/public/pub/adp/pdf/oxyphys.pdf .
- 16.Ministry of Health and Long-Term Care. Home Oxygen Program Policy and Administration Manual [Internet] [[cited: 2011 Nov 7]];[Toronto, ON] Government of Ontario. 2011 May 1; Available from: http://www.health.gov.on.ca/en/pro/programs/adp/information_technology/docs/home_oxygen_m_anual.pdf . [Google Scholar]
- 17.Canadian Lung Association. Fact sheet: What you need to know about oxygen [Internet] [[cited: 2010 Oct 28]];Ottawa: Canadian Lung Association. 2005 Aug 1; Available from: http://www.lung.ca/_resources/Oxygen_COPD_LungAssoc.pdf . [Google Scholar]
- 18.Make B, Krachman S, Panos RJ, Doherty DE, Stoller JK. Oxygen therapy in advanced COPD: In whom does it work? Semin Respir Crit Care Med. 2010;31(3):334–42. doi: 10.1055/s-0030-1254073. [DOI] [PubMed] [Google Scholar]
- 19.Brinkerhoff S. Oxygen therapy in the home: safety precautions and implications for home healthcare clinicians. Home Healthc Nurse. 2009;27(7):417–20. doi: 10.1097/01.NHH.0000358274.54176.50. [DOI] [PubMed] [Google Scholar]
- 20.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman C. Literature searching and evidence interpretation for assessing health care practices. SBU Report No. 119E. 1996 doi: 10.1017/s0266462300008321. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care. [DOI] [PubMed] [Google Scholar]
- 22.United States National Institutes of Health. Effectiveness of Long-term Oxygen Therapy in Treating People With Chronic Obstructive Pulmonary Disease (The Long-term Oxygen Treatment Trial [LOTT]) [Internet] [[updated 2010 Sep 14; cited 2010 Oct 28]]; Available from: http://clinicaltrials.gov/ct2/show/NCT00692198?term=oxygen+therapy&rank=6 . [Google Scholar]
- 23.Cranston JM, Crockett A, Moss J, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.: CD001744. DOI: 10.1002/14651858.CD001744.pub2. doi: 10.1002/14651858.CD001744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorecka D, Gorzelak K, Sliwinski P, Tobiasz M, Zielinski J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52(8):674–9. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidl P, Clement C, Wiese C, Dellweg D, Kohler D. Long-term oxygen therapy stops the natural decline of endurance in COPD patients with reversible hypercapnia. Respiration. 2004;71(4):342–7. doi: 10.1159/000079637. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher EC, Luckett RA, Goodnight-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis. 1992;145(5):1070–6. doi: 10.1164/ajrccm/145.5.1070. [DOI] [PubMed] [Google Scholar]
- 27.Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Enrhart M, Schott R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14(5):1002–8. doi: 10.1183/09031936.99.14510029. [DOI] [PubMed] [Google Scholar]
- 28.Wilt TJ, Niewoehner D, MacDonald R, Kane RL. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147(9):639–53. doi: 10.7326/0003-4819-147-9-200711060-00009. [DOI] [PubMed] [Google Scholar]
- 29.Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290(17):2301–12. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 30.Eaton T, Fergusson W, Kolbe J, Lewis CA, West T. Short-burst oxygen therapy for COPD patients: a 6-month randomised, controlled study. Eur Respir J. 2006;27(4):697–704. doi: 10.1183/09031936.06.00098805. [DOI] [PubMed] [Google Scholar]
- 31.Eaton T, Garrett JE, Young P, Fergusson W, Kolbe J, Rudkin S, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J. 2002;20(2):306–12. doi: 10.1183/09031936.02.00301002. [DOI] [PubMed] [Google Scholar]
- 32.McDonald CF, Blyth CM, Lazarus MD, Marschner I, Barter CE. Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemia. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1616–9. doi: 10.1164/ajrccm.152.5.7582304. [DOI] [PubMed] [Google Scholar]
- 33.Lacasse Y, Lecours R, Pelletier C, Begin R, Maltais F. Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur Respir J. 2005;25(6):1032–8. doi: 10.1183/09031936.05.00113504. [DOI] [PubMed] [Google Scholar]
- 34.Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation - Systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2(3):241–51. [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–5. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–64. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 37.Goksu E, Oktay C, Kartal M, Oskay A, Sayrac AV. Factors affecting revisit of COPD exacerbated patients presenting to emergency department. Eur J Emerg Med. 2010;17(5):283–5. doi: 10.1097/MEJ.0b013e3283314795. [DOI] [PubMed] [Google Scholar]
- 38.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007;30(5):993–1013. doi: 10.1183/09031936.00082507. [DOI] [PubMed] [Google Scholar]
- 39.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of comorbidities. Eur Respir J. 2006;28(6):1245–57. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 40.Ekstrom M, Franklin KA, Strom KE. Increased relative mortality in women with severe oxygen-dependent COPD. Chest. 2010;137(1):31–6. doi: 10.1378/chest.09-0636. [DOI] [PubMed] [Google Scholar]
- 41.Gross NJ. Chronic obstructive pulmonary disease outcome measurements: what’s important? What’s useful? Proc Am Thorac Soc. 2005;2(4):267–71. doi: 10.1513/pats.200504-036SR. [DOI] [PubMed] [Google Scholar]
- 42.Wedzicha JA. Effects of long-term oxygen therapy on neuropsychiatric function and quality of life. Respir Care. 2000;45(1):119–24. [PubMed] [Google Scholar]
- 43.Agence d’evaluation des technologies et des modes d’intervention en sante (AETMIS) (Report prepared by Susan Law and Pascale Lehoux). Hospital Technology at Home: Portable Oxygen Therapy in COPD [Internet] [[cited: 2011 Jan 19]];Montreal: AETMIS. AETMIS 04-03. 2004 Jul 1; Available from: http://www.aetmis.gouv.qc.ca/site/download.php?f=2c3728e0d8adac6eb66642754ecbdb9b . [Google Scholar]
- 44.Eaton T, Lewis C, Young P, Kennedy Y, Garrett JE, Kolbe J. Long-term oxygen therapy improves health-related quality of life. Respir Med. 2004;98(4):285–93. doi: 10.1016/j.rmed.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Crockett AJ, Cranston JM, Moss JR, Alpers JH. Effects of long-term oxygen therapy on quality of life and survival in chronic airflow limitation. Monaldi Arch Chest Dis. 1999;54(2):193–6. [PubMed] [Google Scholar]
- 46.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 47.Okubadejo AA, Paul EA, Jones PW, Wedzicha JA. Does long-term oxygen therapy affect quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia? Eur Respir J. 1996;9(11):2335–9. doi: 10.1183/09031936.96.09112335. [DOI] [PubMed] [Google Scholar]
- 48.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880–7. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 50.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 51.The National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald CF, Crockett AJ, Young IH. Adult domiciliary oxygen therapy. Position statement of the Thoracic Society of Australia and New Zealand. Med J Aust. 2005;182(12):621–6. doi: 10.5694/j.1326-5377.2005.tb06848.x. [DOI] [PubMed] [Google Scholar]


